In Vitro Regeneration, Acclimatization, Phytochemical Profiling, and Antioxidant Properties of Hong Hoen Sirirugsa (Globba sirirugsae Saensouk & P.Saensouk)
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Plant Growth Regulators on the Micropropagation of Globba sirirugsae
2.1.1. Effect of BA in Combination with NAA on Shoot and Root Production
2.1.2. Effect of Kinetin in Combination with NAA on Shoot and Root Production
2.1.3. Influence of BA or Kinetin in Combinations with NAA on Shoot and Root Production
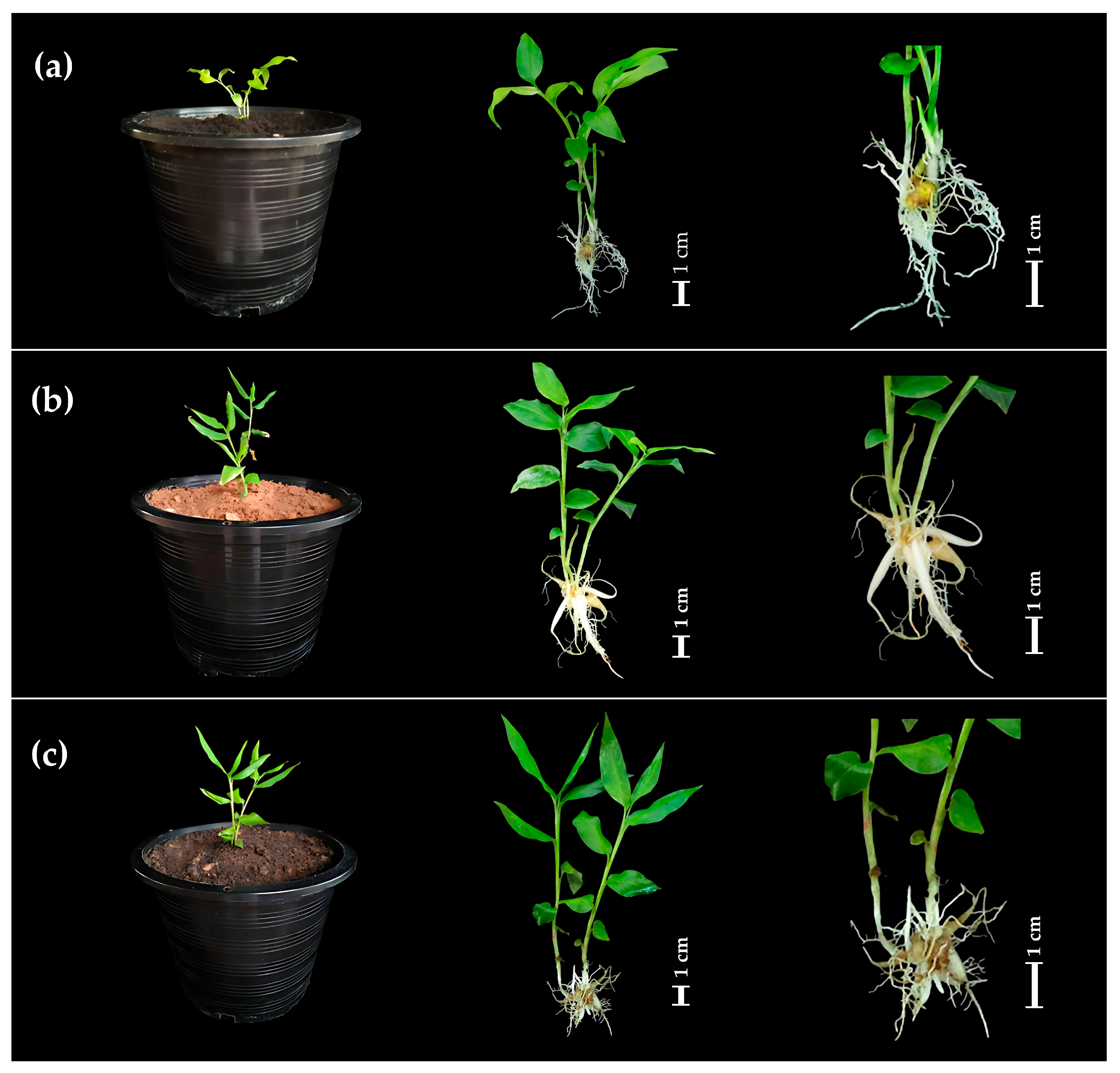
2.2. Transplantation and Acclimatization
2.3. Evaluation of TPC, TFC, and Antioxidant Activity of Globba sirirugsae
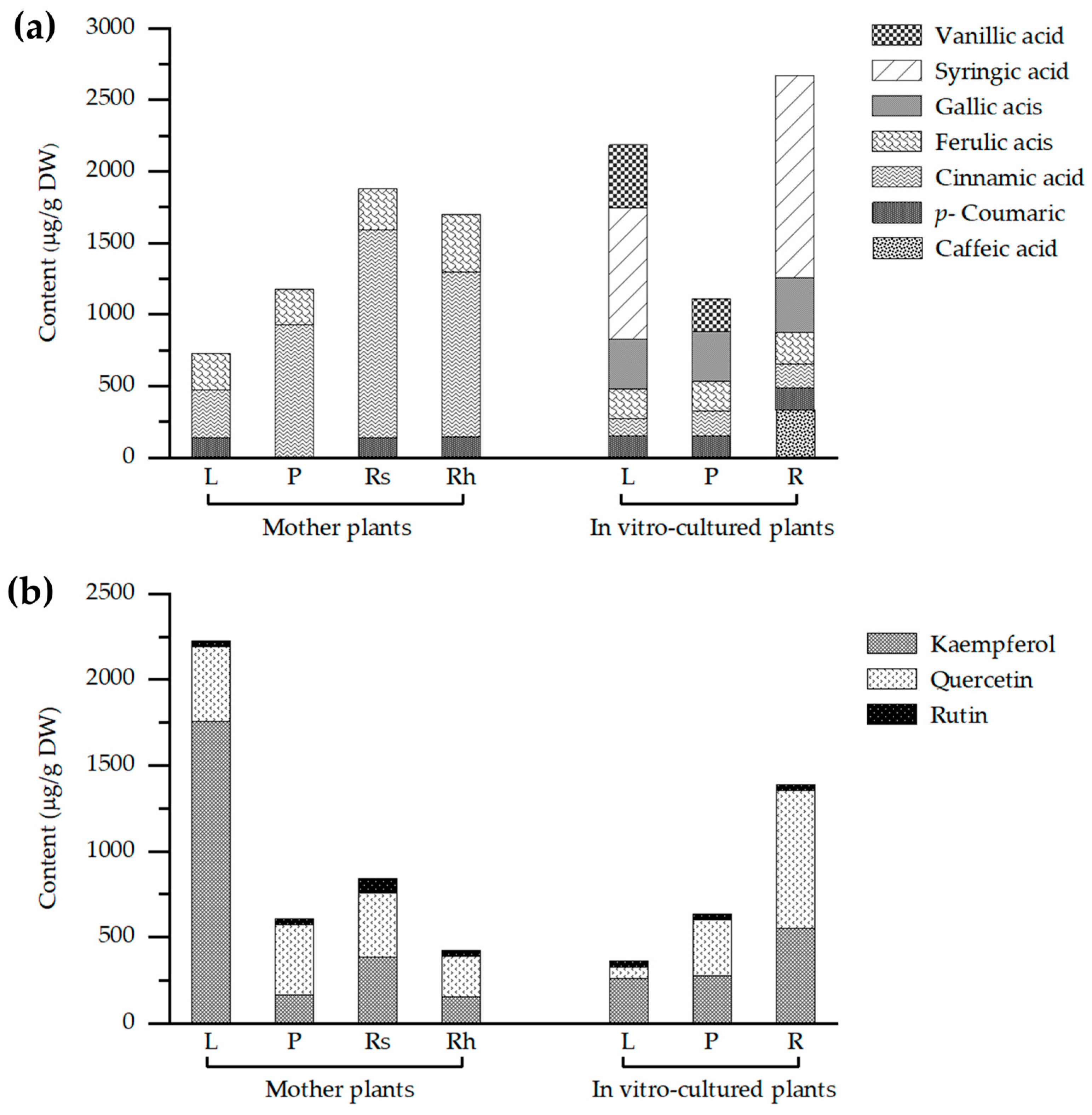
2.4. HPLC-Based Profiling of Phenolic Acids and Flavonoids in Globba sirirugsae
2.5. Determination of Volatile Compounds in Globba sirirugsae by GC-MS Analysis
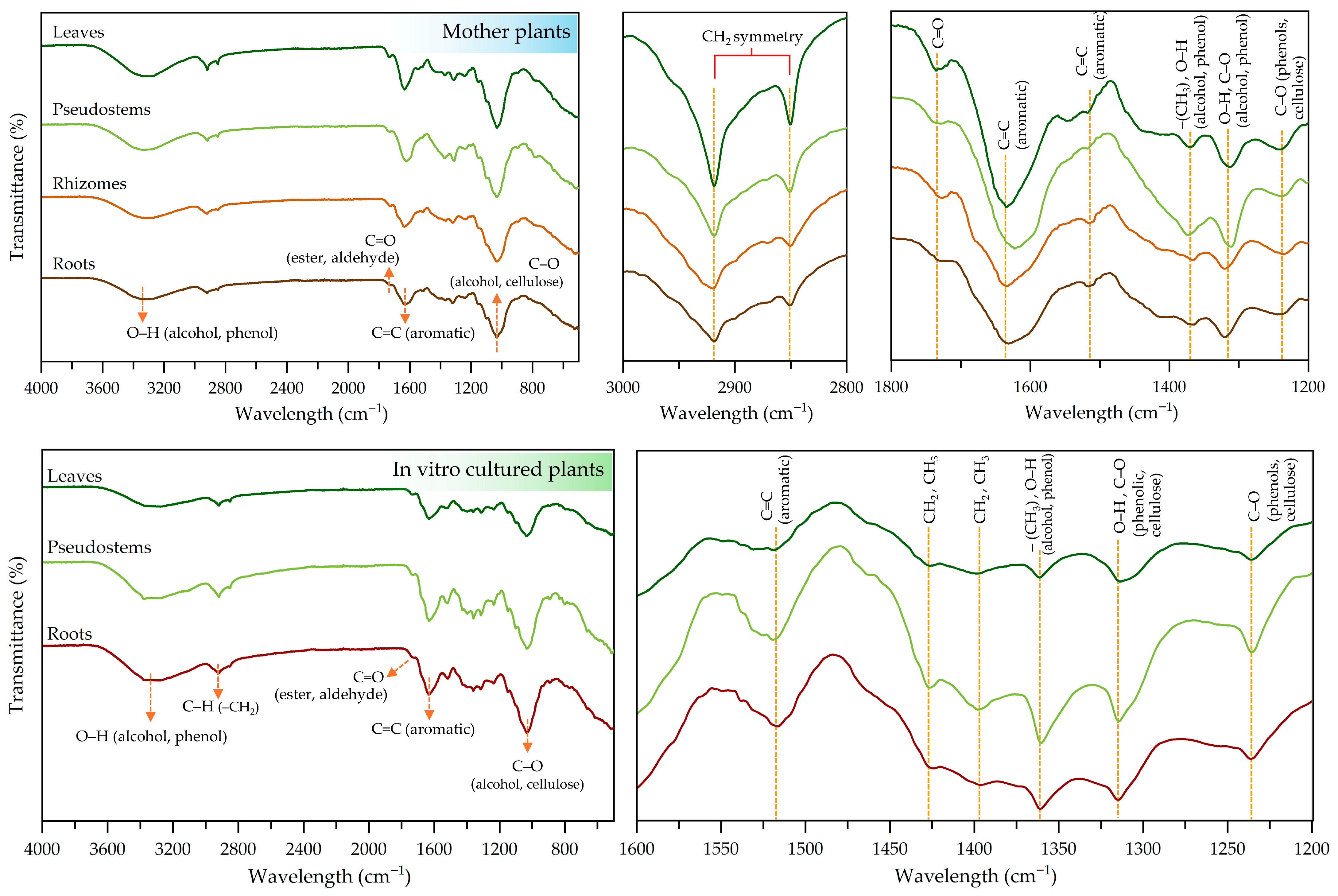
2.6. Screening and Evaluation of Functional Groups in Different Plant Parts by FTIR Analysis
3. Materials and Methods
3.1. Micropropagation of Globba sirirugsae
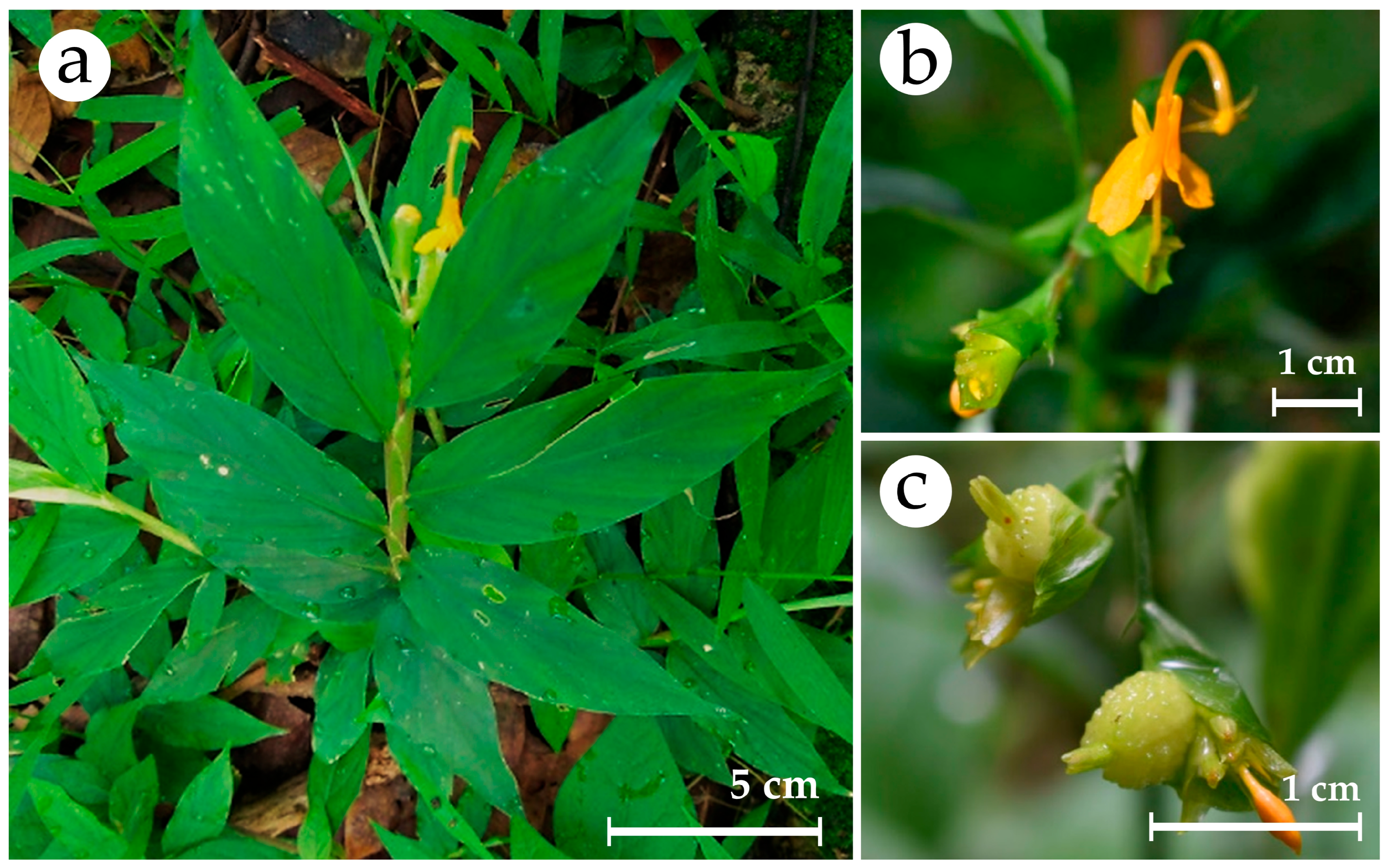
3.1.1. Plant Collection and Explant Tissue Preparation
3.1.2. The Effect of PGRs on Plant Regeneration in MS Medium
3.2. Transplantation and Acclimation
3.3. Determination of Phytochemical Compounds and Antioxidant Activity
3.3.1. Plant Material and Preparation of Plant Extraction
3.3.2. Total Phenolic Content (TPC)
3.3.3. Total Flavonoid Content (TFC)
3.3.4. DPPH Radical Scavenging Assay
3.3.5. ABTS Radical Scavenging Capacity
3.4. Identification and Quantitative Analysis of Phenolic Acids and Flavonoid Components by the HPLC Assay
3.5. GC-MS Analysis of Volatile Components in Globba sirirugsae
3.6. Screening and Evaluation of Molecular Functional Groups of Phytochemicals in Globba sirirugsae by the FTIR Technique
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2025. Available online: https://powo.science.kew.org/ (accessed on 14 August 2025).
- Aslam, M.S.; Ahmad, M.S. Ethnobotanical uses of Globba species: A brief review. BAOJ Pharm. Sci 2017, 3, 035. [Google Scholar]
- Yadav, R.; Gowda, V. Six new species of Globba L. (Zingiberales, Zingiberaceae) from the Eastern Himalayas and Northeast India. Phytokeys 2024, 246, 197–228. [Google Scholar] [CrossRef]
- Sangvirotjanapat, S.; Đăng, T.H.; Newman, M.F. Ten new species of Globba section Globba from continental South-East Asia. Thai For. Bull. (Bot.) 2020, 48, 212–233. [Google Scholar] [CrossRef]
- Saensouk, S.; Saensouk, P. Globba sirirugsae, a new species of Zingiberaceae from Thailand. J. Jpn. Bot. 2020, 95, 327–331. [Google Scholar] [CrossRef]
- Noothalee, S.; Saensouk, S.; Saensouk, P. In vitro propagation of Globba annamensis Gagnep. for conservation of rare plant species in Thailand. KKU Sci. J. 2017, 45, 858–872. [Google Scholar]
- Kho, P.E.; Sani, H.B.; Boyce, P.C.; Sim, S.L. In vitro propagation of Globba brachyanthera K. Schum. Asia Pac. J. Mol. Biol. Biotechnol. 2010, 18, 119–122. [Google Scholar]
- Yaowachai, W.; Saensouk, S.; Saensouk, P. In vitro propagation and determination of total phenolic compounds, flavonoid contents and antioxidative activity of Globba globulifera Gagnep. Pharmacogn. J. 2020, 12, 1740–1747. [Google Scholar] [CrossRef]
- Mawaddah, S.K.; Saputro, N.W.; Lestari, A. The effect of naphthalene acetic acid (NAA) and kinetin of shoot multiplication ginger plant (Globba leucantha var. bicolor Holttum) on in vitro. J. Bioma 2021, 23, 43–50. [Google Scholar] [CrossRef]
- Pimmuen, P.; Saensouk, P.; Saensouk, S. In vitro propagation of Globba marantina L. KKU Res. J. 2014, 19, 596–605. [Google Scholar]
- Phantong, P.; Machikowa, T.; Saensouk, P.; Muangsan, N. Comparing growth and physiological responses of Globba schomburgkii Hook. f. and Globba marantina L. under hydroponic and soil conditions. Emir. J. Food Agric. 2018, 30, 157–164. [Google Scholar] [CrossRef]
- Parida, R.; Mohanty, S.; Nayak, S. In vitro plant regeneration potential of genetically stable Globba marantina L., Zingiberaceous species and its conservation. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 321–327. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Pimmuen, P. In vitro propagation of Globba schomburgkii Hook. f. via bulbil explants. Walailak J. Sci. Technol. (WJST) 2018, 15, 701–710. [Google Scholar] [CrossRef]
- Muktawapai, K.; Wongchaochant, S. Micropropagation and in vitro short-term storage of Globba sherwoodiana WJ Kress & V. Gowda. Agric. Nat. Resour. 2020, 54, 405–414. [Google Scholar] [CrossRef]
- Topoonyanont, N.; Pumisutapon, P.; Klayraung, S.; Poonnoy, P. Temporary immersion bioreactors for large scale Globba micropropagation. Acta Hortic. 2017, 1155, 51–58. [Google Scholar] [CrossRef]
- Verma, C.; Bhatia, S.; Srivastava, S. Traditional medicine of the Nicobarese. Indian J. Tradit. Knowl. 2010, 9, 779–785. [Google Scholar]
- Muthukumarasamy, S.; Mohan, V.R.; Kumaresan, S.; Chelladurai, V. Traditional medicinal practices of Palliyar tribe of Srivilliputhur in antenatal and post-natal care of mother and child. Nat. Prod. Radiance. 2004, 3, 422–426. [Google Scholar]
- Tushar; Basak, S.; Sarma, G.C.; Rangan, L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J. Ethnopharmacol. 2010, 132, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Doungchawee, J.; Kulsing, C.; Suekaew, N.; Na Pombejra, S.; Chavasiri, W.; Plabutong, N.; Thammahong, A.; Khotavivattana, T. Volatile chemical composition, antibacterial and antifungal activities of extracts from different parts of Globba schomburgkii Hook.f. Chem. Biodivers. 2019, 16, e1900057. [Google Scholar] [CrossRef]
- Raj, G.; George, V.; Dan, M.; Sethuraman, M.G. Essential oil composition of Globba schomburgkii Hook. f. and Globba ophioglossa Wight. J. Essent. Oil Res. 2010, 22, 220–222. [Google Scholar] [CrossRef]
- Phuong, N.T.; Thu Thuy, D.T.; Thao, D.T.; Thanh Huyen, D.T.; Hung, L.N.; Anh, N.T.; Ha, L.M. Volatile compounds and biological activities of essential oil of Gobba pendula Roxb. collected at An Giang province. Vietnam J. Sci. Technol. 2022, 58, 434–441. [Google Scholar] [CrossRef]
- Kumar, R.; Prakash, O.; Pant, A.K.; Isidorov, V.A.; Mathela, C.S. Chemical composition, antioxidant and myorelaxant activity of essential oils of Globba sessiliflora Sims. J. Essent. Oil Res. 2012, 24, 385–391. [Google Scholar] [CrossRef]
- Van, H.T.; Dam, S.M.; Phan, U.T.X.; Nguyen, T.N.A.; Nguyen, T.B.T.; Tran, T.L.; Luu, T.N.; Le, V.S.; Huynh, N.T.A. Chemical diversity of essential oils from aerial parts of eight species of Zingiberaceae family from Vietnam. Acta Univ. Agric. Silvic. Mendel. Brun 2022, 70, 273–281. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Pasternak, T.; Steinmacher, D. Plant tissue culture in vitro: A long journey with lingering challenges. Int. J. Plant Biol. 2025, 16, 97. [Google Scholar] [CrossRef]
- Šmeringai, J.; Schrumpfová, P.P.; Pernisová, M. Cytokinins–regulators of de novo shoot organogenesis. Front. Plant Sci. 2023, 14, 1239133. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, C.; Zhang, C.; Shi, B.; Cao, X.; Zhang, T.Q.; Zhao, Z.; Wang, J.W.; Jiao, Y. Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell 2017, 29, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Pokimica, N.; Ćosić, T.; Uzelac, B.; Ninković, S.; Raspor, M. Dissecting the roles of the cytokinin signaling network: The case of de novo shoot apical meristem formation. Biomolecules 2024, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, K.H.; Park, S.J.; Ko, S.R.; Moon, K.B.; Koo, H.; Cho, H.S.; Park, S.U.; Jeon, J.H.; Kim, H.S.; et al. WUSCHEL controls genotype-dependent shoot regeneration capacity in potato. Plant Physiol. 2023, 193, 661–676. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M.; Vasileva, V. The impact of auxin and cytokinin on the growth and development of selected crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, Y.; Li, Y.; Wang, Y.; Jiang, C.; Choisy, P.; Xu, T.; Cai, Y.; Pei, D.; et al. AUXIN RESPONSE FACTOR 18–HISTONE DEACETYLASE 6 module regulates floral organ identity in rose (Rosa hybrida). Plant Physiol. 2021, 186, 1074–1087. [Google Scholar] [CrossRef]
- Saensouk, S.; Benjamin, S.; Chumroenphat, T.; Saensouk, P. In vitro propagation, evaluation of antioxidant activities, and phytochemical profiling of wild and in vitro-cultured plants of Curcuma larsenii Maknoi & Jenjitikul—A rare plant species in Thailand. Horticulturae 2024, 10, 1181. [Google Scholar] [CrossRef]
- Zucco, M.A.; Walters, S.A.; Chong, S.K.; Klubek, B.P.; Masabni, J.G. Effect of soil type and vermicompost applications on tomato growth. Int. J. Recycl. Org. Waste Agric. 2015, 4, 135–141. [Google Scholar] [CrossRef]
- Grace, S.G.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. Lond. B 2000, 355, 1499–1510. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Mora-Vásquez, S.; Puente-Garza, C.A.; García-Lara, S. Recent advances on the use of abiotic stress (water, UV radiation, atmospheric gases, and temperature stress) for the enhanced production of secondary metabolites on in vitro plant tissue culture. Plant Growth Regul. 2022, 97, 1–20. [Google Scholar] [CrossRef]
- Parveen, Z.; Zaidi, S.; Bajguz, A.; Arif, Y.; Hayat, S. Comprehensive insights into flavonoids: Biosynthesis, stress modulation, and plant growth regulation. J. Plant Growth Regul. 2025, 44, 6333–6352. [Google Scholar] [CrossRef]
- Nonthalee, S.; Maneechai, S.; Saensouk, S.; Saensouk, P. Comparative phytochemical profiling (GC-MS and HPLC) and evaluation of antioxidant activities of wild, in vitro cultured and greenhouse plants of Kaempferia grandifolia Saensouk and Jenjitt. and Kaempferia siamensis Sirirugsa; rare plant species in Thailand. Pharmacogn. Mag. 2023, 19, 156–167. [Google Scholar] [CrossRef]
- Saensouk, S.; Yaowachai, W.; Chumroenphat, T.; Nonthalee, S.; Saensouk, P. In vitro regeneration, transplantation and phytochemical profiles of Kaempferia angustifolia Roscoe. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13190. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, S.; Awaeloh, N.; Na-Phatthalung, P.; Kaewmanee, T.; Chusri, S. Exploring antioxidant properties of standardized extracts from medicinal plants approved by the Thai FDA for dietary supplementation. Nutrients 2025, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Chumroenphat, T.; Saensouk, S.; Saensouk, P. Chemical composition and antioxidant activity of three species of Cornukaempferia in Thailand. Biodiversitas 2021, 22, 4036–4044. [Google Scholar] [CrossRef]
- Rahim, N.A.; Roslan, M.N.F.; Muhamad, M.; Seeni, A. Antioxidant activity, total phenolic and flavonoid content and LC–MS profiling of leaves extracts of Alstonia angustiloba. Separations 2022, 9, 234. [Google Scholar] [CrossRef]
- Papayrata, C.; Chumroenphat, T.; Saensouk, P.; Saensouk, S. Diversity of curcuminoids, bioactive compounds and antioxidant activities in three species of Curcuma. Trop. J. Pharm. Res. 2024, 23, 1291–1298. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, X.; Guo, J.; Lu, H.; Xie, J.; Zhang, F.; Yao, C.; Hao, E. Pharmacological potential of cinnamic acid and derivatives: A comprehensive review. Pharmaceuticals 2025, 18, 1141. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Wanyo, P.; Chomnawang, C.; Huaisan, K.; Chamsai, T. Comprehensive analysis of antioxidant and phenolic profiles of Thai medicinal plants for functional food and pharmaceutical development. Plant Foods Hum. Nutr. 2024, 79, 394–400. [Google Scholar] [CrossRef]
- Saensouk, S.; Sonthongphithak, P.; Chumroenphat, T.; Muangsan, N.; Souladeth, P.; Saensouk, P. In vitro multiplication, antioxidant activity, and phytochemical profiling of wild and in vitro-cultured plants of Kaempferia larsenii Sirirugsa—A rare plant species in Thailand. Horticulturae 2025, 11, 281. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Das, S.; Prakash, B. Effect of environmental factors on essential oil biosynthesis, chemical stability, and yields. In Plant Essential Oils; Prakash, B., Dubey, N.K., Freitas Brilhante de São José, J., Eds.; Springer: Singapore, 2024; pp. 225–247. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in plant tissue culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Triet, N.T.; Chen, T.V.; Giang, L.D.; Tran-Trung, H.; An, N.T.G.; Viet, N.T.; Thu, N.V. Rhizome essential oil of Alpinia pinnanensis from Vietnam: Chemical composition and in vitro evaluation of antimicrobial and cytotoxic activities. Pharmacia 2025, 72, 1–7. [Google Scholar] [CrossRef]
- Tran-Trung, H.; Le, D.G.; Hoang, V.T.; Vu, D.C.; Thang, T.D.; Nguyen-Ngoc, H.; Van Chen, T.; Nguyen, T.T.; Tuan, N.H.; Nguyen, T.H.D. Essential oils of two species of Zingiberaceae family from Vietnam: Chemical compositions and α-glucosidase, α-amylase inhibitory effects. Nat. Prod. Commun. 2024, 19, 1934578X241232281. [Google Scholar] [CrossRef]
- Nayak, S.; Jena, A.K.; Mittal, D.K.; Joshi, D. GC-MS analysis of phytocostituents of some wild Zingiberaceae plants methanolic rhizome extracts. Res. Plant Sci. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Annaz, H.; El Fakhouri, K.; Ben Bakrim, W.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Bergamotenes: A comprehensive compile of their natural occurrence, biosynthesis, toxicity, therapeutic merits and agricultural applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 7343–7362. [Google Scholar] [CrossRef] [PubMed]
- Kainat, S.; Gilani, S.R.; Asad, F.; Khalid, M.Z.; Khalid, W.; Ranjha, M.M.A.N.; Bangar, S.P.; Lorenzo, J.M. Determination and comparison of phytochemicals, phenolics, and flavonoids in Solanum lycopersicum using FTIR spectroscopy. Food Anal. Methods 2022, 15, 2931–2939. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Vârban, R.; Crișan, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Ștefan, R. Comparative FT-IR prospecting for cellulose in stems of some fiber plants: Flax, velvet leaf, hemp and jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, J.; Medhi, T.; Kumar, A. Phytochemical screening, quantification, FT-IR analysis, and in silico characterization of potential bio–active compounds identified in HR-LC/MS analysis of the polyherbal formulation from Northeast India. ACS Omega 2022, 7, 33067–33078. [Google Scholar] [CrossRef] [PubMed]
- Maache, M.; Bezazi, A.; Amroune, S.; Scarpa, F.; Dufresne, A. Characterization of a novel natural cellulosic fiber from Juncus effusus L. Carbohydr. Polym. 2017, 171, 163–172. [Google Scholar] [CrossRef]
- Sravan Kumar, S.; Manoj, P.; Giridhar, P. Fourier transform infrared spectroscopy (FTIR) analysis, chlorophyll content and antioxidant properties of native and defatted foliage of green leafy vegetables. J. Food Sci. Technol. 2015, 52, 8131–8139. [Google Scholar] [CrossRef]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Wicharuck, S.; Suang, S.; Chaichana, C.; Chromkaew, Y.; Mawan, N.; Soilueang, P.; Khongdee, N. The implementation of the SPAD-502 Chlorophyll meter for the quantification of nitrogen content in Arabica coffee leaves. MethodsX 2024, 12, 102566. [Google Scholar] [CrossRef] [PubMed]
- Chumroenphat, T.; Sombonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. The diversity of biologically active compounds in the rhizomes of recently discovered Zingiberaceae plants native to Northeastern Thailand. Pharmacogn. J. 2019, 11, 1014–1022. [Google Scholar] [CrossRef]
- Rivero-Pérez, M.D.; Muñiz, P.; Gonzalez-Sanjosé, M.L. Antioxidant profile of red wines evaluated by total antioxidant capacity, scavenger activity, and biomarkers of oxidative stress methodologies. J. Agric. Food Chem. 2007, 55, 5476–5483. [Google Scholar] [CrossRef]
- Payet, B.; Sing, A.S.C.; Smadja, J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: Determination of their polyphenolic and volatile constituents. J. Agric. Food Chem. 2005, 53, 10074–10079. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L.) under freeze–drying and low–temperature drying methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef] [PubMed]
- Siriamornpun, S.; Kaewseejan, N. Quality, bioactive compounds and antioxidant capacity of selected climacteric fruits with relation to their maturity. Sci. Hortic. 2017, 221, 33–42. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. Available online: https://webbook.nist.gov (accessed on 3 September 2025).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]




| BA (mg/L) | NAA (mg/L) | Average No. of Shoots/Explant | Average Shoot Length (cm) | Average No. of Roots/Explant | Average Root Length (cm) |
|---|---|---|---|---|---|
| 0 | 0 | 1.70 ± 0.26 b | 1.47 ± 0.11 b | 1.10 ± 0.62 c | 0.36 ± 0.21 c |
| 1 | 0 | 1.80 ± 0.33 b | 1.83 ± 0.20 ab | 1.51 ± 0.12 c | 0.29 ± 0.03 c |
| 2 | 0 | 3.30 ± 0.42 ab | 1.97 ± 0.11 ab | 1.40 ± 0.37 c | 0.25 ± 0.07 c |
| 3 | 0 | 4.10 ± 0.57 a | 1.86 ± 0.15 ab | 2.10 ± 0.99 bc | 0.30 ± 0.13 c |
| 4 | 0 | 3.80 ± 0.70 ab | 1.68 ± 0.21 ab | 1.10 ± 0.60 c | 0.11 ± 0.05 c |
| 5 | 0 | 3.10 ± 0.55 ab | 1.49 ± 0.22 b | 1.40 ± 0.90 c | 0.19 ± 0.80 c |
| 1 | 0.1 | 3.00 ± 0.52 ab | 1.91 ± 0.16 ab | 7.30 ± 1.02 a | 1.10 ± 0.11 ab |
| 2 | 0.1 | 3.33 ± 0.56 ab | 2.03 ± 0.15 ab | 8.50 ± 2.20 a | 1.53 ± 0.16 a |
| 3 | 0.1 | 3.80 ± 0.98 ab | 1.98 ± 0.16 ab | 5.00 ± 1.15 ab | 0.96 ± 0.21 b |
| 4 | 0.1 | 5.10 ± 1.02 a | 2.17 ± 0.21 a | 7.80 ± 1.53 a | 1.11 ± 0.20 ab |
| 5 | 0.1 | 3.60 ± 0.86 ab | 1.69 ± 0.19 ab | 6.50 ± 1.61 a | 0.86 ± 0.24 b |
| Kinetin (mg/L) | NAA (mg/L) | Average No. of Shoots/Explant | Average Shoot Length (cm) | Average No. of Roots/Explant | Average Root Length (cm) |
|---|---|---|---|---|---|
| 0 | 0 | 1.90 ± 0.35 bc | 2.95 ± 0.35 a | 0.80 ± 0.53 d | 0.21 ± 0.17 d |
| 1 | 0 | 2.00 ± 0.00 bc | 2.93 ± 0.34 a | 1.90 ± 0.43 cd | 0.70 ± 0.10 cd |
| 2 | 0 | 1.60 ± 0.27 bc | 3.42 ± 0.35 a | 0.80 ± 0.36 d | 0.33 ± 0.14 cd |
| 3 | 0 | 2.30 ± 0.42 bc | 3.03 ± 0.33 a | 0.30 ± 0.21 d | 0.20 ± 0.13 d |
| 4 | 0 | 3.90 ± 0.64 a | 2.89 ± 0.21 a | 2.10 ± 0.64 bcd | 0.78 ± 0.15 cd |
| 5 | 0 | 2.10 ± 0.50 bc | 3.67 ± 0.29 a | 2.30 ± 0.73 bcd | 0.81 ± 0.13 c |
| 1 | 0.1 | 1.20 ± 0.13 c | 3.15 ± 0.37 a | 3.30 ± 0.82 bc | 1.41 ± 0.27 b |
| 2 | 0.1 | 1.70 ± 0.21 bc | 2.73 ± 0.34 a | 3.80 ± 0.77 bc | 1.77 ± 0.17 ab |
| 3 | 0.1 | 1.70 ± 0.34 bc | 3.01 ± 0.31 a | 4.50 ± 1.14 b | 2.09 ± 0.33 a |
| 4 | 0.1 | 1.80 ± 0.42 bc | 3.46 ± 0.31 a | 4.40 ± 1.05 b | 1.95 ± 0.23 ab |
| 5 | 0.1 | 2.90 ± 0.64 ab | 2.85 ± 0.23 a | 7.80 ± 1.24 a | 1.93 ± 0.13 ab |
| BA (mg/L) | Kinetin (mg/L) | NAA (mg/L) | Average No. of Shoots/Explant | Average Shoot Length (cm) | Average No. of Roots/Explant | Average Root Length (cm) |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 1.80 ± 0.20 f | 3.93 ± 0.28 ab | 20.70 ± 2.93 c | 1.90 ± 0.15 ab |
| 0.5 | 0 | 0.5 | 3.67 ± 0.24 c | 3.45 ± 0.17 ab | 20.80 ± 0.94 c | 2.03 ± 0.05 a |
| 1 | 0 | 0.5 | 4.40 ± 0.50 ab | 4.20 ± 0.30 a | 16.30 ± 1.84 e | 2.04 ± 0.04 a |
| 2 | 0 | 0.5 | 4.10 ± 0.41 ab | 4.59 ± 0.37 a | 14.30 ± 1.94 e | 2.06 ± 0.13 a |
| 3 | 0 | 0.5 | 4.89 ± 0.51 a | 4.38 ± 0.45 a | 12.00 ± 1.90 f | 1.68 ± 0.10 b |
| 4 | 0 | 0.5 | 5.67 ± 0.46 a | 3.98 ± 0.23 ab | 11.30 ± 1.82 f | 1.90 ± 0.17 ab |
| 5 | 0 | 0.5 | 4.12 ± 0.42 ab | 4.62 ± 0.31 a | 11.70 ± 1.58 f | 1.94 ± 0.12 ab |
| 0.5 | 0 | 1 | 2.80 ± 0.25 de | 3.08 ± 0.27 b | 20.80 ± 0.94 c | 1.76 ± 0.13 ab |
| 1 | 0 | 1 | 3.10 ± 0.18 c | 3.31 ± 0.17 b | 13.90 ± 1.02 f | 2.22 ± 0.22 a |
| 2 | 0 | 1 | 4.20 ± 0.29 ab | 3.18 ± 0.25 b | 16.80 ± 2.22 e | 1.12 ± 0.14 cd |
| 3 | 0 | 1 | 4.10 ± 0.38 ab | 4.31 ± 0.27 a | 19.80 ± 2.78 c | 1.58 ± 0.21 b |
| 4 | 0 | 1 | 4.22 ± 0.36 ab | 4.05 ± 0.33 a | 16.89 ± 2.47 e | 1.21 ± 0.15 cd |
| 5 | 0 | 1 | 4.00 ± 0.30 ab | 4.17 ± 0.26 a | 22.40 ± 1.87 b | 1.53 ± 0.12 b |
| 0 | 0.5 | 0.5 | 2.89 ±0.31 de | 3.10 ± 0.30 b | 19.78 ± 1.26 c | 1.21 ± 0.08 cd |
| 0 | 1 | 0.5 | 3.30 ± 0.33 c | 3.42 ± 0.32 ab | 21.10 ± 3.06 bc | 0.99 ± 0.14 d |
| 0 | 2 | 0.5 | 2.44 ± 0.34 de | 3.87 ± 0.49 a | 20.89 ± 2.68 c | 1.67 ± 0.21 b |
| 0 | 3 | 0.5 | 2.70 ± 0.21 de | 3.81 ± 0.41 a | 17.20 ± 2.32 d | 1.36 ± 0.22 c |
| 0 | 4 | 0.5 | 2.80 ± 0.25 de | 3.68 ± 0.25 a | 20.80 ± 2.34 c | 1.34 ± 0.19 c |
| 0 | 5 | 0.5 | 4.00 ± 0.30 ab | 3.41 ± 0.17 ab | 23.30 ± 1.74 b | 1.48 ± 0.12 c |
| 0 | 0.5 | 1 | 3.20 ± 0.20 c | 2.98 ± 0.22 c | 21.90 ± 1.15 bc | 1.48 ± 0.12 c |
| 0 | 1 | 1 | 2.70 ± 0.40 de | 4.18 ± 0.59 a | 18.10 ± 3.64 d | 0.84 ± 0.19 d |
| 0 | 2 | 1 | 3.90 ± 0.35 ab | 4.32 ± 0.25 a | 35.70 ± 2.67 a | 1.19 ± 0.14 cd |
| 0 | 3 | 1 | 3.22 ± 0.36 c | 3.70 ± 0.47 a | 22.00 ± 4.95 b | 1.23 ± 0.34 cd |
| 0 | 4 | 1 | 3.56 ± 0.18 c | 2.79 ± 0.29 c | 15.44 ± 3.04 e | 0.65 ± 0.13 d |
| 0 | 5 | 1 | 3.67 ± 0.24 c | 2.97 ± 0.23 c | 18.00 ± 2.07 d | 0.92 ± 0.13 d |
| Plant Material | Percentage of Surviving Plantlets (%) | Average No. of Shoots/Explant | Average No. of Leaves/Explant | Average Shoot Length (cm) | Chlorophyll Content (SPAD Unit) |
|---|---|---|---|---|---|
| Soil | 70 | 2.30 ± 0.30 a | 12.80 ± 1.00 a | 8.05 ± 0.88 b | 18.56 ± 2.11 c |
| Sand | 80 | 2.20 ± 0.25 a | 14.50 ± 1.12 a | 10.86 ± 0.79 a | 29.62 ± 1.26 a |
| Soil–sand | 90 | 2.50 ± 0.17 a | 15.40 ± 0.96 a | 8.22 ± 1.01 b | 24.70 ± 1.26 b |
| Condition | Part of Plant | TPC (mg GAE/g DW) | TFC (mg RE/g DW) | DPPH (mg TE/g DW) | ABTS (% Inhibition) |
|---|---|---|---|---|---|
| wild plants | Leaves | 8.24 ± 0.07 a | 3.10 ± 0.22 a | 3.06 ± 0.03 c | 70.35 ± 0.58 c |
| Pseudostems | 4.61 ± 0.06 d | 2.52 ± 0.12 b | 3.60 ± 0.09 bc | 77.75 ± 0.15 b | |
| Roots and storage roots | 7.15 ± 0.25 b | 2.92 ± 0.25 ab | 5.23 ± 0.07 a | 88.85 ± 0.46 a | |
| Rhizomes | 5.96 ± 0.04 c | 1.61 ± 0.14 c | 4.03 ± 0.04 b | 52.20 ± 0.51 f | |
| Tissue- cultured plants | Leaves | 3.98 ± 0.17 e | 1.11 ± 0.03 d | 4.10 ± 0.52 b | 61.31 ± 0.32 d |
| Pseudostems | 3.76 ± 0.05 e | 1.17 ± 0.03 cd | 1.53 ± 0.08 d | 54.98 ± 0.53 e | |
| Roots | 4.45 ± 0.15 d | 1.36 ± 0.09 cd | 0.85 ± 0.08 e | 54.62 ± 1.06 e |
| Condition | Explant | Phenolic Acid Contents (μg/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CA | CMA | CNA | FA | GA | SA | VA | Total | ||
| Wild plants | Leaves | ND | 141.33 ± 0.09 d | 329.73 ± 7.48 d | 258.75 ± 0.28 c | ND | ND | ND | 729.81 ± 2.62 |
| Pseudostems | ND | ND | 926.76 ± 22.65 c | 251.77 ± 0.46 d | ND | ND | ND | 1178.53 ± 11.55 | |
| Roots and storage roots | ND | 141.28 ± 0.07 d | 1447.67 ± 21.45 a | 293.65 ± 0.63 b | ND | ND | ND | 1882.60 ± 12.62 | |
| Rhizomes | 4.69 ± 1.62 b | 138.90 ± 0.01 e | 1152.92 ± 16.50 b | 400.81 ± 0.72 a | ND | ND | ND | 1697.32 ± 8.03 | |
| In vitro-cultured plants | Leaves | ND | 149.09 ± 0.08 c | 125.24 ± 0.60 f | 205.19 ± 0.29 g | 347.67 ± 0.48 b | 917.61 ± 10.81 b | 442.12 ± 1.77 a | 2186.92 ± 2.34 |
| Pseudostems | ND | 152.94 ± 0.03 b | 174.13 ± 0.86 e | 208.91 ± 0.75 f | 343.83 ± 0.51 c | ND | 230.19 ± 2.79 b | 1110.00 ± 1.07 | |
| Roots | 331.19 ±1.29 a | 157.53 ± 0.03 a | 166.95 ± 0.61 e | 220.46 ± 0.78 e | 382.35 ± 0.43 a | 1418.02 ± 2.64 a | ND | 2676.50 ± 1.12 | |
| Condition | Explant | Flavonoid Compound Contents (μg/g DW) | ||||
|---|---|---|---|---|---|---|
| Catechin | Kaempferol | Quercetin | Rutin | Total | ||
| Wild plants | Leaves | ND | 1755.62 ± 26.10 a | 434.29 ± 15.21 b | 36.27 ± 0.17 bc | 2226.18 ± 13.83 |
| Pseudostems | ND | 164.95 ± 2.08 e | 409.99 ± 3.13 c | 31.59 ± 0.05 e | 606.53 ± 1.75 | |
| Roots and storage roots | ND | 383.76 ± 12.28 c | 372.45 ± 1.15 d | 84.76 ± 1.53 a | 840.97 ± 4.99 | |
| Rhizomes | ND | 152.95 ± 0.42 e | 233.87 ± 0.71 f | 37.73 ± 0.01 b | 424.55 ± 0.38 | |
| In vitro- cultured plants | Leaves | ND | 262.79 ± 1.33 d | 65.35 ± 0.41 g | 35.29 ± 0.40 cd | 363.43 ± 0.71 |
| Pseudostems | ND | 275.35 ± 1.36 d | 327.33 ± 1.69 e | 34.12 ± 0.28 d | 636.80 ± 1.11 | |
| Roots | ND | 552.44 ± 0.29 b | 802.55 ± 13.43 a | 35.34 ± 0.52 cd | 1390.33 ± 4.75 | |
| No. | Volatile Compound | RT | RI | MF | MW | Peak Area (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth Conditions | |||||||||||
| Wild Plants | In Vitro-Cultured Plants | ||||||||||
| L | Rh | Rs | L | P | R | ||||||
| Monoterpene Hydrocarbons | |||||||||||
| 1. | α-Pinene | 8.48 | 933 | C10H16 | 136.23 | 19.11 | 35.72 | 32.71 | 5.19 | 4.65 | 19.11 |
| 2. | Camphene | 8.94 | 948 | C10H16 | 136.23 | 0.15 | 6.28 | - | - | 0.35 | - |
| 3. | Sabinene | 9.73 | 969 | C10H16 | 136.23 | 0.22 | - | - | - | - | - |
| 4. | β-Pinene | 9.80 | 976 | C10H16 | 136.23 | 22.38 | 54.71 | 48.61 | 9.04 | 11.50 | - |
| 5. | β-Myrcene | 10.33 | 993 | C10H16 | 136.23 | - | - | - | - | 0.14 | - |
| 6. | D-Limonene | 11.45 | 1030 | C10H16 | 136.23 | - | - | - | - | 0.50 | - |
| Oxygenated monoterpenes | |||||||||||
| 7. | Dehydro-1,8-cineole | 10.27 | 988 | C10H16O | 152.23 | 0.40 | - | - | - | - | - |
| 8. | Eucalyptol | 11.50 | 1032 | C10H18O | 154.25 | 2.11 | - | - | 1.22 | - | - |
| 9. | Bornyl acetate | 19.65 | 1290 | C12H20O2 | 196.29 | - | - | - | - | 0.08 | - |
| 10. | Myrtenyl acetate | 20.83 | 1296 | C12H18O2 | 194.27 | - | - | - | - | 0.07 | - |
| 11. | 2-Oxabicyclo [2.2.2]octan-6-ol, 1,3,3-trimethyl-, acetate | 21.28 | 1347 | C12H20O3 | 212.29 | - | - | - | - | 0.30 | - |
| Sesquiterpene hydrocarbons | |||||||||||
| 12. | δ-Elemene | 21.13 | 1341 | C15H24 | 204.35 | - | - | - | 0.47 | 0.28 | - |
| 13. | α-Cubebene | 21.46 | 1354 | C15H24 | 204.35 | 0.18 | - | - | - | 0.06 | - |
| 14. | Cypera-2,4-diene | 21.85 | 1367 | C15H22 | 202.34 | - | - | - | 0.57 | 2.84 | - |
| 15. | Aristolediene | 22.18 | 1378 | C15H22 | 202.34 | - | - | - | 0.20 | 2.61 | - |
| 16. | Copaene | 22.21 | 1381 | C15H24 | 204.35 | 5.56 | - | - | - | ||
| 17. | β-Elemene | 22.48 | 1389 | C15H24 | 204.35 | - | - | - | 8.93 | 5.09 | - |
| 18. | Cyperene | 22.88 | 1405 | C15H24 | 204.35 | - | - | - | 3.13 | 8.80 | 53.18 |
| 19. | isoledene | 23.29 | 1420 | C15H24 | 204.35 | - | - | - | 0.20 | 0.82 | - |
| 20. | Caryophyllene | 23.42 | 1426 | C15H24 | 204.35 | 0.55 | 0.85 | 18.68 | 22.99 | 8.29 | 0.55 |
| 21. | Selina-5,11-diene | 24.05 | 1450 | C15H24 | 204.35 | 0.71 | - | - | 0.26 | - | - |
| 22. | α-Gurjunene | 24.18 | 1455 | C15H24 | 204.35 | 2.45 | - | - | - | - | - |
| 23. | γ-Maaliene | 24.21 | 1458 | C15H24 | 204.35 | - | - | - | - | 1.13 | - |
| 24. | Humulene | 24.35 | 1460 | C15H24 | 204.35 | - | - | - | 14.71 | 8.09 | - |
| 25. | Alloaromadendrene | 24.52 | 1468 | C15H24 | 204.35 | 2.29 | - | - | - | - | - |
| 26. | cis-Muurola-4(15),5-diene | 24.59 | 1470 | C15H24 | 204.35 | - | - | - | - | 0.56 | - |
| 27. | γ-Gurjunene | 24.88 | 1478 | C15H24 | 204.35 | 2.26 | - | - | - | - | - |
| 28. | α-Gurjunene | 24.89 | 1481 | C15H24 | 204.35 | - | - | - | 2.68 | 4.43 | - |
| 29. | α-Panasinsen | 24.96 | 1484 | C15H24 | 204.35 | - | - | - | - | 1.86 | - |
| 30. | β-Copaene | 25.04 | 1485 | C15H24 | 204.35 | 0.24 | - | - | - | - | - |
| 31. | β-Cubebene | 25.06 | 1488 | C15H24 | 204.35 | - | - | - | 4.38 | 3.24 | - |
| 32. | Aristolochene | 25.13 | 1491 | C15H24 | 204.35 | 2.71 | - | - | 0.93 | 1.20 | - |
| 33. | β-Selinene | 25.18 | 1493 | C15H24 | 204.35 | 4.75 | - | - | 2.10 | 2.44 | - |
| 34. | α-Bergamotene | 25.36 | 1496 | C15H24 | 204.35 | 18.98 | - | - | - | - | - |
| 35. | Eremophylene | 25.39 | 1501 | C15H24 | 204.35 | - | - | - | 2.48 | 4.47 | - |
| 36. | δ-Guaiene | 25.66 | 1510 | C15H24 | 204.35 | 6.05 | - | - | - | - | - |
| 37. | γ-Cadinene | 25.89 | 1521 | C15H24 | 204.35 | - | - | - | 0.40 | 1.83 | - |
| 38. | α-Selinene | 25.99 | 1525 | C15H24 | 204.35 | 2.35 | - | - | 3.15 | 5.25 | - |
| 39. | β-Cadinene | 26.01 | 1526 | C15H24 | 204.35 | 1.08 | - | - | 0.28 | 1.23 | - |
| 40. | α-Guaiene | 26.12 | 1531 | C15H24 | 204.35 | - | - | - | - | 7.09 | - |
| 41. | α--Himachalene | 26.12 | 1531 | C15H24 | 204.35 | - | - | - | 1.33 | - | |
| 42. | α-Cadinene | 26.48 | 1158 | C15H24 | 204.35 | - | - | - | - | 0.07 | - |
| 43. | 1,5-Cyclodecadiene, 1,5-dimethyl-8-(1-methylethylidene)-, (E,E)- | 26.98 | 1566 | C15H24 | 204.35 | - | - | - | - | 0.12 | - |
| 44. | Aromadendrene | 28.77 | 1643 | C15H24 | 204.35 | - | - | - | - | 0.26 | - |
| Oxygenated sesquiterpenes | |||||||||||
| 45. | 10-epi-Elemol | 26.78 | 1558 | C15H26O | 222.37 | - | - | - | - | 0.19 | - |
| 46. | 1-Naphthalenol, 1,2,3,4,4a,7,8,8a-octahydro-1,6-dimethyl-4-(1-methylethyl)-, [1R-(1α,4β,4aβ,8aβ)]- | 27.85 | 1602 | C15H26O | 222.37 | - | - | - | - | 0.11 | - |
| 47. | Epicubenol | 28.37 | 1625 | C15H26O | 222.37 | - | - | - | - | 0.53 | - |
| 48. | tau-Cadinol | 28.63 | 1636 | C15H26O | 222.37 | - | - | - | - | 0.09 | - |
| 49. | Selina-6-en-4-ol | 28.94 | 1650 | C15H26O | 222.37 | - | - | - | - | 0.30 | - |
| 50. | tau-Muurolol | 29.24 | 1663 | C15H26O | 222.37 | - | - | - | - | 0.24 | - |
| 51. | Neointermedeol | 29.31 | 1667 | C15H26O | 222.37 | - | - | - | - | 3.27 | - |
| 52. | 11-Selinene-4-ol | 29.41 | 1671 | C15H26O | 222.37 | - | - | - | - | 4.06 | - |
| 53. | Ambrial | 32.54 | 1816 | C16H26O | 234.38 | - | - | - | - | 0.08 | - |
| Miscellaneous | |||||||||||
| 54. | Butanal, 2-methyl- | 2.59 | 682 | C5H10O | 86.13 | 3.24 | - | - | 2.16 | 0.27 | 26.16 |
| 55. | Acetic acid | 2.92 | 699 | C2H4O2 | 60.05 | - | - | - | 1.97 | 0.30 | - |
| 56. | Pyrrole | 4.16 | 766 | C4H5N | 67.09 | - | - | - | - | 0.11 | - |
| 57. | Octamethyltetrasiloxane | 10.51 | 999 | C8H24O4Si4 | 296.62 | - | - | - | 0.20 | - | - |
| 58. | endo-2-Chlorobornane | 15.70 | 1190 | C10H17Cl | 172.70 | 1.18 | - | - | - | - | |
| 59. | 3-Nonen-5-yne, 4-ethyl- | 21.10 | 1341 | C11H18 | 150.26 | 2.22 | - | - | - | - | - |
| 60. | Tetradecamethylcycloheptasiloxane | 25.31 | 1497 | C14H42O7Si7 | 519.08 | - | - | - | 0.27 | - | - |
| 61. | Octadecamethylcyclononasiloxane | 32.71 | 1824 | C18H54O9Si9 | 667.39 | - | - | - | 1.25 | - | - |
| 62. | Eicosamethyl-cyclodecasiloxane | 35.78 | 1975 | C20H60O10Si10 | 741.54 | - | - | - | 0.14 | - | - |
| Monoterpene hydrocarbons [%] | 41.86 | 96.71 | 81.32 | 14.23 | 17.14 | 19.11 | |||||
| Oxygenated monoterpenes [%] | 2.51 | - | - | 1.22 | 0.45 | - | |||||
| Sesquiterpene hydrocarbons [%] | 50.16 | 0.85 | 18.68 | 69.19 | 72.06 | 53.73 | |||||
| Oxygenated sesquiterpenes [%] | - | - | - | 8.87 | - | - | |||||
| Miscellaneous [%] | 5.46 | 1.18 | - | 5.99 | 0.68 | 26.16 | |||||
| Number of constituents | 22 | 6 | 3 | 27 | 44 | 4 | |||||
| Wavenumber (cm−1) | Vibrational Type | Corresponding Functional Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Wild Plants | MS (2 mg/L BA + 0.1 mg/L NAA) | |||||||
| L | P | Rs | Rh | L | P | R | ||
| 3321 | 3322 | 3330 | 3326 | 3320 | 3332 | 3321 | O–H (Stretching) | Alcohols, Phenols |
| 2929 | 2919 | 2920 | 2919 | 2918 | 2919 | 2920 | C–H (Stretching) | –CH2 (Methylene) (Cellulose) |
| 2850 | 2851 | 2848 | 2851 | 2850 | 2851 | 2852 | C–H (Stretching) | –CH2 (Methylene) (Cellulose) |
| 1735 | 1730 | 1734 | 1730 | 1731 | 1731 | 1735 | C=O (Stretching) | Ester, Aldehyde |
| 1635 | 1623 | 1642 | 1631 | 1632 | 1632 | 1633 | C=C (Stretching) | Aromatic |
| 1521 | 1518 | 1516 | 1516 | 1524 | 1519 | 1517 | C=C (Stretching) | Aromatic |
| - | - | - | - | 1426 | 1426 | 1425 | C–H (bending) | CH2, CH3 |
| - | - | - | - | 1398 | 1398 | 1396 | C–H (bending) | CH2, CH3 |
| 1370 | 1365 | 1365 | 1368 | 1361 | 1360 | 1360 | C–H (bending), O–H (bending) | –CH3, Alcohols, Phenols |
| 1313 | 1311 | 1320 | 1320 | 1314 | 1314 | 1315 | O–H (bending), C–O (Stretching) | Phenolics, Flavonoids, Cellulose |
| 1244 | 1236 | 1236 | 1244 | 1236 | 1235 | 1236 | C–O (Stretching) | Phenols, Cellulose |
| 1033 | 1032 | 1032 | 1032 | 1034 | 1032 | 1032 | C–O (Stretching) | Alcohol, Cellulose |
| No. | PGR Formulation | PGRs (mg/L) | |
|---|---|---|---|
| 1. | BA combined with NAA (11 treatments) | BA | 0, 1, 2, 3, 4, 5 |
| NAA | 0, 0.1 | ||
| 2. | kinetin combined with NAA (11 treatments) | kinetin | 0, 1, 2, 3, 4, 5 |
| NAA | 0, 0.1 | ||
| 3. | BA combined with NAA (13 treatments) | BA | 0, 0.5, 1, 2, 3, 4, 5 |
| NAA | 0, 0.5, 1 | ||
| 4. | kinetin combined with NAA (13 treatments) | kinetin | 0, 0.5, 1, 2, 3, 4, 5 |
| NAA | 0, 0.5, 1 | ||
| Parameter | Operating Conditions |
|---|---|
| Mobile phase | Line A: 0.1% acetic acid in dH2O Line B: acetonitrile |
| Detector | UV-diode array; 280 nm for hydroxybenzoic acid, 320 nm for hydroxy cinnamic acid, 370 nm for flavonoids |
| Column | C–18 |
| Column temperature | 38 °C |
| Flow rate | 0.8 mL/min |
| Sample injection volume | 20 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saensouk, S.; Sonthongphithak, P.; Chumroenphat, T.; Nonthalee, S.; Phrommalee, P.; Muangsan, N.; Keokene, T.; Saensouk, P. In Vitro Regeneration, Acclimatization, Phytochemical Profiling, and Antioxidant Properties of Hong Hoen Sirirugsa (Globba sirirugsae Saensouk & P.Saensouk). Plants 2025, 14, 3544. https://doi.org/10.3390/plants14223544
Saensouk S, Sonthongphithak P, Chumroenphat T, Nonthalee S, Phrommalee P, Muangsan N, Keokene T, Saensouk P. In Vitro Regeneration, Acclimatization, Phytochemical Profiling, and Antioxidant Properties of Hong Hoen Sirirugsa (Globba sirirugsae Saensouk & P.Saensouk). Plants. 2025; 14(22):3544. https://doi.org/10.3390/plants14223544
Chicago/Turabian StyleSaensouk, Surapon, Phiphat Sonthongphithak, Theeraphan Chumroenphat, Sukanya Nonthalee, Phannipha Phrommalee, Nooduan Muangsan, Toulaphone Keokene, and Piyaporn Saensouk. 2025. "In Vitro Regeneration, Acclimatization, Phytochemical Profiling, and Antioxidant Properties of Hong Hoen Sirirugsa (Globba sirirugsae Saensouk & P.Saensouk)" Plants 14, no. 22: 3544. https://doi.org/10.3390/plants14223544
APA StyleSaensouk, S., Sonthongphithak, P., Chumroenphat, T., Nonthalee, S., Phrommalee, P., Muangsan, N., Keokene, T., & Saensouk, P. (2025). In Vitro Regeneration, Acclimatization, Phytochemical Profiling, and Antioxidant Properties of Hong Hoen Sirirugsa (Globba sirirugsae Saensouk & P.Saensouk). Plants, 14(22), 3544. https://doi.org/10.3390/plants14223544








