Arbuscular Mycorrhizal Fungi Enhance the Insecticidal Activity of Annona muricata L. Leaves
Abstract
1. Introduction
2. Results
2.1. Biological Activity of Ethanolic Extracts of Soursop Against Triatoma pallidipennis
2.2. Biological Activity of Ethanolic Extracts of Soursop Against Spodoptera frugiperda
2.3. Annonacin Quantification via HPLC (González-López et al., 2025 [23])
3. Discussion
3.1. Biological Activity of Ethanolic Extracts of Soursop Against Triatoma pallidipennis
3.2. Biological Activity of Ethanolic Extracts of Soursop Against Spodoptera frugiperda
4. Materials and Methods
4.1. Plant Material and Mycorrhizal Inoculation
4.2. Preparation of Soursop Leaf Extracts
4.3. Bioassay in Triatoma pallidipennis Adults
4.3.1. Insects
4.3.2. Application of Extracts
4.4. Bioassay in Spodoptera frugiperda Larvae
4.4.1. Rearing of Spodoptera frugiperda
4.4.2. Application of Extracts
4.5. Determination of Total Annonacins in A. muricata Leaf Extracts
4.5.1. Plant Extract
4.5.2. Annonacin Quantification via HPLC (González-López et al., 2025 [23])
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIATEJ | Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. |
| AMF | Arbuscular mycorrhizal fungi |
| FM | Funneliformis mosseae |
| RI | Rhizophagus intraradices |
| CM | Cerro del Metate |
| AD | Agua Dulce |
| SM | Non-Mycorrhizal Plants |
| SICyT | Secretaría de Innovación Ciencia y Tecnología de Jalisco |
| COECyTJAL | Consejo Estatal de Ciencia y Tecnología de Jalisco |
| NZW | New Zealand White |
| DMSO | Dimethyl Sulfoxide |
| %M | Mortality Percentage |
| DW | Dry Weight |
| LSD | Least Significant Difference |
References
- Hernández-Fuentes, L.M.H.; González, E.M.; Magaña, M.L.G.; Esparza, L.M.A.; González, Y.N.; Villagrán, Z.; Torres, S.G.; Monreal, J.J.V.; Flores, D.A.M. Current situation and perspectives of fruit Annonaceae in Mexico: Biological and agronomic importance and bioactive properties. Plants 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- Septangisih, D.A.; Suparto, I.H.; Achmadi, S.S.; Heryanto, R.; Rafi, M. Untargeted metabolomics using UHPLC-Q-Orbitrap HRMS for identifying cytotoxic compounds on MCF-7 breast cancer cells from Annona muricata Linn leaf extracts as potential anticancer agents. Phytochem. Anal. 2024, 35, ecz3373. [Google Scholar] [CrossRef]
- Tundis, R.; Xiao, J.; Loizzo, M.R. Annona species (Annonaceae): A rich source of potential antitumor agents? Ann. N. Y. Acad. Sci. 2017, 1398, 30–36. [Google Scholar] [CrossRef]
- George, V.C.; Naveen-Kumar, D.R.; Rajkumar, V.; Suresh, P.K.; Ashok-Kumar, R. Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona muricata Linn. in normal and immortalized human cell lines. Asian Pac. J. Cancer Prev. 2012, 13, 699–704. [Google Scholar] [CrossRef]
- Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Rahman, N.M.A.N. Anti-cancer effect of Annona muricata Linn leaves crude extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 311. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Arango, G.J.; González, G.J.; Robledo, S.M.; Vélez, I.D. Cytotoxicity and antileishmanial activity of Annona muricata pericarp. Fitoterapia 2000, 71, 183–186. [Google Scholar] [CrossRef]
- Tchokouaha, L.R.; Tsouh, P.V.; Jiatsa, C.D.; Keumone, R.; Ndjakou, B.L.; Djouonzo, P.T.; Mfopa, A.N.; Legac, J.; Tsabang, N.; Gut, J.; et al. Extracts from Annona muricata L. and Annona reticulata L. (Annonaceae) potently and selectively inhibit Plasmodium falciparum. Medicines 2015, 2, 55–66. [Google Scholar] [CrossRef]
- Abdallah, R.H.; Al-Attar, A.-s.R.; Shehata, Y.M.; Abdel-Fattah, D.M.; Atta, R.M.; Fantoukh, O.I.; Mustafa, A.M. Comprehensive chemical profiling and mechanistic insight into anticancer activity of Annona muricata leaves extract. Pharmaceuticals 2024, 17, 614. [Google Scholar] [CrossRef]
- Isman, M.B.; Seffrin, R. Natural insecticides from the Annonaceae: A unique example for developing biopesticides. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer: Delhi, India, 2015; pp. 21–33. [Google Scholar] [CrossRef]
- Álvarez, O.; Barrachina, I.; Ayala, I.; González, M.C.; Moya, P.; Neske, A.; Bardon, A. Toxic effects of annonaceous acetogenins on Oncopeltus fasciatus. J. Pest Sci. 2008, 81, 85–89. [Google Scholar] [CrossRef]
- Prédes, C.; de Souza, J.; Ferreira, M.; da Silva, P.; Goulart, A. Larvicidal activity and seasonal variation of Annona muricata (Annonaceae) extract on Plutella xylostella (Lepidoptera: Plutellidae). Rev. Colomb. Entomol. 2011, 37, 223–227. [Google Scholar] [CrossRef]
- González-Esquinca, A.R.; Luna-Cazáres, L.M.; Schile, M.; Chacón, I.C.; Hernández, G.L.; Flores, S.; Gerardo, P.M. In vitro larvicidal evaluation of Annona muricata, A. diversifolia and A. lutescens extracts against Anastrepha ludens larvae (Diptera, Tephritidae). Interciencia 2012, 37, 284–289. [Google Scholar]
- Flores-Dávila, M.; González-Villegas, R.; Guerrero-Rodríguez, E.; Mendoza-Villareal, R.; Cárdenas-Elizondo, A.; Cerna-Chávez, C.; Aguirre-Uribe, L. Insecticidal effect of plant extracts on Bactericera cockerelli (Hemiptera: Psyllidae) nymphs. Southwest. Entomol. 2011, 36, 137–144. [Google Scholar] [CrossRef]
- Asmanizar; Djamin, A.; Idris, A.B. Evaluation of Jatropha curcas and Annona muricata seed crude extracts against Sitophilus zeamais infesting stored rice. J. Entomol. 2012, 9, 13–22. [Google Scholar] [CrossRef]
- Grzybowski, A.; Tiboni, M.; Silva, M.A.N.; Chitolina, R.F.; Passos, M.; Fontana, J.D. Synergistic larvicidal effect and morphological alterations induced by ethanolic extracts of Annona muricata and Piper nigrum against the dengue fever vector Aedes aegypti. Pest Manag. Sci. 2013, 69, 589–601. [Google Scholar] [CrossRef]
- Santhosh, S.B.; Ragavendran, C.; Natarajan, D. Spectral and HRTEM analyses of Annona muricata leaf extract-mediated silver nanoparticles and its larvicidal efficacy against three mosquito vectors Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. J. Photochem. Photobiol. B 2015, 153, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.-L.; Zhang, M.-H.; Shi, Z.-Y.; Yang, S.; Zhang, M.-G.; Wang, Z.; Wu, S.-W.; Gao, J.-K. Arbuscular mycorrhizal fungi enhance active ingredients of medicinal plants: A quantitative analysis. Front. Plant Sci. 2023, 14, 1276918. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef]
- González-López, A.M.; Quiñones-Aguilar, E.E.; Guízar-González, C.; Rincón-Enríquez, G. Annonacin accumulation in leaves of Annona muricata L. induced by mycorrhizal colonization. FEMS Microbiol. Lett. 2025, 372, fnaf085. [Google Scholar] [CrossRef]
- Cristóbal-Alejo, J.; Lima-Burgos, A.; Pinzón-López, L.L.; Tun-Suárez, J.M.; Herrera-Parra, E. Hongos micorrízicos arbusculares aceleran el tiempo de crecimiento de portainjertos de guanábana (Annona muricata L.)/Arbuscular mycorrhizal fungi accelerate the growth time of soursop rootstock (Annona muricata L.). Ecosist. Recur. Agropec. 2022, 9, e3226. [Google Scholar]
- González-López, A.M.; Quiñones-Aguilar, E.E.; Aburto-González, C.A.; Alejo-Santiago, G.; Hernández-Cuevas, L.V.; Rincón-Enríquez, G. Mycorrhizal fungi in Annona muricata L. rhizosphere in two agricultural production systems in Nayarit, Mexico. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13850. [Google Scholar] [CrossRef]
- González-López, A.M.; Quiñones-Aguilar, E.E.; Bautista-Cruz, A.; Rincón-Enríquez, G. Influence of arbuscular mycorrhizal fungi on the growth and physiology of Annona muricata (L.) under two irrigation levels in greenhouse conditions. Sci. Hortic. 2025, 344, 114115. [Google Scholar] [CrossRef]
- Secretaria de Salud y Centro Nacional de Programas Preventivos y Control de Enfermedades. In Estrategia de Intervención Nacional Para la Interrupción de la Transmisión Vectorial Intradomiciliaria de la Enfermedad de Chagas en México; Secretaría de Salud: México City, México, 2022; Available online: https://www.gob.mx/cms/uploads/attachment/file/819576/Estrategia_de_intervencio_n_nacional_para_la_Interrupcio_n_de_la_transmisio_n_vectorial_intradomiciliaria_de_la_enfermedad_de_Chagas_en_Me_xico.pdf (accessed on 7 September 2025).
- Barrientos-Gutiérrez, J.E.; Rodríguez-Hernández, C.R.; Zumaquero-Ríos, J.L.; López-Olguín, J.F. Efecto de extractos de semillas de Azadirachta indica aplicado a Meccus pallidipennis (Stål) en condiciones de laboratorio. Southwest. Entomol. 2018, 43, 465–474. [Google Scholar] [CrossRef]
- Parra-Henao, G.; García, C.M.; Cotes, J.M. Actividad insecticida de extractos vegetales sobre Rhodnius prolixus y Rhodnius pallescens (Hemiptera: Reduviidae). Bol. Malariol. Salud Ambient. 2007, 47, 125–137. [Google Scholar]
- Carneiro, A.; Pereira, M.; Galbiati, C. Biocidal activity of Annona coriacea seed extract on Rhodnius neglectus (Hemiptera: Reduviidae). Rev. Biol. Trop. 2013, 61, 419–427. [Google Scholar] [CrossRef]
- Castillo-Sánchez, L.E.; Jiménez-Osornio, J.J.; Delgado-Herrera, M.A. Secondary metabolites of the Annonaceae, Solanaceae, and Meliaceae families used as biological control of insects. Trop. Subtrop. Agroecosyst. 2010, 12, 445–462. [Google Scholar]
- Broglio-Micheletti, S.M.F.; Valente, E.C.N.; Souza, L.A.; Dias, N.S.; Araújo, A.M.N. Extratos de plantas no controle de Rhipicephalus (Boophilus) microplus (Canestrini, 1887) (Acari: Ixodidae) em laboratório. Rev. Bras. Parasitol. Vet. 2009, 18, 44–48. [Google Scholar] [CrossRef]
- Magadum, S.; Mondal, D.B.; Ghosh, S. Comparative efficacy of Annona squamosa and Azadirachta indica extracts against Boophilus microplus Izatnagar isolate. Parasitol. Res. 2009, 105, 1085–1091. [Google Scholar] [CrossRef]
- Ríos, M.Y.; Castrejón, F.; Robledo, N.; León, I.; Rojas, G.; Navarro, V. Chemical composition and antimicrobial activity of the essential oils from Annona cherimola (Annonaceae). J. Mex. Chem. Soc. 2003, 47, 134–142. [Google Scholar]
- González-Coloma, A.; Guadaño, A.; de Inés, C.; Martínez-Díaz, R.; Cortes, D. Selective action of acetogenin mitochondrial complex I inhibitors. Z. Naturforsch. C J. Biosci. 2002, 57, 1028–1034. [Google Scholar] [CrossRef]
- Hazrati, S.; Mohammadi, M.; Mollaei, S.; Ebadi, M.; Pignata, G.; Nicola, S. Nitrogen Fertilization and Glomus Mycorrhizal Inoculation Enhance Growth and Secondary Metabolite Accumulation in Hyssop (Hyssopus officinalis L.). Nitrogen 2025, 6, 60. [Google Scholar] [CrossRef]
- Freitas, A.F.; Pereira, F.F.; Formagio, A.S.N.; Lucchetta, J.T.; Vieira, M.; Mussury, R.M. Effects of methanolic extracts of Annona species on the development and reproduction of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2014, 43, 446–452. [Google Scholar] [CrossRef]
- Di Toto-Blessing, L.; Budeguer, F.; Ramos, J.; Bardon, A.; Díaz, S.; Brovetto, M.; Seoane, G.; Neske, A. Structural factors of annonaceous acetogenins and their semisynthetic analogues related to the toxicity on Spodoptera frugiperda. J. Agric. Chem. Environ. 2015, 4, 56–61. [Google Scholar] [CrossRef]
- Guadaño, A.; Gutiérrez, C.; De la Peña, E.; Cortes, D.; González-Coloma, A. Insecticidal and mutagenic evaluation of two annonaceous acetogenins. J. Nat. Prod. 2000, 63, 773–776. [Google Scholar] [CrossRef]
- de Sousa-Junior, J.C.A.; de Sousa Oliveira, M.; de Araújo Dias, C.H.; Amariz, A.; da Silva Campos, M.A. Effect of inoculation with arbuscular mycorrhizal fungi on larvicidal activity and phenolic compounds in Mimosa tenuiflora cultivated under field conditions. Beni-Suef Univ. J. Basic Appl. Sci. 2025, 14, 41. [Google Scholar] [CrossRef]
- Zubek, S.; Mielcarek, S.; Turnau, K. Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 2012, 22, 149–156. [Google Scholar] [CrossRef]
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular trichomes and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 2006, 16, 485–494. [Google Scholar] [CrossRef]
- Khaosaad, T.; Vierheilig, H.; Nell, M.; Zitterl-Eglseer, K.; Novak, J. Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 2006, 16, 443–446. [Google Scholar] [CrossRef]
- Liu, Y.; Jian, J.; Xu, L.; Meng, L.; Yang, F.; Li, S.; Yan, J. Arbuscular mycorrhizal fungi change the growth and metabolites of Perilla frutescens, with subsequent effects on the development and behavior of Spodoptera exigua. J. Agric. Food Chem. 2025, 73, 18186–18197. [Google Scholar] [CrossRef]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Habibi Machiani, R.; Sadeghpour, A. Arbuscular mycorrhizal fungi and changes in primary and secondary metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef]
- Rahmat, S.; Soheilikhah, Z. Arbuscular Mycorrhizal Fungi and Plant Secondary Metabolism. In Arbuscular Mycorrhizal Fungi and Higher Plants; Ahammed, G.I., Hajiboland, R., Eds.; Springer Nature: Singapore, 2024; pp. 99–120. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Cutler, G.C. Insecticide-induced hormesis and arthropod pest management. Pest Manag. Sci. 2013, 70, 690–697. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, H.; Li, J.; Wang, C.; Li, C.; Gao, Y. Sublethal effects of imidacloprid on the population development of western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Insects 2019, 10, 3. [Google Scholar] [CrossRef]
- Alves, D.S.; Machado, A.R.T.; Campos, V.A.C.; Oliveira, D.F.; Carvalho, G.A. Selection of Annonaceae species for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae) and metabolic Profiling of Duguetia lanceolata using nuclear magnetic resonance spectroscopy. J. Econ. Entomol. 2016, 109, 649–659. [Google Scholar] [CrossRef]
- Ross, D.C.; Brown, T.M. Inhibition of larval growth in Spodoptera frugiperda by sublethal dietary concentrations of insecticides. J. Agric. Food Chem. 1982, 30, 193–196. [Google Scholar] [CrossRef]
- Tasei, J.N.; Sabik, H.; Pirastru, L.; Langiu, E.; Blanche, J.M.; Fournier, J.; Taglioni, J.P. Effects of sublethal doses of Deltamethrin (Decis CE) on Bombus terrestris. J. Apic. Res. 1994, 33, 129–135. [Google Scholar] [CrossRef]
- Medina, C.; Mutis, A.; Bardehle, L.; Hormazabal, E.; Borie, F.; Aguilera, P.; Ortega, F.; Quiroz, A. Arbuscular mycorrhizal fungi enhance monoterpene production in red clover (Trifolium pratense L.): A potential tool for pest control. Nat. Prod. Res. 2023, 37, 981–984. [Google Scholar] [CrossRef]
- Champy, P.; Höglinger, G.U.; Féger, J.; Gleye, C.; Hocquemiller, R.; Laurens, A.; Guérineau, V.; Laprévote, O.; Medja, F.; Lombès, A.; et al. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: Possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem. 2004, 88, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Medina, J.F.; Durán-Prado, A.; Peña del Río, M.Á.; Grageda-Cabrera, O.; Irizar-Garza, M.B.G. Micorriza INIFAP: Biofertilizante para el campo mexicano. In Introducción al Uso y Manejo de Los Biofertilizantes en la Agricultura; Aguado-Santacruz, G.A., Ed.; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP): Celaya, Guanajuato, México, 2012; pp. 219–244. ISBN 978-607-425-861-5. [Google Scholar]
- Trinidad-Cruz, J.R.; Quiñones-Aguilar, E.E.; Hernández-Cuevas, L.V.; López-Pérez, L.; Rincón-Enríquez, G. Hongos micorrízicos arbusculares asociados a la rizósfera de Agave cupreata en regiones mezcaleras del estado de Michoacán, México. Sci. Fungorum 2017, 45, 13–25. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Ryckman, R.E. Laboratory culture of the Triatominae with observations on behavior and a new feeding device. J. Parasitol. 1952, 38, 210–214. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Official Mexican Standard. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Diario Oficial de la Federación: Mexico City, México, 2001. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 27 January 2025).
- Quiñones-Aguilar, E.; Hernández-Hernández, C.; Rincón-Enríquez, G.; López-Pérez, L.; Lobit, P.; Enríquez-Vara, J. Arbuscular mycorrhizal fungi influence on growth of creole maize and larval development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Trop. Subtrop. Agroecosyst. 2023, 26, 2. [Google Scholar] [CrossRef]
- Hardke, J.; Temple, J.; Leonard, B. Laboratory toxicity and field efficacy of selected insecticides against fall armyworm (Lepidoptera: Noctuidae). Fla. Entomol. 2011, 94, 272–278. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 31 July 2025).
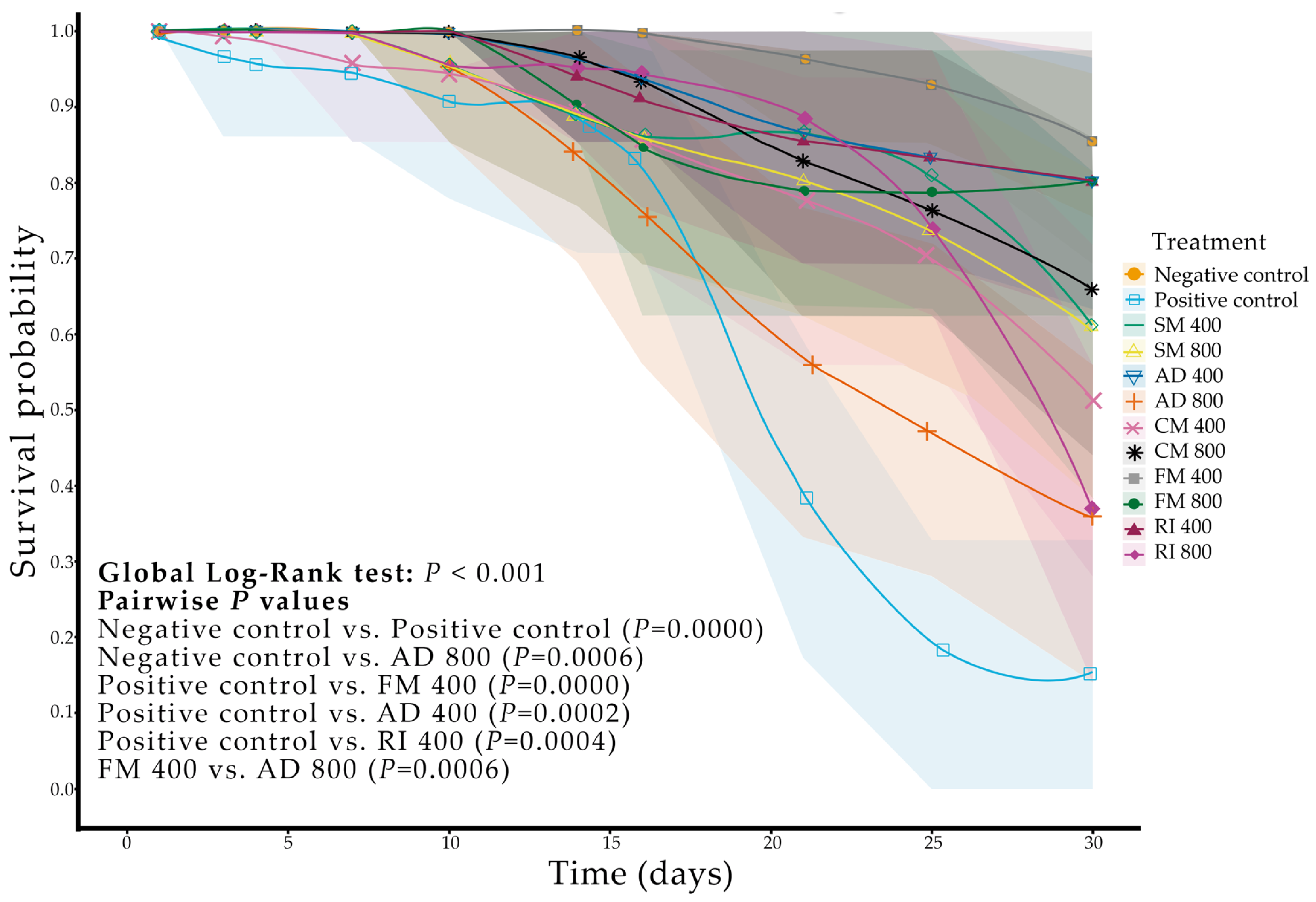
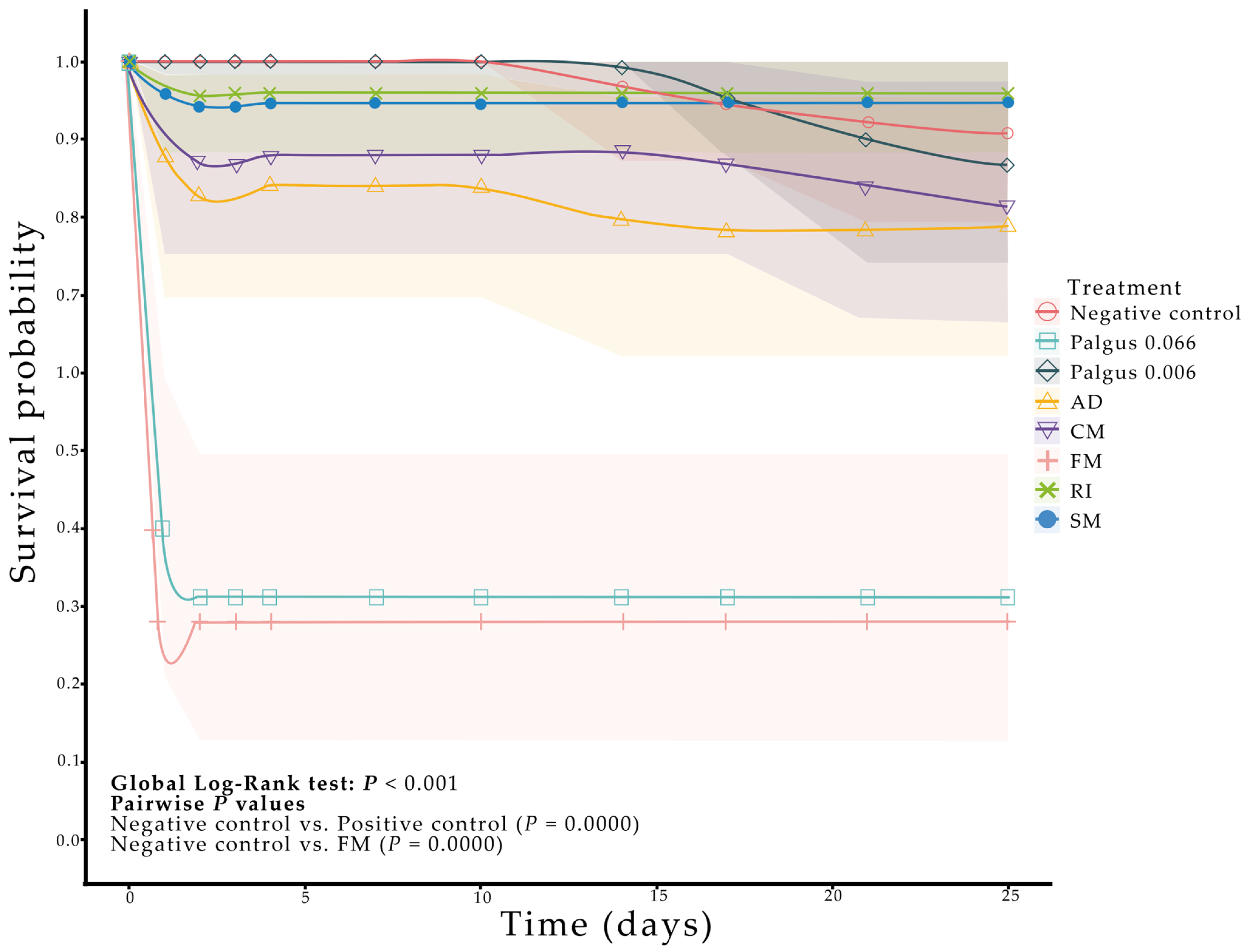
| Treatment † | Extract Concentration (mg·mL−1) | Mortality †† | Egg Number ‡ | Annonacin Concentration ‡‡ (µg·g−1 DW) | Colonization § (%) | ||
|---|---|---|---|---|---|---|---|
| n (♀ + ♂) | (%) | Mean (n) | Total (n) | ||||
| Negative control | -- | 3 (2 + 1) | 15 ± 8.0 | 51.9 ± 35.9 (10) | 519 (10) | -- | -- |
| Positive control | 100 ng | 17 (10 + 7) | 85 ± 8.0 * | 25.5 ± 12.7 (6) | 307 (6) | -- | -- |
| AD | 400 | 4 (2 + 1) | 20 ± 8.9 | 43.9 ± 40.4 (10) | 439 (10) | 1016.0 ± 124.5 ab | 7.6 ± 3.0 cd |
| 800 | 13 (5 + 7) | 65 ± 10.6 * | 42.3 ± 26.1 (9) | 381 (9) | |||
| CM | 400 | 10 (4 + 5) | 50 ± 11.2 | 54.3 ± 26.6 (8) | 489 (8) | 761.8 ± 154.3 ab | 27.8 ± 5.7 ab |
| 800 | 7 (3 + 3) | 35 ± 10.2 | 54.8 ± 35.6 (9) | 494 (9) | |||
| RI | 400 | 5 (3 + 1) | 25 ± 9.7 | 39.6 ± 27.1 (10) | 396 (10) | 1209.0 ± 272.7 a | 41.0 ± 6.4 a |
| 800 | 13 (8 + 5) | 65 ± 10.6 * | 56.4 ± 34.4 (10) | 564 (10) | |||
| FM | 400 | 3 (2 + 1) | 15 ± 8.0 | 66.8 ± 34.0 (10) | 602 (10) | 997.6 ± 130.8 ab | 18.0 ± 6.5 bc |
| 800 | 5 (3 + 2) | 25 ± 9.7 | 39.6 ± 22.3 (10) | 436 (10) | |||
| SM | 400 | 8 (2 + 5) | 40 ± 11.0 | 53.8 ± 26.3 (9) | 485 (9) | 705.1 ± 47.5 b | 2.2 ± 1.8 d |
| 800 | 8 (5 + 2) | 40 ± 11.0 | 40.1 ± 23.4 (9) | 361 (9) | |||
| Treatments † | Weight (mg) | Time (Days) | Mortality †† (%) | ||
|---|---|---|---|---|---|
| Larvae ‡ (n) | Pupa (n) | Larvae to Pupa | Pupa to Adult | ||
| Negative control | 11.3 ± 1.2 (22) a | 227.7 ± 6.8 (19) b | 19.0 ± 0.46 b | 11.0 ± 0.28 a | 12 ± 6.5 |
| Positive control 0.0066 | 53.9 ± 4.2 (25) b | 196.5 ± 5.3 (20) a | 17.5 ± 0.55 c | 11.7 ± 0.40 a | 0 |
| Positive control 0.066 | 0 ± 0 (0) | 0 ± 0 (0) | 0 ± 0 | 0 ± 0 | 100 ± 0 * |
| AD | 9.0 ± 1.3 (15) a | 228.9 ± 9.4 (14) b | 20.1 ± 0.40 a | 11.4 ± 0.28 a | 40 ± 9.8 |
| CM | 8.2 ± 1.2 (19) a | 231.8 ± 7.2 (16) b | 17.8 ± 0.26 c | 12.2 ± 0.28 a | 24 ± 8.5 |
| FM | 44.1 ± 3.7 (7) b | 204.4 ± 9.3 (6) ab | 15.8 ± 0.47 d | 12.3 ± 0.33 a | 72 ± 9.0 * |
| RI | 12.0 ± 2.1 (14) a | 235.9 ± 9.0 (14) b | 20 ± 0.40 a | 10.1 ± 0.24 b | 44 ± 9.9 |
| SM | 12.3 ± 1.2 (19) a | 231.8 ± 4.2 (18) b | 18.8 ± 0.30 ab | 11.0 ± 0.27 a | 24 ± 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-López, A.M.; Quiñones-Aguilar, E.E.; Enríquez-Vara, J.N.; Martínez-Ibarra, J.A.; Rincón-Enríquez, G. Arbuscular Mycorrhizal Fungi Enhance the Insecticidal Activity of Annona muricata L. Leaves. Plants 2025, 14, 3501. https://doi.org/10.3390/plants14223501
González-López AM, Quiñones-Aguilar EE, Enríquez-Vara JN, Martínez-Ibarra JA, Rincón-Enríquez G. Arbuscular Mycorrhizal Fungi Enhance the Insecticidal Activity of Annona muricata L. Leaves. Plants. 2025; 14(22):3501. https://doi.org/10.3390/plants14223501
Chicago/Turabian StyleGonzález-López, Angela Michelle, Evangelina Esmeralda Quiñones-Aguilar, Jhony Navat Enríquez-Vara, José Alejandro Martínez-Ibarra, and Gabriel Rincón-Enríquez. 2025. "Arbuscular Mycorrhizal Fungi Enhance the Insecticidal Activity of Annona muricata L. Leaves" Plants 14, no. 22: 3501. https://doi.org/10.3390/plants14223501
APA StyleGonzález-López, A. M., Quiñones-Aguilar, E. E., Enríquez-Vara, J. N., Martínez-Ibarra, J. A., & Rincón-Enríquez, G. (2025). Arbuscular Mycorrhizal Fungi Enhance the Insecticidal Activity of Annona muricata L. Leaves. Plants, 14(22), 3501. https://doi.org/10.3390/plants14223501





