Exploring Bioactive Polysaccharides in Edible Fruits: A Cross-Biome Perspective
Abstract
1. Introduction
2. Methodology
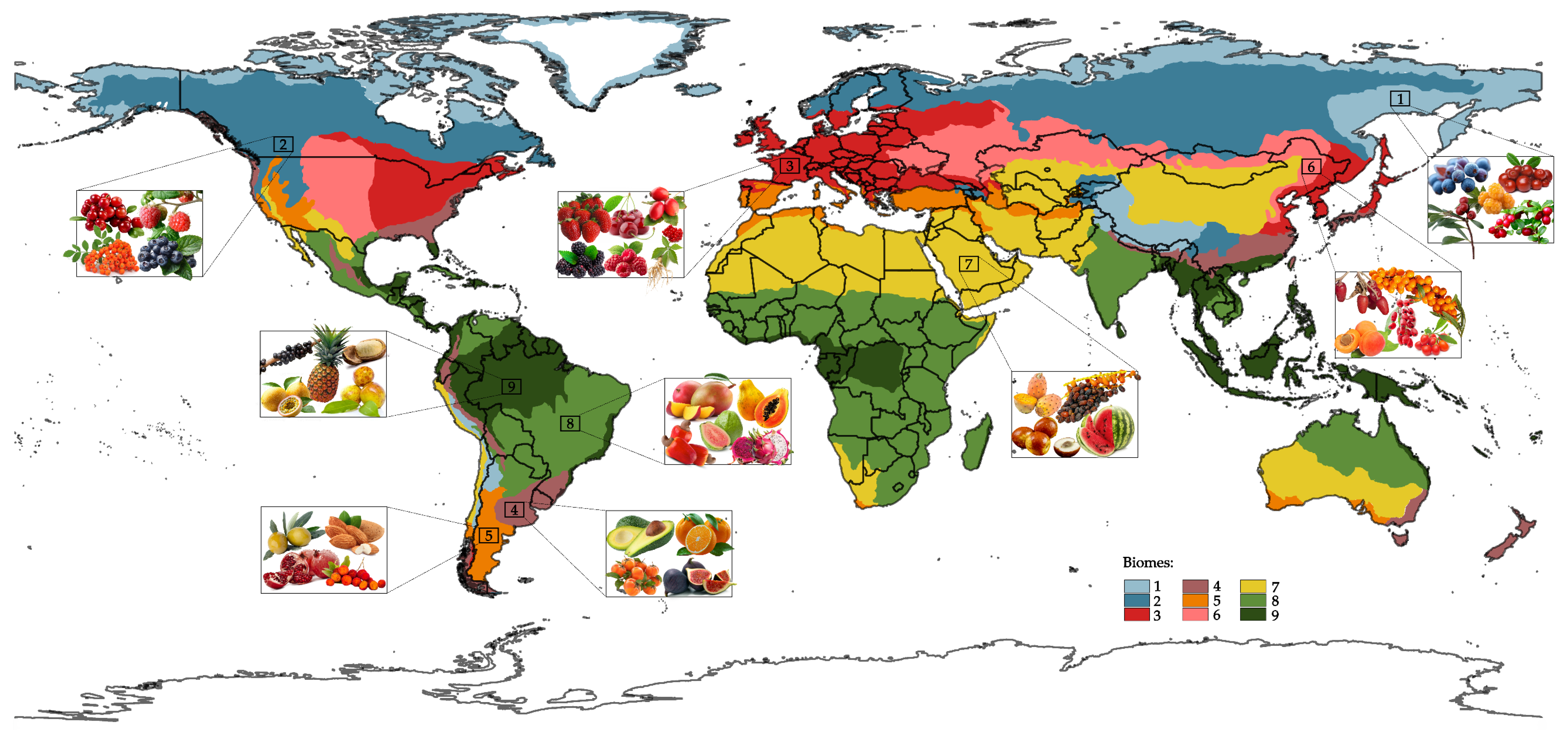
3. Global Biomes and Native Edible Fruits: An Overview
3.1. Biome of the Tundra: Cold-Adapted Fruits and Their Bioactive Polysaccharides
3.2. Biome of the Boreal Forest: Berry Diversity in Northern Ecosystems
3.3. Biome of the Temperate Deciduous Forests: Seasonal Fruits and Polysaccharide Profiles
3.4. Biome of the Temperate Pluvial Evergreen Forest: Rainforest Gems and Their Functional Compounds
3.5. Biome of the Temperate Aridiestival Evergreen Forests: Mediterranean Flora and Polysaccharide Potential
3.6. Biome of the Steppe: Hardy Fruits of Semi-Arid Landscapes
3.7. Biome of the Deserts and Semi-Deserts: Drought-Resilient Species and Bioactive Components
3.8. Biome of the Tropical Pluviseasonal Forests: Seasonal Tropical Fruits and Their Polysaccharides
3.9. Biome of the Tropical Rainforests: Biodiversity Hotspots for Bioactive Compounds
4. Global Diversity of Polysaccharide-Rich Fruits: A Cross-Biome Synthesis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| AISs | Alcohol-Insoluble Solids |
| Ara | Arabinose |
| Araf | Arabinose-furanose |
| CAM | Crassulacean Acid Metabolism |
| CDTA | Chelating Agent |
| DA | Degree of Acetylation |
| DEAE cellulose | Diethylaminoethyl cellulose |
| DM | Degree of Methylation |
| EDTA | Ethylenediaminetetraacetic Acid |
| Fru | Fructose |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| Fuc | Fucose |
| Gal | Galactose |
| GalA | Galacturonic acid |
| GalAp | Galactopyranosyl Acid |
| Galp | Galactopyranose |
| GalpA | Galactopyranose Acid |
| GC/MS | Gas Chromatography–Mass Spectrometry |
| Glc | Glucose |
| GlcA | Glucuronic acid |
| Glcp | Glucose-pyranose |
| GlcpA | Glucuronic-pyranose Acid |
| HC | Hemicellulose |
| HCl | Hydrochloric Acid |
| HG | Homogalacturonan |
| HPAEC-PAD | High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection |
| IDF | Insoluble Dietary Fiber |
| KOH | Potassium Hydroxide |
| Man | Mannose |
| Manp | Mannose-pyranose |
| Me | Methyl |
| MW | Molecular Weight |
| Na2CO3 | Sodium Carbonate |
| NaOAc | Sodium Acetate |
| NMR | Nuclear Magnetic Resonance |
| NOE | Nuclear Overhauser Effect |
| ORAC | Oxygen Radical Absorbance Capacity |
| PAPs | Pineapple Polysaccharides |
| PeSs | Pectin Substances |
| PS-I | Heteropolysaccharide-I of P. guajava |
| RG | Rhamnogalacturonan |
| Rha | Rhamnose |
| Rhap | Rhamnose-pyranose |
| Rib | Ribose |
| SEC-MALLS | Size Exclusion Chromatography coupled with Multi-Angle Light Scattering |
| USA | United States of America |
| VUP-1 | Vaccinium uliginosum polysaccharide |
| WSPs | Water-Soluble Polysaccharides |
| Xyl | Xylose |
| Xylp | Xylose-pyranose |
References
- Pedrosa, L.d.F.; Fabi, J.P. Polysaccharides from Medicinal Plants: Bridging Ancestral Knowledge with Contemporary Science. Plants 2024, 13, 1721. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Guo, Y. Polysaccharides Influence Human Health via Microbiota-Dependent and -Independent Pathways. Front. Nutr. 2022, 9, 1030063. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Liu, X.; Wang, Y.; Yang, X.; Bai, L.; Sun, L.; Zhou, Y.; Cui, S. Structural Characterization and Antioxidant Activity of Pectic Polysaccharides from Veronica peregrina L. Front. Nutr. 2023, 10, 1217862. [Google Scholar] [CrossRef]
- Dion, C.; Chappuis, E.; Ripoll, C. Does Larch Arabinogalactan Enhance Immune Function? A Review of Mechanistic and Clinical Trials. Nutr. Metab. 2016, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Amos, R.A.; Mohnen, D. Critical Review of Plant Cell Wall Matrix Polysaccharide Glycosyltransferase Activities Verified by Heterologous Protein Expression. Front. Plant Sci. 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, M.; Yang, W.; Liu, Y.; Wang, Y.; Chen, G. The Role of Cell Wall Polysaccharides Disassembly and Enzyme Activity Changes in the Softening Process of Hami Melon (Cucumis melo L.). Foods 2022, 11, 841. [Google Scholar] [CrossRef]
- Trandel, M.A.; Johanningsmeier, S.; Schultheis, J.; Gunter, C.; Perkins-Veazie, P. Cell Wall Polysaccharide Composition of Grafted ‘Liberty’ Watermelon with Reduced Incidence of Hollow Heart Defect. Front. Plant Sci. 2021, 12, 623723. [Google Scholar] [CrossRef]
- Ezquer, I.; Salameh, I.; Colombo, L.; Kalaitzis, P. Plant Cell Walls Tackling Climate Change: Biotechnological Strategies to Improve Crop Adaptations and Photosynthesis in Response to Global Warming. Plants 2020, 9, 212. [Google Scholar] [CrossRef]
- Deng, H.; Wang, X.; Wang, Y.; Xiang, Y.; Chen, M.; Zhang, H.; Luo, X.; Xia, H.; Liang, D.; Lv, X.; et al. Dynamic Changes in Cell Wall Polysaccharides during Fruit Development and Ripening of Two Contrasting Loquat Cultivars and Associated Molecular Mechanisms. Foods 2023, 12, 309. [Google Scholar] [CrossRef]
- Mucina, L. Biome: Evolution of a Crucial Ecological and Biogeographical Concept. New Phytol. 2018, 222, 97. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Mohanta, Y.K.; Kaushik, P.; Kumar, J. Physiology, Genomics, and Evolutionary Aspects of Desert Plants. J. Adv. Res. 2023, 58, 63. [Google Scholar] [CrossRef]
- Hengl, T.; Walsh, M.G.; Sanderman, J.; Wheeler, I.; Harrison, S.P.; Prentice, I.C. Global Mapping of Potential Natural Vegetation: An Assessment of Machine Learning Algorithms for Estimating Land Potential. PeerJ 2018, 6, e5457. [Google Scholar] [CrossRef]
- Sukhorukov, A.P.; Sousa-Baena, M.S.; Romanov, M.S.; Wang, X. Editorial: Fruit and Seed Evolution in Angiosperms. Front. Plant Sci. 2023, 14, 1196443. [Google Scholar] [CrossRef]
- Rajewski, A.; Maheepala, D.C.; Le, J.; Litt, A. Multispecies Transcriptomes Reveal Core Fruit Development Genes. Front. Plant Sci. 2022, 13, 954929. [Google Scholar] [CrossRef]
- Neri, L.; Faieta, M.; Di Mattia, C.; Sacchetti, G.; Mastrocola, D.; Pittia, P. Antioxidant Activity in Frozen Plant Foods: Effect of Cryoprotectants, Freezing Process and Frozen Storage. Foods 2020, 9, 1886. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; de Oliveira, K.Á.R.; Queiroga, T.S.; de Souza, E.L. Development and Application of Mucilage and Bioactive Compounds from Cactaceae to Formulate Novel and Sustainable Edible Films and Coatings to Preserve Fruits and Vegetables—A Review. Foods 2024, 13, 3613. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.d.F.; Nascimento, K.R.; Soares, C.G.; de Oliveira, D.P.; de Vos, P.; Fabi, J.P. Unveiling Plant-Based Pectins: Exploring the Interplay of Direct Effects, Fermentation, and Technological Applications in Clinical Research with a Focus on the Chemical Structure. Plants 2023, 12, 2750. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, J.E.; Nelson, E.D.; Sinnott, R.A. Immunomodulatory Dietary Polysaccharides: A Systematic Review of the Literature. Nutr. J. 2010, 9, 54. [Google Scholar] [CrossRef]
- Xu, B.W.; Li, S.S.; Ding, W.L.; Zhang, C.; Rehman, M.U.; Tareen, M.F.; Wang, L.; Huang, S.C. From Structure to Function: A Comprehensive Overview of Polysaccharide Roles and Applications. Food Front. 2025, 6, 15–39. [Google Scholar] [CrossRef]
- Song, S.; Abubaker, M.A.; Akhtar, M.; Elimam, A.M.; Zhu, X.; Zhang, J. Chemical Characterization Analysis, Antioxidants, and Anti-Diabetic Activity of Two Novel Acidic Water-Soluble Polysaccharides Isolated from Baobab Fruits. Foods 2024, 13, 912. [Google Scholar] [CrossRef] [PubMed]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent Advances in Understanding the Roles of Pectin as an Active Participant in Plant Signaling Networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Nazli, Z.I.H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins Functionalized Biomaterials; A New Viable Approach for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef] [PubMed]
- el Fihry, N.; el Mabrouk, K.; Eeckhout, M.; Schols, H.A.; Hajjaj, H. Physicochemical, Structural, and Functional Characterization of Pectin Extracted from Quince and Pomegranate Peel: A Comparative Study. Int. J. Biol. Macromol. 2024, 256, 127957. [Google Scholar] [CrossRef] [PubMed]
- Wachananawat, B.; Kuroha, T.; Takenaka, Y.; Kajiura, H.; Naramoto, S.; Yokoyama, R.; Ishizaki, K.; Nishitani, K.; Ishimizu, T. Diversity of Pectin Rhamnogalacturonan I Rhamnosyltransferases in Glycosyltransferase Family 106. Front. Plant Sci. 2020, 11, 997. [Google Scholar] [CrossRef]
- Yang, H.; Benatti, M.R.; Karve, R.A.; Fox, A.; Meilan, R.; Carpita, N.C.; McCann, M.C. Rhamnogalacturonan-I is a Determinant of Cell–Cell Adhesion in Poplar Wood. Plant Biotechnol. J. 2019, 18, 1027. [Google Scholar] [CrossRef]
- Do Prado, S.B.R.; Melfi, P.R.; Castro-Alves, V.C.; Broetto, S.G.; Araújo, E.S.; Do Nascimento, J.R.O.; Fabi, J.P. Physiological Degradation of Pectin in Papaya Cell Walls: Release of Long Chains Galacturonans Derived from Insoluble Fractions during Postharvest Fruit Ripening. Front. Plant Sci. 2016, 7, 1120. [Google Scholar] [CrossRef]
- Nechita, P.; Mirela, R.; Ciolacu, F. Xylan Hemicellulose: A Renewable Material with Potential Properties for Food Packaging Applications. Sustainability 2021, 13, 13504. [Google Scholar] [CrossRef]
- Ribeiro, A.C.B.; Cunha, A.P.; da Silva, L.M.R.; Mattos, A.L.A.; de Brito, E.S.; de Souza Filho, M.d.S.M.; de Azeredo, H.M.C.; Ricardo, N.M.P.S. From Mango By-Product to Food Packaging: Pectin-Phenolic Antioxidant Films from Mango Peels. Int. J. Biol. Macromol. 2021, 193, 1138–1150. [Google Scholar] [CrossRef]
- Sushytskyi, L.; Synytsya, A.; Čopíková, J.; Lukáč, P.; Rajsiglová, L.; Tenti, P.; Vannucci, L.E. Perspectives in the Application of High, Medium, and Low Molecular Weight Oat β-D-Glucans in Dietary Nutrition and Food Technology—A Short Overview. Foods 2023, 12, 1121. [Google Scholar] [CrossRef]
- Sharma, S.; Wani, K.M.; Mujahid, S.M.; Jayan, L.S.; Rajan, S.S. Review on Pectin: Sources, Properties, Health Benefits and Its Applications in Food Industry. J. Future Foods 2026, 6, 205–219. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lu, W.; Li, W.; Pan, L.; Lu, M.; Cesarino, I.; Li, Z.; Zeng, W. Dietary Fiber in Plant Cell Walls—The Healthy Carbohydrates. Food Qual. Saf. 2022, 6, fyab037. [Google Scholar] [CrossRef]
- McNamara, J.T.; Morgan, J.L.W.; Zimmer, J. A Molecular Description of Cellulose Biosynthesis. Annu. Rev. Biochem. 2015, 84, 895. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, A.; Edwards, C.H.; de Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of Polysaccharides in Food, Digestion, and Health. Crit. Rev. Food Sci. Nutr. 2015, 57, 237. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef]
- Holzwarth, M.; Korhummel, S.; Siekmann, T.; Carle, R.; Kammerer, D.R. Influence of Different Pectins, Process and Storage Conditions on Anthocyanin and Colour Retention in Strawberry Jams and Spreads. LWT—Food Sci. Technol. 2013, 52, 131–138. [Google Scholar] [CrossRef]
- Gafuma, S.; Mugampoza, D.; Byarugaba-Bazirake, G.W. Starch and Pectin Affect Hardness of Cooked Bananas. J. Food Res. 2018, 7, 107. [Google Scholar] [CrossRef]
- Brummell, D.A.; Schröder, R. Xylan Metabolism in Primary Cell Walls. N. Z. J. For. Sci. 2009, 39, 125–143. [Google Scholar]
- Majdoub, H.; Picton, L.; le Cerf, D.; Roudesli, S. Water Retention Capacity of Polysaccharides from Prickly Pear Nopals of Opuntia Ficus Indica and Opuntia Litoralis: Physical–Chemical Approach. J. Polym. Environ. 2010, 18, 451–458. [Google Scholar] [CrossRef]
- Chernova, T.; Mikshina, P.; Petrova, A.; Ibragimova, N.; Ageeva, M.; Gorshkova, T. Rhamnogalacturonan I with β-(1,4)-Galactan Side Chains as an Ever-Present Component of Tertiary Cell Wall of Plant Fibers. Int. J. Mol. Sci. 2023, 24, 17253. [Google Scholar] [CrossRef]
- Qu, X.; Ji, Y.; Long, J.; Zheng, D.; Qiao, Z.; Lin, Y.; Lu, C.; Zhou, Y.; Cheng, H. Immuno- and Gut Microbiota-Modulatory Activities of β-1,6-Glucans from Lentinus edodes. Food Chem. 2025, 466, 142209. [Google Scholar] [CrossRef]
- Yan, C.; Wu, X.; Wang, Y.; Peng, S.; Chen, J.; Zou, L.; McClements, D.J.; Liu, W. Utilization of Polysaccharide-Based High Internal Phase Emulsion for Nutraceutical Encapsulation: Enhancement of Carotenoid Loading Capacity and Stability. J. Funct. Foods 2021, 84, 104601. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef] [PubMed]
- Loidi, J.; Navarro-Sánche, G.; Vynokurov, D. Climatic Definitions of the World’s Terrestrial Biomes. Veg. Classif. Surv. 2022, 3, 231–271. [Google Scholar] [CrossRef]
- Bonannella, C.; Hengl, T.; Parente, L.; de Bruin, S. Biomes of the World under Climate Change Scenarios: Increasing Aridity and Higher Temperatures Lead to Significant Shifts in Natural Vegetation. PeerJ 2023, 11, e15593. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The Role of Light in Regulating Plant Growth, Development and Sugar Metabolism: A Review. Front. Plant Sci. 2024, 15, 1507628. [Google Scholar] [CrossRef]
- Kang, P.; Kim, S.J.; Park, H.J.; Han, S.J.; Kim, I.C.; Lee, H.; Yim, J.H. Trends and Challenges in Plant Cryopreservation Research: A Meta-Analysis of Cryoprotective Agent Development and Research Focus. Plants 2025, 14, 447. [Google Scholar] [CrossRef]
- Guauque-Mellado, D.; Rodrigues, A.; Terra, M.; Mantovani, V.; Yanagi, S.; Diotto, A.; de Mello, C. Evapotranspiration under Drought Conditions: The Case Study of a Seasonally Dry Atlantic Forest. Atmosphere 2022, 13, 871. [Google Scholar] [CrossRef]
- Xia, Y.; Feng, J.; Zhang, H.; Xiong, D.; Kong, L.; Seviour, R.; Kong, Y. Effects of Soil PH on the Growth, Soil Nutrient Composition, and Rhizosphere Microbiome of Ageratina Adenophora. PeerJ 2024, 12, e17231. [Google Scholar] [CrossRef]
- Yadav, V.K.; Kumar, D.; Jha, R.K.; Bairwa, R.K.; Singh, R.; Mishra, G.; Singh, J.P.; Kumar, A.; Vinesh, B.; Jayaswall, K.; et al. Mycorrhizae Set the Stage for Plants to Produce a Higher Production of Biomolecules and Stress-Related Metabolites: A Sustainable Alternative of Agrochemicals to Enhance the Quality and Yield of Beetroot (Beta vulgaris L.). Front. Microbiol. 2023, 14, 1196101. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Pseudomonads Improve Yield, Quality and Nutritional Value of Tomato: A Field Study. Mycorrhiza 2016, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, K.; Muneer, M.A.; Li, C.; Shi, H.; Tang, Y.; Zhang, J.; Ji, B. Soil Moisture and PH Differentially Drive Arbuscular Mycorrhizal Fungal Composition in the Riparian Zone along an Alpine River of Nam Co Watershed. Front. Microbiol. 2022, 13, 994918. [Google Scholar] [CrossRef]
- Szot, I.; Łysiak, G.P. Factors Influencing the Formation, Development of Buds, and Flowering of Temperate Fruit Trees. Agriculture 2025, 15, 1304. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.H.; Li, L.; Li, W.J. Soil Salinity and Drought Tolerance: An Evaluation of Plant Growth, Productivity, Microbial Diversity, and Amelioration Strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Wang, H.; Lv, G.; Cai, Y.; Zhang, X.; Jiang, L.; Yang, X. Determining the Effects of Biotic and Abiotic Factors on the Ecosystem Multifunctionality in a Desert-Oasis Ecotone. Ecol. Indic. 2021, 128, 107830. [Google Scholar] [CrossRef]
- Dellinger, A.S.; Meier, L.; Smith, S.; Sinnott-Armstrong, M. Does the Abiotic Environment Influence the Distribution of Flower and Fruit Colors? Am. J. Bot. 2025, e70044. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, M.M.P.D.; Magnússon, R.; Lara, M.J.; Frost, G.V.; Myers-Smith, I.H.; van Huissteden, J.; Jorgenson, M.T.; Fedorov, A.N.; Epstein, H.E.; Lawrence, D.M.; et al. Tundra Vegetation Change and Impacts on Permafrost. Nat. Rev. Earth Environ. 2022, 3, 68–84. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Forbes, B.C.; Wilmking, M.; Hallinger, M.; Lantz, T.; Blok, D.; Tape, K.D.; MacIas-Fauria, M.; Sass-Klaassen, U.; Lévesque, E.; et al. Shrub Expansion in Tundra Ecosystems: Dynamics, Impacts and Research Priorities. Environ. Res. Lett. 2011, 6, 045509. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Guo, X.; Li, D.; Huo, J.; Yu, Z. Structural and Biochemical Characterization of a Polysaccharide Isolated from Vaccinium uliginosum L. Starch-Staerke 2022, 74, 2100109. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Lingonberry (Vaccinium vitis-idaea L.) Grown in the Pacific Northwest of North America: Anthocyanin and Free Amino Acid Composition. J. Funct. Foods 2012, 4, 213–218. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chemposov, V.V.; Chirikova, N.K. Polymeric Compounds of Lingonberry Waste: Characterization of Antioxidant and Hypolipidemic Polysaccharides and Polyphenol-Polysaccharide Conjugates from Vaccinium Vitis-Idaea Press Cake. Foods 2022, 11, 2801. [Google Scholar] [CrossRef]
- Vaccinium Oxycoccos (Small Cranberry)|Native Plants of North America. Available online: https://www.wildflower.org/plants/result.php?id_plant=VAOX (accessed on 5 September 2025).
- Hu, X.; Yu, C.; Ahmadi, S.; Wang, Y.; Ye, X.; Hou, Z.; Chen, S. Optimization of High-Pressure Processing-Assisted Extraction of Pectic Polysaccharides from Three Berries. Food Qual. Saf. 2022, 6, fyac051. [Google Scholar] [CrossRef]
- Āboliņa, L.; Osvalde, A.; Karlsons, A. Habitat Characteristics and Mineral Nutrition Status of Rubus chamaemorus L. in Latvia. Plants 2023, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Faleva, A.V.; Ul’yanovskii, N.V.; Onuchina, A.A.; Falev, D.I.; Kosyakov, D.S. Comprehensive Characterization of Secondary Metabolites in Fruits and Leaves of Cloudberry (Rubus chamaemorus L.). Metabolites 2023, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Shamilov, A.A.; Nikolaevna Bubenchikova, V.; Chernikov, M.V.; Pozdnyakov, D.I.; Robertovna Garsiya, E.; Larsky, M.V. Bearberry (Arctostaphylos uva-ursi (L.) Spreng.): Chemical Content and Pharmacological Activity. Int. J. Pharm. Excip. 2021, 12, 49–66. [Google Scholar]
- Retallack, G.J. Soil, Soil Processes, and Paleosols. In Encyclopedia of Geology, Volume 1–6, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 690–707. [Google Scholar] [CrossRef]
- Jäderiund, A. Bilberry (Vaccinium myrtillus L.) in a Boreal Forest Ecosystem; Acta Universitatis Agriculturae Sueciae. Silvestria; Department of Forest Vegetation Ecology, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2001; Volume 32. [Google Scholar]
- Hilz, H.; Bakx, E.J.; Schols, H.A.; Voragen, A.G.J. Cell Wall Polysaccharides in Black Currants and Bilberries—Characterisation in Berries, Juice, and Press Cake. Carbohydr. Polym. 2005, 59, 477–488. [Google Scholar] [CrossRef]
- Dale, S. Irruptions of Pine Grosbeaks Pulled by Rowanberry Peaks in Southern Areas. J. Ornithol. 2023, 164, 353–366. [Google Scholar] [CrossRef]
- Zlobin, A.A.; Martinson, E.A.; Litvinets, S.G.; Ovechkina, I.A.; Durnev, E.A.; Ovodova, R.G. Pectin Polysaccharides of Rowan Sorbus aucuparia L. Russ. J. Bioorg. Chem. 2012, 38, 702–706. [Google Scholar] [CrossRef]
- Bell, F.W.; Pitt, D.G. Seasonal susceptibility of boreal plants: Red raspberry phenology as a bioindicator of optimum within-season timing of glyphosate applications. For. Chron. 2007, 83, 607–780. [Google Scholar] [CrossRef]
- Luo, H.; Ying, N.; Zhao, Q.; Chen, J.; Xu, H.; Jiang, W.; Wu, Y.; Wu, Y.; Gao, H.; Zheng, H. A Novel Polysaccharide from Rubus chingii Hu Unripe Fruits: Extraction Optimization, Structural Characterization and Amelioration of Colonic Inflammation and Oxidative Stress. Food Chem. 2023, 421, 136152. [Google Scholar] [CrossRef] [PubMed]
- Vaccinium macrocarpon—FNA. Available online: https://floranorthamerica.org/Vaccinium_macrocarpon (accessed on 5 September 2025).
- Spadoni Andreani, E.; Karboune, S.; Liu, L. Extraction and Characterization of Cell Wall Polysaccharides from Cranberry (Vaccinium macrocarpon Var. Stevens) Pomace. Carbohydr. Polym. 2021, 267, 118212. [Google Scholar] [CrossRef]
- Federation of Eurasian Soil Science Societies. Erasmus Mundus Joint Master Degree in Soil Science (EmiSS) Programme; Federation of Eurasian Soil Science Societies: Atakum, Türkiye, 2023. [Google Scholar]
- Pinheiro, M.N.C.; Gomes, F.; Botelho, G.; Rodrigues, I.; Mariychuk, R.; Symochko, L. Exploring the Multifaceted Aspects of Strawberry Tree (Arbutus unedo L.) Forests in Portugal. Land 2025, 14, 468. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Wagner, C.; Gutierrez, J.; Shaw, O.M. Berries in Microbiome-Mediated Gastrointestinal, Metabolic, and Immune Health. Curr. Nutr. Rep. 2023, 12, 151–166. [Google Scholar] [CrossRef]
- Rincon, S.; Murray, H.; Gössinger, M.; Ginies, C.; Goupy, P.; Dufour, C.; Dangles, O.; Le Bourvellec, C. Characterisation of Phenolic Compounds and Polysaccharides in Strawberry: Cultivar and Harvest Effects and Their Correlation with Nectar Colour Stability. Food Chem. 2025, 473, 143112. [Google Scholar] [CrossRef]
- Cachi, A.M.; Wünsch, A.; Vilanova, A.; Guàrdia, M.; Ciordia, M.; Aletà, N. S-Locus Diversity and Cross-Compatibility of Wild Prunus avium for Timber Breeding. Plant Breed. 2017, 136, 126–131. [Google Scholar] [CrossRef]
- Ross, K.; Siow, Y.; Brown, D.; Isaak, C.; Fukumoto, L.; Godfrey, D. Characterization of Water Extractable Crude Polysaccharides from Cherry, Raspberry, and Ginseng Berry Fruits: Chemical Composition and Bioactivity. Int. J. Food Prop. 2015, 18, 670–689. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere, F.; Venter, A.C.; Vicas, S.I. Phytochemical Composition of Different Botanical Parts of Morus Species, Health Benefits and Application in Food Industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for Ultrasound Extraction of Polysaccharides from Mulberry Fruits with Antioxidant and Hyperglycemic Activity in Vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef]
- Jürgens, A.H.; Seitz, B.; Kowarik, I. Genetic Differentiation of Rosa canina (L.) at Regional and Continental Scales. Plant Syst. Evol. 2007, 269, 39–53. [Google Scholar] [CrossRef]
- Ognyanov, M.; Remoroza, C.; Schols, H.A.; Georgiev, Y.; Kratchanova, M.; Kratchanov, C. Isolation and Structure Elucidation of Pectic Polysaccharide from Rose Hip Fruits (Rosa canina L.). Carbohydr. Polym. 2016, 151, 803–811. [Google Scholar] [CrossRef]
- Williams, C.B.; Sillett, S.C. Epiphyte Communities on Redwood (Sequoia sempervirens) in Northwestern California. Bryologist 2007, 110, 420–452. [Google Scholar] [CrossRef]
- Yahia, E.M.; Woolf, A.B. Avocado (Persea americana Mill.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Cambridge, UK, 2011; pp. 125–185. [Google Scholar] [CrossRef]
- Ford, N.A.; Spagnuolo, P.; Kraft, J.; Bauer, E. Nutritional Composition of Hass Avocado Pulp. Foods 2023, 12, 2516. [Google Scholar] [CrossRef] [PubMed]
- Dongre, P.; Doifode, C.; Choudhary, S.; Sharma, N. Botanical Description, Chemical Composition, Traditional Uses and Pharmacology of Citrus Sinensis: An Updated Review. Pharmacol. Res.-Mod. Chin. Med. 2023, 8, 100272. [Google Scholar] [CrossRef]
- Prabasari, I.; Pettolino, F.; Liao, M.L.; Bacic, A. Pectic Polysaccharides from Mature Orange (Citrus sinensis) Fruit Albedo Cell Walls: Sequential Extraction and Chemical Characterization. Carbohydr. Polym. 2011, 84, 484–494. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, S.; Huang, Q.; Delfianti, M.N.I.; Yuniastuti, E.; Cahyani, V.R. Propagation and Growth of Persimmon (Diospyros kaki L.) in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012037. [Google Scholar] [CrossRef]
- Normakhmatov, R.; Malikova, M.K.; Arifkhodzhaev, A.O.; Rakhimov, D.A. Polysaccharides of Diospyros Kaki. Chem. Nat. Compd. 1999, 35, 94–95. [Google Scholar] [CrossRef]
- de MEDEIROS, A.R.M. Figueira (Ficus carica L.) Do Plantio Ao Processamento Caseiro; Pelotas. Embrapa Clima Temperado: Pelotas, Brazil, 2018. [Google Scholar]
- Zhang, T.; Chen, M.; Li, D.; Zheng, J.; Sun, Y.; Liu, R.; Sun, T. Review of the Recent Advances in Polysaccharides from Ficus carica: Extraction, Purification, Structural Characteristics, Bioactivities and Potential Applications. Int. J. Biol. Macromol. 2024, 281, 136430. [Google Scholar] [CrossRef]
- Durn, G. Terra Rossa in the Mediterranean Region: Parent Materials, Composition and Origin. Geol. Croat. 2003, 56, 83–100. [Google Scholar] [CrossRef]
- El Yamani, M.; Cordovilla, M.d.P. Tolerance Mechanisms of Olive Tree (Olea europaea) under Saline Conditions. Plants 2024, 13, 2094. [Google Scholar] [CrossRef]
- Vierhuis, E.; Schols, H.A.; Beldman, G.; Voragen, A.G.J. Isolation and Characterisation of Cell Wall Material from Olive Fruit (Olea europaea Cv Koroneiki) at Different Ripening Stages. Carbohydr. Polym. 2000, 43, 11–21. [Google Scholar] [CrossRef]
- Dammak, M.I.; Chakroun, I.; Mzoughi, Z.; Amamou, S.; Mansour, H.B.; Le Cerf, D.; Majdoub, H. Characterization of Polysaccharides from Prunus amygdalus Peels: Antioxidant and Antiproliferative Activities. Int. J. Biol. Macromol. 2018, 119, 198–206. [Google Scholar] [CrossRef]
- Dourado, F.; Barros, A.; Mota, M.; Coimbra, M.A.; Gama, F.M. Anatomy and Cell Wall Polysaccharides of Almond (Prunus dulcis D. A. Webb) Seeds. J. Agric. Food Chem. 2004, 52, 1364–1370. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A Review of Composition Studies of Cultivated Almonds: Macronutrients and Micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, C.; Guo, T.; Wei, Y. Punica granatum L. Polysaccharides: A Review on Extraction, Structural Characteristics and Bioactivities. Carbohydr. Res. 2024, 544, 109246. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Khatib, M.; Cecchi, L.; Adessi, A.; Melgarejo, P.; Nunes, C.; Coimbra, M.A.; Mulinacci, N. Pomegranate Peel as a Promising Source of Pectic Polysaccharides: A Multi-Methodological Analytical Investigation. Food Chem. 2022, 397, 133550. [Google Scholar] [CrossRef] [PubMed]
- Harel-Beja, R.; Ophir, R.; Sherman, A.; Eshed, R.; Rozen, A.; Trainin, T.; Doron-Faigenboim, A.; Tal, O.; Bar-Yaakov, I.; Holland, D. The Pomegranate Deciduous Trait Is Genetically Controlled by a PgPolyQ-MADS Gene. Front. Plant Sci. 2022, 13, 870207. [Google Scholar] [CrossRef]
- USDA; NRCS. National Plant Data Center Pacific Madrone: Arbutus menziesii Pursh. In Plant Guide; NRCS: Washington, DC, USA, 2002; pp. 1–4. [Google Scholar]
- Marques, M.P.; Martin, D.; Bosch, M.; Martins, J.; Biswal, A.; Zuzarte, M.; de Carvalho, L.B.; Canhoto, J.; da Costa, R. Unveiling the Compositional Remodelling of Arbutus unedo L. Fruits during Ripening. Sci. Hortic. 2022, 303, 111248. [Google Scholar] [CrossRef]
- Liu, X.; Lee Burras, C.; Kravchenko, Y.S.; Duran, A.; Huffman, T.; Morras, H.; Studdert, G.; Zhang, X.; Cruse, R.M.; Yuan, X. Overview of Mollisols in the World: Distribution, Land Use and Management. Can. J. Soil Sci. 2012, 92, 383–402. [Google Scholar] [CrossRef]
- Li, T.S.C.; Schroeder, W.R. Sea Buckthorn (Hippophae rhamnoides L.): A Multipurpose Plant. Horttechnology 1996, 6, 370–380. [Google Scholar] [CrossRef]
- Teng, H.; He, Z.; Hong, C.; Xie, S.; Zha, X. Extraction, Purification, Structural Characterization and Pharmacological Activities of Polysaccharides from Sea Buckthorn (Hippophae rhamnoides L.): A Review. J. Ethnopharmacol. 2024, 324, 117809. [Google Scholar] [CrossRef]
- Na, X.; Ma, S.; Ma, C.; Liu, Z.; Xu, P.; Zhu, H.; Liang, W.; Kardol, P. Lycium barbarum L. (Goji Berry) Monocropping Causes Microbial Diversity Loss and Induces Fusarium Spp. Enrichment at Distinct Soil Layers. Appl. Soil Ecol. 2021, 168, 104107. [Google Scholar] [CrossRef]
- Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R.J.; Ye, X.; Chen, S. Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. Characterisation of Cell Wall Polysaccharide Profiles of Apricots (Prunus armeniaca L.), Peaches (Prunus persica L.), and Pumpkins (Cucurbita sp.) for the Evaluation of Fruit Product Authenticity. Food Chem. 2008, 106, 421–430. [Google Scholar] [CrossRef]
- Rjeibi, I.; Feriani, A.; Hentati, F.; Hfaiedh, N.; Michaud, P.; Pierre, G. Structural Characterization of Water-Soluble Polysaccharides from Nitraria Retusa Fruits and Their Antioxidant and Hypolipidemic Activities. Int. J. Biol. Macromol. 2019, 129, 422–432. [Google Scholar] [CrossRef]
- Song, L.; Liu, S.; Zhang, L.; Pan, L.; Xu, L. Polysaccharides from Nitraria Retusa Fruit: Extraction, Purification, Structural Characterization, and Antioxidant Activities. Molecules 2023, 28, 1266. [Google Scholar] [CrossRef]
- Pérez-López, A.V.; Lim, S.D.; Cushman, J.C. Humboldt Review: Tissue Succulence in Plants: Carrying Water for Climate Change. J. Plant Physiol. 2023, 289, 154081. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, A.D.M.B.; Fernandes, P.H.C.; Da Silva, E.M. Opuntia ficus-indica (L.) Mill. e as Mudanças Climáticas: Uma Análise a Luz Da Modelagem de Distribuição de Espécies No Bioma Caatinga. Rev. Bras. Meteorol. 2020, 35, 375–385. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The Polysaccharide and Low Molecular Weight Components of Opuntia ficus Indica Cladodes: Structure and Skin Repairing Properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, T.; Pathak, D.; Chopra, H. An Updated Review of Ziziphus Jujube: Major Focus on Its Phytochemicals and Pharmacological Properties. Pharmacol. Res.-Mod. Chin. Med. 2023, 8, 100297. [Google Scholar] [CrossRef]
- Ruan, J.; Han, Y.; Kennedy, J.F.; Jiang, H.; Cao, H.; Zhang, Y.; Wang, T. A Review on Polysaccharides from Jujube and Their Pharmacological Activities. Carbohydr. Polym. Technol. Appl. 2022, 3, 100220. [Google Scholar] [CrossRef]
- Parisa, H.S. Origin of the Dessert Watermelon, Citrullus Lanatus. Acta Hortic. 2017, 1151, 87–93. [Google Scholar] [CrossRef]
- Delgado-Carrillo, O.; Martén-Rodríguez, S.; Ramírez-Mejía, D.; Novais, S.; Quevedo, A.; Ghilardi, A.; Sayago, R.; Lopezaraiza-Mikel, M.; Pérez-Trujillo, E.; Quesada, M. Pollination Services to Crops of Watermelon (Citrullus lanatus) and Green Tomato (Physalis ixocarpa) in the Coastal Region of Jalisco, Mexico. PLoS ONE 2024, 19, e0301402. [Google Scholar] [CrossRef]
- Dammak, M.I.; Ben Salem, Y.; Belaid, A.; Ben Mansour, H.; Hammami, S.; Le Cerf, D.; Majdoub, H. Partial Characterization and Antitumor Activity of a Polysaccharide Isolated from Watermelon Rinds. Int. J. Biol. Macromol. 2019, 136, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Pintaud, J.C.; Ludeña, B.; Aberlenc-Bertossi, F.; Gros-Balthazard, M.; Ivorra, S.; Terral, J.F.; Tengberg, M.; Hernández, I.S.; González-Pérez, M.A.; Sosa, P.; et al. Biogeography of the Date Palm (Phoenix dactylifera L., Arecaceae): Insights on the Origin and on the Structure of Modern Diversity. Acta Hortic. 2013, 994, 19–38. [Google Scholar] [CrossRef]
- Noorbakhsh, H.; Khorasgani, M.R. Date (Phoenix dactylifera L.) Polysaccharides: A Review on Chemical Structure and Nutritional Properties. J. Food Meas. Charact. 2022, 16, 3240–3250. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakuma, T.; Okagawa, N.; Imai, H.; Sato, T.; Yamaguchi, J. Agro-Ecological Condition of the Oxisol-Ultisol Area of the Amazon River System; Report of a Survey from Cerrado to Forest Areas in Brazil; Hokkaido University: Sapporo, Japan, 1989. [Google Scholar]
- Contreras, A. Carica Papaya. In IUCN Red List of Threatened Species; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2016. [Google Scholar] [CrossRef]
- Donadio, J.L.S.; do Prado, S.B.R.; Soares, C.G.; Tamarossi, R.I.; Heidor, R.; Moreno, F.S.; Fabi, J.P. Ripe Papaya Pectins Inhibit the Proliferation of Colon Cancer Spheroids and the Formation of Chemically Induced Aberrant Crypts in Rats Colons. Carbohydr. Polym. 2024, 331, 121878. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Pectic Polysaccharides of Mango (Mangifera indica L): Structural Studies. J. Sci. Food Agric. 2004, 84, 1731–1735. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Arévalo-Marín, E.; Casas, A.; Landrum, L.; Shock, M.P.; Alvarado-Sizzo, H.; Ruiz-Sanchez, E.; Clement, C.R. The Taming of Psidium Guajava: Natural and Cultural History of a Neotropical Fruit. Front. Plant Sci. 2021, 12, 714763. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, S.; Shi, L.; Tan, H. Preparation, Structural Characterization, and Bioactivities of Polysaccharides from Psidium guajava: A Review. Food Chem. 2023, 411, 135423. [Google Scholar] [CrossRef]
- Paula, R.C.M.; Heatley, F.; Budd, P.M. Characterization of Anacardium occidentale Exudate Polysaccharide—De Paula—1998—Polymer International—Wiley Online Library. Polym. Int. 1998, 45, 27–35. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Praciak, A. Hylocereus Undatus (Dragon Fruit); CABI Compendium; CABI: London, UK, 2021. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Wang, L. Structure Characteristics of a Water-Soluble Polysaccharide Purified from Dragon Fruit (Hylocereus undatus) Pulp. Carbohydr. Polym. 2016, 146, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Vitoussek, P.M.; Sanford, R.L. Nutrient Cycling in Moist Tropical Forest. Annu. Rev. Ecol. Syst. 1986, 17, 137–167. [Google Scholar] [CrossRef]
- Flor-Unda, O.; Guanochanga, F.; Samaniego, I.; Arias, V.; Ortiz, B.; Rosales, C.; Palacios-Cabrera, H. Physicochemical Characterization and Antioxidant Capacity of Açaí (Euterpe oleracea) in Ecuadorian Region. Foods 2024, 13, 3046. [Google Scholar] [CrossRef]
- Soares Baal, S.C. 1992-Avaliação in Vitro Da Atividade Antitumoral de Polissacarídeos Provenientes de Açaí (Euterpe Oleracea Mart) e Maracujá (Passiflora Edulis F. Flavicarpa) Sobre Linhagem de Melanoma Murino. 2022. Available online: https://acervodigital.ufpr.br/xmlui/handle/1884/76626 (accessed on 1 May 2025).
- Holderness, J.; Schepetkin, I.A.; Freedman, B.; Kirpotina, L.N.; Quinn, M.T.; Hedges, J.F.; Jutila, M.A. Polysaccharides Isolated from Açaí Fruit Induce Innate Immune Responses. PLoS ONE 2011, 6, e17301. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Mahmoud, A.A.; Ahl, H.A.H.S.-A.; Bratovcic, A.; Tkachenko, K.G.; Kačániová, M.; Rodriguez, R.M. Pineapple (Ananas comosus L. Merr.), Waste Streams, Characterisation and Valorisation: An Overview. Open J. Ecol. 2021, 11, 610–634. [Google Scholar] [CrossRef]
- Wang, L.; Tang, D.Q.; Kuang, Y.; Lin, F.J.; Su, Y. Structural Characteristics of Pineapple Pulp Polysaccharides and Their Antitumor Cell Proliferation Activities. J. Sci. Food Agric. 2015, 95, 2554–2561. [Google Scholar] [CrossRef]
- da Silva, C.V.A.; Salimo, Z.M.; de Souza, T.A.; Reyes, D.E.; Bassicheto, M.C.; de Medeiros, L.S.; Sartim, M.A.; de Carvalho, J.C.; Gonçalves, J.F.C.; Monteiro, W.M.; et al. Cupuaçu (Theobroma grandiflorum): A Multifunctional Amazonian Fruit with Extensive Benefits. Food Res. Int. 2024, 192, 114729. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Polysaccharides from the Pulp of Cupuassu (Theobroma grandiflorum): Structural Characterization of a Pectic Fraction. Carbohydr. Polym. 2009, 77, 72–79. [Google Scholar] [CrossRef]
- Zibadi, S.; Watson, R.R. Passion Fruit (Passiflora edulis). Evid.-Based Integr. Med. 2012, 1, 183–187. [Google Scholar] [CrossRef]
- Silva, D.C.; Freitas, A.L.P.; Barros, F.C.N.; Lins, K.O.A.L.; Alves, A.P.N.N.; Alencar, N.M.N.; De Figueiredo, I.S.T.; Pessoa, C.; De Moraes, M.O.; Costa-Lotufo, L.V.; et al. Polysaccharide Isolated from Passiflora Edulis: Characterization and Antitumor Properties. Carbohydr. Polym. 2012, 87, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.F., Jr.; Da Silva Lédo, A.; Veruska, A.; Da Silva Muniz, C. Hancornia Speciosa—Mangaba. In Plantas Para o Futuro; Procisur: Montevideo, Uruguay, 2017; pp. 177–193. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1096247/1/f6dce1963e4cMangabaPROCISUR.pdf (accessed on 1 August 2025).
- Reis, V.H.d.O.T.; de Melo, V.X.; da Silva, M.L.R.; Filho, P.S.L.; Portugal, L.C.; Sartoratto, A.; Rafacho, B.P.M.; Cazarin, C.B.B.; Cordeiro, L.M.C.; dos Santos, E.F. Insoluble Dietary Fibers from Hancornia speciosa Alleviates Chronic Constipation on Experimental Loperamide-Induced Model. Int. J. Biol. Macromol. 2025, 306, 141215. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, M.A.; Chavarría-Hernández, N.; López-Cuellar, M.d.R.; Rodríguez-Hernández, A.I. A Review of Extracellular Polysaccharides from Extreme Niches: An Emerging Natural Source for Biotechnology. From the Adverse to Diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef]
- Wolf, M.K.; Wiesmeier, M.; Macholdt, J. Importance of Soil Fertility for Climate-Resilient Cropping Systems: The Farmer’s Perspective. Soil Secur. 2023, 13, 100119. [Google Scholar] [CrossRef]
- Bartas, M. Abiotic Stresses in Plants: From Molecules to Environment. Int. J. Mol. Sci. 2024, 25, 8072. [Google Scholar] [CrossRef]
- Videcoq, P.; Garnier, C.; Robert, P.; Bonnin, E. Influence of Calcium on Pectin Methylesterase Behaviour in the Presence of Medium Methylated Pectins. Carbohydr. Polym. 2011, 86, 1657–1664. [Google Scholar] [CrossRef]
- Chialva, M.; Fangel, J.U.; Novero, M.; Zouari, I.; Di Fossalunga, A.S.; Willats, W.G.T.; Bonfante, P.; Balestrini, R. Understanding Changes in Tomato Cell Walls in Roots and Fruits: The Contribution of Arbuscular Mycorrhizal Colonization. Int. J. Mol. Sci. 2019, 20, 415. [Google Scholar] [CrossRef]
- Subedi, T. An Assessment of Mineral Contents in Fruits. Prithvi Acad. J. 2023, 6, 21–31. [Google Scholar] [CrossRef]
- Guillemin, A.; Guillon, F.; Degraeve, P.; Rondeau, C.; Devaux, M.F.; Huber, F.; Badel, E.; Saurel, R.; Lahaye, M. Firming of Fruit Tissues by Vacuum-Infusion of Pectin Methylesterase: Visualisation of Enzyme Action. Food Chem. 2008, 109, 368–378. [Google Scholar] [CrossRef][Green Version]
- Fonseca, V.A.; Dos Santos, M.R.; da Silva, J.A.; Donato, S.L.R.; Rodrigues, C.S.; Brito, C.F.B. Morpho-Physiology, Yield, and Water-Use Efficiency of Opuntia Ficus-Indica Irrigated with Saline Water. Acta Sci. Agron. 2019, 41, e42631. [Google Scholar] [CrossRef]
- Sevgi, A.; Özçelik, M.; Yılmaz, T. Extraction, Characterization, and Rheology of Opuntia ficus Indica Cladode Polysaccharides. J. Food Process. Preserv. 2021, 46, e16196. [Google Scholar] [CrossRef]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Takahashi, D.; Soga, K.; Kikuchi, T.; Kutsuno, T.; Hao, P.; Sasaki, K.; Nishiyama, Y.; Kidokoro, S.; Sampathkumar, A.; Bacic, A.; et al. Structural Changes in Cell Wall Pectic Polymers Contribute to Freezing Tolerance Induced by Cold Acclimation in Plants. Curr. Biol. 2024, 34, 958–968.e5. [Google Scholar] [CrossRef]
- Messeder, J.V.S.; Silveira, F.A.O.; Cornelissen, T.G.; Fuzessy, L.F.; Guerra, T.J. Frugivory and Seed Dispersal in a Hyperdiverse Plant Clade and Its Role as a Keystone Resource for the Neotropical Fauna. Ann. Bot. 2021, 127, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Boanares, D.; Ferreira, B.G.; Kozovits, A.R.; Sousa, H.C.; Isaias, R.M.S.; França, M.G.C. Pectin and Cellulose Cell Wall Composition Enables Different Strategies to Leaf Water Uptake in Plants from Tropical Fog Mountain. Plant Physiol. Biochem. 2018, 122, 57–64. [Google Scholar] [CrossRef]
- Kutsuno, T.; Chowhan, S.; Kotake, T.; Takahashi, D. Temporal Cell Wall Changes during Cold Acclimation and Deacclimation and Their Potential Involvement in Freezing Tolerance and Growth. Physiol. Plant 2023, 175, e13837. [Google Scholar] [CrossRef]
- Lei, B.; Cui, J.; Newman, C.; Buesching, C.D.; Xie, Z.; MacDonald, D.W.; Zhou, Y. Seed Dispersers Shape the Pulp Nutrients of Fleshy-Fruited Plants. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210817. [Google Scholar] [CrossRef]
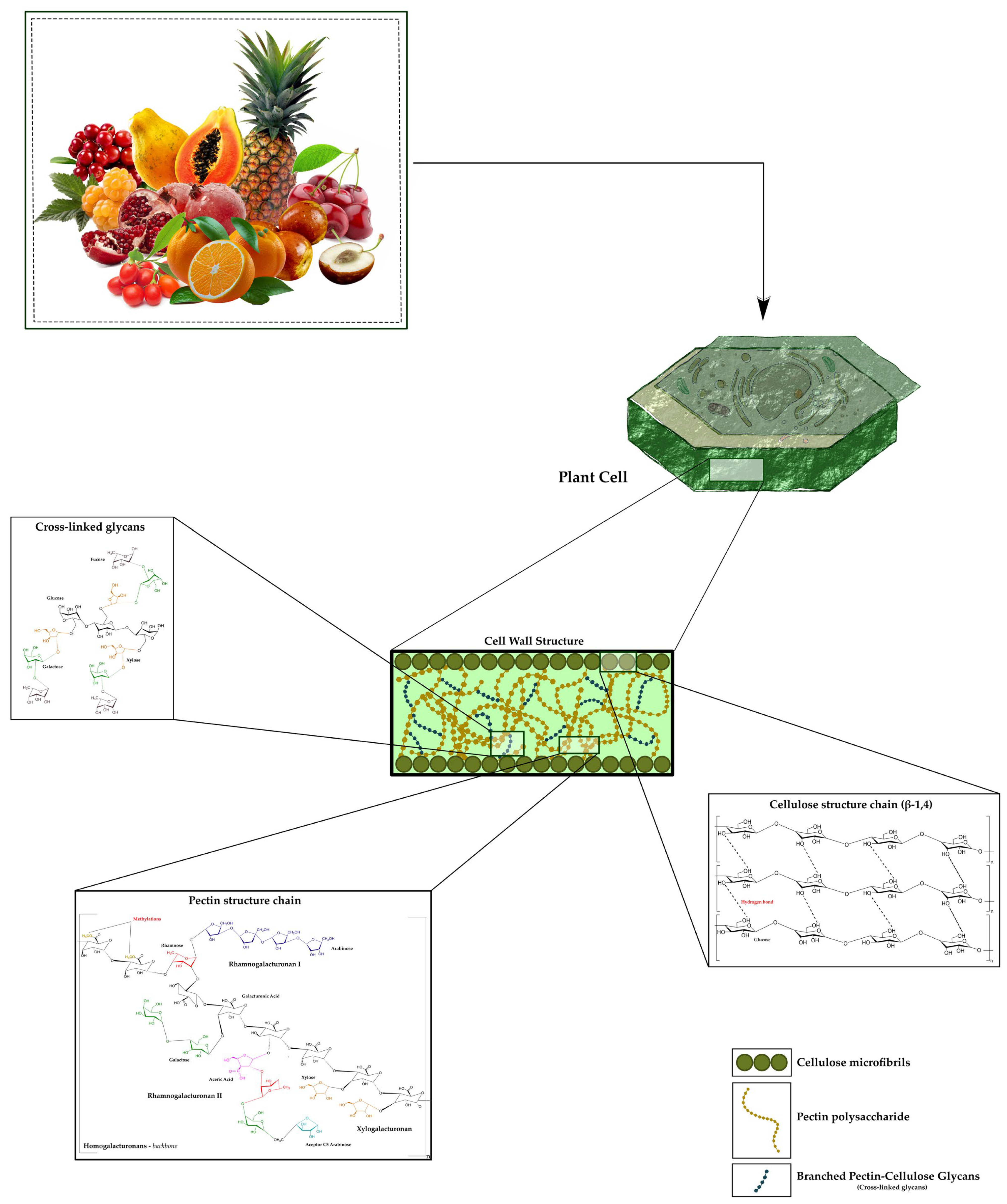

| Fruits | General Functional Implications |
|---|---|
| Vaccinium uliginosum (Arctic blueberry) | Cryoprotective polysaccharides aiding water stabilization and freeze tolerance. |
| Vaccinium vitis-idaea (Lingonberry) | Polyphenol–polysaccharide conjugates providing antioxidant defense and cold adaptation. |
| Vaccinium oxycoccos (Cranberry) | RG-I–rich pectins improving gelation and resilience to freezing. |
| Rubus chamaemorus (Cloudberry) | Free monosaccharides supporting osmotic regulation under low temperature. |
| Arctostaphylos uva-ursi (Bearberry) | Phenolic-linked polymers contributing to stress resistance (leaves documented). |
| Fruits | General Functional Implications |
|---|---|
| Vaccinium myrtillus (Bilberry) | Acetylated xylans and methylated pectins increasing rigidity and cold endurance. |
| Sorbus aucuparia (Rowanberry) | Arabinan–polyphenol interactions providing antioxidant buffering. |
| Rubus chingii (Asian raspberry) | Branched RG-I polysaccharides conferring flexibility and cold tolerance. |
| Vaccinium macrocarpon (Cranberry) | HG and RG-I pectins balancing mechanical strength and water retention. |
| Fruits | General Functional Implications |
|---|---|
| Fragaria ananassa (Strawberry) | Highly methylated HG pectin maintaining firmness during ripening. |
| Prunus avium (Sweet cherry) | Acidic heteropolysaccharides modulating texture and antioxidant potential. |
| Rubus idaeus (Raspberry) | Glucan- and arabinogalactan-rich polymers enhancing stress response. |
| Morus nigra (Mulberry) | Uronic acid–rich polysaccharides with hypoglycemic and antioxidant activity. |
| Rosa canina (Rose hip) | HG–RG-I hybrid pectin with antioxidant and immunomodulatory effects. |
| Fruits | General Functional Implications |
|---|---|
| Persea americana (Avocado) | Mannoheptulose and perseitol aiding osmoregulation and postharvest metabolism. |
| Citrus sinensis (Orange) | HG–RG-I pectin matrix enhancing hydration and tissue firmness. |
| Diospyros kaki (Persimmon) | Polysaccharide heterogeneity supporting water balance and nutraceutical potential. |
| Ficus carica (Fig fruit) | Branched heteropolysaccharides contributing to hydration and soft texture. |
| Fruits | General Functional Implications |
|---|---|
| Olea europaea (Olive) | De-esterified pectins and xyloglucans maintaining firmness and drought tolerance. |
| Prunus amygdalus (Almond) | Ara- and Gal-rich pectins reinforcing structure under arid stress. |
| Punica granatum (Pomegranate) | Highly esterified HG pectins providing antioxidant protection and stability. |
| Arbutus unedo | Lignified xylan–cellulose networks ensuring drought resistance and firmness. |
| Fruits | General Functional Implications |
|---|---|
| Hippophae rhamnoides (Sea buckthorn) | HG pectins and arabinogalactans enhancing heat and drought tolerance. |
| Berberis vulgaris (Barberry) | Pectin heteropolymers supporting antioxidant and mechanical stability. |
| Lycium barbarum (Goji) | RG-I and HG fractions mediating osmotic protection and immunomodulation. |
| Prunus armeniaca (Apricot) | RG-I–rich pectins improving solubility and stabilizing bioactivity. |
| Nitraria retusa | Branched arabinogalactans aiding salt tolerance and antioxidant defense. |
| Fruits | General Functional Implications |
|---|---|
| Opuntia ficus-indica (Prickly pear) | Mucilaginous arabinogalactans enabling water retention and desiccation resistance. |
| Ziziphus jujuba (Jujube) | RG-I pectins and arabinans contributing to drought adaptation and antioxidant activity. |
| Citrullus lanatus (Watermelon) | Branched RG-I and arabinogalactan networks ensuring hydration control. |
| Phoenix dactylifera (Date palm) | Hemicellulosic xylans and galactomannans strengthening seed structure and water balance. |
| Fruits | General Functional Implications |
|---|---|
| Carica papaya (Papaya) | Dynamic HG–RG-I remodeling regulating softening and enzymatic ripening. |
| Mangifera indica (Mango) | Arabinogalactan–RG complex ensuring cell wall flexibility and antioxidant defense. |
| Psidium guajava (Guava) | Ara- and Gal-rich heteropolysaccharides improving stress tolerance and digestibility. |
| Anacardium occidentale (Cashew) | Branched β-D-galactans conferring hydration stability and antimicrobial barrier. |
| Hylocereus undatus (Dragon fruit) | Acidic heteropolysaccharides maintaining cell wall integrity in dry habitats. |
| Fruits | General Functional Implications |
|---|---|
| Euterpe oleracea (Açaí) | Arabinogalactans and pectins providing elasticity and antioxidant protection. |
| Ananas comosus (Pineapple) | Man-rich heteropolysaccharides aiding tissue flexibility and enzymatic defense. |
| Theobroma grandiflorum (Cupuaçu) | HG–RG-I pectins supporting water regulation and pathogen resistance. |
| Passiflora edulis (Passion fruit) | Low-esterified pectins modulating viscosity and antimicrobial defense. |
| Hancornia speciosa (Mangaba) | Pectic polysaccharides with arabinogalactan branches contributing to high water retention, antioxidant activity, and cell wall elasticity under humid conditions. |
| Biomes | Fruits | Identified Polysaccharides | Structure | Ref. |
|---|---|---|---|---|
| Tundra | Arctic Blueberry (Vaccinium uliginosum) | VUP-1 (heteropolysaccharide) | Ara, Man, GalA, Glc and Gal | [62] |
| Lingonberry (Vaccinium vitis-idaea) | Acidic polymers and neutral arabinogalactans esterified with hydroxycinnamates | Glc, Ara, Gal, GalA, GlaA, alongside trace amounts of Rha, Fuc, Xyl, Rib | [63,64] | |
| Cranberry (Vaccinium oxycoccos) | RG-I pectic polysaccharides | GalA, homogeneous HG domains, branched RG-I with arabinogalactan side chains | [65,66] | |
| Cloudberry (Rubus chamaemorus) | No structural polysaccharides have been characterized to date | Carbohydrate fraction: Gly, Fru, Xyl, Gal and Ara | [67,68] | |
| Bearberry (Arctostaphylos uva-ursi) | No structural polysaccharides have been characterized to date | - | [69] | |
| Boreal Forest | Bilberry (Vaccinium myrtillus) | Glc-rich HCs, cellulose and low pectin constitution | HG with methyl esterification; RG-I with arabinan side chains | [73,74] |
| Rowanberry (Sorbus aucuparia) | Water-soluble pectin | High GalA; HG backbone + RG-I domains; Araf and Galp side chains | [73,74] | |
| Raspberry (Rubus chingii) | Acidic heteropolysaccharide (pRCP) | Backbone of →3,6)-β-D- Galp + →5)-α-L-Araf; Ara (39.76%) and Gal (39.43%) | [75,76] | |
| Cranberry (Vaccinium macrocarpon) | Stratified pectic polysaccharides | Methyl-esterified HG (75%) + arabinan/galactan side chains; RG-I with arabinogalactan substitutions (Ara + Gal/Rha = 11.5:1) | [77,78] | |
| Temperate Deciduous Forests | Strawberries (Fragaria ananassa) | Pectins (GalA-rich) and HCs | High methylation (60%); HG-dominated regions with arabinogalactan side chains | [80,81,82] |
| Sweet cherries (Prunus avium) | Ara/Gal-rich heteropolysaccharides | Ara, Gal, Glc and uronic acids | [83,84] | |
| Raspberries (Rubus idaeus) | Ara-rich polysaccharides | (1→4)-α-glucans; enzyme-resistant RG-I fragments | [84] | |
| American ginseng berries (Panax quinquefolius) | Gal-rich heteropolysaccharides | Low uronic acids; protein-polysaccharide interactions | [84] | |
| Mulberry (Morus nigra) | Ultrasound-assisted extracted polysaccharides | Glc, Ara, Gal and uronic acid | [85,86] | |
| Rose hip (Rosa canina) | GalA-rich pectin | HG backbone with methylesterification and acetylation, with RG-I segments; unique oligomers, including unsaturated pentamers with dual methyl and acetyl substitutions | [87,88] | |
| Temperate Pluvial Evergreen Forest | Avocado (Persea americana) | Pectin | Cellulose/hemicellulose, pectin; C7 sugars (mannoheptulose, perseitol) | [90,91] |
| Orange (Citrus sinensis) | Methyl-esterified HG + RG-I | Albedo pectin (83–85% GalA); branched RG-I with Ara/Gal side chains | [92,93] | |
| Persimmon (Diospyros kaki) | Water-soluble polysaccharides | Ara-rich side chains, Gal, Glc and GalA | [94,95] | |
| Fig fruit (Ficus carica) | Heteropolysaccharides | Ara, Gal, Gly; Branched α-1,4/β-1,3,6 linkages; HG (pectin) + xyloglucans (hemicellulose) | [96,97] | |
| Temperate Aridiestival Evergreen Forests | Olive (Olea europaea) | HG + RG-I pectins | Reduced methyl esterification/acetylation during ripening; increased RG-I branching | [99,100] |
| Almond (Prunus amygdalus) | Pectic polysaccharides | Peel: HG and RG-I domains; HCl-soluble pectin; GalA, Ara, Gal, Man, acid uronic. Seed: Ara-rich, xyloglucans and acidic xylans embedded in cellulose-hemicellulose matrix | [101,102,103] | |
| Pomegranate (Punica granatum) | Pectic polysaccharides | HG pectins: 46–68% GalA; high methylation + acylation; branched Ara (1→5)-α-L-Araf | [104,105,106] | |
| Arbutus unedo | Cellulose + HCs (xylans/xyloglucans) | Glc, Xyl and Ara; Lignified matrix with scleroids; reduced HG esterification during ripening | [107,108] | |
| Steppe | Sea Buckthorn (Hippophae Rhamnoides) | Hot water, ultrasonic-, microwave- and ethanol-assisted extracted polysaccharides | GalA, Glc, Gal, Ara, Rha, Xyl and Man; β-(1→4)-galactan backbone and α-(1→5)-Araf with Xyl substitutions | [111] |
| Barberry (Berberis vulgaris) | Pectic polysaccharides | High GalA, Ara/Gal-dominated side chains; branched RG-I + HG domains | [110,111] | |
| Goji (Lycium barbarum) | Pectic polysaccharides | Alkali-extracted RG-I (Ara + Gal/Rha = 7.77); acid-extracted HG with low branching | [112,113] | |
| Apricots (Prunus armeniaca) | Pectic polysaccharides | RG-I: Ara, Gal; Arabinogalactan-protein conjugates; high Man in hemicellulose | [114] | |
| Nitraria retusa | Heteropolysaccharides | Ara, Gal, Glc; α-(1→6)-galactan + branched arabinan; antioxidant activity linked to uronic acids | [115,116] | |
| Deserts and Semi-Deserts | Prickly pear (Opuntia ficus-indica) | Heteropolysaccharides | Linear β-(1→4)-galactan backbone; complex α-arabinan with Xyl substitutions. (α-(1→5)-arabinan with 2,3,5-linked branches.) | [118,119] |
| Jujube (Ziziphus jujuba) | RG-I (arabinogalactan) + arabinan | α-(1→4)-GalA backbone + β-(1→4)-galactan/α-(1→5)-arabinan side chains. | [120,121] | |
| Watermelon (Citrullus lanatus) | RG-I pectin (arabinogalactan) | β-(1→6)-galactan + α-L-Araf side chains; compact branched conformation | [122,123,124] | |
| Date palm (Phoenix dactylifera) | Heteropolysaccharides | Mesocarp: (1→3)-β-D-Glcp backbone with (1→6)-linked branches; Seed xylan: β-(1→4)-Xylp with Ara/Gal substitutions | [125,126] | |
| Tropical Pluviseasonal Forests | Papaya (Carica papaya) | Pectic polysaccharides | HG pectins: High GalA (69–74%), reduced RG-I during ripening; β-glucans released from cellulose during ripening | [128,129] |
| Mango (Mangifera indica) | Heteropolysaccharides | Arabinogalactan, RG; 1→4-β-galactan backbone + 1→5-α-Araf | [130,131] | |
| Guava (Psidium guajava) | Heteropolysaccharides | Ara-rich, Glc; (1→5)-α-L-Ara backbone; branched (1→3,6)-β-D-Gal | [132,133] | |
| Cashew (Anacardium occidentale) | Branched heteropolysaccharides | β-(1→3,6)-Galp; side chains with GlcA, Ara, Rha | [134] | |
| Dragon fruit (Hylocereus undatus) | Heteropolysaccharides | GlcA, Gal, Rha; backbone: →4-β-GlcA + →6-β-Gal; side chains: α-L-Araf-(1→5)-α-L-Araf | [135,136] | |
| Tropical Rainforests | Açaí (Euterpe oleracea) | Arabinogalactan + HG pectin | β-(1,3)-galactan backbone + Ara/Gal side chains; partial esterification | [138,139,140] |
| Pineapple (Ananas comosus) | Man-rich heteropolysaccharides | Ara, Xyl, Man, Glc, Gal, Rha, GalA; →4)-α-D-Manp backbone; Ara/Gal side chains + methyl-esterified GalA | [141,142] | |
| Cupuassu (Theobroma grandiflorum) | Pectic polysaccharides | High methyl esterification, low acetylation; RG-I backbone contained →4)-α-D- Galactopyranosyl Acid (GalAp)-(1→ and →2,4)-α-L-Rhap-(1→ linkages, substituted at O-4 of Rha with side chains rich in Gal and Ara | [143,144] | |
| Passion fruit (Passiflora edulis) | Pectic polysaccharides | GalA, Ara, Rha; (1→4)-GalA backbone; low esterification + acetyl groups. | [145,146] | |
| Mangaba (Hancornia speciosa) | Heteropolysaccharides | Arabinan (RG-I-associated); (1→5)-α-L-Araf units; (1→4)-β-D-Galp residues. | [147,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, K.R.; Júnior, L.T.; Sogayar, M.C.; Fabi, J.P. Exploring Bioactive Polysaccharides in Edible Fruits: A Cross-Biome Perspective. Plants 2025, 14, 3515. https://doi.org/10.3390/plants14223515
Nascimento KR, Júnior LT, Sogayar MC, Fabi JP. Exploring Bioactive Polysaccharides in Edible Fruits: A Cross-Biome Perspective. Plants. 2025; 14(22):3515. https://doi.org/10.3390/plants14223515
Chicago/Turabian StyleNascimento, Karen Rebouças, Leandro Teodoro Júnior, Mari Cleide Sogayar, and João Paulo Fabi. 2025. "Exploring Bioactive Polysaccharides in Edible Fruits: A Cross-Biome Perspective" Plants 14, no. 22: 3515. https://doi.org/10.3390/plants14223515
APA StyleNascimento, K. R., Júnior, L. T., Sogayar, M. C., & Fabi, J. P. (2025). Exploring Bioactive Polysaccharides in Edible Fruits: A Cross-Biome Perspective. Plants, 14(22), 3515. https://doi.org/10.3390/plants14223515









