Green Extraction of Bioactive Compounds from Plant-Based Agri-Food Residues: Advances Toward Sustainable Valorization
Abstract
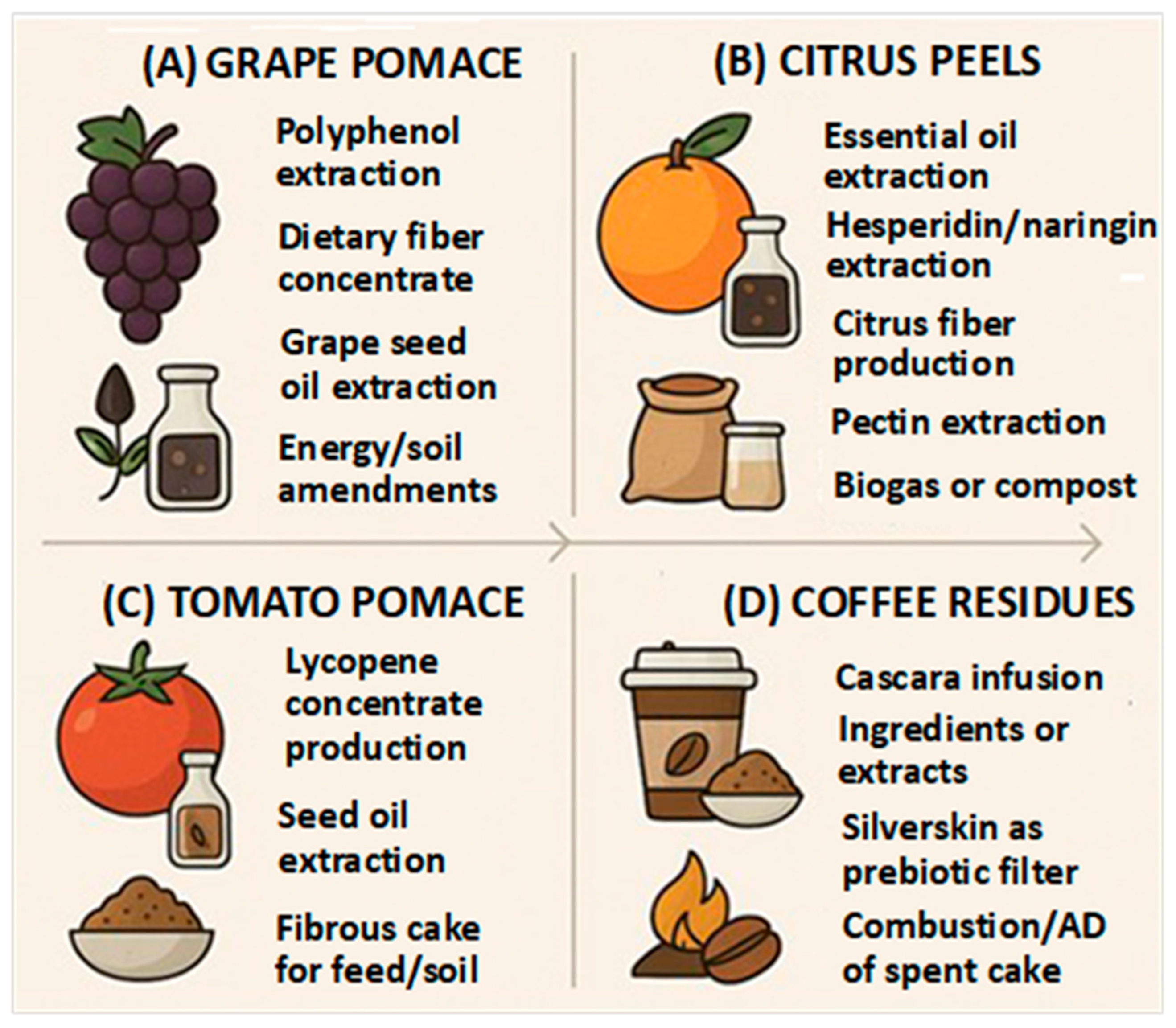
1. Introduction
2. Methodology
3. Bioactive Compounds
4. Green Extraction Technologies
4.1. Ultrasound-Assisted Extraction (UAE)
4.2. Microwave-Assisted Extraction (MAE)
4.3. Pressurized Liquid Extraction (PLE)
4.4. Supercritical CO2 Extraction (SFE)
4.5. Natural Deep Eutectic Solvents (NADES)
4.6. Enzyme-Assisted Extraction (EAE)
5. In Vitro Bioactivity Evidence for Agri-Food Residues
6. In Vivo Bioactivity Evidence for Agri-Food Residues
7. Critical Appraisal and Translational Considerations
| Chemical Class/Identified Bioactives | Residue (Origin) and Circularity/LCA Notes/Green Extraction | Hypothetical Human Dose Calculated According to a Translation Formula Based on Surface Area [77] | In Vivo Model and Outcomes/Intended Application | Ref. |
|---|---|---|---|---|
| Polyphenol mixture (anthocyanins, flavanols, phenolic acids. Catechins, quercetin, gallic/caffeic acids, procyanidins | 400 mg/kg body weight grape pomace (wine coproduct)—upcycled ingredient; valorizes winery waste; potential greenhouse gas reduction. Hydroethanolic extraction; spray-dry | 32 mg/kg; human dose for a 70 kg individual = 2.24 g | High-fat diet mice: reduction of body-weight gain; increase short-chain fatty acids; improved microbiota composition. Weight-management functional ingredient | [68] |
| Ellagitannins and derivatives. Punicalagin, ellagic acid → urolithins | 150 mg/kg body weight pomegranate peel (juice waste)—cardiometabolic protection. High-phenolic density from peel; biorefinery node. Hydroethanolic extraction; purification | 24 mg/kg; human dose for a 70 kg individual = 1.68 g | Diabetic rats: reduction NLRP3/caspase-1/IL-1β; improved lipid profile; histological protection. Cardiometabolic nutraceutical | [69] |
| Phenolics and fiber (coffee by-product). Chlorogenic acids (minor), fiber-bound oligosaccharides | 1 g/kg body weight coffee silverskin (roasting by-product) prebiotic. Avoid landfilling/incineration of silverskin. Green UAE; low-energy drying | 162 mg/kg; human dose for a 70 kg individual = 11.34 g | Rats: ↑ SCFAs; improved metabolic readouts; microbiota shifts. Prebiotic ingredient | [59] |
| Polyphenol mixture (apple). Chlorogenic acid, phloridzin, quercetin glycosides | 100 mg/kg body weight apple pomace (juice/cider residue) neuroprotective candidate. Pomace biorefinery (polyphenols + pectin). Aqueous ethanol extraction; stabilization | 8.1 mg/kg; human dose for a 70 kg individual = 0.57 g | Mice: reversal of MK-801-induced memory impairment; hippocampal gene modulation. Cognitive-health dietary ingredient | [74] |
| Soluble fibers (pectin). High-methoxyl pectin; minor phenolics | 10% diet banana peels (fruit processing waste) anti-obesity fiber. Supports zero-waste in banana chain. Hot-water extraction; ethanol precipitation | - | Obese hypercholesterolemic mice: improved adiposity and lipid profile. Fiber supplement; fat-reduction aid | [29] |
| Xanthones and phenolics. Mangiferin; quercetin derivatives | 5 g/kg body weight mango peel (juice/drying waste)—standardized extract. Revenue from peels complements fruit value chain. Hydroethanolic extraction; standardization | 810.8 mg/kg; human dose for a 70 kg individual = 56.8 g | Prediabetic rats: improved glycemia and lipids; enzyme inhibition (α-amylase/α-glucosidase)/Metabolic-health nutraceutical | [31] |
| Proantho-cyanidins (A-type). Procyanidin A1 | 300 mg/kg body weight peanut skins (blanching waste)—polyphenol extract. Requires allergen controls in scale-up. Ethanolic extraction; enrichment | 24 mg/kg; human dose for a 70 kg individual = 1.68 g | Type 2 diabetes mice: improved gut barrier (tight junctions); anti-inflammatory effects/Gut-barrier/anti-inflammatory nutraceutical | [79] |
| Mixed phenolics. Anacardic acids, carotenoids, phenolic acids | 500 mg/kg body weight cashew apple bagasse (juice residue)/standardized extract. Valorizes bagasse in cashew processing. Hydroethanolic extraction; spray-dry | 40.5 mg/kg; human dose for a 70 kg individual = 2.83 g | DSS-colitis in mice: improved disease activity. immunomodulatory protection. Anti-inflammatory (preclinical inflammatory bowel disease) | [73] |
8. Implications of Valorizing Agri-Food Residues for Bioactive Recovery or Human Nutrition and Health
9. Circularity, Applications, and Life Cycle Assessment (LCA) Perspectives
10. Market Translation, Economic Drivers, and Policy Frameworks for Residue-Derived Bioactives
10.1. Introductory Considerations
10.2. Regulatory Footholds and Global Alignment
10.3. Eco-Labels, Upcycled Certification, and Consumer Perception
10.4. Public Procurement, Innovation Funding, and Bioeconomy Clusters
11. Research Gaps and Future Directions
11.1. Introdutory Considerations
11.2. Need for Standardized Human Trials
11.3. Harmonized LCA Methodologies for Bioactive Extraction
11.4. Industrial Scalability of Green Extraction Systems
11.5. Long-Term Safety, Bioavailability, and Microbiome Interactions
12. Commercial Deployment and Patent Landscape
12.1. General Aspects
12.2. Olive By-Products → Hydroxytyrosol (HT)
12.3. Brewer’s Spent Grain (BSG) → Proteins and Fibers
12.4. Citrus Peels → Citrus Fiber and Flavanones
12.5. Coffee Residues → Cascara and Silverskin
12.6. Tomato Pomace/Peels → Lycopene Concentrates
12.7. Grape Seeds/Pomace → Polyphenol Concentrates
12.8. Patent to Market Landscape
13. New Perspectives in Research on Residue Valorization for Bioactive Recovery
14. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANVISA | Brazil’s National Health Surveillance Agency |
| BSG | Brewers’ spent grain |
| EAE | Enzyme-assisted extraction |
| EFSA | European Food Safety Authority |
| EU | European Union |
| FDA | U.S. Food and Drug Administration |
| GRAS | Generally Recognized as Safe |
| HT | Hydroxytyrosol |
| LCA | Life cycle assessment |
| MAE | Microwave-assisted extraction |
| NADES | Natural deep eutectic solvents |
| PLE | Pressurized liquid extraction |
| RCT | Randomized controlled trial |
| SCFAs | Short-chain fatty acids |
| SFE | Supercritical CO2 extraction |
| UAE | Ultrasound-assisted extraction |
References
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-Industrial by-Products: Valuable Sources of Bioactive Compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A New Sustainability Paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sowjanya, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process—A Sustainable Symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Bisht, A.; Sahu, S.C.; Kumar, A.; Maqsood, S.; Barwant, M.M.; Jaiswal, S.G. Recent Advances in Conventional and Innovative Extraction Techniques for Recovery of High-Added Value Compounds for Food Additives and Nutraceuticals. Food Phys. 2025, 2, 100047. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical Analysis of Green Extraction Techniques Used for Botanicals: Trends, Priorities, and Optimization Strategies—A Review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Araújo, M.A.; Rodrigues Morais, B.; da Silva Santos, J.P.; de Jesus, L.K.; Aurélio Lomba, K.; do Nascimento, G.C.; Soares, M.A.; Neves, N.d.A.; Andressa, I.; Pedrosa Silva Clerici, M.T.; et al. Green Chemistry and Multivariate Optimization in the Extraction of Phenolic Compounds: The Potential of NaDES in Alternative Raw Materials for Expanded Extrudates. Methods Protoc. 2025, 8, 82. [Google Scholar] [CrossRef]
- Marianne, L.-C.; Lucía, A.-G.; de Jesús, M.-S.M.; Eric Leonardo, H.-M.; Mendoza-Sánchez, M. Optimization of the Green Extraction Process of Antioxidants Derived from Grape Pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- Lopes, J.d.C.; Madureira, J.; Margaça, F.M.A.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Li, Z.; Liu, D.; Fan, J. A Review of the Polyphenols Extraction from Apple Pomace: Novel Technologies and Techniques of Cell Disintegration. Crit. Rev. Food Sci. Nutr. 2023, 63, 9752–9765. [Google Scholar] [CrossRef]
- Wang, C.; You, Y.; Huang, W.; Zhan, J. The High-Value and Sustainable Utilization of Grape Pomace: A Review. Food Chem. X 2024, 24, 101845. [Google Scholar] [CrossRef]
- Zoccatelli, G.; Ciulu, M. Perspectives on the Use of Coffee Silverskin in Food Formulations. J. Agric. Food Res. 2025, 21, 101975. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Casertano, M.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Torres-León, C. Recent Advances in Bio-Based Extraction Processes for the Recovery of Bound Phenolics from Agro-Industrial by-Products and Their Biological Activity. Crit. Rev. Food Sci. Nutr. 2024, 64, 10643–10667. [Google Scholar] [CrossRef]
- Areti, H.A.; Muleta, M.D.; Abo, L.D.; Hamda, A.S.; Adugna, A.A.; Edae, I.T.; Daba, B.J.; Gudeta, R.L. Innovative Uses of Agricultural By-Products in the Food and Beverage Sector: A Review. Food Chem. Adv. 2024, 5, 100838. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2023, 12, 38. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Kato-Schwartz, C.G.; Corrêa, R.C.G.; de Souza Lima, D.; de Sá-Nakanishi, A.B.; de Almeida Gonçalves, G.; Seixas, F.A.V.; Haminiuk, C.W.I.; Barros, L.; Ferreira, I.C.F.R.; Bracht, A.; et al. Potential Anti-Diabetic Properties of Merlot Grape Pomace Extract: An in Vitro, in Silico and in Vivo Study of α-Amylase and α-Glucosidase Inhibition. Food Res. Int. 2020, 137, 109462. [Google Scholar] [CrossRef]
- Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, M.T.; Romani, A.; Bernini, R.; Sweeney, T. Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants. Nutrients 2019, 11, 548. [Google Scholar] [CrossRef]
- Raya-Morquecho, E.M.; Aguilar-Zarate, P.; Sepúlveda, L.; Michel, M.R.; Iliná, A.; Aguilar, C.N.; Ascacio-Valdés, J.A. Ellagitannins and Their Derivatives: A Review on the Metabolization, Absorption, and Some Benefits Related to Intestinal Health. Microbiol. Res. 2025, 16, 113. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Esposito, L.; Molina-Hernandez, J.B.; Pérez-Álvarez, J.Á.; Martuscelli, M.; Chaves-López, C. Cocoa Shell Infusion: A Promising Application for Added-Value Beverages Based on Cocoa’s Production Coproducts. Foods 2023, 12, 2442. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, T.G.; Saricaoglu, B.; Ozkan, G.; Tomas, M.; Capanoglu, E. Valorization of Tea Waste: Composition, Bioactivity, Extraction Methods, and Utilization. Food Sci. Nutr. 2024, 12, 3112–3124. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A Comparative Study on Different Extraction Techniques to Recover Red Grape Pomace Polyphenols from Vinification Byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules 2021, 26, 4495. [Google Scholar] [CrossRef]
- Ntzimani, A.; Tsevdou, M.; Katsouli, M.; Thanou, I.; Tsimogiannis, D.; Giannakourou, M.; Taoukis, P. Recovery of Carotenoids via Novel Extraction Technologies for the Valorization of Tomato By-Products. Processes 2025, 13, 2964. [Google Scholar] [CrossRef]
- Macias-Garbett, R.; Serna-Hernández, S.O.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Phenolic Compounds From Brewer’s Spent Grains: Toward Green Recovery Methods and Applications in the Cosmetic Industry. Front. Sustain. Food Syst. 2021, 5, 681684. [Google Scholar] [CrossRef]
- Bagabaldo, P.A.A.; Atienza, L.M.; Castillo-Israel, K.A.T.; Estacio, M.A.C.; Gaban, P.J.V.; Maniwang, J.R.C.; Gapasin, R.P.; Estribillo, A.G.M.; Cena-Navarro, R.B. ‘Saba’ Banana (Musa Acuminata x Balbisiana BBB Group) Peel Pectin Supplementation Improves Biomarkers of Obesity and Associated Blood Lipid Disorders in Obese Hypercholesterolemic Mice. Curr. Res. Food Sci. 2022, 5, 251–260. [Google Scholar] [CrossRef]
- Fang, W.; Peng, W.; Qi, W.; Zhang, J.; Song, G.; Pang, S.; Wang, Y. Ferulic Acid Combined with Different Dietary Fibers Improve Glucose Metabolism and Intestinal Barrier Function by Regulating Gut Microbiota in High-Fat Diet-Fed Mice. J. Funct. Foods 2024, 112, 105919. [Google Scholar] [CrossRef]
- Preciado-Saldaña, A.M.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Astiazaran-Garcia, H.F.; Montiel-Herrera, M.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Wall-Medrano, A. Mango “Ataulfo” Peel Extract Improves Metabolic Dysregulation in Prediabetic Wistar Rats. Life 2022, 12, 532. [Google Scholar] [CrossRef]
- Chong, Y.K.; Gan, W.K.; Tan, J.B.L.; Mohd Jaaffar, A.K.H.; Baharum, Z.; Yeong, K.Y. Exploring the Antimicrobial Activity of Fermented and Non-Fermented Cocoa Bean Shell Extracts through Metabolomics Analysis and Synergistic Studies. J. Sci. Food Agric. 2025, 105, 6495–6505. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Cañas, S.; Cobeta, I.M.; Rebollo-Hernanz, M.; Rodríguez-Rodríguez, P.; Benítez, V.; Arribas, S.M.; Martín-Cabrejas, M.A.; Aguilera, Y. Uncovering Cocoa Shell as a Safe Bioactive Food Ingredient: Nutritional and Toxicological Breakthroughs. Future Foods 2024, 10, 100461. [Google Scholar] [CrossRef]
- Amin, A.; Ullah, N.; Khan, M.A.; Elsadek, M.F.; Elshikh, M.S.; Hasnain, S.Z.U.; Baloch, R.; Chaman, S.; Makhkamov, T.; Yuldashev, A.; et al. Mango Peel Extracts and Mangiferin Chromatographic Fourier-Transform Infrared Correlation with Antioxidant, Antidiabetic, and Advanced Glycation End Product Inhibitory Potentials Using in Silico Modeling and in Vitro Assays. Biomed. Chromatogr. 2024, 38, e5936. [Google Scholar] [CrossRef] [PubMed]
- Ligarda-Samanez, C.A.; Huamán-Carrión, M.L.; Calsina-Ponce, W.C.; Cruz, G.D.l.; Calderón Huamaní, D.F.; Cabel-Moscoso, D.J.; Garcia-Espinoza, A.J.; Sucari-León, R.; Aroquipa-Durán, Y.; Muñoz-Saenz, J.C.; et al. Technological Innovations and Circular Economy in the Valorization of Agri-Food By-Products: Advances, Challenges and Perspectives. Foods 2025, 14, 1950. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Khan, M.I.; Kumar, V.; Shang, X.; Lee, J.-H.; Ko, E.-Y. Bioactive Compounds of Agro-Industrial By-Products: Current Trends, Recovery, and Possible Utilization. Antioxidants 2025, 14, 650. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Mesquita, L.M.d.S.; Contieri, L.S.; e Silva, F.A.; Bagini, R.H.; Bragagnolo, F.S.; Strieder, M.M.; Sosa, F.H.B.; Schaeffer, N.; Freire, M.G.; Ventura, S.P.M.; et al. Path2Green: Introducing 12 Green Extraction Principles and a Novel Metric for Assessing Sustainability in Biomass Valorization. Green Chem. 2024, 26, 10087–10106. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Joković, N.; Matejić, J.; Zvezdanović, J.; Stojanović-Radić, Z.; Stanković, N.; Mihajilov-Krstev, T.; Bernstein, N. Onion Peel as a Potential Source of Antioxidants and Antimicrobial Agents. Agronomy 2024, 14, 453. [Google Scholar] [CrossRef]
- Şen, E.; Göktürk, E.; Hajiyev, V.; Uğuzdoğan, E. Comparisons of Pulsed Ultrasound-Assisted and Hot-Acid Extraction Methods for Pectin Extraction under Dual Acid Mixtures from Onion (Allium cepa L.) Waste. Food Sci. Nutr. 2023, 11, 7320–7329. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Rajabzadeh, Q.; Tavakoli, R.; Ibrahim, S.A.; Jafari, S.M. Ultrasound-Assisted Extraction of Saffron Bioactive Compounds; Separation of Crocins, Picrocrocin, and Safranal Optimized by Artificial Bee Colony. Ultrason. Sonochem. 2022, 86, 105971. [Google Scholar] [CrossRef]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized Microwave-Assisted Extraction of Phenolic Acids from Citrus Mandarin Peels and Evaluation of Antioxidant Activity in Vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Jurić, M.; Golub, N.; Galić, E.; Radić, K.; Maslov Bandić, L.; Vitali Čepo, D. Microwave-Assisted Extraction of Bioactive Compounds from Mandarin Peel: A Comprehensive Biorefinery Strategy. Antioxidants 2025, 14, 722. [Google Scholar] [CrossRef]
- Tufail, T.; Bader Ul Ain, H.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Villacís-Chiriboga, J.; Voorspoels, S.; Uyttebroek, M.; Ruales, J.; Van Camp, J.; Vera, E.; Elst, K. Supercritical CO2 Extraction of Bioactive Compounds from Mango (Mangifera indica L.) Peel and Pulp. Foods 2021, 10, 2201. [Google Scholar] [CrossRef]
- Aniceto, J.P.S.; Rodrigues, V.H.; Portugal, I.; Silva, C.M. Valorization of Tomato Residues by Supercritical Fluid Extraction. Processes 2022, 10, 28. [Google Scholar] [CrossRef]
- de Lima, N.D.; da Silva Monteiro Wanderley, B.R.; Andrade Ferreira, A.L.; Pereira-Coelho, M.; da Silva Haas, I.C.; Vitali, L.; dos Santos Madureira, L.A.; Müller, J.M.; Fritzen-Freire, C.B.; de Mello Castanho Amboni, R.D. Green Extraction of Phenolic Compounds from the By-Product of Purple Araçá (Psidium myrtoides) with Natural Deep Eutectic Solvents Assisted by Ultrasound: Optimization, Comparison, and Bioactivity. Food Res. Int. 2024, 191, 114731. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, J.; Huang, Z.; Guo, Y. Sustainable Recovery and Recycling of Natural Deep Eutectic Solvent for Biomass Fractionation via Industrial Membrane-Based Technique. Ind. Crops Prod. 2023, 194, 116351. [Google Scholar] [CrossRef]
- Fujimoto, H.; Narita, Y.; Iwai, K.; Hanzawa, T.; Kobayashi, T.; Kakiuchi, M.; Ariki, S.; Wu, X.; Miyake, K.; Tahara, Y.; et al. Bitterness Compounds in Coffee Brew Measured by Analytical Instruments and Taste Sensing System. Food Chem. 2021, 342, 128228. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, K.-T.; Kim, H.J.; Chung, M.-S.; Chang, P.-S.; Park, H.; Pai, H.-D. Antioxidant Activities of Onion (Allium cepa L.) Peel Extracts Produced by Ethanol, Hot Water, and Subcritical Water Extraction. Food Sci. Biotechnol. 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the Phytochemicals from Coffee and Cocoa By-Products with Their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate Peel Polyphenols Inhibits Inflammation in LPS-Induced RAW264.7 Macrophages via the Suppression of TLR4/NF-κB Pathway Activation. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- de Oliveira Raphaelli, C.; dos Santos Pereira, E.; Camargo, T.M.; Vinholes, J.; Rombaldi, C.V.; Vizzotto, M.; Nora, L. Apple Phenolic Extracts Strongly Inhibit α-Glucosidase Activity. Plant Foods Hum. Nutr. 2019, 74, 430–435. [Google Scholar] [CrossRef]
- Sri Harsha, P.S.C.; Lavelli, V. Grape Pomace as a Source of Phenolics for the Inhibition of Starch Digestion Enzymes: A Comparative Study and Standardization of the Efficacy. Foods 2024, 13, 4103. [Google Scholar] [CrossRef]
- Sarkar, D.; Christopher, A.; Shetty, K. Phenolic Bioactives From Plant-Based Foods for Glycemic Control. Front. Endocrinol. 2022, 12, 727503. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients 2019, 11, 2693. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Kazem, A.E.; Campanella, O.H.; Cano, M.P.; Hamaker, B.R.; Serna-Saldívar, S.O.; Welti-Chanes, J. In Vitro Fecal Fermentation of High Pressure-Treated Fruit Peels Used as Dietary Fiber Sources. Molecules 2019, 24, 697. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.d.L.; Cebollón-Juárez, A.; Bosquez-Molina, E.; Totosaus, A. Mango Peel Flour and Potato Peel Flour as Bioactive Ingredients in the Formulation of Functional Yogurt. Food Sci. Technol. 2022, 42, e38220. [Google Scholar] [CrossRef]
- Radulescu, C.; Olteanu, R.L.; Buruleanu, C.L.; Tudorache, M.N.; Dulama, I.D.; Stirbescu, R.M.; Bucurica, I.A.; Stanescu, S.G.; Banica, A.L. Polyphenolic Screening and the Antioxidant Activity of Grape Pomace Extracts of Romanian White and Red Grape Varieties. Antioxidants 2024, 13, 1133. [Google Scholar] [CrossRef]
- Mozafari, L.; Cano-Lamadrid, M.; Martínez-Zamora, L.; Bueso, M.C.; Kessler, M.; Artés-Hernández, F. Pulsed Ultrasound-Assisted Extraction of Lycopene and β-Carotene from Industrial Grated Tomato by-Products. LWT 2024, 204, 116462. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Benítez, J.J.; Guzmán-Puyol, S.; Porras-Vázquez, J.M.; Hierrezuelo, J.; Grifé-Ruiz, M.; Romero, D.; Di Capua, A.; Bochicchio, R.; Laurenza, S.; et al. Enhanced Extraction of Bioactive Compounds from Tea Waste for Sustainable Polylactide-Based Bioplastic Applications in Active Food Packaging. Food Packag. Shelf Life 2024, 46, 101410. [Google Scholar] [CrossRef]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Ofrydopoulou, A.; Shiels, K.; Saha, S.K.; Tsoupras, A. In Vitro Antioxidant, Antithrombotic and Anti-Inflammatory Properties of the Amphiphilic Bioactives from Greek Organic Starking Apple Juice and Its By-Products (Apple Pomace). Appl. Sci. 2025, 15, 2807. [Google Scholar] [CrossRef]
- Machado, M.; Galrinho, M.F.; Passos, C.P.; Espírito Santo, L.; Simona Chiș, M.; Ranga, F.; Puga, H.; Palmeira, J.; Coimbra, M.A.; Oliveira, M.B.P.P.; et al. Prebiotic Potential of a Coffee Silverskin Extract Obtained by Ultrasound-Assisted Extraction on Lacticaseibacillus paracasei Subsp. Paracasei. J. Funct. Foods 2024, 120, 106378. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Chongsuwat, R.; Phosat, C.; Butacnum, A. Rice Bran Oil Containing Gamma-Oryzanol Improves Lipid Profiles and Antioxidant Status in Hyperlipidemic Subjects: A Randomized Double-Blind Controlled Trial. J. Altern. Complement. Med. 2019, 25, 353–358. [Google Scholar] [CrossRef]
- Han, Y.; Yang, C.; Tian, X.; Shi, X.; Wang, H.; Li, H. Grape Pomace Polyphenol Extract Alleviates Obesity in Mice and Improves Gut Microbiota and Short Chain Fatty Acids. Foods 2025, 14, 2823. [Google Scholar] [CrossRef]
- Abo-Saif, M.A.; Ragab, A.E.; Ibrahim, A.O.; Abdelzaher, O.F.; Mehanyd, A.B.M.; Saber-Ayad, M.; El-Feky, O.A. Pomegranate Peel Extract Protects against the Development of Diabetic Cardiomyopathy in Rats by Inhibiting Pyroptosis and Downregulating LncRNA-MALAT1. Front. Pharmacol. 2023, 14, 1166653. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Pérez-Jiménez, J.; Ramos, S.; Portillo, M.P.; Martín, M.Á.; Mateos, R. Hydroxytyrosol Supplementation Improves Antioxidant and Anti-Inflammatory Status in Individuals with Overweight and Prediabetes: A Randomized, Double-Blind, Placebo-Controlled Parallel Trial. Clin. Nutr. 2025, 52, 17–26. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; He, B.; Zhou, G.; Cui, Y. Effects of Citrus Flavanone Hesperidin Extracts or Purified Hesperidin Consumption on Risk Factors for Cardiovascular Disease: Evidence From an Updated Meta-Analysis of Randomized Controlled Trials. Curr. Dev. Nutr. 2024, 8, 102055. [Google Scholar] [CrossRef]
- Heidari, Z.; Farahmandpour, F.; Bazyar, H.; Pashayee-Khamene, F. Effects of Hesperidin Supplementation on Cardiometabolic Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2025, 83, e1014–e1033. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.G.; Braga, L.E.d.O.; de Oliveira, E.C.S.; de Carvalho, J.E.; Lazarini, J.G.; Rosalen, P.L.; Dionísio, A.P.; Ruiz, A.L.T.G. Evaluation of a Standardized Extract Obtained from Cashew Apple (Anacardium occidentale L.) Bagasse in DSS-Induced Mouse Colitis. Foods 2023, 12, 3318. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Shimada, M.; Maeda, H.; Narumi, T.; Ichita, J.; Itoku, K.; Nakajima, A. Apple Pomace Extract Improves MK-801-Induced Memory Impairment in Mice. Nutrients 2024, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Gondi, M.; Basha, S.A.; Bhaskar, J.J.; Salimath, P.V.; Prasada Rao, U.J.S. Anti-Diabetic Effect of Dietary Mango (Mangifera Indica L.) Peel in Streptozotocin-Induced Diabetic Rats. J. Sci. Food Agric. 2015, 95, 991–999. [Google Scholar] [CrossRef]
- D’Amico, D.; Olmer, M.; Fouassier, A.M.; Valdés, P.; Andreux, P.A.; Rinsch, C.; Lotz, M. Urolithin A Improves Mitochondrial Health, Reduces Cartilage Degeneration, and Alleviates Pain in Osteoarthritis. Aging Cell 2022, 21, e13662. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Yasuda, T.; Takagi, T.; Asaeda, K.; Hashimoto, H.; Kajiwara, M.; Azuma, Y.; Kitae, H.; Hirai, Y.; Mizushima, K.; Doi, T.; et al. Urolithin A-Mediated Augmentation of Intestinal Barrier Function through Elevated Secretory Mucin Synthesis. Sci. Rep. 2024, 14, 15706. [Google Scholar] [CrossRef]
- Liu, M.; Huang, B.; Wang, L.; Lu, Q.; Liu, R. Peanut Skin Procyanidins Ameliorate Insulin Resistance via Modulation of Gut Microbiota and Gut Barrier in Type 2 Diabetic Mice. J. Sci. Food Agric. 2022, 102, 5935–5947. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 432/2012; Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. European Union (EU): Brussels, Belgium, 2012.
- FDA GRAS Notice, No. 943; Citrus Fiber as a Food Ingredient. U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Resolução RDC no 778/2023; Procedures for Novel Food Approval in Brazil. ANVISA: Brasília, Brazil, 2023.
- Yan, B.; Fang, Z.; Shen, L.; Qu, H. Root Cause Analysis of Quality Defects Using HPLC–MS Fingerprint Knowledgebase for Batch-to-Batch Quality Control of Herbal Drugs. Phytochem. Anal. 2015, 26, 261–268. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Martínez-Maqueda, D.; Hereu, M.; Amézqueta, S.; Torres, J.L.; Pérez-Jiménez, J. Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial. Foods 2020, 9, 1279. [Google Scholar] [CrossRef]
- Schiano, E.; Vaccaro, S.; Scorcia, V.; Carnevali, A.; Borselli, M.; Chisari, D.; Guerra, F.; Iannuzzo, F.; Tenore, G.C.; Giannaccare, G.; et al. From Vineyard to Vision: Efficacy of Maltodextrinated Grape Pomace Extract (MaGPE) Nutraceutical Formulation in Patients with Diabetic Retinopathy. Nutrients 2024, 16, 2850. [Google Scholar] [CrossRef]
- Hikal, W.M.; Said-Al Ahl, H.A.H.; Bratovcic, A.; Tkachenko, K.G.; Sharifi-Rad, J.; Kačániová, M.; Elhourri, M.; Atanassova, M. Banana Peels: A Waste Treasure for Human Being. Evid.-Based Complement. Altern. Med. 2022, 2022, 7616452. [Google Scholar] [CrossRef]
- Yari, Z.; Movahedian, M.; Imani, H.; Alavian, S.M.; Hedayati, M.; Hekmatdoost, A. The Effect of Hesperidin Supplementation on Metabolic Profiles in Patients with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Eur. J. Nutr. 2020, 59, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.-J.; Tomas, M.; et al. Functional Implications of Bound Phenolic Compounds and Phenolics–Food Interaction: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muñoz, C.; Vaillant, F. Metabolic Fate of Ellagitannins: Implications for Health, and Research Perspectives for Innovative Functional Foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A Improves Muscle Strength, Exercise Performance, and Biomarkers of Mitochondrial Health in a Randomized Trial in Middle-Aged Adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.-J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In Vitro Colonic Metabolism of Coffee and Chlorogenic Acid Results in Selective Changes in Human Faecal Microbiota Growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef]
- Precup, G.; Marini, E.; Zakidou, P.; Beneventi, E.; Consuelo, C.; Fernández-Fraguas, C.; Garcia Ruiz, E.; Laganaro, M.; Magani, M.; Mech, A.; et al. Novel Foods, Food Enzymes, and Food Additives Derived from Food by-Products of Plant or Animal Origin: Principles and Overview of the EFSA Safety Assessment. Front. Nutr. 2024, 11, 1390734. [Google Scholar] [CrossRef]
- Noviana, E.; Indrayanto, G.; Rohman, A. Advances in Fingerprint Analysis for Standardization and Quality Control of Herbal Medicines. Front. Pharmacol. 2022, 13, 853023. [Google Scholar] [CrossRef]
- Ali Redha, A.; Kodikara, C.; Cozzolino, D. Does Encapsulation Improve the Bioavailability of Polyphenols in Humans? A Concise Review Based on In Vivo Human Studies. Nutrients 2024, 16, 3625. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and Delivery of Bioactive Citrus Pomace Polyphenols: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Physicochemical Properties and Bioaccessibility of Phenolic Compounds of Dietary Fibre Concentrates from Vegetable By-Products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef] [PubMed]
- Melios, S.; Johnson, H.; Grasso, S. Sensory Quality and Regulatory Aspects of Upcycled Foods: Challenges and Opportunities. Food Res. Int. 2025, 199, 115360. [Google Scholar] [CrossRef]
- Sánchez Macarro, M.; Martínez Rodríguez, J.P.; Bernal Morell, E.; Pérez-Piñero, S.; Victoria-Montesinos, D.; García-Muñoz, A.M.; Cánovas García, F.; Castillo Sánchez, J.; López-Román, F.J. Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients 2020, 12, 1475. [Google Scholar] [CrossRef]
- Bianchi, S.; Vannini, M.; Sisti, L.; Marchese, P.; Mallegni, N.; Rodríguez, Ó.; Kohnen, S.; Tchoumtchoua, J.; Cinelli, P.; Celli, A. Valorization of Coffee Silverskin by Cascade Extraction of Valuable Biomolecules: Preparation of Eco-Friendly Composites as the Ultimate Step. Biofuels Bioprod. Biorefin. 2024, 18, 524–542. [Google Scholar] [CrossRef]
- Prado-Acebo, I.; Cubero-Cardoso, J.; Lu-Chau, T.A.; Eibes, G. Integral Multi-Valorization of Agro-Industrial Wastes: A Review. Waste Manag. 2024, 183, 42–52. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef]
- Schmidt Rivera, X.C.; Gallego-Schmid, A.; Najdanovic-Visak, V.; Azapagic, A. Life Cycle Environmental Sustainability of Valorisation Routes for Spent Coffee Grounds: From Waste to Resources. Resour. Conserv. Recycl. 2020, 157, 104751. [Google Scholar] [CrossRef]
- Voccia, D.; Milvanni, G.; Leni, G.; Lamastra, L. Life Cycle Assessment and Circular Economy Evaluation of Extraction Techniques: Energy Analysis of Antioxidant Recovery from Wine Residues. Energies 2025, 18, 4851. [Google Scholar] [CrossRef]
- Geetha, R. Circular Economy through Integrated Industrial Ecology: Innovations in Resource Recovery and Process Re-Design. Biotechnol. Notes 2025, 6, 245–259. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Dried Coffee Husk (Cascara) from Coffea Arabica L. as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07085. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Schmidt, L.M.; Reynolds, W.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Biorefinery Cascade Processing for Creating Added Value on Tomato Industrial By-Products from Tunisia. Biotechnol. Biofuels 2016, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rawson, A.; Kumar, A.; CK, S.; Vignesh, S.; Venkatachalapathy, N. Lycopene Extraction from Industrial Tomato Processing Waste Using Emerging Technologies, and Its Application in Enriched Beverage Development. Int. J. Food Sci. Technol. 2023, 58, 2141–2150. [Google Scholar] [CrossRef]
- Eckhardt, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Coffee Cherry (Cascara) Fruit Products for Flour Replacement and Other Alternative Food Uses. Molecules 2022, 27, 8435. [Google Scholar] [CrossRef]
- Vanyan, L.; Cenian, A.; Trchounian, K. Biogas and Biohydrogen Production Using Spent Coffee Grounds and Alcohol Production Waste. Energies 2022, 15, 5935. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent Coffee Ground Characterization, Pelletization Test and Emissions Assessment in the Combustion Process. Sci. Rep. 2021, 11, 5119. [Google Scholar] [CrossRef]
- Fernandes, F.; Delerue-Matos, C.; Grosso, C. Unveiling the Potential of Agrifood By-Products: A Comprehensive Review of Phytochemicals, Bioactivities and Industrial Applications. Waste Biomass Valor. 2025, 16, 2715–2748. [Google Scholar] [CrossRef]
- Orive, M.; Cebrián, M.; Amayra, J.; Zufía, J.; Bald, C. Techno-Economic Assessment of a Biorefinery Plant for Extracted Olive Pomace Valorization. Process Saf. Environ. Prot. 2021, 147, 924–931. [Google Scholar] [CrossRef]
- Cao, Y.; Jang, Y. Consumer perception of upcycled food labels. Br. Food J. 2025, 127, 3230–3245. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA Health Claim on Olive Oil Polyphenols: Acid Hydrolysis Validation and Total Hydroxytyrosol and Tyrosol Determination in Italian Virgin Olive Oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef] [PubMed]
- Kull, A.-K.; Lachenmeier, D.W. Comprehensive Update on European Union Labeling Standards for Coffee and Its By-Products. Proceedings 2024, 109, 19. [Google Scholar] [CrossRef]
- Gatto, F.; Savini, C.; Sacco, M.G.; Vinciguerra, D.; Buttinger, G.; Corbisier, P.; Mazzara, M.; Emons, H. Single and Multi-Laboratory Validation of a Droplet Digital PCR Method. Food Control 2022, 140, 109117. [Google Scholar] [CrossRef]
- Roberts, A.; Haighton, L.A. A Hard Look at FDA’s Review of GRAS Notices. Regul. Toxicol. Pharmacol. 2016, 79, S124–S128. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O. Communicating Upcycled Foods: Frugality Framing Supports Acceptance of Sustainable Product Innovations. Food Qual. Prefer. 2022, 100, 104596. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O. Consumer Understanding of Upcycled Foods—Exploring Consumer-Created Associations and Concept Explanations across Five Countries. Food Qual. Prefer. 2023, 112, 105033. [Google Scholar] [CrossRef]
- Bastounis, A.; Buckell, J.; Hartmann-Boyce, J.; Cook, B.; King, S.; Potter, C.; Bianchi, F.; Rayner, M.; Jebb, S.A. The Impact of Environmental Sustainability Labels on Willingness-to-Pay for Foods: A Systematic Review and Meta-Analysis of Discrete Choice Experiments. Nutrients 2021, 13, 2677. [Google Scholar] [CrossRef]
- Ruiz Sierra, A.; Zika, E.; Lange, L.; Ruiz de Azúa, P.L.; Canalis, A.; Mallorquín Esteban, P.; Paiano, P.; Mengal, P. The Bio-Based Industries Joint Undertaking: A High Impact Initiative That Is Transforming the Bio-Based Industries in Europe. New Biotechnol. 2021, 60, 105–112. [Google Scholar] [CrossRef]
- Molin, E.; Lingegård, S.; Martin, M.; Björklund, A. Sustainable Public Food Procurement: Criteria and Actors’ Roles and Influence. Front. Sustain. Food Syst. 2024, 8, 1360033. [Google Scholar] [CrossRef]
- Molin, E.; Martin, M.; Björklund, A. Addressing Sustainability within Public Procurement of Food: A Systematic Literature Review. Sustainability 2021, 13, 13395. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering According to Urolithin Metabotype Explains the Interindividual Variability in the Improvement of Cardiovascular Risk Biomarkers in Overweight-Obese Individuals Consuming Pomegranate: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- Amponsah, L.; Chuck, C.; Parsons, S. Life Cycle Assessment of a Marine Biorefinery Producing Protein, Bioactives and Polymeric Packaging Material. Int. J. Life Cycle Assess. 2024, 29, 174–191. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and Health: Interactions between Fibre, Plant Polyphenols and the Gut Microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; DeGeorge, D.; Diehn, D.; Galatz, A.; Garza, J.; McGowan, L.; Drake, M.; Amin, S.; Lammert, A. Upcycled vs. Sustainable: Identifying Consumer Segments and Recognition of Sustainable and Upcycled Foods Within the United States. Foods 2025, 14, 3508. [Google Scholar] [CrossRef]
- Batarfi, W.A.; Yunus, M.H.M.; Hamid, A.A.; Lee, Y.T.; Maarof, M. Hydroxytyrosol: A Promising Therapeutic Agent for Mitigating Inflammation and Apoptosis. Pharmaceutics 2024, 16, 1504. [Google Scholar] [CrossRef]
- EverGrain. EverGrain Ingredients. Available online: https://evergrainingredients.com/ (accessed on 1 November 2025).
- Fonseca, Y.A.d.; Fernandes, A.R.A.C.; Gurgel, L.V.A.; Baêta, B.E.L. Comparative Life Cycle Assessment of Early-Stage Technological Layouts for Brewers’ Spent Grain Upcycling: A Sustainable Approach for Adding Value to Waste. J. Water Process Eng. 2024, 66, 105904. [Google Scholar] [CrossRef]
- Khorasanian, A.S.; Fateh, S.T.; Gholami, F.; Rasaei, N.; Gerami, H.; Khayyatzadeh, S.S.; Shiraseb, F.; Asbaghi, O. The Effects of Hesperidin Supplementation on Cardiovascular Risk Factors in Adults: A Systematic Review and Dose–Response Meta-Analysis. Front. Nutr. 2023, 10, 1177708. [Google Scholar] [CrossRef]
- Machado, M.; Fernandes, I.; Fernandes, A.; Espírito Santo, L.; Passos, C.; Santamarina, A.; Cardelle-Cobas, A.; Coimbra, M.A.; Oliveira, M.B.P.P.; Ferreira, H.; et al. Impact of In Vitro Gastrointestinal Digestion on the Chemical Composition and Prebiotic Potential of Coffee Silverskin. Plant Foods Hum. Nutr. 2025, 80, 154. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral Valorisation of Tomato By-Products towards Bioactive Compounds Recovery: Human Health Benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sahadev, S.; Wei, X.; Henninger, C.E. Modelling the Antecedents of Consumers’ Willingness to Pay for Eco-Labelled Food Products. Int. J. Consum. Stud. 2023, 47, 1256–1272. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Chen, H.-S. Understanding Consumers’ Intentions to Purchase Clean Label Products: Evidence from Taiwan. Nutrients 2022, 14, 3684. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Chávez, J.; Dufoo-Hurtado, E.; Santos-Zea, L.; Ramírez-Jiménez, A.K. Looking into New Sources of Bioactives: Seasonal Variation in Bioactive Compounds and Dietary Fiber of Agave Bagasse from Mezcal Production. Foods 2025, 14, 1632. [Google Scholar] [CrossRef]
- Marcu Spinu, S.; Dragoi Cudalbeanu, M.; Major, N.; Goreta Ban, S.; Palčić, I.; Ortan, A.; Rosu, P.M.; Babeanu, N.E. Box–Behnken Design Optimization of Green Extraction from Tomato Aerial Parts and Axillary Shoots for Enhanced Recovery of Rutin and Complementary Bioactive Compounds. Antioxidants 2025, 14, 1062. [Google Scholar] [CrossRef]
- Drescher, A.; Schwingshackl, L.; Kienberger, M. Identification of Molecules from Tomato Plant Residues Using Sustainable Green Chemicals. Biomass Conv. Bioref. 2025, 15, 14387–14398. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Mora, Z.V.l.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Ma, S.; Cai, C.; Lu, Q.; Tan, Z. A Review of Green Solvents for the Extraction and Separation of Bioactive Ingredients from Natural Products. Food Chem. 2025, 478, 143703. [Google Scholar] [CrossRef]
- Alloun, W.; Calvio, C. Bio-Driven Sustainable Extraction and AI-Optimized Recovery of Functional Compounds from Plant Waste: A Comprehensive Review. Fermentation 2024, 10, 126. [Google Scholar] [CrossRef]
- Lee, S.Y.; Stuckey, D.C. Separation and Biosynthesis of Value-Added Compounds from Food-Processing Wastewater: Towards Sustainable Wastewater Resource Recovery. J. Clean. Prod. 2022, 357, 131975. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Allaw, M.; Hellström, J.; Granato, D.; Manconi, M. Jabuticaba (Myrciaria jaboticaba) Peel as a Sustainable Source of Anthocyanins and Ellagitannins Delivered by Phospholipid Vesicles for Alleviating Oxidative Stress in Human Keratinocytes. Molecules 2021, 26, 6697. [Google Scholar] [CrossRef]
- Seguenka, B.; do Nascimento, L.H.; Feiden, T.; Fernandes, I.A.; Magro, J.D.; Junges, A.; Valduga, E.; Steffens, J. Ultrasound-Assisted Extraction and Concentration of Phenolic Compounds from Jabuticaba Sabará (Plinia peruviana (Poir.) Govaerts) Peel by Nanofiltration Membrane. Food Chem. 2024, 453, 139690. [Google Scholar] [CrossRef] [PubMed]
- Castilho, P.A.; Bracht, L.; Barros, L.; Albuquerque, B.R.; Dias, M.I.; Ferreira, I.C.F.R.; Comar, J.F.; Silva, T.B.V.d.; Peralta, R.M.; de Sá-Nakanishi, A.B.; et al. Effects of a Myrciaria Jaboticaba Peel Extract on Starch and Triglyceride Absorption and the Role of Cyanidin-3-O-Glucoside. Food Funct. 2021, 12, 2644–2659. [Google Scholar] [CrossRef] [PubMed]
- Barroso, T.L.C.T.; Castro, L.E.N.; da Rosa, R.G.; Brackmann, R.; Goldbeck, R.; Forster-Carneiro, T. Obtaining Value-Added Products from Cashew Apple Bagasse: A Sustainable Alternative Achieved through a Hydrothermal Process. Food Res. Int. 2025, 208, 116276. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Moncada, O.D.; López-Cuellar, L.; Sánchez-Garzón, F.S.; Forero-Doria, O.; Bolivar-Pineda, L.M.; Nerio, L.S. Optimization of Microwave-Assisted Extraction of Pectin from Cupuassu (Theobroma grandiflorum) Pod Husk as an Alternative for Waste Valorization of Amazonian Fruits. Food Meas. 2025, 19, 7931–7945. [Google Scholar] [CrossRef]
- Melo, J.O.F.; Conchinhas, B.; Leitão, A.E.B.; Ramos, A.L.C.C.; Sousa, I.M.N.d.; de Seixas Boavida Ferreira, R.M.; Ribeiro, A.C.; Batista-Santos, P. Phenolic Compounds Characterization of Caryocar Brasiliense Peel with Potential Antioxidant Activity. Plants 2024, 13, 2016. [Google Scholar] [CrossRef]
- Carlos de Sousa, W.; Alves Morais, R.; Damian Giraldo Zuniga, A. Buriti (Mauritia flexuosa) Shell Flour: Nutritional Composition, Chemical Profile, and Antioxidant Potential as a Strategy for Valuing Waste from Native Brazilian Fruits. Food Res. Int. 2024, 190, 114578. [Google Scholar] [CrossRef]
- da Silva, L.R.; Feitosa, L.H.F.; e Silva, M.B.; Silva, G.d.S.; de Souza, E.G.T.; Marques, A.D.J.d.F.; Sacilotto, E.S.; Arcanjo, N.M.d.O.; Lima, M.d.S.; Pacheco, M.T.B.; et al. Upcycled Beverage From Roasted Açaí (Euterpe oleracea) Seeds: Antioxidant Capacity and Cytoprotection Through Gastrointestinal Simulation. Mol. Nutr. Food Res. 2025, early view, e70270. [Google Scholar] [CrossRef]
- Garcia, J.A.A.; Corrêa, R.C.G.; Barros, L.; Pereira, C.; Abreu, R.M.V.; Alves, M.J.; Calhelha, R.C.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Chemical Composition and Biological Activities of Juçara (Euterpe edulis Martius) Fruit by-Products, a Promising Underexploited Source of High-Added Value Compounds. J. Funct. Foods 2019, 55, 325–332. [Google Scholar] [CrossRef]
- da Silva Donadone, D.B.; de Castro França, I.A.; Silva, D.L.G.; Faria, M.G.I.; Ruiz, S.P.; Barros, B.C.B. Enhancement of Phenolic Recovery by Probe-Type Ultrasound-Assisted Extraction of Acerola By-Product and Evaluation of Antioxidant and Antibacterial Activities. Appl. Sci. 2025, 15, 9154. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of Waste Mango Peels for Extraction of Polyphenolic Antioxidants by Ultrasound-Assisted Natural Deep Eutectic Solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential Extraction of Phenolics and Pectin from Mango Peel Assisted by Ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.V.; Silva, V.D.M.; Ramos, A.L.C.C.; Coelho, T.; de Melo, A.C.; de Seixas Boavida Ferreira, R.M.; Augusti, R.; de Lucena, R.F.P.; Melo, J.O.F.; de Araújo, R.L.B. Investigating the Chemical Profile of Underexplored Parts of Dipteryx Alata (Baru) Using the PS–MS Technique. Plants 2024, 13, 1833. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Silva, G.F.; de Oliveira, V.L.P.; Kubijan, L.A.C.; Costa, L.G.; de Melo, A.M.; Teófilo, M.N.G.; Morgado, C.M.A.; de Campos, A.J.; Peixoto, J.d.C.; et al. Bioinputs from Eugenia Dysenterica DC. (Myrtaceae): Optimization of Ultrasound-Assisted Extraction and Assessment of Antioxidant, Antimicrobial, and Antibiofilm Activities. Molecules 2025, 30, 1115. [Google Scholar] [CrossRef]
- Leite, P.I.P.; Barreto, S.M.A.G.; Freitas, P.R.; de Araújo, A.C.J.; Paulo, C.L.R.; de Almeida, R.S.; de Assis, C.F.; Padilha, C.E.A.; Ferrari, M.; de Sousa Junior, F.C. Extraction of Bioactive Compounds from Buriti (Mauritia flexuosa L.) Fruit by Eco-Friendly Solvents: Chemical and Functional Characterization. Sustain. Chem. Pharm. 2021, 22, 100489. [Google Scholar] [CrossRef]
- Carvalho, A.V.; da Silveira, T.F.; de Sousa, S.H.B.; de Moraes, M.R.; Godoy, H.T. Phenolic Composition and Antioxidant Capacity of Bacaba-de-Leque (Oenocarpus distichus Mart.) Genotypes. J. Food Compos. Anal. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- da Silva Pires, C.; da Silva, M.; Tormen, L.; Bainy, E.M. Application of Guabiroba (Campomanesia xanthocarpa) Peel Extracts as Antioxidant Agents in Tilapia Pâtés. J. Culin. Sci. Technol. 2025, 23, 303–323. [Google Scholar] [CrossRef]
- Conceição, N.; Albuquerque, B.R.; Pereira, C.; Corrêa, R.C.G.; Lopes, C.B.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. By-Products of Camu-Camu [Myrciaria dubia (Kunth) McVaugh] as Promising Sources of Bioactive High Added-Value Food Ingredients: Functionalization of Yogurts. Molecules 2020, 25, 70. [Google Scholar] [CrossRef]
- Viganó, J.; de Aguiar, A.C.; Veggi, P.C.; Sanches, V.L.; Rostagno, M.A.; Martínez, J. Techno-Economic Evaluation for Recovering Phenolic Compounds from Acai (Euterpe oleracea) by-Product by Pressurized Liquid Extraction. J. Supercrit. Fluids 2022, 179, 105413. [Google Scholar] [CrossRef]
- Ribeiro, L.d.O.; de Freitas, B.P.; Lorentino, C.M.A.; Frota, H.F.; dos Santos, A.L.S.; Moreira, D.d.L.; Amaral, B.S.D.; Jung, E.P.; Kunigami, C.N. Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds. Molecules 2022, 27, 410. [Google Scholar] [CrossRef]
- Tornuk, F.; Akman, P.K. Recent Developments in the Valorization of Agri-Food Waste and Byproducts by Fermentation. J. Sci. Food Agric. 2025. early view. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Saccharomyces Cerevisiae Biomass as a Source of Next-Generation Food Preservatives: Evaluating Potential Proteins as a Source of Antimicrobial Peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4450–4479. [Google Scholar] [CrossRef] [PubMed]
- Nikkilä, I.; Waldén, M.; Maina, N.H.; Tenkanen, M.; Mikkonen, K.S. Fungal Cell Biomass From Enzyme Industry as a Sustainable Source of Hydrocolloids. Front. Chem. Eng. 2020, 2, 574072. [Google Scholar] [CrossRef]
- Wu, K.-K.; Xu, P.-P.; Zhao, L.; Ren, N.-Q.; Zhang, Y.-F. Microbial Conversion of Carbon Dioxide into Premium Medium-Chain Fatty Acids: The Progress, Challenges, and Prospects. npj Mater. Sustain. 2024, 2, 4. [Google Scholar] [CrossRef]
- Madjarov, J.; Soares, R.; Paquete, C.M.; Louro, R.O. Sporomusa Ovata as Catalyst for Bioelectrochemical Carbon Dioxide Reduction: A Review Across Disciplines From Microbiology to Process Engineering. Front. Microbiol. 2022, 13, 913311. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, X. Microbial Conversion of CO2 to Organic Compounds. Energy Environ. Sci. 2024, 17, 7017–7034. [Google Scholar] [CrossRef]
| Extraction Technology | Working Principle/Main Bioactives Recovered | Operational Advantages | Limitations/Challenges |
|---|---|---|---|
| Ultrasound-Assisted Extraction (UAE) | Acoustic cavitation generates microbubble formation, collapse, and cell disruption/Polyphenols, phenolic acids, pigments | Low energy demand; short extraction times; compatible with aqueous–ethanolic solvents; effective as a pre-treatment | Limited scalability at industrial volumes; uneven cavitation in large reactors |
| Microwave-Assisted Extraction (MAE) | Dielectric heating induces rapid molecular rotation and internal heating, enhancing cell rupture/Polyphenols, flavonoids, alkaloids | Very fast extraction; higher yields; reduced solvent use; suitable for polar matrices | Non-uniform heating in batch systems requires matrices with dielectric properties |
| Pressurized Liquid Extraction (PLE/ASE) | High pressure + moderate temperature increase solvent diffusivity while keeping it in liquid state/Phenolic acids, flavonoids, carotenoids | High extraction efficiency; reproducible; >70% solvent reduction; suitable for multi-step biorefineries | Elevated temperatures may affect thermolabile compounds |
| Supercritical CO2 Extraction (SFE) | CO2 above its critical point offers gas-like diffusivity and liquid-like solvating power/Carotenoids, sterols, lipophilic phenolics | Solvent-free extracts; high purity; recyclable CO2; environmentally safe | High capital cost; lower efficiency for highly polar compounds unless co-solvents are added |
| Natural Deep Eutectic Solvents (NADES) | Hydrogen-bonded natural components form tunable, biodegradable solvents/Polyphenols, flavonoids, alkaloids | High selectivity; biodegradable; 20–50% higher yields than aqueous ethanol | High viscosity; solvent recovery still challenging |
| Enzyme-Assisted Extraction (EAE) | Hydrolytic enzymes degrade polysaccharide matrices and release bound compounds/Bound phenolics, ferulates, encapsulated bioactives | Mild temperatures; reduced chemical use; enhanced release of bound phenolics; synergistic with UAE/PLE | Enzyme cost; longer processing times |
| Chemical Class | Identified Bioactives | Residue (Origin) and Circularity | Green Extraction Method and In Vitro Findings | Intended Application Circularity/LCA Notes | Ref. |
|---|---|---|---|---|---|
| Polyphenols (anthocyanins, flavanols, phenolic acids) | Catechin, epicatechin, gallic and caffeic acids | Grape pomace (wine coproduct), reused as antimicrobial/antioxidant ingredient | Aqueous/ethanolic UAE. Antioxidant and antibacterial activity vs. pathogens; antibiofilm effects | Food preservative, antioxidant system. Valorizes winery waste; replaces synthetic antioxidants | [62] |
| Ellagitannins/phenolics | Punicalagin, punicalin, ellagic acid | Pomegranate peel (juice waste), anti-inflammatory extract | Hydroethanolic extraction/NADES Reduced TNF-α–driven inflammation in Caco-2 cells; antioxidant capacity | Nutraceutical, anti-inflammatory High phenolic yield; integrates with juice lines | [21] |
| Flavonols | Quercetin, kaempferol | Onion skins (processing waste), pigment recovery | Ethanol-based UAE or MAE Strong antioxidant and antimicrobial activity | Natural colorant and antioxidant Utilizes outer skins; replaces synthetic dyes | [40] |
| Bound and free phenolics (hydroxy-cinnamates) | Ferulic, p-coumaric, vanillic, syringic acids | Brewers’ spent grain (brewery by-product), food/cosmetic use | Enzymatic hydrolysis + UAE. Tyrosinase inhibition; antioxidant activity | Cosmetic antioxidant, food ingredient High-volume residue; enzyme-assisted recovery | [28] |
| Polyphenols and methylxanthines | Theobromine, caffeine, catechins | Cocoa bean shells (chocolate industry waste) | Hydroethanolic extraction. Antimicrobial vs. oral and foodborne pathogens; antioxidant effect | Oral-care, food antimicrobial Upcycles cocoa shells; reduces waste | [32] |
| Carotenoids and phenolics | Lycopene, β-carotene, rutin | Tomato pomace (canning waste)—natural colorant | Pulsed ultrasound-assisted. Antioxidant protection against lipid oxidation | Colorant and antioxidant for foods Valorizes tomato residues; circular pigment source | [63] |
| Polyphenols Catechin | Epigalocatequina-3-galato, epicocatequina-3-galato, epigalocatequina, and epicocatequina | Tea pomace (spent leaves), nano-delivery system | Aqueous ethanolic extraction. Retained antioxidant activity in packaging composites | Active packaging, antioxidant release Reduces plastic waste; biodegradable systems | [64] |
| Polyphenols (apple) | Chlorogenic acid, phloridzin, quercetin | Apple pomace (juice residue), anti-inflammatory source | Cold Press-assisted solvent extraction. Antioxidant, anti-inflammatory, anti-thrombotic effects | Functional food ingredient Combined with pectin recovery | [65] |
| Coffee by-product fibers and phenolics | Chlorogenic acids, xylans | Coffee silverskin (roasting by-product) | Green UAE. Antioxidant and prebiotic activity; supports probiotics | Prebiotic ingredient Enables circular coffee chain | [66] |
| Fibers and phenolics | Insoluble fiber-bound polyphenols | Rice bran (milling residue) | Enzymatic and aqueous extraction. Antioxidant and lipid-peroxidation inhibition | Functional fiber ingredient Promotes zero-waste rice valorization | [67] |
| Chemical Class/Identified Bioactives | Residue Origin and Circularity/LCA Notes/Green Extraction | In Vivo Model and Outcomes/Intended Application | Ref. |
|---|---|---|---|
| Simple phenols (olive by-products). Hydroxytyrosol (HT) | Olive pomace/extra virgin olive oil side streams—purified HT. Adds value to olive pomace; aligns with European Union health claim context. Phenolics purified from side streams | Randomized controlled trial (RCT) (overweight/prediabetes): improved antioxidant/anti-inflammatory status Clinical-grade nutraceutical | [70] |
| Fibers and phenolics Insoluble fiber-bound polyphenols | Rice bran (milling residue) Enzymatic and aqueous extraction | Antioxidant and lipid-peroxidation inhibition Functional fiber ingredient Promotes zero-waste rice valorization | [67] |
| Citrus flavanones. Hesperidin, naringin | Citrus peels (juice industry)—standardized flavanones. Valorization with essential oils/pectin co-streams. Hydroethanolic extraction; standardization | Human RCTs/meta-analysis: improved lipids, blood pressure, and inflammatory markers. Cardiometabolic support | [71] |
| Maltodextrinated grape pomace extract | Randomized controlled clinical trial (99 patients) with grape pomace extract for diabetic retinopathy. | The grape pomace extract group showed improvement in best-corrected visual acuity | [85] |
| Grape pomace polyphenols (29.6%) | Randomized cross-over clinical trial was conducted (49 patients exhibiting at least two metabolic syndrome factors were supplemented with a daily dose of 8 g for 6 weeks, with an equivalent control (CTL) period. | The reduction in insulin levels in subjects at cardiometabolic risk upon grape pomace supplementation appears not to be induced by changes in the major subgroups of gut microbiota. | [84] |
| Industrial Areas | Bioactives Derived from Food Processing by-Products |
|---|---|
| Food and beverages | Extracts can serve as natural antioxidants, color stabilizers (e.g., lycopene from tomato pomace, anthocyanins from berry pomace), texturizers (e.g., citrus fiber, pectins from fruit peels), and prebiotic ingredients (e.g., silverskin from coffee, bran from grains) |
| Nutraceuticals and medical foods | Standardized extracts with specified marker compound concentrations (e.g., punicalagin in pomegranate extract, hesperidin in citrus extract, hydroxytyrosol in olive extract) can be used in nutraceutical formulations and medical foods |
| Cosmetics and personal care | Anti-oxidative and anti-inflammatory actives from sources like pomegranate peel, olive pomace, grape pomace, and cocoa shells can be incorporated into cosmetic and personal care products. Cocoa shell polyphenols can also be used as oral-care antimicrobials |
| Active packaging and biomaterials | Catechins from tea pomace and phenolic compounds from fruit peels can be embedded in biodegradable films for controlled release and oxidation control in active packaging applications |
| Residue/Waste Stream | Bioactive(s)/Functional Target | Key Findings/Highlights | Ref. |
|---|---|---|---|
| Jabuticaba (Plinia peruviana (Poir.) Govaerts) peel | Anthocyanins, total phenolics; antioxidant | Optimized probe-UAE and concentration of phenolics by up to 45%; identified cyanidin-3-glucoside and related anthocyanins. | [147] |
| Jabuticaba [Myrciaria jaboticaba (Vell.) O. Berg.] peel | Anthocyanins | The jabuticaba peel extract inhibited starch and very strongly triglyceride absorption in mice. | [148] |
| Jabuticaba (Myrciaria/Plinia jaboticaba). peel (and bagasse/seed in some works) | Anthocyanins (e.g., cyanidin-3-O-glucoside), ellagitannins, phenolic acids | Conventional solvent extraction; comparative seasonal profiling; nanoencapsulation in phospholipid vesicles Antioxidant capacity; in vitro anti-cancer/keratinocyte oxidative stress models; techno-functional flours | [146] |
| Cashew (Anacardium occidentale L.) apple bagasse | Phenolics, food-grade pectin | Hydrothermal/pressurized water processes enable simultaneous valorization (phenolics + pectin) from cashew apple bagasse; scalable route indicated. | [149] |
| Cupuaçu [Theobroma grandiflorum (Willd. ex Spreng.) Schum.] | Pectin | Microwave-assisted extraction of pectin from cupuaçu pod husk | [150] |
| Pequi (Caryocar brasiliense Camb.) peel | Phenolics; antioxidant | Characterization confirmed high phenolic content in pequi peel extracts; supports residue as source of functional ingredients | [151] |
| Buriti (Mauritia flexuosa L.) shell flour | Phenolics | Shell flour showed relevant phytochemicals and antioxidant potential; proposes waste-to-ingredient pathway for native Brazilian fruit residues. | [152] |
| Buriti (Mauritia flexuosa) shell flour | Carotenoids | High content of carotenoids, mainly β-carotene (27.18–62.94 µg/100 g) and α-carotene (18.23–60.28 µg/100 g) | [152] |
| Açaí (Euterpe oleracea Mart.) seeds (roasted)—upcycled beverage | Phenolics (e.g., chlorogenic acids, procyanidins); functional beverage | Roasted açaí seed proposed as caffeine-free “coffee” with characterized phenolics; illustrates food-grade upcycling of a major residue stream | [153] |
| Juçara (Euterpe edulis Mart.) fruit by-products | Phenolics, antioxidant, and antibacterial potential | The peel flour presented antioxidant and antibacterial potentials | [154] |
| Acerola (Malpighia emarginata DC) by-products | Phenolics; antioxidant and antibacterial | Probe-UAE optimized by Box–Behnken design increased total phenolic compounds recovery/antioxidant activity; antibacterial activity validated | [155] |
| Mango peel (Mangifera indica L.) | Phenolics and terpenoids; green solvent extraction | NADES-based UAE demonstrated as a green/efficient route for phenolics/terpenoids from peel. | [156] |
| Mango peel (Mangifera indica L.) | Phenolics and pectin | Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. | [157] |
| Baru (Dipteryx alata Vogel). peel/pulp/endocarp (under-used parts) | Diverse phenolics by Paper Spray Mass Spectrometry fingerprinting | Paper-spray mass spectrometry (profiling) + conventional extracts. Chemical mapping to support residue valorization. | [158] |
| Cagaita (Eugenia dysenterica DC) Peel + seed (by-products) | Catechin, epicatechin, quercetin (High-Performance Liquid Chromatography with Diode Array Detection), total phenolics | UAE was optimized by Response Surface Methodology. Antioxidant, antimicrobial, antibiofilm activities. | [159] |
| Buriti (Mauritia Flexuosa L.) Peel and pulp (including shell) | Carotenoids (β-carotene), phenolic compounds | Eco-friendly supramolecular solvents (octanoic-acid based) and ethanol, also with PLE. Antioxidant capacity; antibacterial/modulatory effects reported for extracts. | [160] |
| Bacaba (Oenocarpus bacaba Mart./Oenocarpus distichus Mart.). peel/residue | Anthocyanins, rutin, epicatechin; high total phenolics | Optimized solvent extraction; compositional profiling. High antioxidant capacity in residues; food prototype uses (e.g., beverages). | [161] |
| Guabiroba (Campomanesia xanthocarpa O. Berg.). Peel extract used as additive | Phenolics (tannins, flavonoids) | Hydroalcoholic extraction; incorporation study. Natural antioxidant/clean-label preservative in tilapia pâté. | [162] |
| Purple araçá (Psidium myrtoides O. Berg.) by-products (peel/seed) | Phenolic acids and flavonoids | NADES + UAE (green extraction) Antioxidant and in vitro antidiabetic activity | [49] |
| Camu-camu [Myrciaria dubia (Kunth) McVaugh] peel and seed (industrial by-residues) | Ellagic acid derivatives, procyanidins, other phenolics | Conventional/optimized extractions; response-surface optimization for seed coat. Antioxidant; anti-diabetic/anti-hypertensive/antiproliferative in vitro; food fortification. | [163] |
| Açaí (Euterpe oleracea Mart.) Seeds (major processing waste) | Procyanidins (B type), catechin/epicatechin | PLE; tech-economic assessed. Antioxidant extracts; feasibility for industrial recovery. | [164] |
| Umbu (Spondias tuberosa Arruda) peel | Phenolics (Ultra-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry) | Thermal-assisted solid–liquid extraction optimized by Response Surface Methodology. Antioxidant, antimicrobial; α-amylase inhibition. | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagueyam, S.S.; dos Santos Filho, J.R.; Contato, A.G.; de Souza, C.G.M.; Castoldi, R.; Corrêa, R.C.G.; Conte Junior, C.A.; Yamaguchi, N.U.; Bracht, A.; Peralta, R.M. Green Extraction of Bioactive Compounds from Plant-Based Agri-Food Residues: Advances Toward Sustainable Valorization. Plants 2025, 14, 3597. https://doi.org/10.3390/plants14233597
Kagueyam SS, dos Santos Filho JR, Contato AG, de Souza CGM, Castoldi R, Corrêa RCG, Conte Junior CA, Yamaguchi NU, Bracht A, Peralta RM. Green Extraction of Bioactive Compounds from Plant-Based Agri-Food Residues: Advances Toward Sustainable Valorization. Plants. 2025; 14(23):3597. https://doi.org/10.3390/plants14233597
Chicago/Turabian StyleKagueyam, Samanta Shiraishi, José Rivaldo dos Santos Filho, Alex Graça Contato, Cristina Giatti Marques de Souza, Rafael Castoldi, Rúbia Carvalho Gomes Corrêa, Carlos Adam Conte Junior, Natália Ueda Yamaguchi, Adelar Bracht, and Rosane Marina Peralta. 2025. "Green Extraction of Bioactive Compounds from Plant-Based Agri-Food Residues: Advances Toward Sustainable Valorization" Plants 14, no. 23: 3597. https://doi.org/10.3390/plants14233597
APA StyleKagueyam, S. S., dos Santos Filho, J. R., Contato, A. G., de Souza, C. G. M., Castoldi, R., Corrêa, R. C. G., Conte Junior, C. A., Yamaguchi, N. U., Bracht, A., & Peralta, R. M. (2025). Green Extraction of Bioactive Compounds from Plant-Based Agri-Food Residues: Advances Toward Sustainable Valorization. Plants, 14(23), 3597. https://doi.org/10.3390/plants14233597












