Searching for Hypoglycemic Compounds from Brazilian Medicinal Plants Through UPLC-HRMS and Molecular Docking
Abstract
1. Introduction
2. Results
2.1. Screening for α-Glycosidase Inhibition Activity of Extracts from Brazilian Medicinal Plants
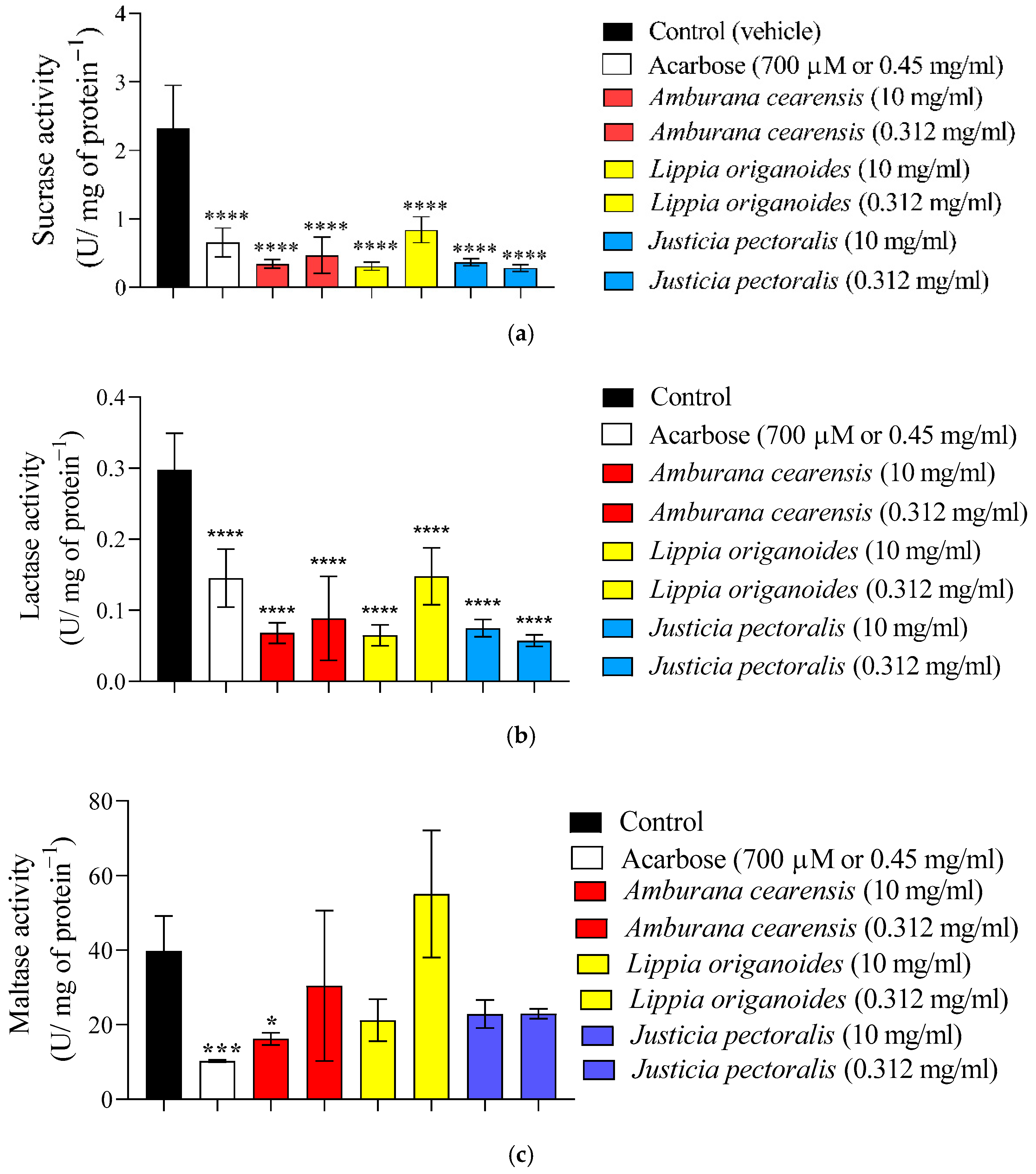
2.2. Toxicological Evaluation of the Bioactive Extracts
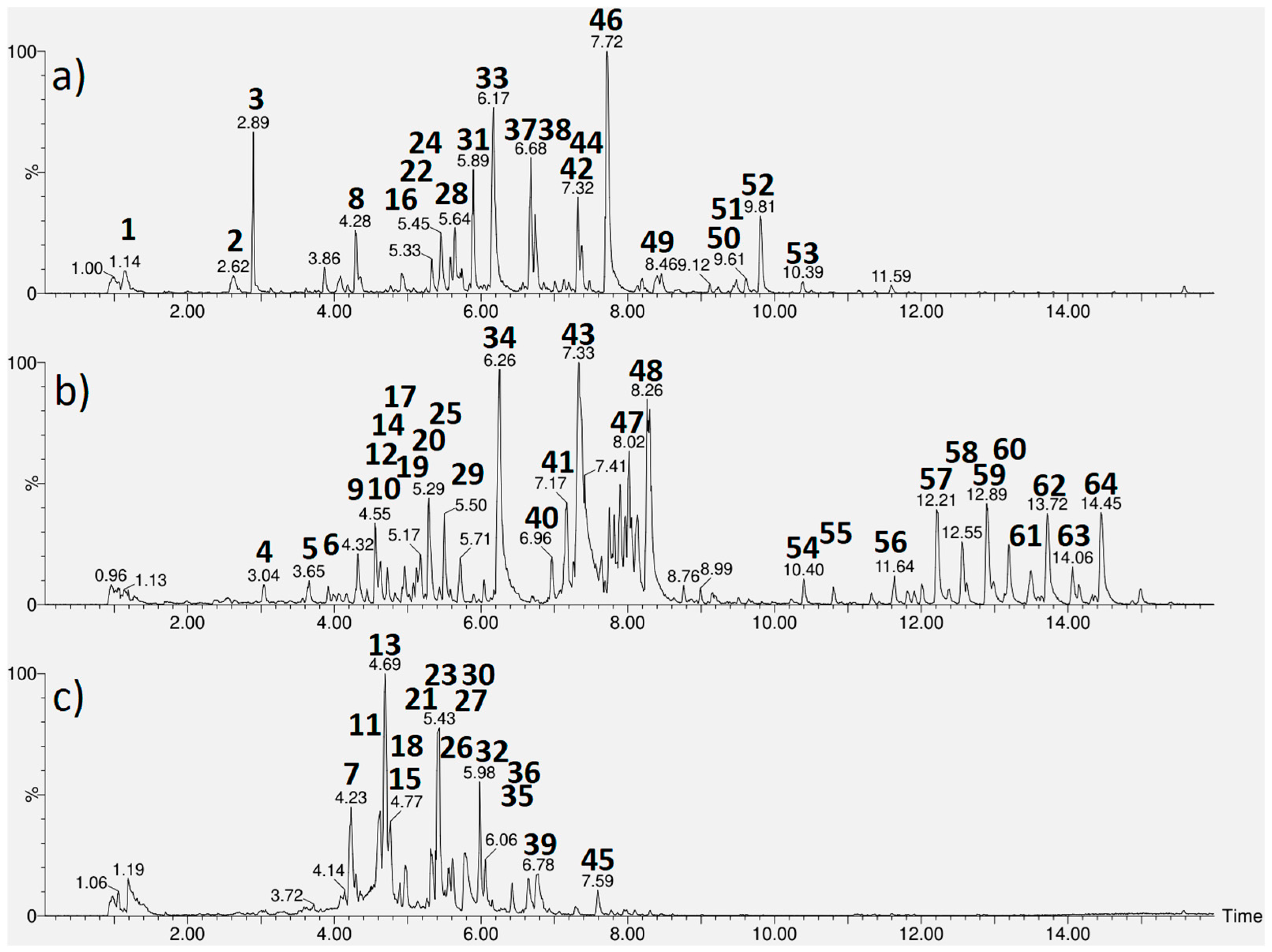
2.3. Chemical Characterization of the Bioactive Extracts
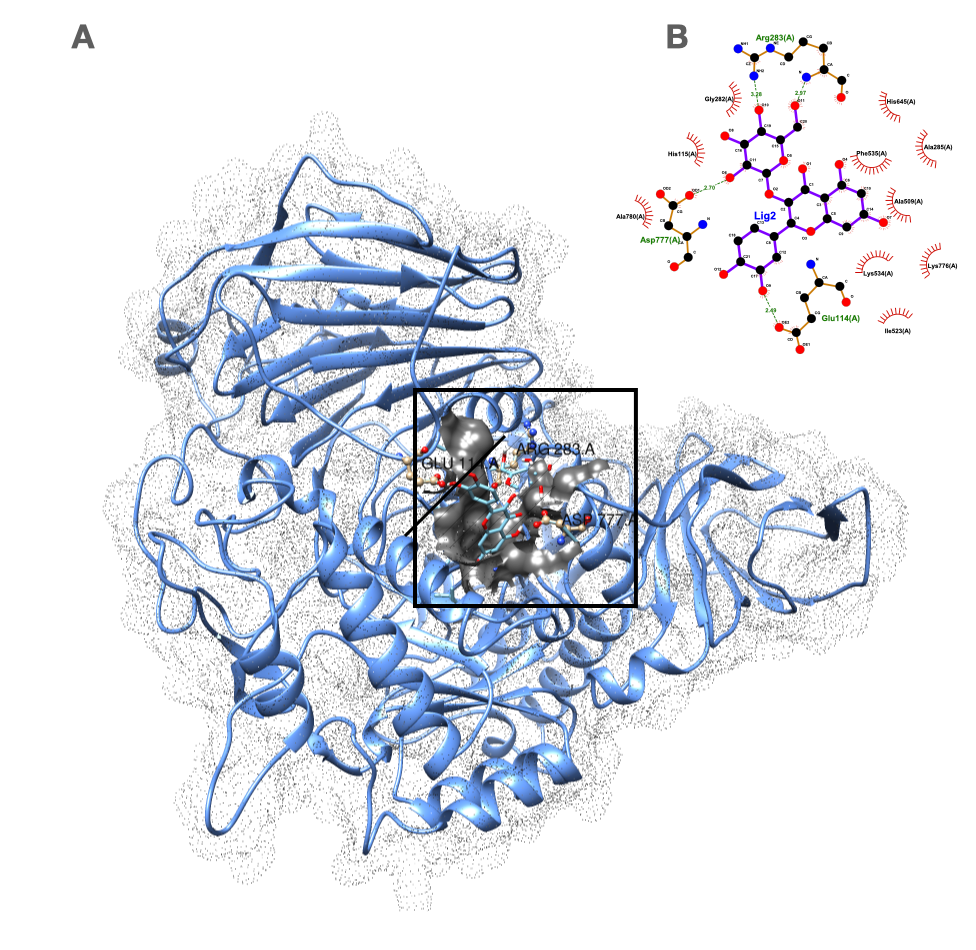
2.4. Molecular Docking of the Bioactive Compounds and Their ADME Properties
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Plant Material and Preparation of Plant Extracts

4.3. In Vitro α-Glucosidase Inhibition Assay
4.4. Animals
4.5. Ex Vivo Intestinal Disaccharidases Inhibition Assay
4.6. Zebrafish Acute Toxicity Assay
4.7. Statistical Analyses
4.8. UPLC-HRMS Analyses
4.9. Molecular Docking Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | One-way analysis of variance |
| IC50 | half maximal inhibitory concentration |
| IL-1β | interleukin-1β |
| IL- | interleukin-6 |
References
- Yedjou, C.G.; Grigsby, J.; Mbemi, A.; Nelson, D.; Mildort, B.; Latinwo, L.; Tchounwou, P.B. The Management of Diabetes Mellitus Using Medicinal Plants and Vitamins. Int. J. Mol. Sci. 2023, 24, 9085. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-Derived Non-Phenolic α-Amylase and α-Glucosidase Inhibitors for Controlling Starch Digestion Rate and Guiding Diabetes-Friendly Recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.L.; Liu, C.; Wang, X.T.; Liu, X.D.; Xie, L. Insulin Deficiency Induces Abnormal Increase in Intestinal Disaccharidase Activities and Expression under Diabetic States, Evidences from in Vivo and in Vitro Study. Biochem. Pharmacol. 2011, 82, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef] [PubMed]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An Overview on the Role of Bioactive α-Glucosidase Inhibitors in Ameliorating Diabetic Complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Dong, Y.; Sui, L.; Yang, F.; Ren, X.; Xing, Y.; Xiu, Z. Reducing the Intestinal Side Effects of Acarbose by Baicalein through the Regulation of Gut Microbiota: An in Vitro Study. Food Chem. 2022, 394, 133561. [Google Scholar] [CrossRef]
- Zanzabil, K.Z.; Hossain, M.S.; Hasan, M.K. Diabetes Mellitus Management: An Extensive Review of 37 Medicinal Plants. Diabetology 2023, 4, 186–234. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef]
- Meunier, M.; Schinkovitz, A.; Derbré, S. Current and Emerging Tools and Strategies for the Identification of Bioactive Natural Products in Complex Mixtures. Nat. Prod. Rep. 2024, 41, 1766–1786. [Google Scholar] [CrossRef]
- Leal, L.K.A.M.; Silva, A.H.; de Barros Viana, G.S. Justicia pectoralis, a Coumarin Medicinal Plant Have Potential for the Development of Antiasthmatic Drugs? Rev. Bras. Farmacogn. 2017, 27, 794–802. [Google Scholar] [CrossRef]
- Moura, C.T.M.; Batista-Lima, F.J.; Brito, T.S.; Silva, A.A.V.; Ferreira, L.C.; Roque, C.R.; Aragão, K.S.; Havt, A.; Fonseca, F.N.; Leal, L.K.A.M.; et al. Inhibitory Effects of a Standardized Extract of Justicia pectoralis in an Experimental Rat Model of Airway Hyper-Responsiveness. J. Pharm. Pharmacol. 2017, 69, 722–732. [Google Scholar] [CrossRef]
- Pinto, N.d.O.F.; Rodrigues, T.H.S.; Pereira, R.d.C.A.; Silva, L.M.A.e.; Cáceres, C.A.; Azeredo, H.M.C.d.; Muniz, C.R.; Brito, E.S.d.; Canuto, K.M. Production and Physico-Chemical Characterization of Nanocapsules of the Essential Oil from Lippia sidoides Cham. Ind. Crops Prod. 2016, 86, 279–288. [Google Scholar] [CrossRef]
- Guedes, J.A.C.; Santiago, Y.G.; dos Reis Luz, L.; Silva, M.F.S.; Ramires, C.M.C.; de Lima, M.A.C.; de Oliveira, V.R.; do Ó Pessoa, C.; Canuto, K.M.; de Brito, E.S.; et al. Comparative Analyses of Metabolic Fingerprint Integrated with Cytotoxic Activity and in Silico Approaches of the Leaves Extract of Spondias mombin L. and Spondias tuberosa Arr. Cam. from Northeast, Brazil. Phytochem. Lett. 2020, 40, 26–36. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-Based Metabolite Profiling of Methanolic Extracts from the Medicinal and Aromatic Species Mentha Pulegium and Origanum Majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Manca, M.L.; Manconi, M.; Caddeo, C.; Vázquez, J.A.; Lozano-Sánchez, J.; Escribano-Ferrer, E.; Arráez-Román, D.; Segura-Carretero, A. Incorporation of Lippia Citriodora Microwave Extract into Total-Green Biogelatin-Phospholipid Vesicles to Improve Its Antioxidant Activity. Nanomaterials 2020, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.; Peng, Y.; Fu, X.; Wang, Y.; Xiao, W.; Song, S. Screening and Identification of Multi-Components in Re Du Ning Injections Using LC/TOF-MS Coupled with UV-Irradiation. J. Chromatogr. Sci. 2015, 53, 778–786. [Google Scholar] [CrossRef]
- Barizão, É.O.; Boeing, J.S.; Rotta, E.M.; Volpato, H.; Nakamura, C.V.; Maldaner, L.; Visentainer, J.V. Phenolic Composition of Dipteryx alata Vogel Pulp + Peel and Its Antioxidant and Cytotoxic Properties. J. Braz. Chem. Soc. 2021, 32, 2206–2214. [Google Scholar] [CrossRef]
- Sousa, A.D.; Maia, A.I.V.; Rodrigues, T.H.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Cassia Alves Pereira, R.; Vieira, R.F.; de Brito, E.S. Ultrasound-Assisted and Pressurized Liquid Extraction of Phenolic Compounds from Phyllanthus amarus and Its Composition Evaluation by UPLC-QTOF. Ind. Crops Prod. 2016, 79, 91–103. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Gouveia, S.; Castilho, P.C. Analysis of Phenolic Compounds in Leaves from Endemic Trees from Madeira Island. A Contribution to the Chemotaxonomy of Laurisilva Forest Species. Ind. Crops Prod. 2015, 64, 135–151. [Google Scholar] [CrossRef]
- Rastrelli, L.; Caceres, A.; Morales, C.; De Simone, F.; Aquino, R. Iridoids from Lippia Graveolens. Phytochemistry 1998, 49, 1829–1832. [Google Scholar] [CrossRef]
- Leal, C.M.; Borges, R.M.; Simas, R.C.; Costa, F.N.; Leitão, G.G. A New Tetraglycosylated Flavonoid from Leaves of Platycyamus regnellii Benth. Isolated by High-Speed Countercurrent Chromatography. J. Braz. Chem. Soc. 2019, 30, 2561–2566. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kite, G.C.; Lewis, G.P.; Forest, F.; Nyirenda, S.P.; Belmain, S.R.; Sileshi, G.W.; Veitch, N.C. Distinct Chemotypes of Tephrosia Vogelii and Implications for Their Use in Pest Control and Soil Enrichment. Phytochemistry 2012, 78, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Gigliobianco, M.R.; Cortese, M.; Nannini, S.; Di Nicolantonio, L.; Peregrina, D.V.; Lupidi, G.; Vitali, L.A.; Bocchietto, E.; Di Martino, P.; Censi, R. Chemical, Antioxidant, and Antimicrobial Properties of the Peel and Male Flower By-Products of Four Varieties of Punica granatum L. Cultivated in the Marche Region for Their Use in Cosmetic Products. Antioxidants 2022, 11, 768. [Google Scholar] [CrossRef]
- Gondim, C.N.F.L.; Bezerra, D.A.C.; Sampaio, N.F.L.; de Alencar, M.A.S.; da Costa, R.H.S.; Abreu, L.S.; Raimundo.e.Silva, J.P.; Pereira Junior, F.N.; Tavares, J.F.; da Silva, M.S.; et al. HPLC-DAD-ESI-MS Profile, Antibacterial Activity, and Modulation of the Activity of Antibiotics by Carica papaya L. against Escherichia coli Serotypes. Phytomedicine Plus 2022, 2, 100306. [Google Scholar] [CrossRef]
- Huang, S.-T.; Wang, C.-Y.; Yang, R.-C.; Wu, H.-T.; Yang, S.-H.; Cheng, Y.-C.; Pang, J.-H.S. Ellagic Acid, the Active Compound of Phyllanthus Urinaria, Exerts In Vivo Anti-Angiogenic Effect and Inhibits MMP-2 Activity. Evid. Based Complement. Altern. Med. 2011, 2011, 215035. [Google Scholar] [CrossRef]
- Cunha, A.G.; Brito, E.S.; Moura, C.F.H.; Ribeiro, P.R.V.; Miranda, M.R.A. UPLC-qTOF-MS/MS-Based Phenolic Profile and Their Biosynthetic Enzyme Activity Used to Discriminate between Cashew Apple (Anacardium occidentale L.) Maturation Stages. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1051, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.M.; de Marqui, S.R.; Andrade, T.d.J.A.S.; Silva, D.H.S. Phytochemical, Metabolic Profiling and Antiparasitic Potential from Inga semialata Leaves (Fabaceae). Nat. Prod. Res. 2022, 36, 1898–1903. [Google Scholar] [CrossRef]
- Canuto, K.M.; Silveira, E.R.; Bezerra, A.M.E. Estudo fitoquímico de espécimens cultivados de cumaru (Amburana cearensis A. C. smith). Quimica Nova 2010, 33, 662–666. [Google Scholar] [CrossRef]
- Mohd Jusoh, N.H.; Subki, A.; Yeap, S.K.; Yap, K.C.; Jaganath, I.B. Pressurized Hot Water Extraction of Hydrosable Tannins from Phyllanthus Tenellus Roxb. BMC Chem. 2019, 13, 134. [Google Scholar] [CrossRef]
- Bento, J.A.C.; Ribeiro, P.R.V.; Bassinello, P.Z.; Brito, E.S.d.; Zocollo, G.J.; Caliari, M.; Soares Júnior, M.S. Phenolic and Saponin Profile in Grains of Carioca Beans during Storage. LWT 2021, 139, 110599. [Google Scholar] [CrossRef]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica. Molecules 2021, 26, 6493. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Ak, G.; Sinan, K.I.; Elbasan, F.; Ferrarese, I.; Sut, S.; Yıldıztugay, E.; Peron, G.; Schievano, E.; Nancy Picot-Allain, M.C.; et al. Hypericum Triquetrifolium and H. Neurocalycinum as Sources of Antioxidants and Multi-Target Bioactive Compounds: A Comprehensive Characterization Combining In Vitro Bioassays and Integrated NMR and LC-MS Characterization by Using a Multivariate Approach. Front. Pharmacol. 2021, 12, 660735. [Google Scholar] [CrossRef] [PubMed]
- Canuto, K.M.; Lima, M.A.S.; Silveira, E.R. Amburosides C-H and 6-O-protocatechuoyl coumarin from Amburana cearensis. J. Braz. Chem. Soc. 2010, 21, 1746–1753. [Google Scholar] [CrossRef][Green Version]
- Stander, M.A.; Redelinghuys, H.; Masike, K.; Long, H.; Van Wyk, B.-E. Patterns of Variation and Chemosystematic Significance of Phenolic Compounds in the Genus Cyclopia (Fabaceae, Podalyrieae). Molecules 2019, 24, 2352. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Sieniawska, E.; Sinan, K.I.; Nancy Picot-Allain, M.C.; Yerlikaya, S.; Cengiz Baloglu, M.; Altunoglu, Y.C.; Senkardes, I.; Rengasamy, K.R.; Zengin, G. Utilisation of Rhododendron Luteum Sweet Bioactive Compounds as Valuable Source of Enzymes Inhibitors, Antioxidant, and Anticancer Agents. Food Chem. Toxicol. 2020, 135, 111052. [Google Scholar] [CrossRef]
- Ismail, W.M.; Ezzat, S.M.; El-Mosallamy, A.E.M.K.; El Deeb, K.S.; El-Fishawy, A.M. In Vivo Antihypertensive Activity and UHPLC-Orbitrap-HRMS Profiling of Cuphea ignea A. DC. ACS Omega 2022, 7, 46524–46535. [Google Scholar] [CrossRef] [PubMed]
- Jurič, A.; Gašić, U.; Brčić-Karačonji, I.; Jurica, K.; Milojković-Opsenica, D. The Phenolic Profile of Strawberry Tree (Arbutus unedo L.) Honey. J. Serbian Chem. Soc. 2020, 85, 1011–1019. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef]
- Almeida, M.C.S.d.; Alves, L.A.; Souza, L.G.d.S.; Machado, L.L.; Matos, M.C.d.; Oliveira, M.C.F.d.; Lemos, T.L.G.; Braz-Filho, R. Flavonoides e outras substâncias de Lippia sidoides e suas atividades antioxidantes. Quimica Nova 2010, 33, 1877–1881. [Google Scholar] [CrossRef]
- Garcia-Carrasco, M.; Picos-Corrales, L.A.; Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; Licea-Claverie, A.; Heredia, J.B. Loading and Release of Phenolic Compounds Present in Mexican Oregano (Lippia graveolens) in Different Chitosan Bio-Polymeric Cationic Matrixes. Polymers 2022, 14, 3609. [Google Scholar] [CrossRef]
- Bianco, G.; Pascale, R.; Carbone, C.F.; Acquavia, M.A.; Cataldi, T.R.I.; Schmitt-Kopplin, P.; Buchicchio, A.; Russo, D.; Milella, L. Determination of Soyasaponins in Fagioli Di Sarconi Beans (Phaseolus vulgaris L.) by LC-ESI-FTICR-MS and Evaluation of Their Hypoglycemic Activity. Anal. Bioanal. Chem. 2018, 410, 1561–1569. [Google Scholar] [CrossRef]
- Mecha, E.; Erny, G.L.; Guerreiro, A.C.L.; Feliciano, R.P.; Barbosa, I.; Bento da Silva, A.; Leitão, S.T.; Veloso, M.M.; Rubiales, D.; Rodriguez-Mateos, A.; et al. Metabolomics Profile Responses to Changing Environments in a Common Bean (Phaseolus vulgaris L.) Germplasm Collection. Food Chem. 2022, 370, 131003. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular Docking as a Tool for the Discovery of Molecular Targets of Nutraceuticals in Diseases Management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Abudurexiti, A.; Zhang, R.; Zhong, Y.; Tan, H.; Yan, J.; Bake, S.; Ma, X. Identification of α-Glucosidase Inhibitors from Mulberry Using UF-UPLC-QTOF-MS/MS and Molecular Docking. J. Funct. Foods 2023, 101, 105362. [Google Scholar] [CrossRef]
- Li, H.; He, Z.; Shen, Q.; Fan, W.; Tan, G.; Zou, Y.; Mei, Q.; Qian, Z. Rapid Screening Alpha-Glucosidase Inhibitors from Polygoni Vivipari Rhizoma by Multi-Step Matrix Solid-Phase Dispersion, Ultrafiltration and HPLC. Molecules 2021, 26, 6111. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Joubert, E. Critical Assessment of In Vitro Screening of α-Glucosidase Inhibitors from Plants with Acarbose as a Reference Standard. Planta Med. 2022, 88, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Miranda, V.C.; Pereira, Y.L.G.; da Paz, A.P.S.; de Souza, K.R.; da Silva, M.C.F.; Muto, N.A.; Monteiro, P.R.; Santos, A.V.; Hamoy, M.; de Medeiros, M.d.G.F.; et al. Hypoglycemic and Hypolipidemic Effects of Lippia origanoides Kunth in Diabetic Rats. Food Sci. Nutr. 2024, 12, 5131–5146. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin Inhibits α-Glucosidase Activity: A Promising Strategy for the Regulation of Postprandial Hyperglycemia in High Fat Diet Fed Streptozotocin Induced Diabetic Rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Liu, C.-T.; Liu, Q.-B.; Ren, J.; Li, L.-Z.; Huang, X.-X.; Wang, Z.-Z.; Song, S.-J. Iridoid Glycosides from the Flower Buds of Lonicera japonica and Their Nitric Oxide Production and α-Glucosidase Inhibitory Activities. J. Funct. Foods 2015, 18, 512–519. [Google Scholar] [CrossRef]
- Yu, K.; Geng, X.; Chen, M.; Zhang, J.; Wang, B.; Ilic, K.; Tong, W. High Daily Dose and Being a Substrate of Cytochrome P450 Enzymes Are Two Important Predictors of Drug-Induced Liver Injury. Drug Metab. Dispos. 2014, 42, 744–750. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, H.-J.; Li, P.-F.; Yang, Y.-B.; Wu, L.-H.; Chou, G.-X.; Wang, Z.-T. Diterpenoids and Phenylethanoid Glycosides from the Roots of Clerodendrum bungei and Their Inhibitory Effects against Angiotensin Converting Enzyme and α-Glucosidase. Phytochemistry 2014, 103, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Chike-Ekwughe, A.; Adegboyega, A.E.; Johnson, T.O.; Adebayo, A.H.; Ogunlana, O.O. In Vitro and in Silico Inhibitory Validation of Tapinanthus Cordifolius Leaf Extract on Alpha-Glucosidase in the Management of Type 2 Diabetes. J. Biomol. Struct. Dyn. 2024, 42, 2512–2524. [Google Scholar] [CrossRef]
- Sancheti, S.; Sancheti, S.; Bafna, M.; Seo, S.-Y. 2,4,6-Trihydroxybenzaldehyde as a Potent Antidiabetic Agent Alleviates Postprandial Hyperglycemia in Normal and Diabetic Rats. Med. Chem. Res. 2011, 20, 1181–1187. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in Dietary Polyphenols as α-Glucosidases Inhibitors: A Review on Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Boulis, A.G.; El Zalabani, S.M.; Ghaly, N.S.; Sabry, O.M.; El-Manawaty, M.A.; Afifi, A.H.; Melek, F.R. Laxilignans A-C from the Leaves of Terminalia laxiflora Engl. and Their α-Glucosidase Inhibitory Activity. Phytochem. Lett. 2024, 59, 79–86. [Google Scholar] [CrossRef]
- Goh, B.H.; Lee Tan, J.B. Geraniin: A Promising Multifunctional Nutraceutical for Diabetes Management. Food Rev. Int. 2025, 41, 578–614. [Google Scholar] [CrossRef]
- Seong, S.H.; Kim, B.-R.; Park, J.-S.; Jeong, D.Y.; Kim, T.-S.; Im, S.; Jeong, J.-W.; Cho, M.L. Phytochemical Profiling of Symplocos tanakana Nakai and S. sawafutagi Nagam. Leaf and Identification of Their Antioxidant and Anti-Diabetic Potential. J. Pharm. Biomed. Anal. 2023, 233, 115441. [Google Scholar] [CrossRef]
- Vinholes, J.; Grosso, C.; Andrade, P.B.; Gil-Izquierdo, A.; Valentão, P.; Pinho, P.G.d.; Ferreres, F. In Vitro Studies to Assess the Antidiabetic, Anti-Cholinesterase and Antioxidant Potential of Spergularia Rubra. Food Chem. 2011, 129, 454–462. [Google Scholar] [CrossRef]
- Filho, R.R.B.X.; Ribeiro, P.R.V.; Silva, L.M.A.e.; Pereira, L.L.; Freire, G.A.; Martins, C.B.R.; Wong, D.V.T.; Dionísio, A.P.; de Alencar, N.M.N.; Frederico, M.J.S.; et al. Chemical Composition and Antidiabetic Potential of a Phenolic-Rich Extract from Cashew Fiber. ACS Food Sci. Technol. 2025, 5, 1687–1698. [Google Scholar] [CrossRef]
- Magalhães, F.E.A.; de Sousa, C.Á.P.B.; Santos, S.A.A.R.; Menezes, R.B.; Batista, F.L.A.; Abreu, Â.O.; de Oliveira, M.V.; Moura, L.F.W.G.; Raposo, R.d.S.; Campos, A.R. Adult Zebrafish (Danio rerio): An Alternative Behavioral Model of Formalin-Induced Nociception. Zebrafish 2017, 14, 422–429. [Google Scholar] [CrossRef]
- Dahlqvist, A. Assay of Intestinal Disaccharidases. Scand. J. Clin. Lab. Investig. 1984, 44, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Frederico, M.J.S.; Cipriani, A.; Heim, J.B.A.; Mendes, A.K.B.; Aragón, M.; Gaspar, J.M.; De Alencar, N.M.N.; Silva, F.R.M.B. Electrophilic Agonists Modulate the Transient Receptor Potential Ankyrin-1 Channels Mediated by Insulin and Glucagon-like Peptide-1 Secretion for Glucose Homeostasis. Pharmaceuticals 2023, 16, 1167. [Google Scholar] [CrossRef]
- Collymore, C.; Rasmussen, S.; Tolwani, R.J. Gavaging Adult Zebrafish. J. Vis. Exp. 2013. [Google Scholar] [CrossRef]
- Arellano-Aguilar, O.; Solis-Angeles, S.; Serrano, L.; Morales-Sierra, E.; Mendez-Serrano, A.; Montero-Montoya, R. Use of the Zebrafish Embryo Toxicity Test for Risk Assessment Purpose: Case Study. Fish. Sci. 2015, 9, 052–062. [Google Scholar]
- Guedes, I.A.; Pereira da Silva, M.M.; Galheigo, M.; Krempser, E.; de Magalhães, C.S.; Correa Barbosa, H.J.; Dardenne, L.E. DockThor-VS: A Free Platform for Receptor-Ligand Virtual Screening. J. Mol. Biol. 2024, 436, 168548. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
| Extracts | Inhibition | IC50 (mg/mL) |
|---|---|---|
| Lippia origanoides | Yes | 0.485 ± 0.0851 |
| Justicia pectoralis | Yes | 0.812 ± 0.0486 |
| Amburana cearensis | Yes | 1.579 ± 0.478 |
| Libidibia ferrea | No | - |
| Spondias mombin | No | - |
| Acarbose (positive control) | Yes | 0.159 ± 0.0059 |
| Peak a | Rt Min. | [M − H]− Observed | [M − H]− Calculated | MS/MS Fragments b | Empirical Formula | Ppm (Error) | Putative Name | Extract | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.14 | 195.0502 | 195.0505 | - | C6H11O7 | −1.5 | Quinic acid # | A | [15] |
| 2 | 2.62 | 467.1188 | 467.1190 | 305 | C21H23O12 | −0.4 | Epigallocatechin-O-hexoside | A | [16] |
| 3 | 2.89 | 391.1230 | 391.1240 | 229, 211, 185, 167 | C16H23O11 | −2.6 | Shanzhiside | A | [17,18] |
| 4 | 3.04 | 329.0883 | 329.0873 | 167 | C14H17O9 | 3.0 | Vanilic acid hexoside | B | [19] |
| 5 | 3.65 | 209.0300 | 209.0297 | 191 | C6H9O8 | 1.4 | Mucic acid isomer | B | [20] |
| 6 | 3.86 | 481.1338 | 481.1346 | 319 | C22H25O12 | −1.7 | Unknown | A | - |
| 7 | 4.23 | 353.0871 | 353.0873 | 191 | C16H17O9 | −0.6 | Chlorogenic acid # | C | [20] |
| 8 | 4.28 | 389.1075 | 389.1084 | 345, 209, 183, 165 | C16H21O11 | −2.3 | Secologanoside | A | [21,22] |
| 9 | 4.32 | 209.0297 | 209.0297 | 191 | C6H9O8 | 0.0 | Mucic acid isomer | B | [20] |
| 10 | 4.55 | 355.0659 | 355.0665 | 209, 191 | C15H15O10 | −1.7 | Caffeic acid glucuronide | B | [19] |
| 11 | 4.62 | 951.0710 | 951.0740 | 301 | C41H27O27 | −3.2 | Geraniin # | C | [20] |
| 12 | 4.63 | 885.2689 | 885.2665 | 739, 593, 285 | C39H49O23 | 2.7 | Kaempferol-O-hexosyl-O-rhamnopyranoside-O-rhamnopyranoside-O-rhamnopyranoside | B | [23] |
| 13 | 4.69 | 633.0724 | 633.0728 | 463 | C27H21O18 | −0.6 | Corilagin # | C | [20] |
| 14 | 4.72 | 915.2777 | 915.2770 | 739, 593, 285 | C40H51O24 | 0.8 | Methoxykaempferol-O-hexosyl-O-rhamnopyranoside-O-rhamnopyranoside-O-rhamnopyranoside | B | [24] |
| 15 | 4.77 | 935.0778 | 935.0791 | 633, 301 | C41H27O26 | −1.4 | Galloyl-bis-HHDP-hexoside isomer | C | [25] |
| 16 | 4.92 | 387.1651 | 387.1655 | 225 | C18H27O9 | −1.0 | Tuberonic acid-O-hexoside | A | [17] |
| 17 | 4.96 | 755.2014 | 755.2035 | 609, 301 | C33H39O20 | −2.8 | Quercetin rhamnosylrutinoside | B | [19] |
| 18 | 4.97 | 935.0763 | 935.0791 | 633, 301 | C41H27O26 | −3.0 | Galloyl-bis-HHDPhexoside isomer | C | [25] |
| 19 | 5.17 | 583.1667 | 583.1663 | 421, 259 | C26H31O15 | 0.7 | Unknown | B | - |
| 20 | 5.29 | 739.2065 | 739.2086 | 593, 285 | C33H39O19 | −2.8 | Kaempferol-O-(α-L-rhamnosyl)-rutinoside | B | [26] |
| 21 | 5.31 | 433.0818 | 433.0807 | 301 | C19H13O12 | 2.5 | Ellagic acid pentoside | C | [25] |
| 22 | 5.32 | 179.0346 | 179.0344 | 135 | C9H7O4 | 1.1 | Caffeic acid # | A | [15] |
| 23 | 5.43 | 953.0896 | 953.0896 | 301, 169 | C41H30O27 | 0.0 | Chebulagic acid | C | [27] |
| 24 | 5.45 | 463.0863 | 463.0877 | 301, 300 | C21H19O12 | −3.0 | Quercetin-3-O-galactose (hyperoside/hyperin) | A | [28] |
| 25 | 5.50 | 593.1493 | 593.1506 | 285 | C27H29O15 | −2.2 | Kaempferol-O-rutinoside | B | [26] |
| 26 | 5.57 | 985.1158 | 985.1125 | 300, 169 | C42H33O28 | −3.3 | Ellagic acid derivative | C | - |
| 27 | 5.61 | 965.0858 | 965.0896 | 300, 169 | C42H29O27 | −3.8 | Ellagic acid derivative | C | - |
| 28 | 5.64 | 1035.3279 | 1035.3236 | - | C57H55O21 | 4.0 | Unknown | A | - |
| 29 | 5.71 | 211.0601 | 211.0606 | 167 | C10H11O5 | −2.4 | Eudesmic acid | B | [29] |
| 30 | 5.79 | 300.9977 | 300.9984 | - | C14H5O8 | −2.3 | Ellagic acid # | C | [25] |
| 31 | 5.89 | 537.1600 | 537.1608 | - | C25H29O13 | −1.5 | Lippioside I | A | [17] |
| 32 | 5.98 | 463.0871 | 463.0877 | 301 | C21H19O12 | −1.3 | Quercetin-3-O-glucose (isoquercitrin) # | C | [20] |
| 33 | 6.17 | 623.1981 | 623.1976 | 461, 315 | C29H35O15 | 0.8 | Verbascoside | A | [17] |
| 34 | 6.26 | 421.1139 | 421.1135 | 153 | C20H21O10 | 0.0 | Amburoside A # | B | [30] |
| 35 | 6.43 | 447.0937 | 447.0927 | 285 | C21H19O11 | 2.2 | Kaempferol hexoside | C | [31] |
| 36 | 6.65 | 315.0141 | 315.0141 | - | C15H7O8 | 0.0 | Methylellagic acid | C | - |
| 37 | 6.68 | 451.1246 | 451.1240 | 285 | C21H23O11 | 1.3 | Catechin-O-hexoside | A | [32] |
| 38 | 6.74 | 505.0981 | 505.0982 | 301 | C23H21O13 | −0.2 | Quercetin acetylhexoside | A | [33] |
| 39 | 6.78 | 451.1048 | 451.1029 | 341, 323, 217 | C24H19O9 | 4.2 | Cinchonain-Ib | C | [34] |
| 40 | 6.96 | 463.1242 | 463.1242 | 301 | C22H23O11 | −0.4 | Amburoside D | B | [35] |
| 41 | 7.17 | 447.1281 | 447.1291 | 285 | C22H23O10 | −2.2 | Isosakuranetin-hexoside | B | [36] |
| 42 | 7.32 | 369.1552 | 369.1549 | 165 | C18H25O8 | 0.8 | Unknown | A | - |
| 43 | 7.33 | 435.1280 | 435.1291 | 273, 167 | C21H23O10 | −1.1 | Amburoside B # | B | [35,37] |
| 44 | 7.37 | 435.1297 | 435.1291 | 273 | C21H23O10 | 1.4 | Phloretin-O-hexoside | A | [38] |
| 45 | 7.59 | 613.1400 | 613.1405 | 503 | C26H29O17 | −0.8 | Unknown | C | - |
| 46 | 7.72 | 489.1039 | 489.1033 | 285 | C23H21O12 | 1.2 | Kaempferol-O-acetylhexoside | A | [39] |
| 47 | 8.02 | 163.0396 | 163.0395 | 119 | C9H7O3 | 0.6 | Coumaric acid | B | [40] |
| 48 | 8.26 | 193.0502 | 193.0501 | 134 | C10H9O4 | 0.5 | Ferulic acid # | B | |
| 49 | 8.40 | 285.0408 | 285.0399 | - | C15H9O6 | 3.2 | Kaempferol # | A | [39] |
| 50 | 9.48 | 271.0607 | 271.0606 | - | C15H11O5 | 0.4 | Naringenin # | A | [41] |
| 51 | 9.61 | 299.0552 | 299.0556 | 284 | C16H11O6 | −3.5 | Kaempferide | A | [42] |
| 52 | 9.81 | 353.1587 | 353.1600 | 149 | C18H25O7 | −3.7 | Unknown | A | - |
| 53 | 10.39 | 595.1808 | 595.1816 | 433, 283 | C31H31O12 | −1.3 | Unknown | A | - |
| 54 | 10.40 | 939.4971 | 939.4953 | 921, 877, 551, 455 | C48H75O18 | 1.9 | Soyasaponin Be | B | [43] |
| 55 | 10.80 | 909.4828 | 909.4848 | 891, 763, 455 | C47H73O17 | −2.0 | Soyasaponin Bg | B | |
| 56 | 11.64 | 1065.5343 | 1065.5329 | 955 | C47H85O26 | 1.3 | Unknown saponin | B | - |
| 57 | 12.21 | 923.5007 | 923.5004 | - | C48H75O17 | 0.3 | Unknown saponin | B | - |
| 58 | 12.55 | 925.5173 | 925.5161 | 907, 833, 599 | C48H77O17 | 1.3 | Hydroxyoleanenyl-deoxy-mannopyranosyl-hexopyranosyl-glucopyranosiduronic acid | B | [44] |
| 59 | 12.89 | 893.4922 | 893.4899 | - | C48H77O17 | 1.3 | Unknown saponin | B | - |
| 60 | 13.19 | 895.5078 | 895.5055 | - | C47H75O16 | 2.6 | Unknown saponin | B | - |
| 61 | 13.50 | 1051.5511 | 747 | - | C54H83O20 | 3.1 | Unknown saponin | B | - |
| 62 | 13.72 | 1049.5350 | 1049.5321 | 1019 | C54H81O20 | 2.8 | Unknown saponin | B | - |
| 63 | 14.06 | 1021.5373 | 1021.5372 | - | C53H81O19 | 0.1 | Unknown saponin | B | - |
| 64 | 14.45 | 1019.5243 | 1019.5216 | - | C53H79O19 | 2.6 | Unknown saponin | B | - |
| Compounds | CID | Score | Intermolecular Energy | vdW Energy | Electrostatic Energy |
|---|---|---|---|---|---|
| Acarbose | 9811704 | −7.465 | −75.493 | −9.318 | −66.175 |
| Quercetin-3-O-galactose (hyperoside) | 5281643 | −7.994 | −44.180 | −16.492 | −27.688 |
| Quercetin-3-O-glucose (isoquercitrin) | 25203368 | −7.514 | −41.106 | −20.638 | −20.468 |
| Trimethoxy benzoic acid (eudesmic acid) | 8357 | −6.391 | −39.873 | 2.921 | −42.794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, E.M.; Freire, G.A.; Martins, C.B.R.; Xavier Filho, R.R.B.; Silva, G.S.d.; Alencar, N.M.N.d.; Ribeiro, P.R.V.; Martins, N.F.; Milhome, Y.A.; Santos, H.S.d.; et al. Searching for Hypoglycemic Compounds from Brazilian Medicinal Plants Through UPLC-HRMS and Molecular Docking. Plants 2025, 14, 3517. https://doi.org/10.3390/plants14223517
Batista EM, Freire GA, Martins CBR, Xavier Filho RRB, Silva GSd, Alencar NMNd, Ribeiro PRV, Martins NF, Milhome YA, Santos HSd, et al. Searching for Hypoglycemic Compounds from Brazilian Medicinal Plants Through UPLC-HRMS and Molecular Docking. Plants. 2025; 14(22):3517. https://doi.org/10.3390/plants14223517
Chicago/Turabian StyleBatista, Elisabeth Mariano, Gabriela Araújo Freire, Caio Bruno Rodrigues Martins, Raimundo Rigoberto Barbosa Xavier Filho, Gisele Silvestre da Silva, Nylane Maria Nunes de Alencar, Paulo Riceli Vasconcelos Ribeiro, Natalia Florencio Martins, Yasmim Aquino Milhome, Helcio Silva dos Santos, and et al. 2025. "Searching for Hypoglycemic Compounds from Brazilian Medicinal Plants Through UPLC-HRMS and Molecular Docking" Plants 14, no. 22: 3517. https://doi.org/10.3390/plants14223517
APA StyleBatista, E. M., Freire, G. A., Martins, C. B. R., Xavier Filho, R. R. B., Silva, G. S. d., Alencar, N. M. N. d., Ribeiro, P. R. V., Martins, N. F., Milhome, Y. A., Santos, H. S. d., Frederico, M. J. S., Oliveira, L. d. S., & Canuto, K. M. (2025). Searching for Hypoglycemic Compounds from Brazilian Medicinal Plants Through UPLC-HRMS and Molecular Docking. Plants, 14(22), 3517. https://doi.org/10.3390/plants14223517








