Industrial Applications of Different Parts of Flatland Polygonum cuspidatum by Combining Microwave-Assisted Extraction and Fermentation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Materials
2.2. MAE of P. cuspidatum
- (1)
- Extraction temperature (x1, °C)
- (2)
- Extraction time (x2, s)
- (3)
- L/S (x3, mL/g)
2.3. Fermentation of P. cuspidatum Flower Extract
2.4. Measurement of Extracellular Tyrosinase Activity
2.5. Measurement of Tyrosinase Activity and Melanin Content in Human HEMn Cells
2.6. Assessment of TPC and TFC
2.7. Assessment of DPPH and ABTS Free Radical Scavenging Activities
2.8. Measurement of Antiaging Activity
2.9. Measurement of Antimicrobial Activity
2.10. Measurement of Anti-Inflammatory Activity
2.11. Assessment of Cell Survival and Wound Healing Ability
2.12. Analysis of Chemical Composition
2.13. Statistical Analysis
3. Results and Discussion
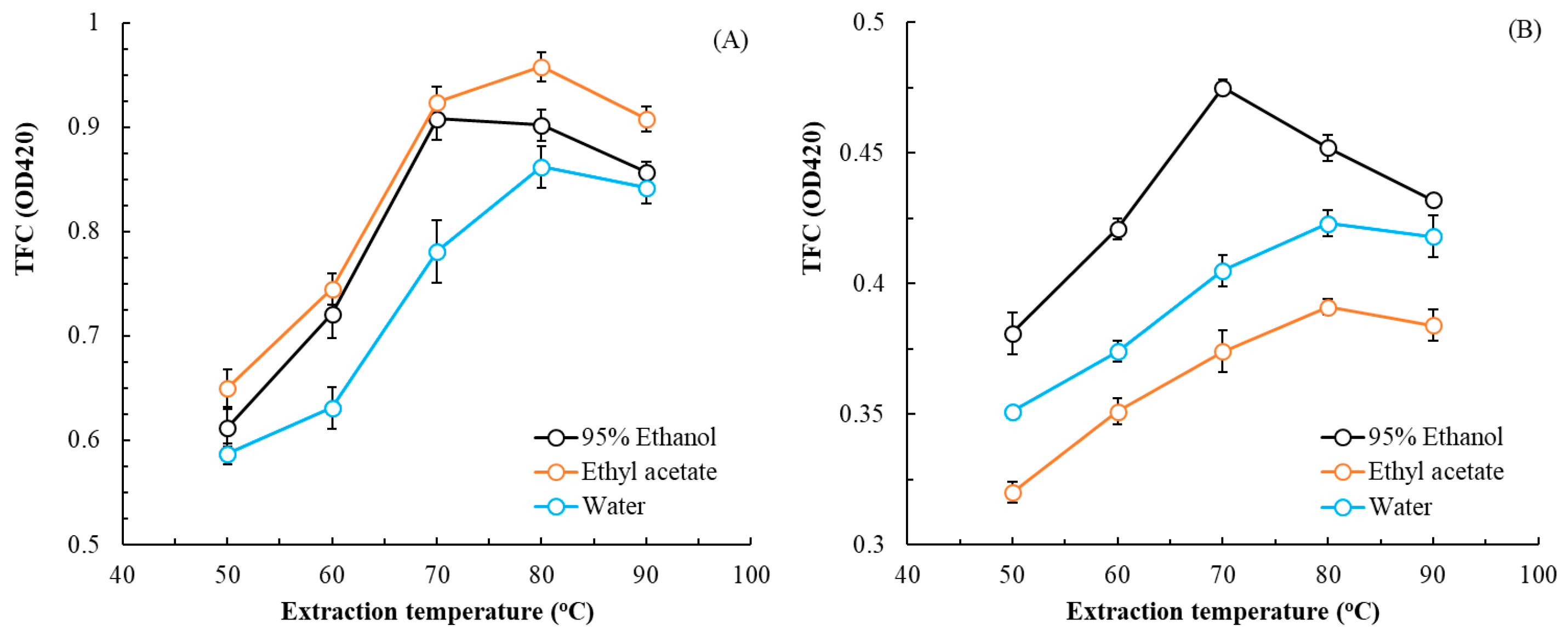
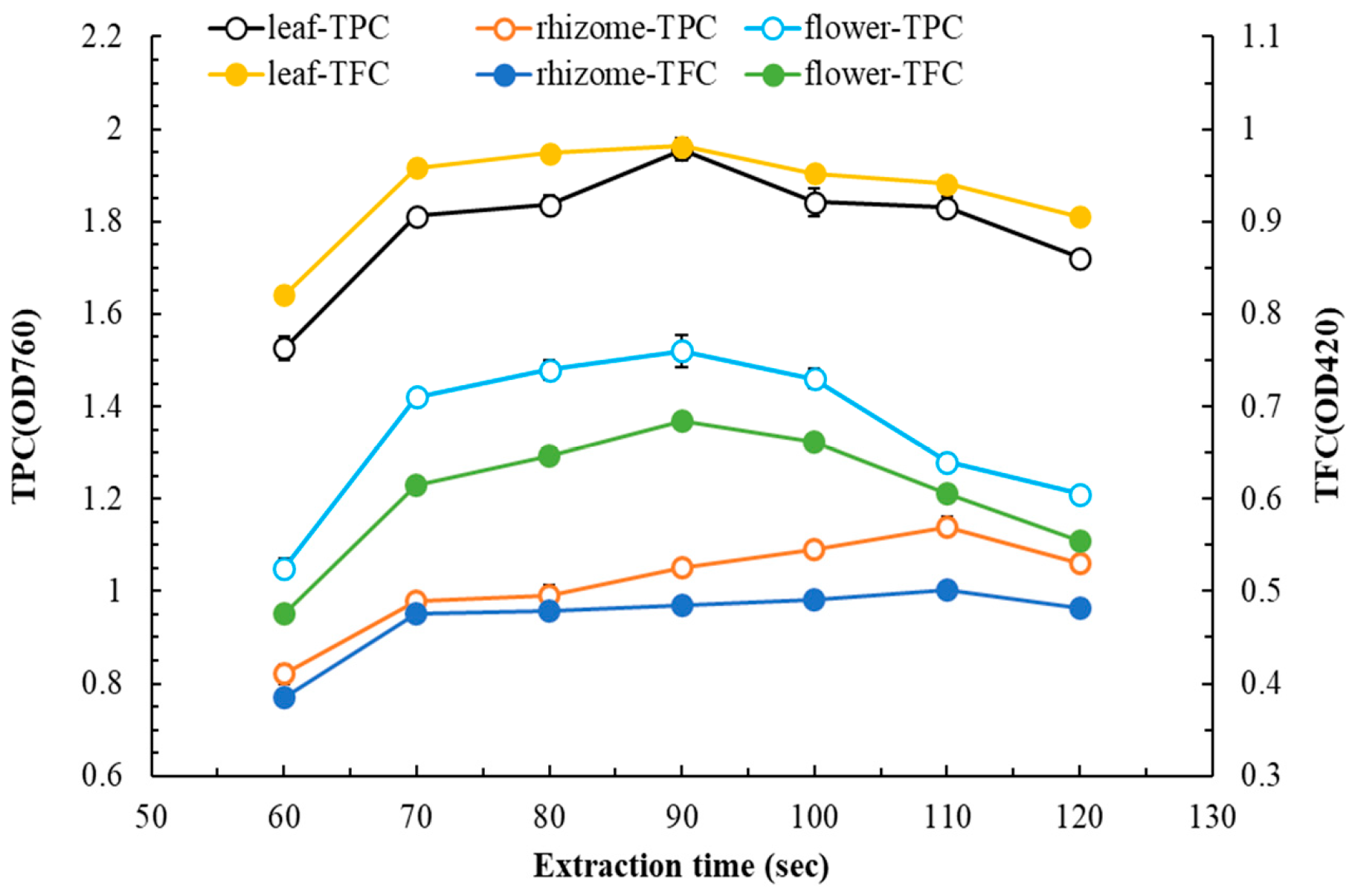
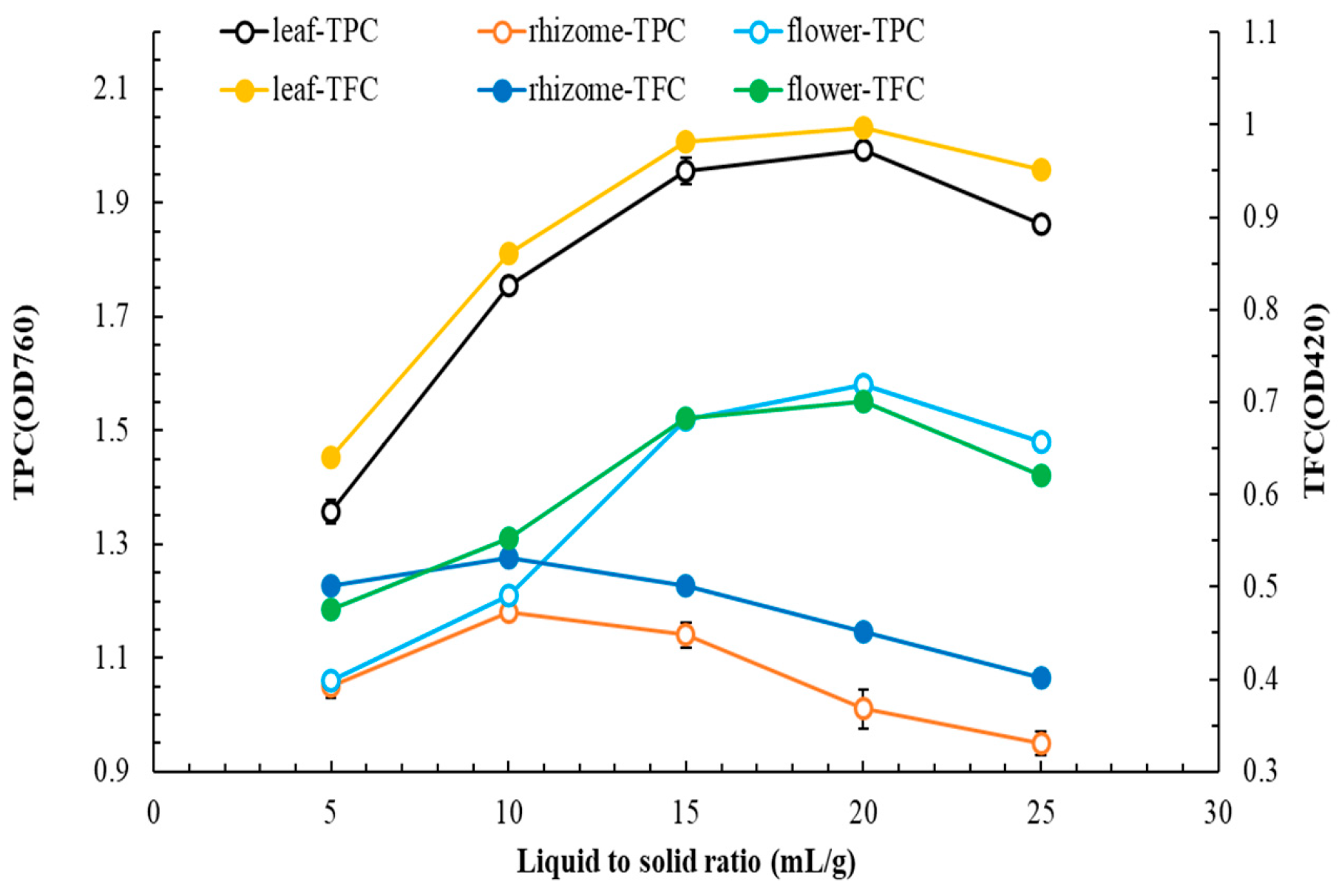
3.1. MAE of Flatland P. cuspidatum—Single-Factor Experiments
3.2. MAE of Flatland P. cuspidatum—Experimental Design
3.3. Whitening and Antioxidant Effects of Flatland P. cuspidatum Extracts
3.4. Antiaging and Antimicrobial Effects of Flatland P. cuspidatum Extract
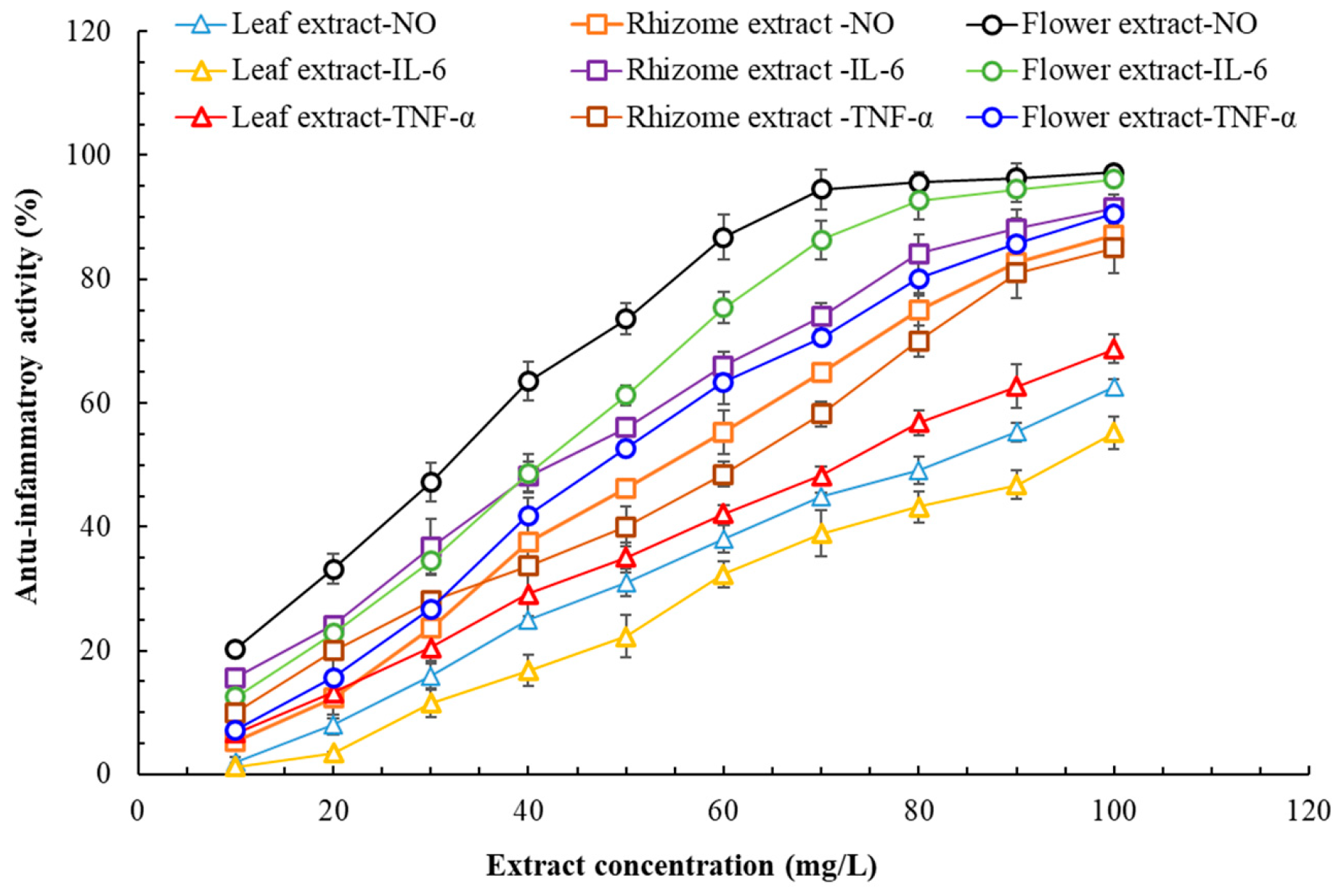
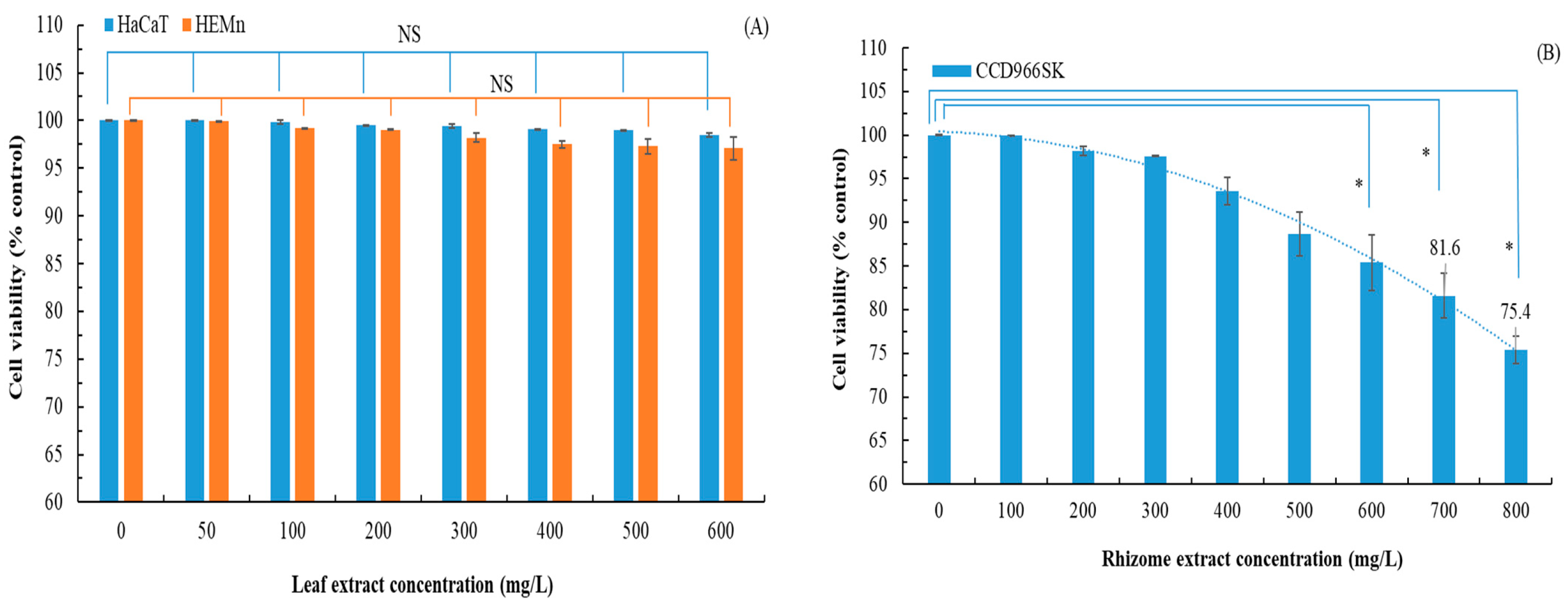
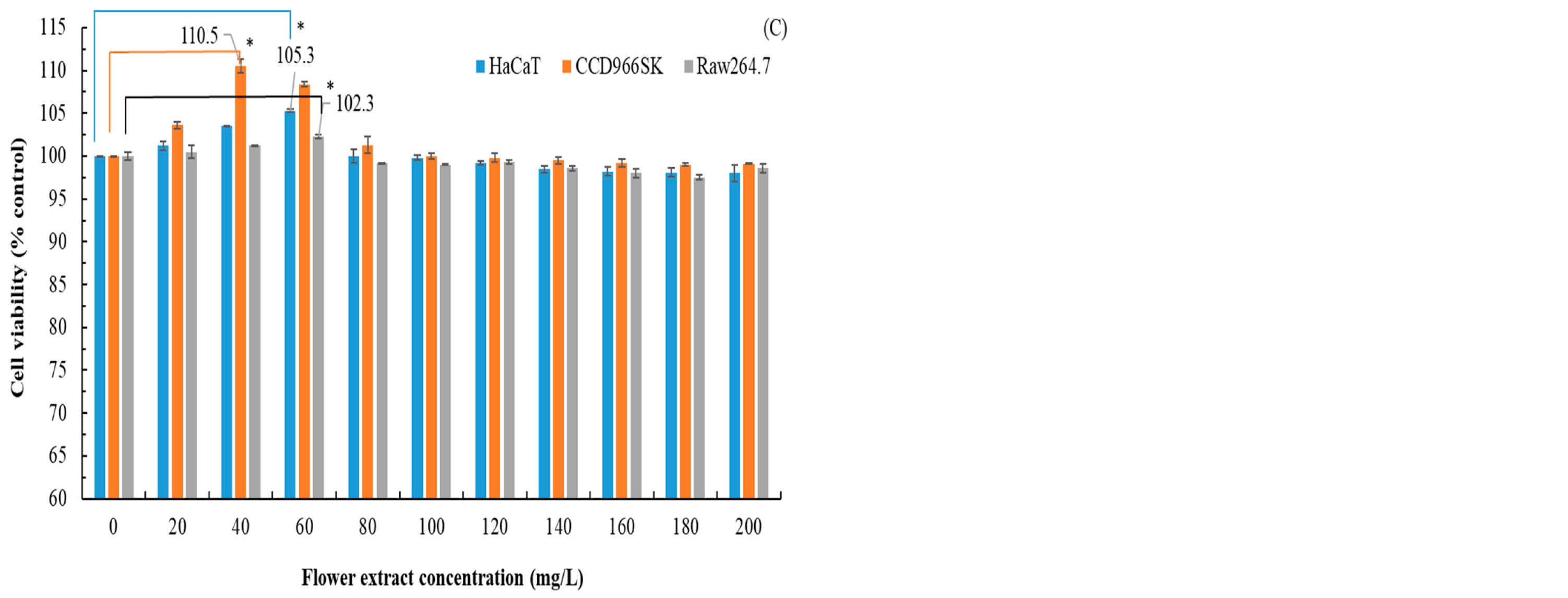
3.5. Anti-Inflammatory Effects, Wound Healing Ability, and Cytotoxicity of Flatland P. cuspidatum Extracts
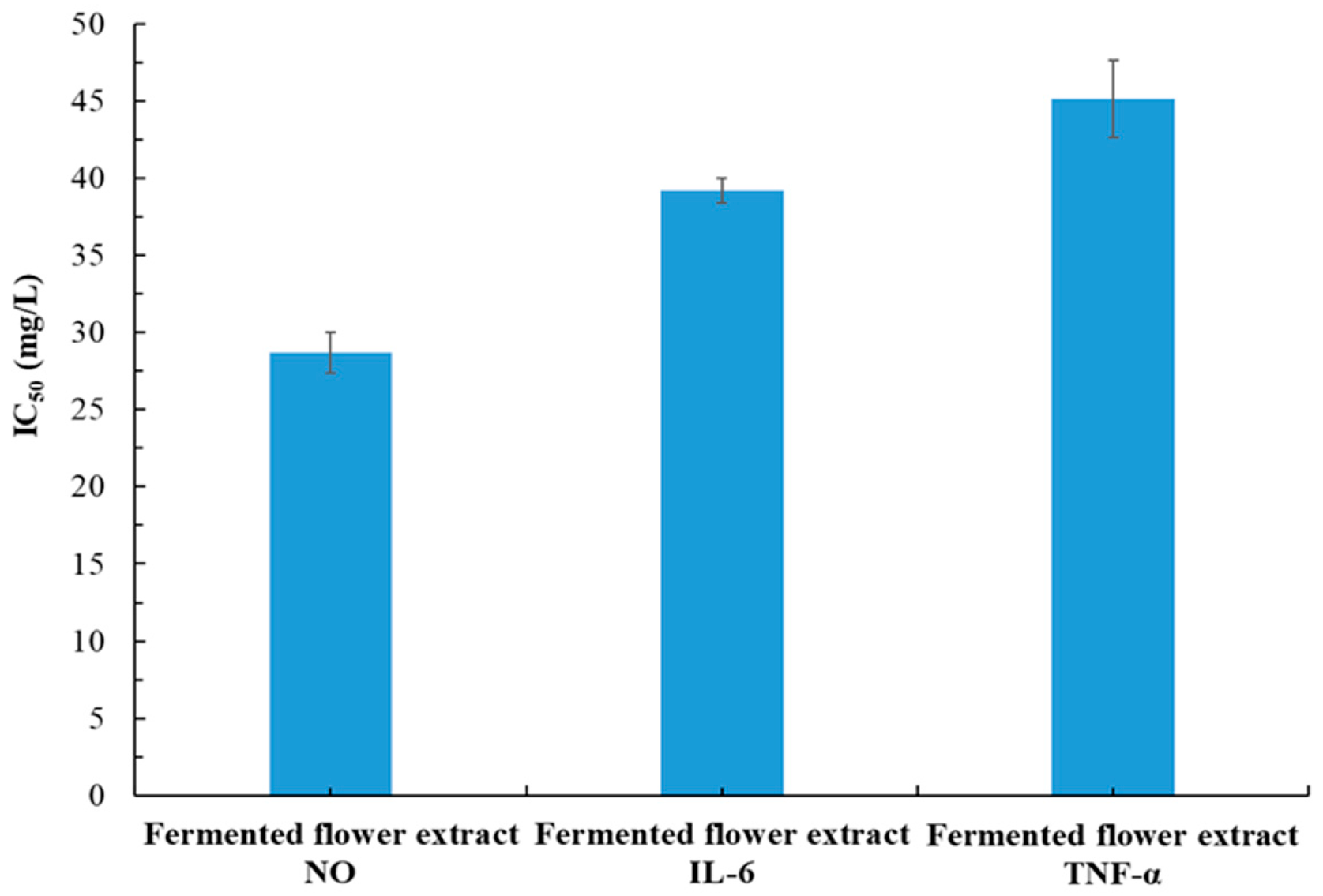
3.6. Effects of Fermentation on Pharmacological Activities of P. cuspidatum Flower Extract
3.7. Major Chemical Compositions and Contents of P. cuspidatum Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Li, C.; Kwok, S.T.; Zhang, Q.W.; Chan, S.W. A review of the pharmacological effects of the dried root of Polygonum cuspidatum (Hu Zhang) and its constituents. Evid. Based. Complement. Alternat. Med. 2012, 2013, 208349. [Google Scholar]
- Ke, J.; Li, M.T.; Xu, S.; Ma, J.; Liu, M.Y.; Han, Y. Advances for pharmacological activities of Polygonum cuspidatum—A review. Pharm. Biol. 2023, 61, 177–188. [Google Scholar] [CrossRef]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany phytochemistry pharmacology potential application of Polygonum cuspidatum Siebet Zucc: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Zhang, Q.; Liu, Z.; Zhang, T.; Wang, L.; Liu, J.; He, T.; Chen, Y.; Feng, J.; Chen, Y. Polygonum cuspidatum extract exerts antihyperlipidemic effects by regulation of PI3K/Akt/Foxo3 signaling pathway. Oxid. Med. Cell. Longev. 2021, 2021, 3830671. [Google Scholar] [CrossRef]
- Choi, D.H.; Han, J.H.; Yu, K.H.; Hong, M.; Lee, S.Y.; Park, K.H.; Lee, S.U.; Kwon, T.H. Antioxidant and anti-obesity activities of Polygonum cuspidatum extract through alleviation of lipid accumulation on 3T3-L1 adipocytes. J. Microbiol. Biotechnol. 2020, 30, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, T.T.; Zhang, T.; Jia, H.M.; Li, J.J.; Zhang, H.W.; Zou, Z.M. Anti-inflammatory constituents in the root and rhizome of Polygonum cuspidatum by UPLC-PDA-QTOF/MS and lipopolysaccharide-activated RAW264.7 macrophages. J. Pharm. Biomed. Anal. 2021, 195, 113839. [Google Scholar] [CrossRef]
- Sharma, K.R.; Adhikari, S. Phytochemical analysis biological activities of Artemisia vulgaris grown in different altitudes of Nepal. Int, J. Food Prop. 2023, 26, 414–427. [Google Scholar] [CrossRef]
- Wang, G.H.; Lin, Y.M.; Kuo, J.T.; Lin, C.P.; Chang, C.F.; Hsieh, M.C.; Cheng, C.Y.; Chung, Y.C. Comparison of biofunctional activity of Asparagus cochinchinensis (Lour.) Merr. extract before and after fermentation with Aspergillus oryzae. J. Biosci. Bioeng. 2019, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Ye, L.; Chen, X.; Meng, Y.; Zhou, L.; Chen, Q.; Zheng, G.; Hu, J.; Shi, Z. Screening of key components for melanogenesis inhibition of Polygonum cuspidatum extract based on the spectrum–effect relationship and molecular docking. Molecules 2024, 29, 857. [Google Scholar] [CrossRef]
- Park, B.; Jo, K.; Lee, T.G.; Lee, I.S.; Kim, J.S.; Kim, C.S. Polygonum cuspidatum stem extract (PSE) ameliorates dry eye disease by inhibiting inflammation apoptosis. J. Exerc. Nutr. Biochem. 2019, 23, 14–22. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Song, S.; Xiao, Z.; Chen, X.; Huang, B.; Sun, M.; Su, G.; Zhou, D.; Wang, G.; et al. Effective inhibition of coronavirus replication by Polygonum cuspidatum. Front. Biosci. 2021, 2, 789–798. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and evaluation of dermo-cosmetic activities of the invasive plant species Polygonum cuspidatum. Plants 2022, 12, 83. [Google Scholar] [CrossRef]
- Fletes-Vargas, G.; Rodríguez-Rodríguez, R.; Pacheco, N.; Pérez-Larios, A.; Espinosa-Andrews, H. Evaluation of the biological properties of an optimized extract of Polygonum cuspidatum using ultrasonic-assisted extraction. Molecules 2023, 8, 4079. [Google Scholar] [CrossRef] [PubMed]
- Ruan, N.; Jiao, Z.; Tang, L. Response surface methodology to optimize supercritical carbon dioxide extraction of Polygonum cuspidatum. J. AOAC Int. 2022, 105, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High biological value compounds extraction from citrus waste with non-conventional methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Bagade, S.B.; Patil, M. Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: A review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. The comparative influence of novel extraction technologies on in vitro prebiotic-inducing chemical properties of fucoidan extracts from Ascophyllum nodosum. Food Hydrocoll. 2019, 90, 462–471. [Google Scholar] [CrossRef]
- Wang, G.H.; Chen, C.Y.; Tsai, T.H.; Chen, C.K.; Cheng, C.Y.; Huang, Y.H.; Hsieh, M.C.; Chung, Y.C. Evaluation of tyrosinase inhibitory and antioxidant activities of Angelica dahurica root extracts for four different probiotic bacteria fermentations. J. Biosci. Bioeng. 2017, 123, 679–684. [Google Scholar] [CrossRef]
- Wu, L.; Chen, C.Y.; Cheng, C.Y.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y.C. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed. Res. Int. 2018, 2018, 5201786. [Google Scholar]
- Chen, C.Y.; Wang, G.H.; Chang, Y.C.; Liu, S.; Lin, Y.T.; Lai, Y.L.; Chung, Y.C. Cosmeceutical application of extracts from the flowers, stems, and leaves of Buddleja davidii grown at different altitudes. Front. Pharmacol. 2025, 16, 1551134. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Chen, C.Y.; Hu, C.Y.; Chen, Y.H.; Li, Y.T.; Chung, Y.C. Submerged fermentation with Lactobacillus brevis significantly improved the physiological activities of Citrus aurantium flower extract. Heliyon 2022, 8, e10498. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Geurts, N.; Martens, E.; Van den Steen, P.E.; Jonghe, S.D.; Herdewijn, P.; Opdenakker, G. Gelatin degradation assay reveals MMP-9 inhibitors and function of O-glycosylated domain. World J. Biol. Chem. 2011, 2, 14–24. [Google Scholar] [CrossRef]
- Millar, B.C.; Rao, J.R.; Moore, J.E. Fighting antimicrobial resistance (AMR): Chinese herbal medicine as a source of novel antimicrobials-an update. Lett. Appl. Microbiol. 2021, 73, 400–407. [Google Scholar] [CrossRef]
- Varthaliti, A.; Lygizos, V.; Fanaki, M.; Pergialiotis, V.; Papapanagiotou, A.; Pappa, K.; Theodora, M.; Daskalaki, M.A.; Antsaklis, P.; Daskalakis, G. The role of IL-6 and TNF-α as early biomarkers in the prediction and diagnosis of gestational diabetes mellitus. Biomedicines 2025, 13, 1627. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric oxide: Physiological functions, delivery, and biomedical applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Cornara, L.; Burlando, B.; Cascini, A.; Denaro, M.; Smeriglio, A.; Trombetta, D. Carpobrotus edulis (L) NEBr extract as a skin preserving agent: From traditional medicine to scientific validation. J. Integr. Med. 2021, 19, 526–536. [Google Scholar] [CrossRef]
- Li, L.; Song, X.; Yin, Z.; Jia, R.; Li, Z.; Zhou, X.; Zou, Y.; Li, L.; Yin, L.; Yue, G.; et al. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol. Res. 2016, 186, 139–145. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Wen, D.; Guo, J.; Xiong, Q.; Li, P.; Zhao, L.; Wong, J.; Wu, C.; Dong, L. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immun. Inflamm. Dis. 2021, 9, 959–970. [Google Scholar] [CrossRef]
- Hadzik, J.; Choromańska, A.; Karolewicz, B.; Matkowski, A.; Dominiak, M.; Złocińska, A.; Nawrot-Hadzik, I. Oral wound healing potential of Polygoni Cuspidati Rhizoma et Radix decoction-in vitro study. Pharmaceuticals 2023, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Luo, X.Q.; Gu, S.Y.; Xu, J.H. The effects of Polygonum cuspidatum extract on wound healing in rats. J. Ethnopharmacol. 2012, 141, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.V.B.; Queiroz, L.D.; Barcelos, R.; Primo, L.S.G.; Maia, L.C.; Alves, G.G. MTT versus other cell viability assays to evaluate the biocompatibility of root canal filling materials: A systematic review. Int. Endod. J. 2020, 53, 1348–1373. [Google Scholar] [CrossRef]
- He, X.J.; Fu, J.X.; Jiao, J.; Gai, Q.Y.; Fu, Y.J.; Feng, X.; Wang, Y. Semi-solid-state fermentation of Polygonum cuspidatum roots by a novel endophytic fungus Penicillium rubens with capabilities of cell wall hydrolysis and polydatin deglycosylation to improve the yield of high-value resveratrol. Ind. Crop. Prod. 2024, 209, 117964. [Google Scholar] [CrossRef]
- Zeng, H.J.; Li, Q.Y.; Ma, J.; Yang, R.; Qu, L.B. A comparative study on the effects of resveratrol and oxyresveratrol against tyrosinase activity and their inhibitory mechanism. Spectrochim. Acta. Mol. Biomol. Spectrosc. 2021, 251, 119405. [Google Scholar] [CrossRef]
- Chen, J.; Ye, W. Molecular mechanisms underlying Tao-Hong-Si-Wu decoction treating hyperpigmentation based on network pharmacology, Mendelian randomization analysis, and experimental verification. Pharm. Biol. 2024, 62, 296–313. [Google Scholar] [CrossRef]
- Blom van Staden, A.; Oosthuizen, C.B.; Lall, N. The effect of Aspalathus linearis (Burm.f.) R. Dahlgren and its compounds on tyrosinase and melanogenesis. Sci. Rep. 2021, 11, 7020. [Google Scholar] [CrossRef]
- Besrour, N.; Oludemi, T.; Mandim, F.; Pereira, C.; Dias, M.I.; Soković, M.; Stojković, D.; Ferreira, O.; Ferreira, I.C.F.R.; Barros, L. Valorization of Juglans regia leaves as cosmeceutical ingredients: Bioactivity evaluation and final formulation development. Antioxidants 2022, 11, 677. [Google Scholar] [CrossRef]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb leaves. Food Chem. 2013, 138, 327–333. [Google Scholar] [CrossRef]
- Aires, A.; Dias, C.; Carvalho, R.; Saavedra, M.J. Analysis of glycosylated flavonoids extracted from sweet-cherry stems, as antibacterial agents against pathogenic Escherichia coli isolates. Acta Biochim. Pol. 2017, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Molee, W.; Phanumartwiwath, A.; Kesornpun, C.; Sureram, S.; Ngamrojanavanich, N.; Ingkaninan, K.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Naphthalene derivatives and quinones from Ventilago denticulata and their nitric oxide radical scavenging, antioxidant, cytotoxic, antibacterial, and phosphodiesterase inhibitory activities. Chem. Biodivers. 2018, 15, e1700537. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Xu, J.; Liu, S.; Song, Z.; Chen, T.; Deng, X.; Wang, J.; Lv, Q. Quercitrin is a novel inhibitor of Salmonella enterica serovar Typhimurium type III secretion system. Molecules 2023, 28, 5455. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.H.; Chen, Y.J.; Chung, T.Y.; Lin, N.H.; Chen, W.Y.; Chen, C.Y.; Lee, M.R.; Chou, C.C.; Tzen, J.T. Emoghrelin, a unique emodin derivative in Heshouwu, stimulates growth hormone secretion via activation of the ghrelin receptor. J. Ethnopharmacol. 2015, 159, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Sisubalan, N.; Wang, S.; Kesika, P.; Chaiyasut, C. Exploring the therapeutic potential of green tea (Camellia sinensis L.) in Anti-aging: A comprehensive review of mechanisms and findings. Mini Rev. Med. Chem. 2025, 25, 403–424. [Google Scholar] [CrossRef]
- Fan, P.; Zhang, T.; Hostettmann, K. Anti-inflammatory Activity of the invasive neophyte Polygonum cuspidatum Sieb. and Zucc. (Polygonaceae) and the chemical comparison of the invasive and native varieties with regard to resveratrol. J. Tradit. Complement. Med. 2013, 3, 182–187. [Google Scholar] [CrossRef]
- XunLi Liu, Y.; Chu, S.; Yang, S.; Peng, Y.; Ren, S.; Wen, B.; Chen, N. Physcion and physcion 8-O-β-glucopyranoside: A review of their pharmacology, toxicities and pharmacokinetics. Chem. Biol. Interact. 2019, 310, 108722. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Ritmejerytė, E.; Ryan, R.Y.M.; Byatt, B.J.; Peck, Y.; Yeshi, K.; Daly, N.L.; Zhao, G.; Crayn, D.; Loukas, A.; Pyne, S.G.; et al. Anti-inflammatory properties of novel galloyl glucosides isolated from the Australian tropical plant Uromyrtus metrosideros. Chem. Biol. Interact. 2022, 368, 110124. [Google Scholar] [CrossRef]
- Georgiou, N.; Kakava, M.G.; Routsi, E.A.; Petsas, E.; Stavridis, N.; Freris, C.; Zoupanou, N.; Moschovou, K.; Kiriakidi, S.; Mavromoustakos, T. Quercetin: A potential polydynamic drug. Molecules 2023, 28, 8141. [Google Scholar] [CrossRef]
- Bagheri Bavandpouri, F.S.; Azizi, A.; Abbaszadeh, F.; Kiani, A.; Farzaei, M.H.; Mohammadi-Noori, E.; Fakhri, S.; Echeverría, J. Polydatin attenuated neuropathic pain and motor dysfunction following spinal cord injury in rats by employing its anti-inflammatory and antioxidant effects. Front. Pharmacol. 2024, 15, 1452989. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, X.; Sallam, A.S.; Dong, W.; Sun, Y.; Zeng, X.; Liu, Z. Extraction, isolation, identification and bioactivity of anthraquinones from Aspergillus cristatus derived from Fuzhaun brick tea. Food Chem. 2025, 474, 143104. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; He, Z.; Tan, G.; Zou, Y.; Xie, J.; Qian, Z. Screening of tyrosinase, xanthine oxidase, and α-glucosidase inhibitors from Polygoni Cuspidati Rhizoma et Radix by ultrafiltration and HPLC analysis. Molecules 2023, 28, 4170. [Google Scholar] [CrossRef]
- Yu, H.R.; Chen, B.H. Analysis of Phenolic acids and flavonoids in rabbiteye blueberry leaves by UPLC-MS/MS and preparation of nanoemulsions and extracts for improving antiaging effects in mice. Foods 2023, 12, 1942. [CrossRef] [PubMed]
- Hatano, T.; Uebayashi, H.; Ito, H.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 1999, 47, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Kengne, I.C.; Feugap, L.D.T.; Njouendou, A.J.; Ngnokam, C.D.J.; Djamalladine, M.D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Tamokou, J.D. Antibacterial, antifungal and antioxidant activities of whole plant chemical constituents of Rumex abyssinicus. BMC Complement. Med. Ther. 2021, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, R.; Li, Q.; Peñalver, P.; Belmonte-Reche, E.; Andrés-Bilbao, M.; Lucas, R.; de Paz, M.V.; Kuipers, O.P.; Morales, J.C. Chemically tuning resveratrol for the effective killing of gram-positive pathogens. J. Nat. Prod. 2022, 85, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, H.; Wang, Y. Investigation of Chinese herbal decoctions with enzymatic hydrolysis and sequential fermentation as potential nutrient supplements. Appl. Sci. 2023, 13, 2154. [Google Scholar] [CrossRef]
- Fang, C.; Wang, J.; Ma, S.; Huang, W.; Liu, X.; He, M.; He, F.; Fu, J. Valorization of the non-medicinal parts of Polygonatum sibiricum and Gentiana scabra Bunge from Liaoning via solid-state co-fermentation: Synergistic antibacterial enhancement. Fermentation 2025, 11, 643. [Google Scholar] [CrossRef]



| In Vitro Antityrosinase Activity | In Vivo Antityrosinase Activity | Melanin Content in HEMn Cells | |
|---|---|---|---|
| Leaf extract | 58.4 ± 2.6 | 66.8 ± 9.2 | 41.3 ± 5.8 |
| Rhizome extract | 72.6 ± 11.7 | 81.5 ± 15.5 | 80.7 ± 26.8 |
| Flower extract | 417.1 ± 36.5 | 436.7 ± 41.3 | 435.1 ± 34.7 |
| Fermented flower extract | − | − | 57.9 ± 7.1 |
| DPPH (IC50, mg/L) | ABTS (IC50, mg/L) | TPC (mg-GAE/g-DW) | TFC (mg-RE/g-DW) | |
|---|---|---|---|---|
| Leaf extract | 62.7 ± 9.2 | 12.7 ± 3.9 | 127.5 ± 14.6 | 460.4 ± 37.2 |
| Rhizome extract | 17.4 ± 2.3 | 40.6 ± 5.6 | 396.3 ± 21.8 | 255.5 ± 19.4 |
| Flower extract | 105.2 ± 5.8 | 18.5 ± 2.3 | 102.4 ± 10.1 | 323.5 ± 26.7 |
| Fermented flower extract | − | − | 360.5 ± 19.2 | 446.4 ± 30.6 |
| MMP-1 | Collagenase | Elastase | |
|---|---|---|---|
| Leaf extract | 92.1 ± 10.4 | 225.7 ± 23.4 | 49.6 ± 3.7 |
| Rhizome extract | 63.2 ± 7.5 | 201.4 ± 30.6 | 276.5 ± 12.5 |
| Flower extract | 95.6 ± 8.2 | 263.1 ± 14.3 | 102.6 ± 15.4 |
| Fermented flower extract | 54.6 ± 7.2 | 200.8 ± 27.3 | 55.2 ± 6.3 |
| Leaf Extract | Rhizome Extract | Flower Extract | Fermented Flower Extract | ||
|---|---|---|---|---|---|
| Bacteria (MBC, mg/L) | |||||
| B. subtilis | 200 ± 35.4 | 300 ± 35.4 | 800 ± 70.7 | 400 ± 0.0 | |
| G(+) | S. aureus | 150 ± 35.4 | 367 ± 20.4 | 700 ± 35.4 | 200 ± 0.0 |
| E. faecalis | 200 ± 0.0 | 400 ± 0.0 | 750 ± 35.4 | 400 ± 0.0 | |
| C. acnes | 233 ± 20.4 | 300 ± 35.4 | 1000 ± 70.7 | 367 ± 20.4 | |
| E. coli | 200 ± 0.0 | 517 ± 54.0 | 450 ± 35.4 | 200 ± 0.0 | |
| G(−) | P. aeruginosa | 217 ± 20.4 | 1700 ± 141.4 | 450 ± 0.0 | 200 ± 0.0 |
| A. baumannii | 400 ± 35.4 | 1500 ± 0.0 | 500 ± 0.0 | 217 ± 20.4 | |
| Fungi (MFC, mg/L) | |||||
| C. albicans | 350 ± 0.0 | 500 ± 70.7 | 1500 ± 70.7 | 350 ± 35.4 | |
| A. brasiliensis | 300 ± 0.0 | 833 ± 73.6 | – * | 500 ± 70.7 | |
| E. floccosum | 350 ± 35.4 | 1000 ± 0.0 | – | 500 ± 70.7 | |
| Chemical Compounds | RT (min) | Leaf Extract | Rhizome Extract | Flower Extract | Fermented Flower Extract |
|---|---|---|---|---|---|
| Relative Content (%) | |||||
| Galloyl glucoside | 3.41 | 0 | 0.13 | 0.23 | 0.25 |
| 3-O-Caffeoylquinic acid | 3.85 | 3.12 | 0 | 0.15 | 0.78 |
| p-coumaroylquinic acid | 4.32 | 2.89 | 0 | 0.21 | 0.26 |
| 5-O-caffeoylquinic acid | 4.56 | 5.41 | 1.45 | 2.14 | 2.25 |
| Decursin | 5.81 | 0.57 | 3.82 | 0.25 | 1.05 |
| Catechin-5-O-glucoside | 7.12 | 2.28 | 0.12 | 1.71 | 1.02 |
| Catechin | 8.13 | 3.45 | 0.31 | 1.23 | 1.03 |
| Epicatechin | 10.72 | 3.15 | 0.33 | 1.62 | 1.14 |
| Resveratroloside | 11.57 | 0.57 | 1.74 | 2.14 | 0.95 |
| Polydatin | 11.83 | 7.32 | 0.48 | 10.62 | 10.37 |
| Polydatin gallate | 12.05 | 0.18 | 0.62 | 0.53 | 0.48 |
| Quercetin | 12.27 | 2.34 | 0 | 6.12 | 7.35 |
| Quercetin 3-O-pentoside | 12.54 | 5.12 | 0 | 5.63 | 4.86 |
| Quercitrin | 13.71 | 10.34 | 2.87 | 4.68 | 5.27 |
| Kaempferol | 13.85 | 3.65 | 0 | 8.57 | 9.24 |
| Resveratrol | 14.52 | 17.62 | 13.63 | 12.74 | 13.68 |
| Epicatechin gallate | 15.87 | 0.23 | 0.63 | 0 | 0.35 |
| Resveratrol-3-O-D-(2-galloyl)-glucopyranoside | 16.48 | 0 | 3.21 | 2.08 | 2.94 |
| Resveratrol-4′-O-β-D-glucoside | 17.26 | 0 | 0.12 | 0.71 | 0.48 |
| Emodin-1-O-β-D-glucoside | 18.52 | 0.35 | 7.72 | 0.92 | 0.71 |
| Resveratrol-4′-O-β-D-glucoside (isomer) | 19.08 | 0.71 | 0.66 | 0.27 | 0.38 |
| Apigenin | 19.35 | 2.45 | 0.28 | 0.42 | 0.61 |
| Luteoin-7-O-glucoside | 20.01 | 0 | 0.24 | 0.31 | 0.22 |
| Emodin-O-(galloyl)-glucoside | 20.28 | 0 | 0.29 | 1.92 | 1.51 |
| Torachrysone-8-O-glucoside | 21.37 | 0 | 8.23 | 0.83 | 0.62 |
| Emodin-8-O-β-D-glucoside | 21.68 | 1.12 | 18.11 | 2.72 | 2.09 |
| Emodin-O-(galloyl)-glucoside | 23.14 | 0 | 0.18 | 1.07 | 0.84 |
| Acetylemodin-O-glucoside | 24.18 | 0 | 0.84 | 0 | 0 |
| Emodin-8-O-(6′-O-malonyl)-glucoside | 23.57 | 0.95 | 0.83 | 0.43 | 0.72 |
| Emodin-6-O-glucoside | 24.62 | 0.32 | 0.24 | 0.51 | 0.38 |
| Emodin-1-questin | 24.81 | 0.27 | 1.54 | 0.36 | 0.13 |
| Hydroxyl aloe-emodin | 24.99 | 0 | 0.18 | 0.24 | 0.15 |
| Torachrysone-8-O-(acetyl)-glucoside | 25.34 | 0 | 0.42 | 0.57 | 0.31 |
| citreorosein | 25.92 | 5.17 | 1.06 | 16.61 | 15.39 |
| Physcion glucoside | 26.14 | 0 | 0.52 | 2.61 | 2.04 |
| Physcion | 27.35 | 0.27 | 0.37 | 1.24 | 1.93 |
| emodin-3-methyl ether | 28.71 | 7.91 | 1.52 | 1.67 | 1.24 |
| Emodin | 30.67 | 12.24 | 22.55 | 5.41 | 6.03 |
| Torachrysone | 31.51 | 0 | 4.76 | 0.53 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Wang, G.-H.; Kuo, J.-T.; Hsu, P.-N.; Shen, Y.-C.; Chen, Y.-H.; Chung, Y.-C. Industrial Applications of Different Parts of Flatland Polygonum cuspidatum by Combining Microwave-Assisted Extraction and Fermentation Process. Plants 2025, 14, 3572. https://doi.org/10.3390/plants14233572
Chen C-Y, Wang G-H, Kuo J-T, Hsu P-N, Shen Y-C, Chen Y-H, Chung Y-C. Industrial Applications of Different Parts of Flatland Polygonum cuspidatum by Combining Microwave-Assisted Extraction and Fermentation Process. Plants. 2025; 14(23):3572. https://doi.org/10.3390/plants14233572
Chicago/Turabian StyleChen, Chih-Yu, Guey-Horng Wang, Jong-Tar Kuo, Pei-Ning Hsu, Yu-Chen Shen, Yen-Hsun Chen, and Ying-Chien Chung. 2025. "Industrial Applications of Different Parts of Flatland Polygonum cuspidatum by Combining Microwave-Assisted Extraction and Fermentation Process" Plants 14, no. 23: 3572. https://doi.org/10.3390/plants14233572
APA StyleChen, C.-Y., Wang, G.-H., Kuo, J.-T., Hsu, P.-N., Shen, Y.-C., Chen, Y.-H., & Chung, Y.-C. (2025). Industrial Applications of Different Parts of Flatland Polygonum cuspidatum by Combining Microwave-Assisted Extraction and Fermentation Process. Plants, 14(23), 3572. https://doi.org/10.3390/plants14233572








