Insights from Structure–Function Studies into Perception of Fatty Acid-Derived Defense Signals
Abstract
1. Introduction
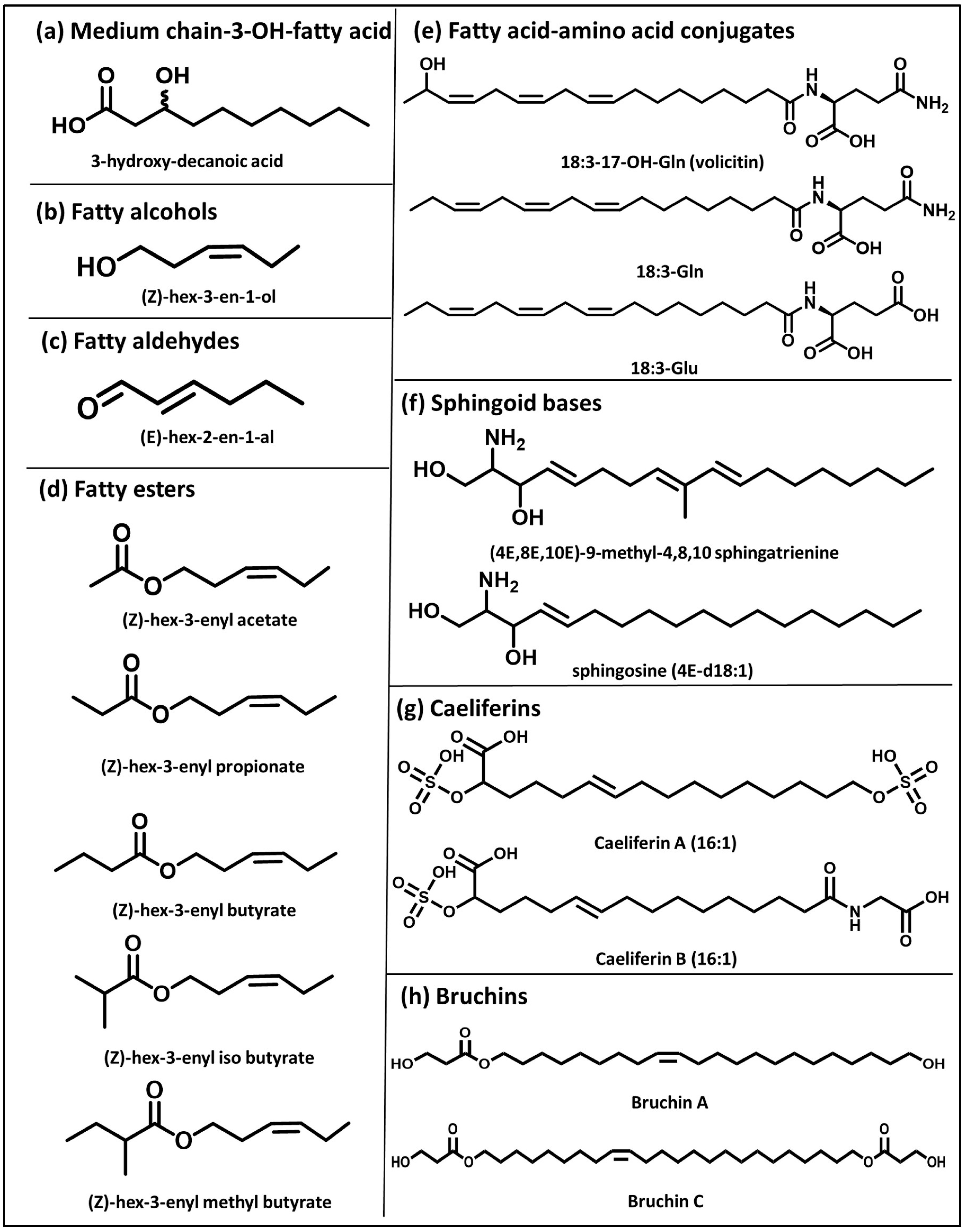
2. Medium-Chain Fatty Acids
3. Green Leaf Volatiles and Derivatives
3.1. Structure–Function Correlations Among the Three GLV Groups
| GLVs Tested | Gene Expression Method | Species | Expressed Genes (Functional Categories) | Reference |
|---|---|---|---|---|
| E2-HAL, hexanol, Z3-HOL, Z3-HAC | 10 marker genes | A. thaliana | JA biosynthesis and response, phenyl- propanoid synthesis (no defense-pathogens) | Bate and Rothstein, 1998 [43] |
| E2-HAL Z3-HAL Z3-HOL, Z3-HAC | microarray | A. thaliana | abiotic stress, defense (pathogens, SA) defense (pathogens, SA) defense (pathogens, SA), abiotic stress | Yamauchi, 2015 and 2018 [44,45] |
| E2-HAL, Z3-HAL, Z3-HOL | 6 marker genes | A. thaliana | JA synthesis and response | Kishimoto, 2005 [46] |
| E2-HAL, Z3-HAL | 5 marker genes | A. thaliana | JA synthesis and response (no defense-pathogens) | Kishimoto, 2006 [47] |
| E2-HAL | microarray & 12 marker genes | A. thaliana | defense (pathogens, SA) | Mirabella, 2015 [48] |
| Z3-HAL, E2-HOL, Z3-HOL, E2-HAC, Z3-HAC | 12 marker genes | A. thaliana | no response | Mirabella, 2015 [48] |
| E2-HAL | proteomics, RNAseq | S. lycopersicum | defense (pathogens) | Zhang, 2023 [52] |
| Z3-HAC Z3-HOL, hexanol | 3 defense marker genes | S. lycopersicum | JA-related | Perez-Hedo, 2021 [53] |
| Z3-HOL | microarray | Z. mays | defense (herbivores), JA synthesis and response | Engelberth 2013 [54] |
| Z3-HAL Z3-HOL | 3 marker genes | Z. mays | JA-related JA related | Tanaka 2023 [55] |
| GLVs from cut grass | RNAseq | L. temulentum | defense (herbivores), JA synthesis and response | Dombrowski, 2019 [56] |
| Z3-HOL, Z3-HAC | phospho- proteomics | S. peruvianum | defense (herbivores), DAMP signaling | Tanarsuwongkul, 2024 [58] |
3.2. Fatty Alcohols
3.3. Fatty Aldehydes
3.4. Fatty Esters
| C6:1 Isomer and Ester Group 2 | Bioassay | Species | Activity Compared to Z3-C2 (cetate) 1 | Reference |
|---|---|---|---|---|
| E2-C2 (acetate) | JA accumulation extrafloral nectar secretion | Z. mays P. lunatus | > = 3 (1) 5 | Engelberth, 2011 [64] Heil, 2008 [67] |
| E3-C2 (acetate) | extrafloral nectar secretion | P. lunatus | =(1) | Heil, 2008 [67] |
| 5-C2 (acetate) | JA accumulation extrafloral nectar secretion | Z. mays P. lunatus | inactive <(1,2) | Engelberth, 2011 [64] Heil, 2008 [67] |
| Z3-C3 (propionate) | defense gene expression pathogen defense 5 | S. lycopersicum S. lycopersicum | >(1) >(2) | Pérez-Hedo, 2021 [53] López-Gresa, 2018 [72] |
| Z3-C4 (butyrate) | extrafloral nectar secretion defense gene expression pathogen defense | P. lunatus S. lycopersicum S. lycopersicum | <(1,2) >(1,2) >(1) | Heil, 2008 [67] Pérez-Hedo, 2021 [53] López-Gresa, 2018 [72] |
| Z3-C4 (iso-butyrate) | pathogen defense | S. lycopersicum | >(3) | López-Gresa, 2018 [72] |
| C4 (butyrate) 4 | defense gene expression | S. lycopersicum | >(1,2) | Pérez-Hedo, 2021 [53] |
| Z3-C5 (methyl- butyrate) | extrafloral nectar secretion | P. lunatus | <(1,2) | Heil, 2008 [67] |
4. Other Aliphatic Elicitors of Defense Responses
4.1. Fatty Acid–Amino Acid Conjugates
4.2. Sphingoid Bases
4.3. Caeliferins
4.4. Bruchins
5. Effect of Physicochemical Properties of Small Molecules on Perception
6. Perception of Small Molecule Elicitors by Membrane-Bound Receptors
7. Unknown Mechanisms of Perception of Small Molecule Elicitors
7.1. Fatty Alcohols (FAlcs)
7.2. Fatty Aldehydes (FAlds)
7.3. Fatty Esters (FEs)
7.4. All GLVs
7.5. Caeliferins
7.6. Bruchins
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DAMP | damage-associated molecular pattern |
| FAC | fatty acid–amino acid conjugate |
| FAlc | fatty alcohol |
| FAld | fatty aldehyde |
| FE | fatty ester |
| GLV | green leaf volatile |
| HAC | hexenyl acetate |
| HAL | hexenal |
| HOL | hexenol |
| H/MAMP | herbivore/microbe-associated molecular pattern |
| MAPK | mitogen-activated protein kinase |
| mcFA | medium-chain fatty acid |
| PRR | pattern recognition recptor |
| VOC | volatile organic compound |
References
- Sugimoto, K.; Matsui, K.; Iijima, Y.; Akakabe, Y.; Muramoto, S.; Ozawa, R.; Uefune, M.; Sasaki, R.; Alamgir, K.M.; Akitake, S.; et al. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc. Natl. Acad. Sci. USA 2014, 111, 7144–7149. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant Biol. 2017, 38, 68–77. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Zipfel, C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol. Cell 2021, 81, 3449–3467. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, A.; Dawid, C.; Gisch, N.; Schmid, C.; Raasch, L.; Gerster, T.; Schäffer, M.; Smakowska-Luzan, E.; Belkhadir, Y.; Vlot, A.C.; et al. Bacterial medium-chain 3-hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science 2019, 364, 178–181. [Google Scholar] [CrossRef]
- Ranf, S.; Gisch, N.; Schäffer, M.; Illig, T.; Westphal, L.; Knirel, Y.A.; Sánchez-Carballo, P.M.; Zähringer, U.; Hückelhoven, R.; Lee, J.; et al. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 2015, 16, 426–433. [Google Scholar] [CrossRef]
- Kato, H.; Nemoto, K.; Shimizu, M.; Abe, A.; Asai, S.; Ishihama, N.; Matsuoka, S.; Daimon, T.; Ojika, M.; Kawakita, K.; et al. Recognition of pathogen-derived sphingolipids in Arabidopsis. Science 2022, 376, 857–860. [Google Scholar] [CrossRef]
- Truitt, C.L.; Wei, H.-X.; Pare, P.W. A Plasma Membrane Protein from Zea mays Binds with the Herbivore Elicitor Volicitin. Plant Cell 2004, 16, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Poretsky, E.; Ruiz, M.; Ahmadian, N.; Steinbrenner, A.D.; Dressano, K.; Schmelz, E.A.; Huffaker, A. Comparative analyses of responses to exogenous and endogenous antiherbivore elicitors enable a forward genetics approach to identify maize gene candidates mediating sensitivity to herbivore-associated molecular patterns. Plant J. 2021, 108, 1295–1316. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, J.R.; Jaini, R.; Morgan, J.A.; Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant Sci. 2015, 20, 545–550. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Schaller, G.E. The Mechanism of Ethylene Perception. Plant Physiol. 1996, 111, 653–660. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Z.; La Clair, J.J.; Chory, J.; Noel, J.P. Smoke-derived karrikin perception by the α/β-hydrolase KAI2 from Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 8284–8289. [Google Scholar] [CrossRef]
- Stirling, S.A.; Guercio, A.M.; Patrick, R.M.; Huang, X.-Q.; Bergman, M.E.; Dwivedi, V.; Kortbeek, R.W.J.; Liu, Y.-K.; Sun, F.; Tao, W.A.; et al. Volatile communication in plants relies on a KAI2-mediated signaling pathway. Science 2024, 383, 1318–1325. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; He, L.; Huang, F.; Zhang, D.; Wang, Y.; Wei, X.; Han, M.; Deng, H.; Luo, L.; et al. Molecular basis of methyl-salicylate-mediated plant airborne defence. Nature 2023, 622, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, A.; Higaki, T.; Koeduka, T.; Ishigami, K.; Hosokawa, S.; Watanabe, H.; Matsui, K.; Hasezawa, S.; Touhara, K. Transcriptional regulators involved in responses to volatile organic compounds in plants. J. Biol. Chem. 2019, 294, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, D.; Jiang, R.T.; Taly, A.; Chataigneau, T.; Specht, A.; Grutter, T. Ligand-Gated Ion Channels: New Insights into Neurological Disorders and Ligand Recognition. Chem. Rev. 2012, 112, 6285–6318. [Google Scholar] [CrossRef]
- Pantoja, O. Recent Advances in the Physiology of Ion Channels in Plants. Annu. Rev. Plant Biol. 2021, 72, 463–495. [Google Scholar] [CrossRef]
- Simon, A.A.; Navarro-Retamal, C.; Feijó, J.A. Merging Signaling with Structure: Functions and Mechanisms of Plant Glutamate Receptor Ion Channels. Annu. Rev. Plant Biol. 2023, 74, 415–452. [Google Scholar] [CrossRef]
- Tapken, D.; Anschütz, U.; Liu, L.H.; Huelsken, T.; Seebohm, G.; Becker, D.; Hollmann, M. A Plant Homolog of Animal Glutamate Receptors Is an Ion Channel Gated by Multiple Hydrophobic Amino Acids. Sci. Signal. 2013, 6, ra47. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.I.; Naeem, M.; Aftab, T. From Neurotransmitter to Plant Protector: The Intricate World of GABA Signaling and its Diverse Functions in Stress Mitigation. J. Plant Growth Regul. 2025, 44, 403–418. [Google Scholar] [CrossRef]
- Liao, P.; Maoz, I.; Shih, M.L.; Lee, J.H.; Huang, X.Q.; Morgan, J.A.; Dudareva, N. Emission of floral volatiles is facilitated by cell-wall non-specific lipid transfer proteins. Nat. Commun. 2023, 14, 330. [Google Scholar] [CrossRef]
- Juen, Z.; Lu, Z.Y.; Yu, R.H.; Chang, A.N.; Wang, B.; Fitzpatrick, A.W.P.; Zuker, C.S. The structure of human sweetness. Cell 2025, 188, 4141–4153. [Google Scholar] [CrossRef]
- Yang, L.; Cui, M.; Liu, B. Current Progress in Understanding the Structure and Function of Sweet Taste Receptor. J. Mol. Neurosci. 2021, 71, 234–244. [Google Scholar] [CrossRef]
- Gotkhindikar, A.; Chakravorty, D.; Sengupta, D.; Joshi, M.; Assmann, S.M. Is GCR1 the GPR157 of plants? Plant Physiol. 2025, 197, kiaf057. [Google Scholar] [CrossRef]
- Chini, A.; Monte, I.; Fernández-Barbero, G.; Boter, M.; Hicks, G.; Raikhel, N.; Solano, R. A small molecule antagonizes jasmonic acid perception and auxin responses in vascular and nonvascular plants. Plant Physiol. 2021, 187, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tan, X.; Zheng, N.; Hatate, T.; Kimura, Y.; Kepinski, S.; Nozaki, H. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 5632–5637. [Google Scholar] [CrossRef] [PubMed]
- Lumba, S.; Holbrook-Smith, D.; McCourt, P. The perception of strigolactones in vascular plants. Nat. Chem. Biol. 2017, 13, 599–606. [Google Scholar] [CrossRef]
- Matsui, K.; Engelberth, J. Green Leaf Volatiles-The Forefront of Plant Responses Against Biotic Attack. Plant Cell Physiol. 2022, 63, 1378–1390. [Google Scholar] [CrossRef]
- Schuman, M.C. Where, When, and Why Do Plant Volatiles Mediate Ecological Signaling? The Answer Is Blowing in the Wind. Annu. Rev. Plant Biol. 2023, 74, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, S.; Guayazan-Palacios, N.; Steinbrenner, A.D. Molecular tug-of-war: Plant immune recognition of herbivory. Plant Cell 2022, 34, 1497–1513. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-mediated plant-plant interactions: Volatile organic compounds as modulators of receiver plant defence, growth, and reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Jones, A.C.; Felton, G.W.; Tumlinson, J.H. The dual function of elicitors and effectors from insects: Reviewing the ‘arms race’ against plant defenses. Plant Mol. Biol. 2022, 109, 427–445. [Google Scholar] [CrossRef]
- Mwenda, C.M.; Matsui, K. The importance of lipoxygenase control in the production of green leaf volatiles by lipase-dependent and independent pathways. Plant Biotechnol. 2014, 31, 445–452. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green Leaf Volatiles: A Plant’s Multifunctional Weapon against Herbivores and Pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Gorman, Z.; Tolley, J.P.; Koiwa, H.; Kolomiets, M.V. The Synthesis of Pentyl Leaf Volatiles and Their Role in Resistance to Anthracnose Leaf Blight. Front. Plant Sci. 2021, 12, 719587. [Google Scholar] [CrossRef]
- Heil, M.; Silva Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Heil, M.; Ton, J. Long-distance signalling in plant defence. Trends Plant Sci. 2008, 13, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Duran-Flores, D.; Heil, M. Sources of specificity in plant damaged-self recognition. Curr. Opin. Plant Biol. 2016, 32, 77–87. [Google Scholar] [CrossRef]
- Meents, A.K.; Mithofer, A. Plant-Plant Communication: Is There a Role for Volatile Damage-Associated Molecular Patterns? Front. Plant Sci. 2020, 11, 583275. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J. Green Leaf Volatiles: A New Player in the Protection against Abiotic Stresses? Int. J. Mol. Sci. 2024, 25, 9471. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. Reactivity of Z-3-Hexenal with Amino Groups Provides a Potential Mechanism for Its Direct Effects on Insect Herbivores. Insects 2025, 16, 582. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007, 49, 194–207. [Google Scholar] [CrossRef]
- Bate, N.J.; Rothstein, S.J. C-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998, 16, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimoto, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015, 5, 8030. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Matsuda, A.; Matsuura, N.; Mizutani, M.; Sugimoto, Y. Transcriptome analysis of Arabidopsis thaliana treated with green leaf volatiles: Possible role of green leaf volatiles as self-made damage-associated molecular patterns. J. Pestic. Sci. 2018, 43, 207–213. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile C6-aldehydes and Allo-ocimene Activate Defense Genes and Induce Resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1093–1102. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. ETR1-, JAR1- and PAD2-dependent signaling pathways are involved in C6-aldehyde-induced defense responses of Arabidopsis. Plant Sci. 2006, 171, 415–423. [Google Scholar] [CrossRef]
- Mirabella, R.; Rauwerda, H.; Allmann, S.; Scala, A.; Spyropoulou, E.A.; de Vries, M.; Boersma, M.R.; Breit, T.M.; Haring, M.A.; Schuurink, R.C. WRKY40 and WRKY6 act downstream of the green leaf volatile E-2-hexenal in Arabidopsis. Plant J. 2015, 83, 1082–1096. [Google Scholar] [CrossRef]
- Mirabella, R.; Rauwerda, H.; Struys, E.A.; Jakobs, C.; Triantaphylides, C.; Haring, M.A.; Schuurink, R.C. The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. Plant J. 2008, 53, 197–213. [Google Scholar] [CrossRef]
- Scala, A.; Mirabella, R.; Goedhart, J.; de Vries, M.; Haring, M.A.; Schuurink, R.C. Forward genetic screens identify a role for the mitochondrial HER2 in E-2-hexenal responsiveness. Plant Mol. Biol. 2017, 95, 399–409. [Google Scholar] [CrossRef]
- Aratani, Y.; Uemura, T.; Hagihara, T.; Matsui, K.; Toyota, M. Green leaf volatile sensory calcium transduction in Arabidopsis. Nat. Commun. 2023, 14, 6236. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Du, S.; Deng, Z.; Liang, Q.; Song, G.; Wang, H.; Yan, M.; Wang, X. Transcriptomic and proteomic analysis reveals (E)-2-hexenal modulates tomato resistance against Botrytis cinerea by regulating plant defense mechanism. Plant Mol. Biol. 2023, 111, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hedo, M.; Alonso-Valiente, M.; Vacas, S.; Gallego, C.; Rambla, J.L.; Navarro-Llopis, V.; Granell, A.; Urbaneja, A. Eliciting tomato plant defenses by exposure to herbivore induced plant volatiles. Entomol. Gen. 2021, 41, 209–218. [Google Scholar] [CrossRef]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early transcriptome analyses of Z-3-Hexenol-treated Zea mays revealed distinct transcriptional networks and anti-herbivore defense potential of green leaf volatiles. PLoS ONE 2013, 8, e77465. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Fujita, K.; Date, M.; Watanabe, B.; Matsui, K. Structure-activity relationship of volatile compounds that induce defense-related genes in maize seedlings. Plant Signal. Behav. 2023, 18, 2234115. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, J.E.; Kronmiller, B.A.; Hollenbeck, V.G.; Rhodes, A.C.; Henning, J.A.; Martin, R.C. Transcriptome analysis of the model grass Lolium temulentum exposed to green leaf volatiles. BMC Plant Biol. 2019, 19, 222. [Google Scholar] [CrossRef]
- Dombrowski, J.E.; Hind, S.R.; Martin, R.C.; Stratmann, J.W. Wounding systemically activates a mitogen-activated protein kinase in forage and turf grasses. Plant Sci. 2011, 180, 686–693. [Google Scholar] [CrossRef]
- Tanarsuwongkul, S.; Fisher, K.W.; Mullis, B.T.; Negi, H.; Roberts, J.; Tomlin, F.; Wang, Q.; Stratmann, J.W. Green leaf volatiles co-opt proteins involved in molecular pattern signalling in plant cells. Plant Cell Environ. 2024, 47, 928–946. [Google Scholar] [CrossRef]
- Asai, N.; Nishioka, T.; Takabayashi, J.; Furuichi, T. Plant volatiles regulate the activities of Ca2+-permeable channels and promote cytoplasmic calcium transients in Arabidopsis leaf cells. Plant Signal. Behav. 2009, 4, 294–300. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci. 2012, 196, 93–100. [Google Scholar] [CrossRef]
- Kikuta, Y.; Ueda, H.; Nakayama, K.; Katsuda, Y.; Ozawa, R.; Takabayashi, J.; Hatanaka, A.; Matsuda, K. Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. Plant Cell Physiol. 2011, 52, 588–596. [Google Scholar] [CrossRef]
- Hu, L.F.; Ye, M.; Erb, M. Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defence and resistance. Plant Cell Environ. 2019, 42, 959–971. [Google Scholar] [CrossRef]
- Hu, L.F. Integration of multiple volatile cues into plant defense responses. New Phytol. 2022, 233, 618–623. [Google Scholar] [CrossRef]
- Engelberth, J. Selective inhibition of jasmonic acid accumulation by a small α, β-unsaturated carbonyl and phenidone reveals different modes of octadecanoid signalling activation in response to insect elicitors and green leaf volatiles in Zea mays. BMC Res. Notes 2011, 4, 377. [Google Scholar] [CrossRef]
- Dombrowski, J.E.; Martin, R.C. Activation of MAP kinases by green leaf volatiles in grasses. BMC Res. Notes 2018, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Negi, H.; Cole, O.; Tomlin, F.; Wang, Q.; Stratmann, J.W. Structure-Function Analysis of Volatile (Z)-3-Fatty Alcohols in Tomato. J. Chem. Ecol. 2025, 51, 6. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Lion, U.; Boland, W. Defense-inducing volatiles: In search of the active motif. J. Chem. Ecol. 2008, 34, 601–604. [Google Scholar] [CrossRef]
- Brambilla, A.; Sommer, A.; Ghirardo, A.; Wenig, M.; Knappe, C.; Weber, B.; Amesmaier, M.; Lenk, M.; Schnitzler, J.P.; Vlot, A.C. Immunity-associated volatile emissions of beta-ionone and nonanal propagate defence responses in neighbouring barley plants. J. Exp. Bot. 2022, 73, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Elizarraraz-Martinez, I.J.; Rojas-Raya, M.A.; Feregrino-Perez, A.A.; Partida-Martinez, L.P.; Heil, M. Immunity priming and biostimulation by airborne nonanal increase yield of field-grown common bean plants. Front. Plant Sci. 2024, 15, 1451864. [Google Scholar] [CrossRef]
- Giron-Calva, P.S.; Molina-Torres, J.; Heil, M. Volatile dose and exposure time impact perception in neighboring plants. J. Chem. Ecol. 2012, 38, 226–228. [Google Scholar] [CrossRef]
- Huang, P.C.; Berg-Falloure, K.M.; Gao, X.; Meeley, R.; Kolomiets, M.V. Pentyl leaf volatiles promote insect and pathogen resistance via enhancing ketol-mediated defense responses. Plant Physiol. 2025, 197, kiae646. [Google Scholar] [CrossRef]
- Lopez-Gresa, M.P.; Paya, C.; Ozaez, M.; Rodrigo, I.; Conejero, V.; Klee, H.; Belles, J.M.; Lison, P. A New Role For Green Leaf Volatile Esters in Tomato Stomatal Defense Against Pseudomonas syringe pv. tomato. Front. Plant Sci. 2018, 9, 1855. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Alborn, H.T.; Nakanishi, T.; Suckling, D.M.; Nishida, R.; Tumlinson, J.H.; Mori, N. Fatty Acid-amino Acid Conjugates Diversification in Lepidopteran Caterpillars. J. Chem. Ecol. 2010, 36, 319–325. [Google Scholar] [CrossRef]
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An Elicitor of Plant Volatiles from Beet Armyworm Oral Secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Alborn, H.T.; Loughrin, J.H.; Tumlinson, J.H. Volicitin, An Elicitor of Maize Volatiles in Oral Secretion of Spodoptera exigua: Isolation and Bioactivity. J. Chem. Ecol. 2000, 26, 189–202. [Google Scholar] [CrossRef]
- Ling, X.; Gu, S.; Tian, C.; Guo, H.; Degen, T.; Turlings, T.C.J.; Ge, F.; Sun, Y. Differential Levels of Fatty Acid-Amino Acid Conjugates in the Oral Secretions of Lepidopteran Larvae Account for the Different Profiles of Volatiles. Pest Manag. Sci. 2021, 77, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; Tumlinson, J.H.; Teal, P.E.A. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA 2009, 106, 653–657. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Ishikawa, C.; Seidl-Adams, I.; Bosak, E.; Aboshi, T.; Tumlinson, J.H.; Mori, N. N-(18-Hydroxylinolenoyl)-L-Glutamine: A Newly Discovered Analog of Volicitin in Manduca sexta and its Elicitor Activity in Plants. J. Chem. Ecol. 2014, 40, 484–490. [Google Scholar] [CrossRef]
- Grissett, L.; Ali, A.; Coble, A.-M.; Logan, K.; Washington, B.; Mateson, A.; McGee, K.; Nkrumah, Y.; Jacobus, L.; Abraham, E.; et al. Survey of Sensitivity to Fatty Acid-Amino Acid Conjugates in the Solanaceae. J. Chem. Ecol. 2020, 46, 330–343. [Google Scholar] [CrossRef]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [CrossRef]
- Krempl, C.; Joussen, N.; Reichelt, M.; Kai, M.R.; Vogel, H.; Heckel, D.G. Consumption of gossypol increases fatty acid-amino acid conjugates in the cotton pests Helicoverpa armigera and Heliothis virescens. Arch. Insect Biochem. Physiol. 2021, 108, e21843. [Google Scholar] [CrossRef]
- VanDoorn, A.; Kallenbach, M.; Borquez, A.A.; Baldwin, I.T.; Bonaventure, G. Rapid modification of the insect elicitor N-linolenoyl-glutamate via a lipoxygenase-mediated mechanism on Nicotiana attenuata leaves. BMC Plant Biol. 2010, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999, 18, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Meindl, T.; Boller, T.; Felix, G. The plant wound hormone systemin binds with the N-terminal part to its receptor but needs the C-terminal part to activate it. Plant Cell 1998, 10, 1561–1570. [Google Scholar] [CrossRef]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981. [Google Scholar] [CrossRef] [PubMed]
- Doss, R.P.; Oliver, J.E.; Proebsting, W.M.; Potter, S.W.; Kuy, S.; Clement, S.L.; Williamson, R.T.; Carney, J.R.; DeVilbiss, E.D. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA 2000, 97, 6218–6223. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Doss, R.P.; Marquez, B.; DeVilbiss, E.D. Bruchins, plant mitogens from weevils: Structural requirements for activity. J. Chem. Ecol. 2002, 28, 2503–2513. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Shih, M.L.; Morgan, J.A.; Dudareva, N. Two-way communication: Volatile emission and uptake occur through the same barriers. Mol. Plant 2023, 16, 1–3. [Google Scholar] [CrossRef]
- Köhler, H.-R.; Gräff, T.; Schweizer, M.; Blumhardt, J.; Burkhardt, J.; Ehmann, L.; Hebel, J.; Heid, C.; Kundy, L.; Kuttler, J.; et al. LogD-based modelling and ΔlogD as a proxy for pH-dependent action of ionizable chemicals reveal the relevance of both neutral and ionic species for fish embryotoxicity and possess great potential for practical application in the regulation of chemicals. Water Res. 2023, 235, 119864. [Google Scholar] [CrossRef]
- Felle, H.H. pH: Signal and messenger in plant cells. Plant Biol. 2001, 3, 577–591. [Google Scholar] [CrossRef]
- Li, N.; Xu, C.; Li-Beisson, Y.; Philippar, K. Fatty Acid and Lipid Transport in Plant Cells. Trends Plant Sci. 2016, 21, 145–158. [Google Scholar] [CrossRef]
- Liu, N.-J.; Hou, L.-P.; Bao, J.-J.; Wang, L.-J.; Chen, X.-Y. Sphingolipid metabolism, transport, and functions in plants: Recent progress and future perspectives. Plant Commun. 2021, 2, 100214. [Google Scholar] [CrossRef]
- Kinoshita, T.; Cano-Delgado, A.; Seto, H.; Hiranuma, S.; Fujioka, S.; Yoshida, S.; Chory, J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 2005, 433, 167–171. [Google Scholar] [CrossRef]
- Bi, G.Z.; Zhou, Z.Y.; Wang, W.B.; Li, L.; Rao, S.F.; Wu, Y.; Zhang, X.J.; Menke, F.L.H.; Chen, S.; Zhou, J.M. Receptor-Like Cytoplasmic Kinases Directly Link Diverse Pattern Recognition Receptors to the Activation of Mitogen-Activated Protein Kinase Cascades in Arabidopsis. Plant Cell 2018, 30, 1543–1561. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Haj Ahmad, F.; Fuglsang, A.T.; Steppuhn, A.; Stintzi, A.; Schaller, A. Poltergeist-Like 2 (PLL2)-dependent activation of herbivore defence distinguishes systemin from other immune signalling pathways. Nat. Plants 2025, 11, 1270–1281. [Google Scholar] [CrossRef]
- Ryan, C.A. The systemin signaling pathway: Differential activation of plant defensive genes. Biochim. Biophys. Acta 2000, 1477, 112–121. [Google Scholar] [CrossRef]
- Wang, L.; Einig, E.; Almeida-Trapp, M.; Albert, M.; Fliegmann, J.; Mithofer, A.; Kalbacher, H.; Felix, G. The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants 2018, 4, 152–156. [Google Scholar] [CrossRef]
- Wang, L.; Maier, L.-P.; Pham, N.; Wang, Y.L.; Wang, X.; Schaller, A.; Fliegmann, J.; Erb, M.; Boller, T.; Felix, G. A receptor antagonist counterbalances multiple systemin phytocytokines in tomato. Cell 2025, 188, 6509–6518. [Google Scholar] [CrossRef] [PubMed]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Ferrari, S.; Ausubel, F.M.; Dewdney, J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant 2008, 1, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Ketter, A.P. Cellular Events Conditioned by the Np Gene of Pisum sativum L. in Response to Reduced UV Light, Weevil Oviposition, and Bruchins. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2006. [Google Scholar]
- Shiu, S.-H.; Bleecker, A.B. Expansion of the Receptor-Like Kinase/Pelle Gene Family and Receptor-Like Proteins in Arabidopsis. Plant Physiol. 2003, 132, 530–543. [Google Scholar] [CrossRef]
- Vaid, N.; Pandey, P.K.; Tuteja, N. Genome-wide analysis of lectin receptor-like kinase family from Arabidopsis and rice. Plant Mol. Biol. 2012, 80, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Cañeque, T.; Müller, S.; Rodriguez, R. Visualizing biologically active small molecules in cells using click chemistry. Nat. Rev. Chem. 2018, 2, 202–215. [Google Scholar] [CrossRef]
- Kaschani, F.; Verhelst, S.H.L.; Van Swieten, P.F.; Verdoes, M.; Wong, C.-S.; Wang, Z.; Kaiser, M.; Overkleeft, H.S.; Bogyo, M.; Van Der Hoorn, R.A.L. Minitags for small molecules: Detecting targets of reactive small molecules in living plant tissues using ‘click chemistry’. Plant J. 2009, 57, 373–385. [Google Scholar] [CrossRef]
- Yu, H.; Buchholz, A.; Pullinen, I.; Saarela, S.; Li, Z.; Virtanen, A.; Blande, J.D. Biogenic secondary organic aerosol participates in plant interactions and herbivory defense. Science 2024, 385, 1225–1230. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Kivimäenpää, M.; Nizkorodov, S.A. Plant-derived Secondary Organic Material in the Air and Ecosystems. Trends Plant Sci. 2017, 22, 744–753. [Google Scholar] [CrossRef]
- Jain, S.; Zahardis, J.; Petrucci, G.A. Soft Ionization Chemical Analysis of Secondary Organic Aerosol from Green Leaf Volatiles Emitted by Turf Grass. Environ. Sci. Technol. 2014, 48, 4835–4843. [Google Scholar] [CrossRef]
- Harvey, R.M.; Zahardis, J.; Petrucci, G.A. Establishing the contribution of lawn mowing to atmospheric aerosol levels in American suburbs. Atmos. Chem. Phys. 2014, 14, 797–812. [Google Scholar] [CrossRef]
- Hamilton, J.F.; Lewis, A.C.; Carey, T.J.; Wenger, J.C.; Borrás i Garcia, E.; Muñoz, A. Reactive oxidation products promote secondary organic aerosol formation from green leaf volatiles. Atmos. Chem. Phys. 2009, 9, 3815–3823. [Google Scholar] [CrossRef]
| Falc 2 | Bioassay | Species | Activity Compared to Z3-C6:1 1 | Reference |
|---|---|---|---|---|
| C6:0 | JA accumulation JA-related genes MAPK activation LOX-1 gene expr. pH change | Z. mays Z. mays L. temulentum A. thaliana S. peruvianum | inactive inactive = 3 > > | Engelberth, 2011 [64] Tanaka, 2023 [55] Dombrowski, 2018 [65] Bate, 1998 [43] Fisher, 2025 [66] |
| E2-C6:1 | MAPK activation LOX-1 gene expr. pH change | L. temulentum A. thaliana S. peruvianum | = = > | Dombrowski, 2018 [65] Bate, 1998 [43] Fisher, 2025 [66] |
| E3-C6:1 | JA-related genes MAPK activation | Z. mays L. temulentum | < = | Tanaka, 2023 [55] Dombrowski, 2018 [65] |
| Z3-C4:1 | pH change MAPK activation root growth inhibition | S. peruvianum S. peruvianum S. lycopersicum | inactive inactive > | Fisher, 2025 [66] Fisher, 2025 [66] Fisher, 2025 [66] |
| Z2-C5:1 | JA-related genes | Z. mays | inactive | Tanaka, 2023 [55] |
| Z3-C7:1 | JA-related genes pH change MAPK activation root growth inhibition | Z. mays S. peruvianum S. peruvianum S. lycopersicum | inactive > (<C8:1/C9:1) > (<C8:1/C9:1) > (<C8:1/C9:1) | Tanaka, 2023 [55] Fisher, 2025 [66] Fisher, 2025 [66] Fisher, 2025 [66] |
| Z3-C8:1 | JA-related genes pH change MAPK activation root growth inhibition | Z. mays S. peruvianum S. peruvianum S. lycopersicum | inactive > (>C7:1; <C9:1) > (>C7:1; <C9:1) > (>C7:1/C9:1) | Tanaka, 2023 [55] Fisher, 2025 [66] Fisher, 2025 [66] Fisher, 2025 [66] |
| Z3-C9:1 | JA-related genes pH change MAPK activation root growth inhibition | Z. mays S. peruvianum S. peruvianum S. lycopersicum | inactive > (>C7:1/C8:1) > (>C7:1/C8:1) > (>C7:1; <C8:1) | Tanaka, 2023 [55] Fisher, 2025 [66] Fisher, 2025 [66] Fisher, 2025 [66] |
| Fald 2 | Bioassay | Species | Activity Compared to E2-C6:1 1 | Reference |
|---|---|---|---|---|
| C6:0 | root growth inhibition Ca2+-fluxes MAPK activation | A. thaliana A. thaliana L. temulentum | inactive inactive = 3 | Mirabella, 2008 [49] Aratani, 2023 [51] Dombrowski, 2018 [65] |
| Z3-C6:1 | JA accumulation HSFA2 TF expression Ca2+-fluxes MAPK activation | Z. mays A. thaliana A. thaliana L. temulentum | < (>C8:1) inactive > = | Engelberth, 2011 [64] Yamauchi, 2015 [44] Aratani, 2023 [51] Dombrowski, 2018 [65] |
| C3:1 (acrolein) | JA accumulation HSFA2 TF expression | Z. mays A. thaliana | inactive inactive | Engelberth, 2011 [64] Yamauchi, 2015 [44] |
| E2-C4:1 | JA accumulation HSFA2 TF expression | Z. mays A. thaliana | inactive = | Engelberth, 2011 [64] Yamauchi, 2015 [44] |
| E2-C5:1 | HSFA2 TF expression root growth inhibition | A. thaliana A. thaliana | = < (>C7:1) | Yamauchi, 2015 [44] Mirabella, 2008 [49] |
| E2-C7:1 | HSFA2 TF expression root growth inhibition | A. thaliana A. thaliana | = < (<C5:1) | Yamauchi, 2015 [44] Mirabella, 2008 [49] |
| E2-C8:1 | JA accumulation HSFA2 TF expression | Z. mays A. thaliana | < (<Z3-6:1) > | Engelberth, 2011 [64] Yamauchi, 2015 [44] |
| C9:0 | MAPK activation | L. temulentum | = | Dombrowski, 2018 [65] |
| E2-C9:1 | JA accumulation HSFA2 TF expression root growth inhibition | Z. mays A. thaliana A. thaliana | inactive = inactive | Engelberth, 2011 [64] Yamauchi, 2015 [44] Mirabella, 2008 [49] |
| C10:0 | MAPK activation | L. temulentum | = | Dombrowski, 2018 [65] |
| E2-C10:1 | HSFA2 TF expression | Z. mays | inactive | Yamauchi, 2015 [44] |
| FAC | Bioassay | Species | Activity, Ranked 1 | Reference |
|---|---|---|---|---|
| 18:3-17-OH-L-Gln 2 18:3-17-OH-D-Gln 18:3-L-Gln Gln (no FA) 18:3-17-OH (no amino acid) | competition for [3H]-L-18:3-OH binding | Z. mays (membrane preps) | 1 no competitor 2 no competitor no competitor | Truitt, 2004 [8] |
| 18:3-17-OH-L-Gln 18:3-17-OH-D-Gln 18:3-L-Gln Gln (no FA) 18:3-17-OH (no amino acid) | VOC release assay | Z. mays (seedlings) | 1 inactive 2 inactive inactive | Truitt, 2004 [8] |
| 18:3-Glu 18:2-Glu 18:1-Gln 16:1-Gln | VOC release assay (when added to oral secretions) | Z. mays (seedlings) | active active active active | Ling, 2021 [76] |
| 18:3-17-OH-L-Gln 18:3-Gln | ethylene and JA synthesis | Z. mays, S. melongena, G. max | active active | Schmelz, 2009 [77] |
| 18:3-17-OH-L-Gln 18:3-Gln | ethylene and JA synthesis | A. thaliana, V. unguiculata, S. lycopersicum | inactive inactive | Schmelz, 2009 [77] |
| 18:3-17-OH-L-Gln 18:3-Gln 18:3-Glu 18:2-Gln 18:2-Glu | ethylene and JA synthesis | G. max | active inactive inactive inactive inactive | Schmelz, 2009 [77] |
| 18:3-17-OH-L-Gln 18:3-18-OH-L-Gln 18:3-Gln 18:3-Glu 18:3-18-OH-L-Glu | VOC release assay | Z. mays | 1 2 2 3 3 | Yoshinaga, 2014 [78] |
| 18:3-17-OH-L-Gln 18:3-18-OH-L-Gln 18:3-Gln 18:3-Glu 18:3-18-OH-L-Glu | VOC release assay | S. melongena, N. tabacum | 1 1 2 2 2 | Yoshinaga, 2014 [78] |
| 18:3-17-OH-L-Gln 18:3-Gln 18:3-Glu | MAPK activation | seven species in the tomato clade | insensitive insensitive insensitive | Grissett, 2020 [79] |
| 18:3-17-OH-L-Gln 18:3-Gln 18:3-Glu | MAPK activation | N. alata, N. sylvestris, N. knightiana | insensitive insensitive insensitive | Grissett, 2020 [79] |
| 18:3-17-OH-L-Gln 18:3-Gln 18:3-Glu | MAPK activation | N. tabacum, N. benthamiana | sensitive sensitive sensitive | Grissett, 2020 [79] |
| 18:3-17-OH-L-Gln 18:3-Gln 18:3-Glu | MAPK activation | C. annuum | insensitive sensitive sensitive | Grissett, 2020 [79] |
| 18:3-17-OH-L-Gln 18:3-Gln 18:3-Glu | MAPK activation | S. melongena | sensitive sensitive sensitive | |
| 18:3-17-OH-L-Gln 18:3-Gln 18:3-Glu | MAPK activation | P. hybrida | insensitive sensitive sensitive | Grissett, 2020 [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratmann, J.W.; Negi, H.; Wang, Q. Insights from Structure–Function Studies into Perception of Fatty Acid-Derived Defense Signals. Plants 2025, 14, 3518. https://doi.org/10.3390/plants14223518
Stratmann JW, Negi H, Wang Q. Insights from Structure–Function Studies into Perception of Fatty Acid-Derived Defense Signals. Plants. 2025; 14(22):3518. https://doi.org/10.3390/plants14223518
Chicago/Turabian StyleStratmann, Johannes W., Harshita Negi, and Qian Wang. 2025. "Insights from Structure–Function Studies into Perception of Fatty Acid-Derived Defense Signals" Plants 14, no. 22: 3518. https://doi.org/10.3390/plants14223518
APA StyleStratmann, J. W., Negi, H., & Wang, Q. (2025). Insights from Structure–Function Studies into Perception of Fatty Acid-Derived Defense Signals. Plants, 14(22), 3518. https://doi.org/10.3390/plants14223518







