Kenyan Orthosiphon schimperi Benth. Essential Oil: Chemical Composition and Cytotoxic Activity on HeLa Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Gas Chromatography and Mass Spectrometry (GC-MS) Analysis of the Essential Oil
| Taxa | Parts | Origins | Main Components (%) | Compoud Classes | Ref. |
|---|---|---|---|---|---|
| O. aristatus var. aristatus | lv | Commercial | Palmitic acid (9.51%) β-Selinene (7.84%) β-Caryophyllene (6.45%) δ-Cadinene (6.33%) Caryophyllene oxide (6.05%) Hexahydrofarnesyl acetone (5.76%) β-Elemene (4.92%) α-Humulene (3.37%) | O SH SH SH OS O SH SH | [27] |
| O. diffusus (Benth) Benth. * | lv | India | n-Eicosane (19.5%) β-Caryophyllene (18.6%) n-Octocosane (12.2%) Limonene (11.6%) β-Ocimene (4.2) | O SH O MH MH | [28] |
| O. pallidus Royle, ex Benth | ap | India | β-Caryophyllene (17.4%) 7-epi-α-Selinene (15.2%) Terpinolene (6.9%) β-Pinene (6.8%) β-Elemene (5.1%) α-Humulene (4.9%) α-Copaene (4.8%) epi-Cubebol (4.5%) Zonarene (3.9%) | SH SH MH MH SH SH SH OS SH | [29] |
| O. stamineus Benth. ** | ap | Malaysia | β-Caryophyllene (26.31%) Bicyclogermacrene (7.7%) Eugenol methyl ether (7.4%) α-Humulene (5.06%) α-Copaene (2.26%) | SH SH OM SH SH | [30] |
| lv | Malaysia | β-Caryophyllene (24.0%) α-Humulene (14.2%) β-Elemene (11.1%) 1-Octen-3-ol (8.2%) β-Bourbonene (3.4%) | SH SH SH O SH | [31] | |
| st | Malaysia | β-Caryophyllene (35.1%) α-Humulene (18.4%) β-Elemene (8.5%) 1-Octen-3-ol (7.0%) β-Bourbonene (3.0%) | SH SH SH O SH | [3] | |
| O. thymiflorus (Roth) Sleesen | lv | India | 2-Isopropyl-5-methyl-9-methylene-bicyclo-1-decene (4.4.0) (42.62%) Carotol (16.48%) α-Cadinol (6.14%) δ-3-Carene (5.15%) α-Humulene (3.61%) γ-Cadinene (3.34%) | SH OS OS MH SH SH | [32] |
| ap, r | India | Isobornyl acetate (55.36%) Nerolidol (7.19%) Camphene (5.52%) Eugenol (4.15%) α-Pinene (4.07%) | O OS SH OM MH | [33] |
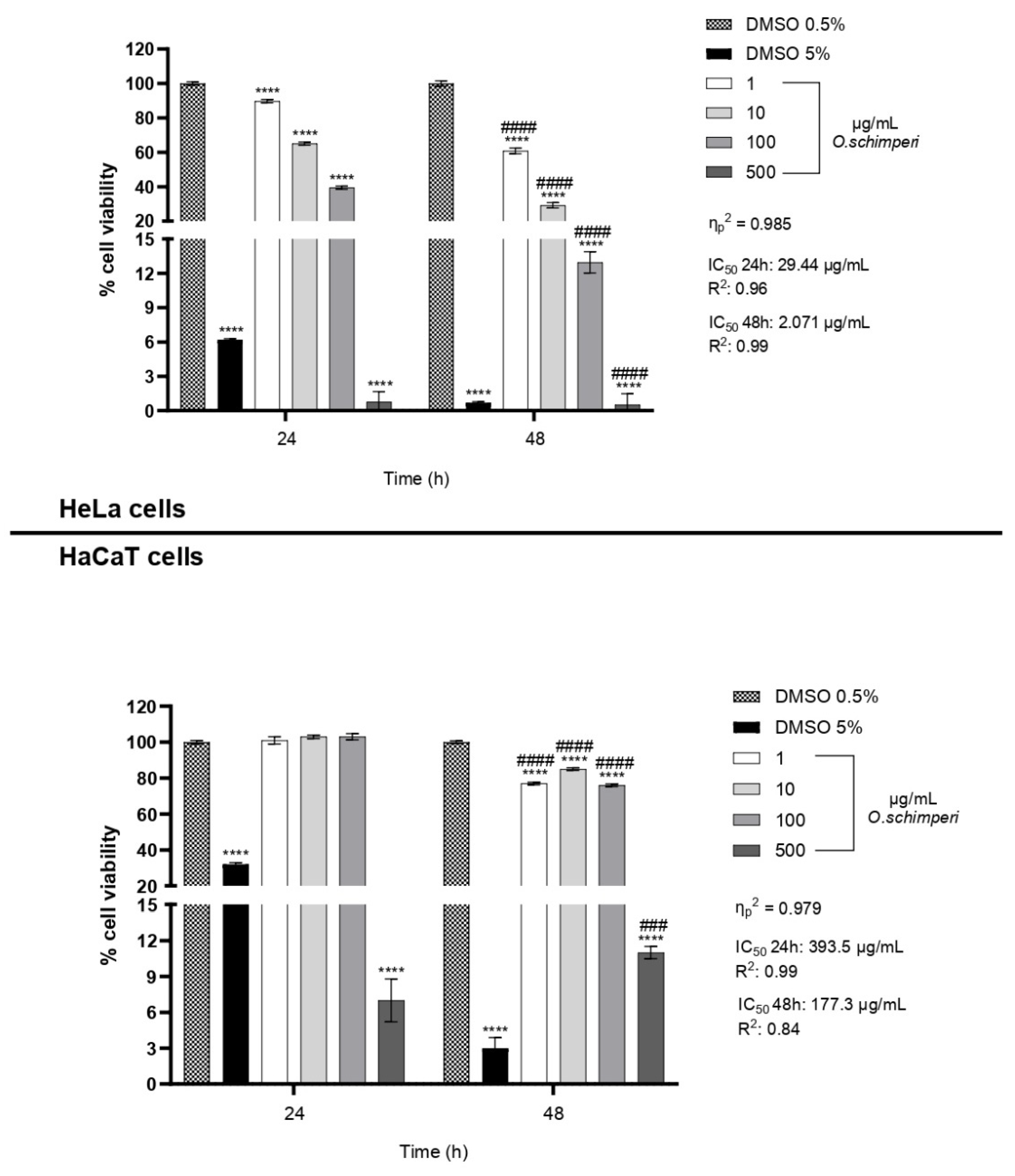
2.2. Cytotoxicity of OS
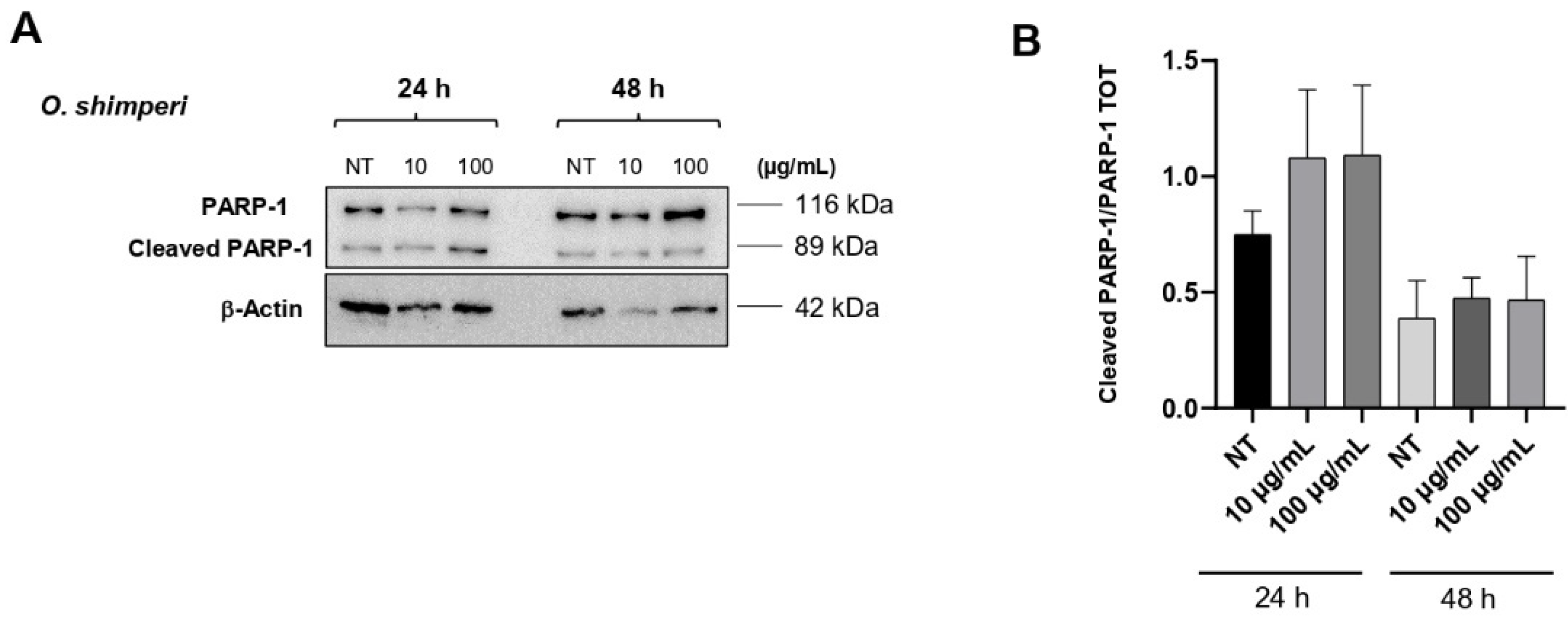
2.3. OS Induces PARP-1 Cleavage in HeLa Cells
3. Materials and Methods
3.1. Plant Materials
3.2. Isolation of EO
3.3. Chemicals
3.4. GC-MS Analysis
3.5. Cell Viability Assay (CCK-8)
3.6. Western Blot Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Data Availability Statement
Conflicts of Interest
References
- Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 22 March 2025).
- Ameer, O.Z.; Salman, I.M.; Asmawi, M.Z.; Ibraheem, Z.O.; Yam, M.F. Orthosiphon stamineus: Traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food 2012, 15, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Sundarammal, S.; Thirugnanasampandan, R.; Selvi, M.T. Chemical composition analysis and antioxidant activity evaluation of essential oil from Orthosiphon thymiflorus (Roth) Sleesen. Asian Pac. J. Trop. Biomed. 2012, 2 (Suppl. 1), S112–S115. [Google Scholar] [CrossRef]
- Sini, K.R.; Haribabu, Y.; Sajith, M.S.; Sreekumar, K.S. In-vitro cytotoxic activity of Orthosiphon thymiflorus Roth.) sleensen leaf extract against dalton lymphoma ascites cell line. J. Chem. Pharmaceut. Res. 2012, 4, 917–921. [Google Scholar]
- Kavimani, S.; Ilango, R.; Thangadurai, J.; Jaykar, B.; Majumdar, U.; Gupta, M. Diuretic activity of aqueous extract of Orthosiphon thymiflorus in rats. Indian J. Pharmaceut. Sci. 1997, 59, 96–98. [Google Scholar]
- Kovendan, K.; Murugan, K.; Vincent, S.; Barnard, D.R. Mosquito larvicidal properties of Orthosiphon thymiflorus (Roth) Sleesen. (Family: Labiatae) against mosquito vectors, Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2012, 5, 299–305. [Google Scholar] [CrossRef]
- Singh, U.; Wadhwani, A.M.; Johri, B.M. Dictionary of economic plants in India; Indian Council of Agriculture Research: New Delhi, India, 1996; p. 159. [Google Scholar]
- Kiruthika, A.; Meenakshi, S.M. Anticancer studies on Orthosiphon pallidus Royle. and Peristrophe bicalyculata Nees. J. Pharm. Res. 2011, 4, 2654–2656. [Google Scholar]
- Watt, J.M.; Brandwijk-Breyer, M.G. Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; E. & S. Livingstone Ltd.: Edinburgh, UK, 1962. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants. An Illustrated Dictionary; Springer Science: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef]
- Basu, N.K.; Singh, H. Investigation of Orthosiphon pallidus Royle I. Preliminary chemical study and isolation of Orthosiphonol. J. Am. Pharmaceut. Assoc. 1956, 9, 595–598. [Google Scholar] [CrossRef]
- Regina, K.M.M.; Adama, H.; Jeanne, M.; Odile, N. Ethnobotany and ethnopharmacognosy of Lamiaceae species from Central Burkina Faso: Leucas martinicensis (Jacquin) R. Brown, Hoslundia opposita Vahl and Orthosiphon pallidus Royle Ex Benth. Am. J. Ethnomed. 2015, 2, 219–232. [Google Scholar]
- Ghaffari, H.; Venkataramana, M.; Nayaka, S.C.; Ghassam, B.J.; Angaswamy, N.; Shekar, S.; Kumara, K.K.S.; Prakash, H.S. Hepatoprotective action of Orthosiphon diffusus (Benth.) methanol active fraction through antioxidant mechanisms: An in vivo and in vitro evaluation. J. Ethnopharmacol. 2013, 149, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Masuda, K.; Shiragami, S.; Jitoe, A.; Nakatani, N. Orthosiphol A and B, novel diterpenoid inhibitors of TPA (12-O-tetradecanoylphorbol-13-acetate)-induced inflammation, from Orthosiphon stamineus. Tetrahedron 1992, 48, 6787–6792. [Google Scholar] [CrossRef]
- Matsubara, T.; Bohgaki, T.; Watarai, M.; Suzuki, H.; Ohashi, K.; Shibuya, H. Antihypertensive actions of methylripariochromene A from Orthosiphon aristatus, an Indonesian traditional medicinal plant. Biol. Pharm. Bull. 1999, 22, 1083–1088. [Google Scholar] [CrossRef]
- Ohashi, K.; Bohgaki, T.; Matsubara, T.; Shibuya, H. Indonesian medicinal plants. XXIII. Chemical structures of two new migrated pimarane-type diterpenes, neoorthosiphols A and B and suppressive effects on rat thoracic aorta of chemical constituents isolated from the leaves of Orthosiphon aristatus (Lamiaceae). Chem. Pharm. Bull. 2000, 48, 433–435. [Google Scholar] [CrossRef]
- Prajapati, N.D.; Purohit, S.S.; Sharma, A.K.; Kumar, T. A Handbook of Medicinal Plants: A Complete Source Book; Agrobios: Jodhpur, India, 2003; p. 373. [Google Scholar]
- Suroowan, S.; Mahomoodally, M.F. A comparative ethnopharmacological analysis of traditional medicine used against respiratory tract diseases in Mauritius. J. Ethnopharmacol. 2016, 177, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Adnyana, I.K.; Setiawan, F.; Insanu, M. From ethnopharmacology to clinical study of Orthosiphon stamineus Benth. Int. J. Pharm. Pharm. Sci. 2013, 5, 66–73. [Google Scholar]
- Omwenga, E.O.; Hensel, A.; Shitandi, A.; Goycoolea, F.M. Ethnobotanical survey of traditionally used medicinal plants for infections of skin, gastrointestinal tract, urinary tract and the oral cavity in Borabu sub-county, Nyamira county, Kenya. J. Ethnopharmacol. 2015, 176, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Gidwani, B.; Gupta, A.; Dhongade, H.; Kaur, C.D.; Kashyap, P.P.; Tripathi, D.K. A review of the medicinal plants of genus Orthosiphon (Lamiaceae). Int. J. Biol. Chem. 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Abirami, K.; Revathi, P.; Thenmozhi, K.; Sowndhararajan, K. Comprehensive Review on Wild Basil Genus Orthosiphon of Lamiaceae. In Bioprospecting of Tropical Medicinal Plants; Arunachalam, K., Yang, X., Puthanpura Sasidharan, S., Eds.; Springer: Cham, Switzerland, 2023; pp. 409–426. [Google Scholar] [CrossRef]
- Wang, R.; Qiu, L.; Zhang, Q.W.; Lin, L. Ethnopharmocology, phytochemistry and pharmacology of the genus Orthosiphon: A review. Phytomed. Plus 2025, 5, 100748. [Google Scholar] [CrossRef]
- Kalusalingam, A.; Hasnu, D.N.; Khan, A.; Tan, C.S.; Menon, B.; Narayanan, V.; Goh, K.W.; Ikmal, A.M.; Talip, N.; Subramanian, P.; et al. An Updated review of ethnobotany, ethnopharmacology, phytochemistry and pharmacological activities of Orthosiphon stamineus Benth. Malays. Appl. Biol. 2024, 53, 1–18. [Google Scholar] [CrossRef]
- Available online: https://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=150420 (accessed on 22 March 2025).
- Schut, G.A.; Zwaving, J.H. Content and composition of the essential oil of Orthosiphon aristatus. Planta Med. 1986, 52, 240–241. [Google Scholar] [CrossRef]
- Sadashiva, C.T.; Sharanappa, P.; Naidoo, Y.; Sulaimon, C.T.; Balachandran, I. Chemical composition of essential oil from Orthosiphon diffuses Benth. J. Med. Plants Res. 2013, 7, 170–172. [Google Scholar] [CrossRef]
- Joshi, R.K. GC-MS analysis of the volatile constituents of Orthosiphon pallidus Royle, ex Benth. Nat. Prod. Res. 2020, 34, 441–444. [Google Scholar] [CrossRef]
- Azizan, N.; Mohd Said, S.; Abidin, Z.Z.; Jantan, I. Composition and antibacterial activity of the essential oils of Orthosiphon stamineus Benth and Ficus deltoidea Jack against pathogenic oral bacteria. Molecules 2017, 22, 2135. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Ismail, Z.; Rahman, A.; Kang, S.C. Chemical composition and anti-fungal properties of the essential oils and crude extracts of Orthosiphon stamineus Benth. Ind. Crops Prod. 2008, 27, 328–334. [Google Scholar] [CrossRef]
- Yogesh, U.; Sudarshan, S.; Rajesh, D.; Bothara Sunil, B. GC-MS analysis and antibacterial activity of the essential oil from Orthosiphon thymiflorurs Roth. In Proceedings of the International Conference on Industrial Pharmacy (ICIP 2014) Kuantan, Pahang, Malaysia, 16–17 August 2014. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid. Based Compl. Altern. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Medical Principles and Practice 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A natural compound with versatile pharmacological actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gartzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2017, 210, 402–414. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Harris, R. Synergism in the essential oil world. Int. J. Aromather. 2002, 12, 179–186. [Google Scholar] [CrossRef]
- Khodaei, N.; Houde, M.; Bayen, S.; Karboune, S. Exploring the synergistic effects of essential oil and plant extract combinations to extend the shelf life and the sensory acceptance of meat products: Multi-antioxidant systems. J. Food Sci. Technol. 2023, 60, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Połeć, K.; Wyżga, B.; Olechowska, K.; Hąc-Wydro, K. On the synergy/antagonism of selected terpenes in the effect on lipid membranes studied in model systems. J. Mol. Liq. 2022, 349, 118473. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlockm, G.E.; Schulz, H.; et al. Essential oils as multicomponent mixtures and their potential for human health and well-being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef]
- Brandes, A.; Dunning, M.; Langland, J. Antimicrobial activity of individual volatile compounds from various essential oils. Molecules 2024, 29, 1811. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell. Mol. Biol. 2017, 63, 42–47. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia 10.3; Determination of Essential Oils in Herbal Drugs; Council of Europe: Strasbourg, France, 2020; p. 307. [Google Scholar]
- Delicato, A.; Montuori, E.; Angrisano, T.; Pollice, A.; Calabrò, V. YB-1 oncoprotein controls PI3K/Akt pathway by reducing pten protein level. Genes 2012, 12, 1551. [Google Scholar] [CrossRef]
- Chianese, T.; Trinchese, G.; Leandri, R.; De Falco, M.; Mollica, M.P.; Scudiero, R.; Rosati, L. Glyphosate Exposure Induces Cytotoxicity, Mitochondrial Dysfunction and Activation of ERα and ERβ Estrogen Receptors in Human Prostate PNT1A Cells. Int. J. Mol. Sci. 2024, 25, 7039. [Google Scholar] [CrossRef] [PubMed]

| No. | Classes and Compounds a | LRI b | LRI c | Area (%) |
|---|---|---|---|---|
| ∑ Monoterpene Hydrocarbons (MH) | 3.06 ± 0.09 | |||
| 1 | α-Pinene | 929 | 931 | 0.39 ± 0.01 |
| 2 | β-Pinene | 970 | 975 | 1.57 ± 0.06 |
| 3 | β-Myrcene | 989 | 990 | 0.11 ± 0.00 |
| 4 | α-Terpinene | 1012 | 1017 | 0.13 ± 0.00 |
| 5 | p-Cymene | 1019 | 1018 | 0.11 ± 0.00 |
| 6 | Limonene | 1024 | 1030 | 0.32 ± 0.01 |
| 7 | cis-β-Ocimene | 1037 | 1035 | 0.27 ± 0.01 |
| 8 | trans-β-Ocimene | 1047 | 1046 | 0.09 ± 0.00 |
| 9 | γ-Terpinene | 1055 | 1053 | 0.07 ± 0.00 |
| ∑ Oxygenated Monoterpenes (OM) | 83.47 ± 3.71 | |||
| 10 | Linalool | 1099 | 1097 | 2.74 ± 0.11 |
| 11 | Isoborneol | 1172 | 1160 | 0.17 ± 0.00 |
| 12 | α-Terpineol | 1185 | 1190 | 0.42 ± 0.01 |
| 13 | Estragole | 1193 | 1195 | 0.21 ± 0.01 |
| 14 | Eugenol methyl ether | 1413 | 1401 | 79.38 ± 3.56 |
| 15 | Isoeugenol methyl ether | 1495 | 1492 | 0.55 ± 0.02 |
| ∑ Sesquiterpene Hydrocarbons (SH) | 1.51 ± 0.04 | |||
| 16 | α-Cubebene | 1345 | 1346 | 0.10 ± 0.00 |
| 17 | α-Copaene | 1371 | 1373 | 0.63 ± 0.02 |
| 18 | α-Bourbonene | 1379 | 1376 | 0.36 ± 0.01 |
| 19 | α-Bergamotene | 1439 | 1435 | 0.42 ± 0.01 |
| ∑ Oxygenated Sesquiterpenes (OS) | 8.64 ± 0.38 | |||
| 20 | Caryophyllene oxide | 1578 | 1578 | 8.00 ± 0.36 |
| 21 | Alloaromadendrene oxide | 1630 | 1631 | 0.64 ± 0.02 |
| Total | 96.68 ± 4.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalamenti, N.; Maresca, V.; Porrello, A.; Ilardi, V.; Postiglione, A.; Dentato, M.; De Marino, E.; Pollice, A.; Bruno, M. Kenyan Orthosiphon schimperi Benth. Essential Oil: Chemical Composition and Cytotoxic Activity on HeLa Cells. Plants 2025, 14, 3513. https://doi.org/10.3390/plants14223513
Badalamenti N, Maresca V, Porrello A, Ilardi V, Postiglione A, Dentato M, De Marino E, Pollice A, Bruno M. Kenyan Orthosiphon schimperi Benth. Essential Oil: Chemical Composition and Cytotoxic Activity on HeLa Cells. Plants. 2025; 14(22):3513. https://doi.org/10.3390/plants14223513
Chicago/Turabian StyleBadalamenti, Natale, Viviana Maresca, Antonella Porrello, Vincenzo Ilardi, Alessia Postiglione, Martina Dentato, Elena De Marino, Alessandra Pollice, and Maurizio Bruno. 2025. "Kenyan Orthosiphon schimperi Benth. Essential Oil: Chemical Composition and Cytotoxic Activity on HeLa Cells" Plants 14, no. 22: 3513. https://doi.org/10.3390/plants14223513
APA StyleBadalamenti, N., Maresca, V., Porrello, A., Ilardi, V., Postiglione, A., Dentato, M., De Marino, E., Pollice, A., & Bruno, M. (2025). Kenyan Orthosiphon schimperi Benth. Essential Oil: Chemical Composition and Cytotoxic Activity on HeLa Cells. Plants, 14(22), 3513. https://doi.org/10.3390/plants14223513










