Encapsulation and Digestive Evaluation of Infusion Extracts from Semi-Desert Mexican Plants: Phytochemical Profiling and Bioactivities
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Studied Plants
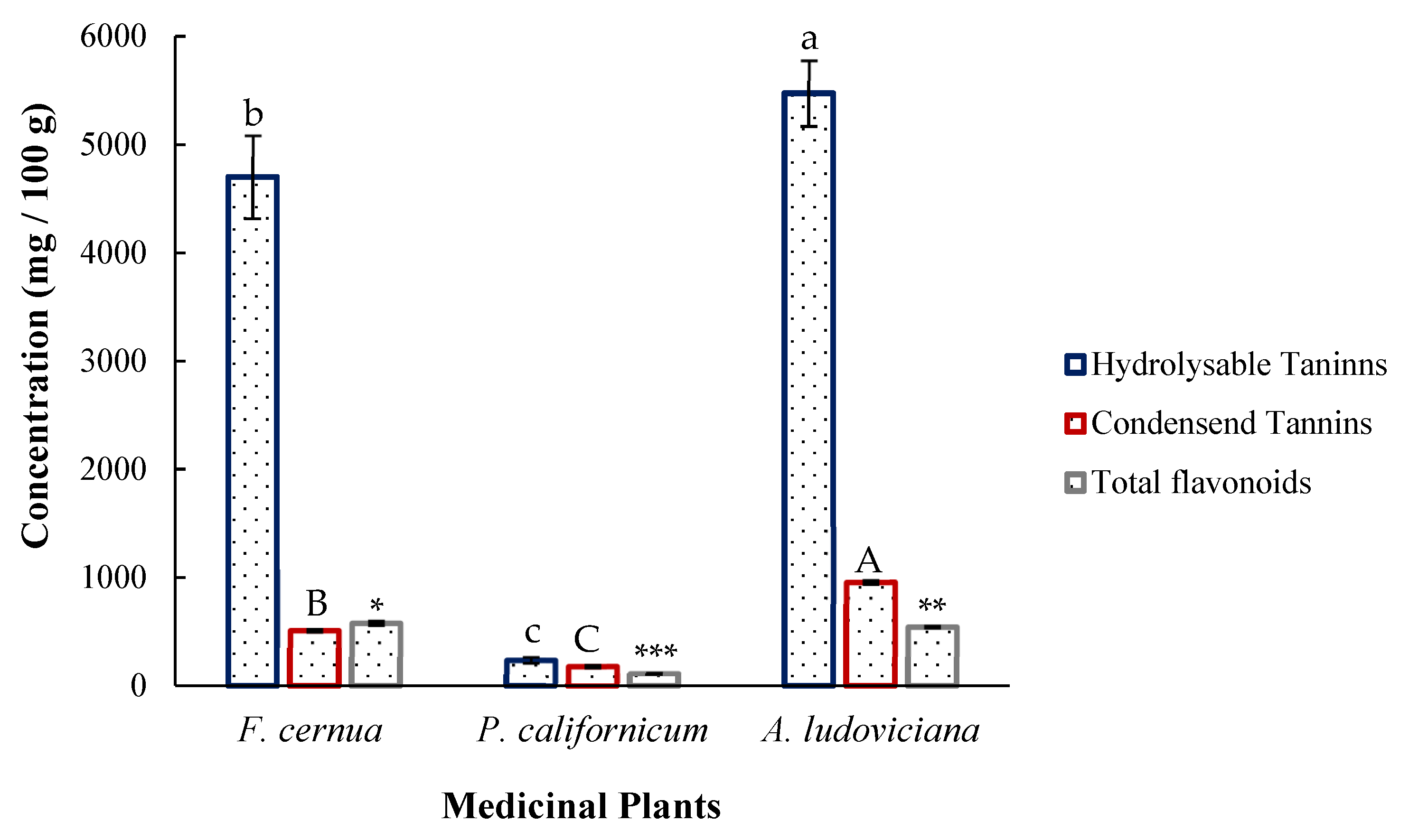
2.2. Phytochemical Composition
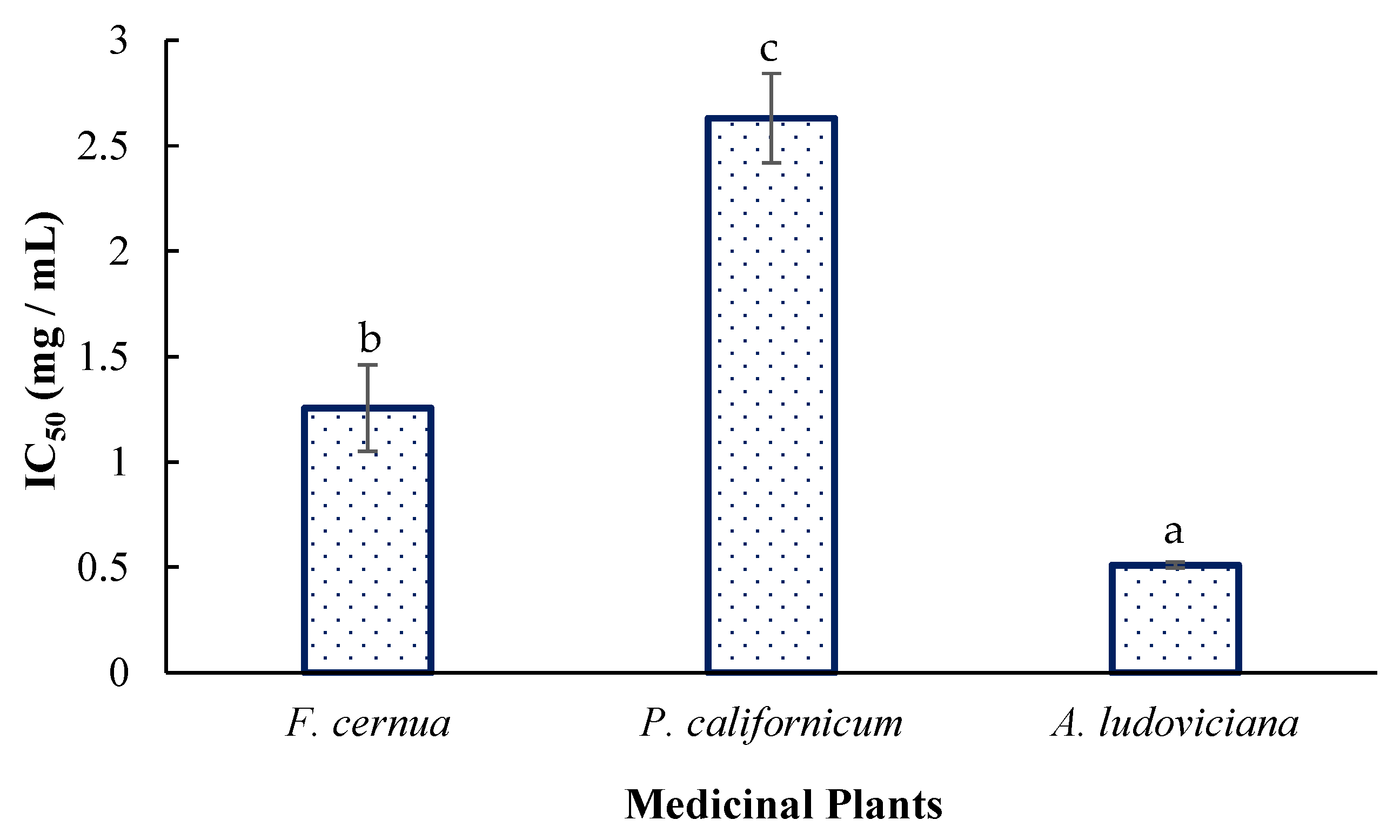
2.3. Free Radical–Scavenging Activity
2.4. Antiparasitic Activity
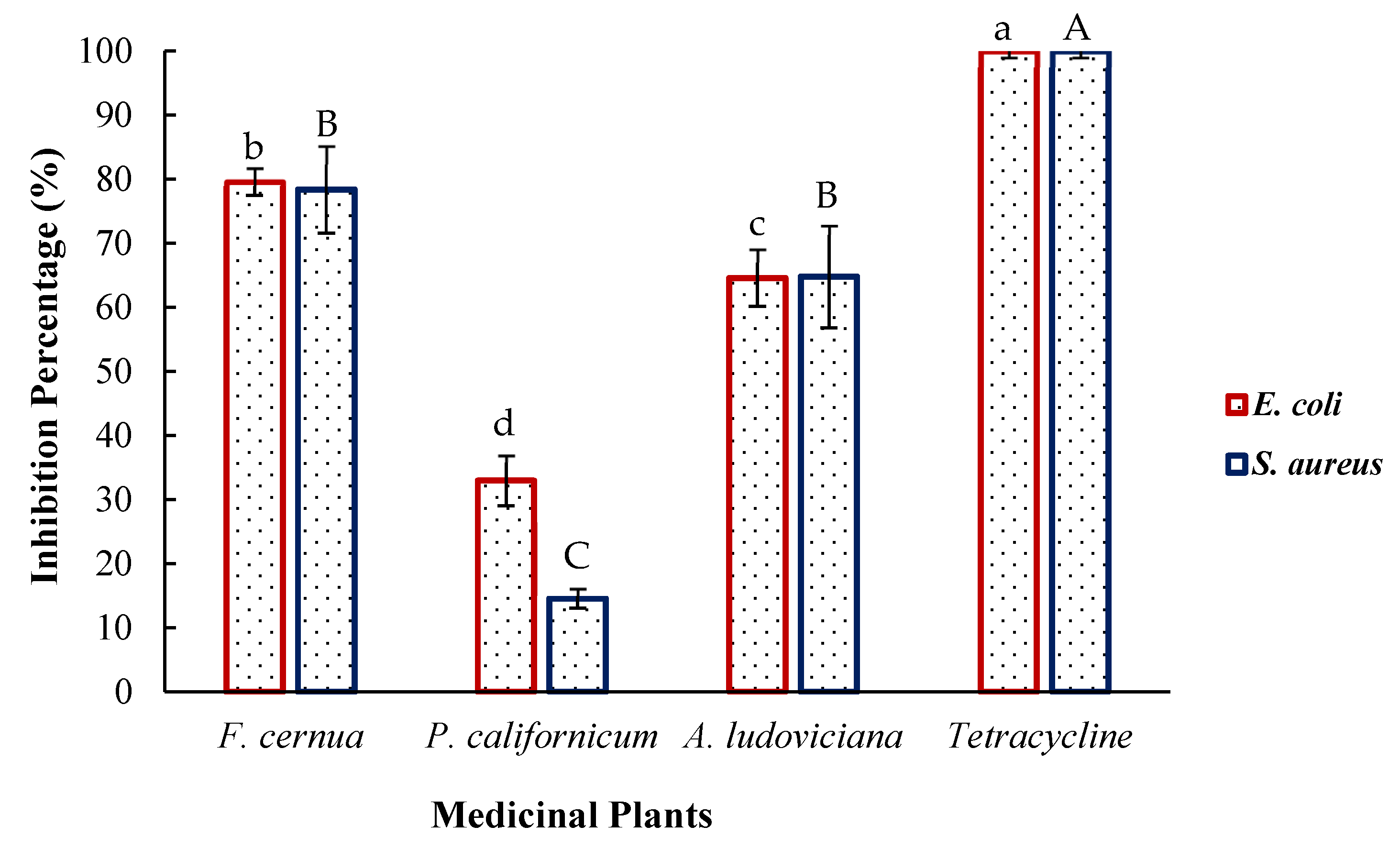
2.5. Inhibition of Bacterial Growth
2.6. HPLC–MS Analysis
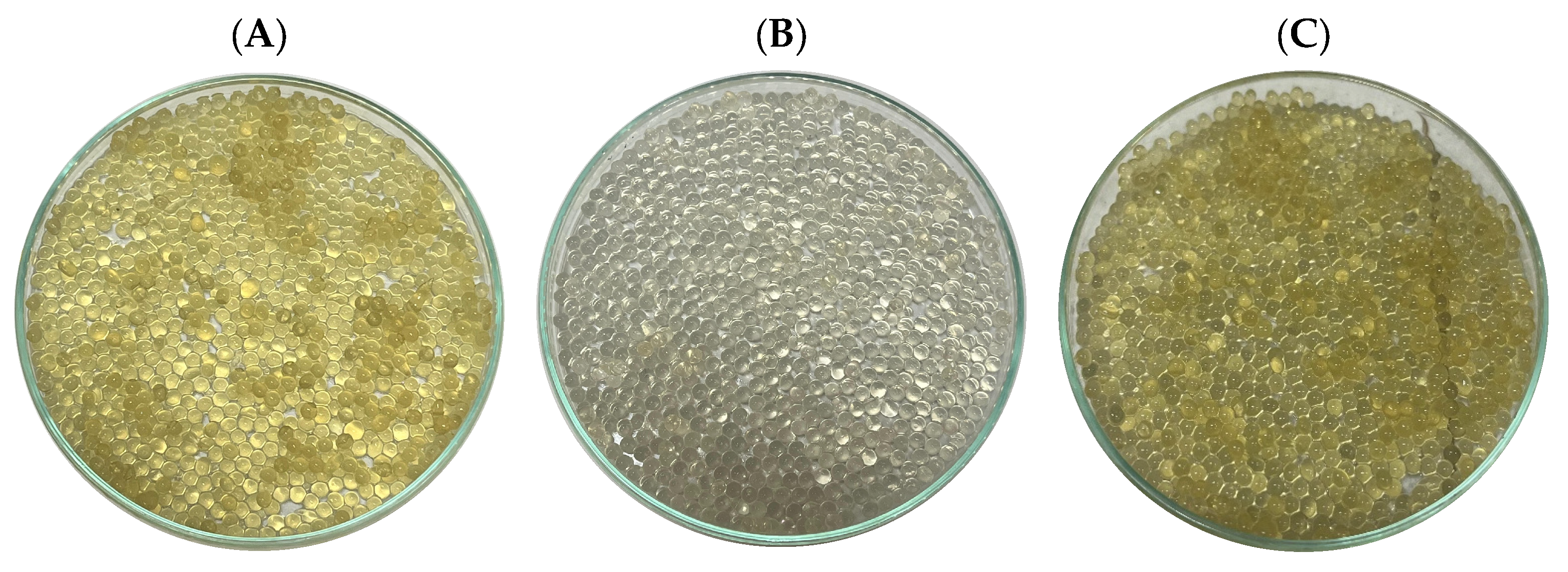
2.7. Agar-Agar Beads
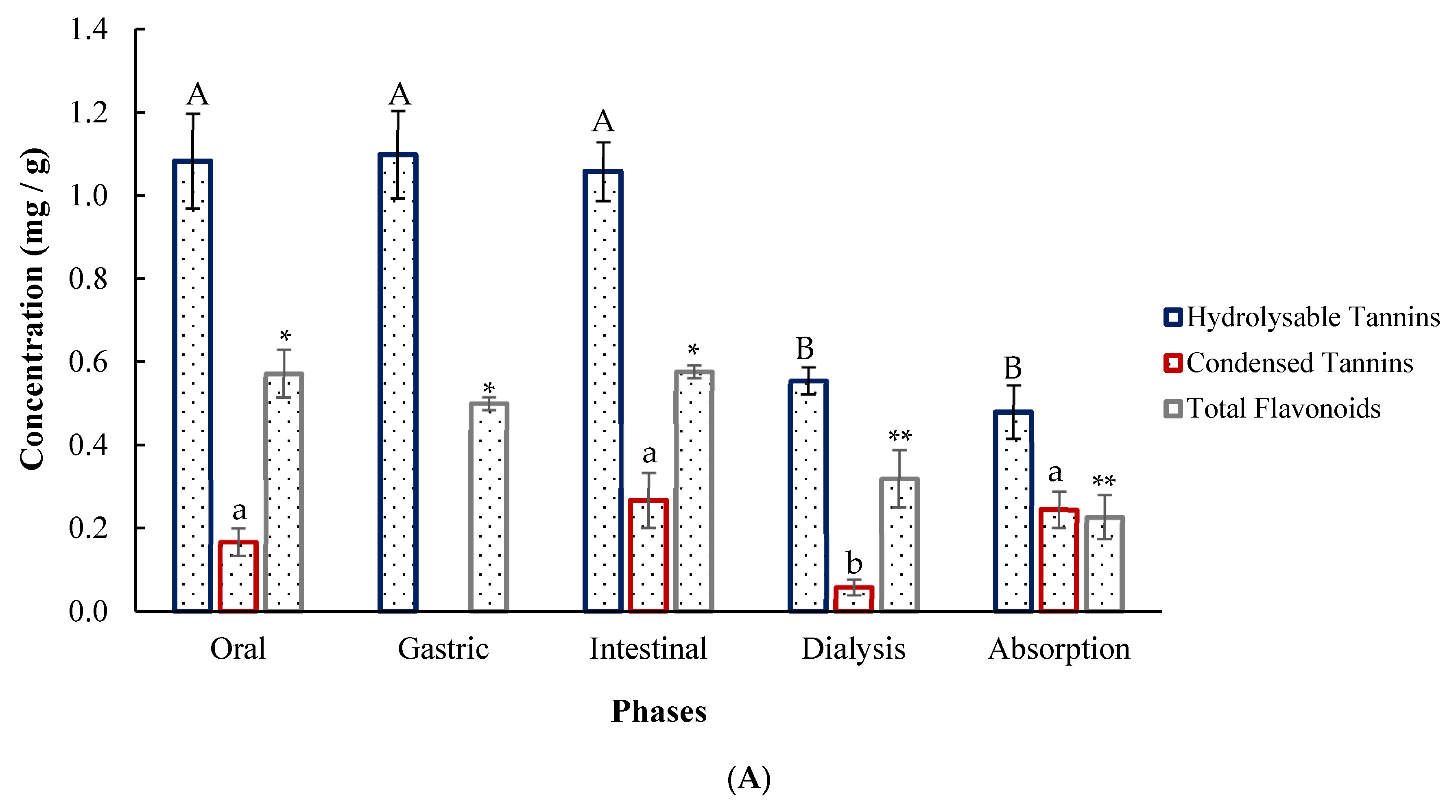
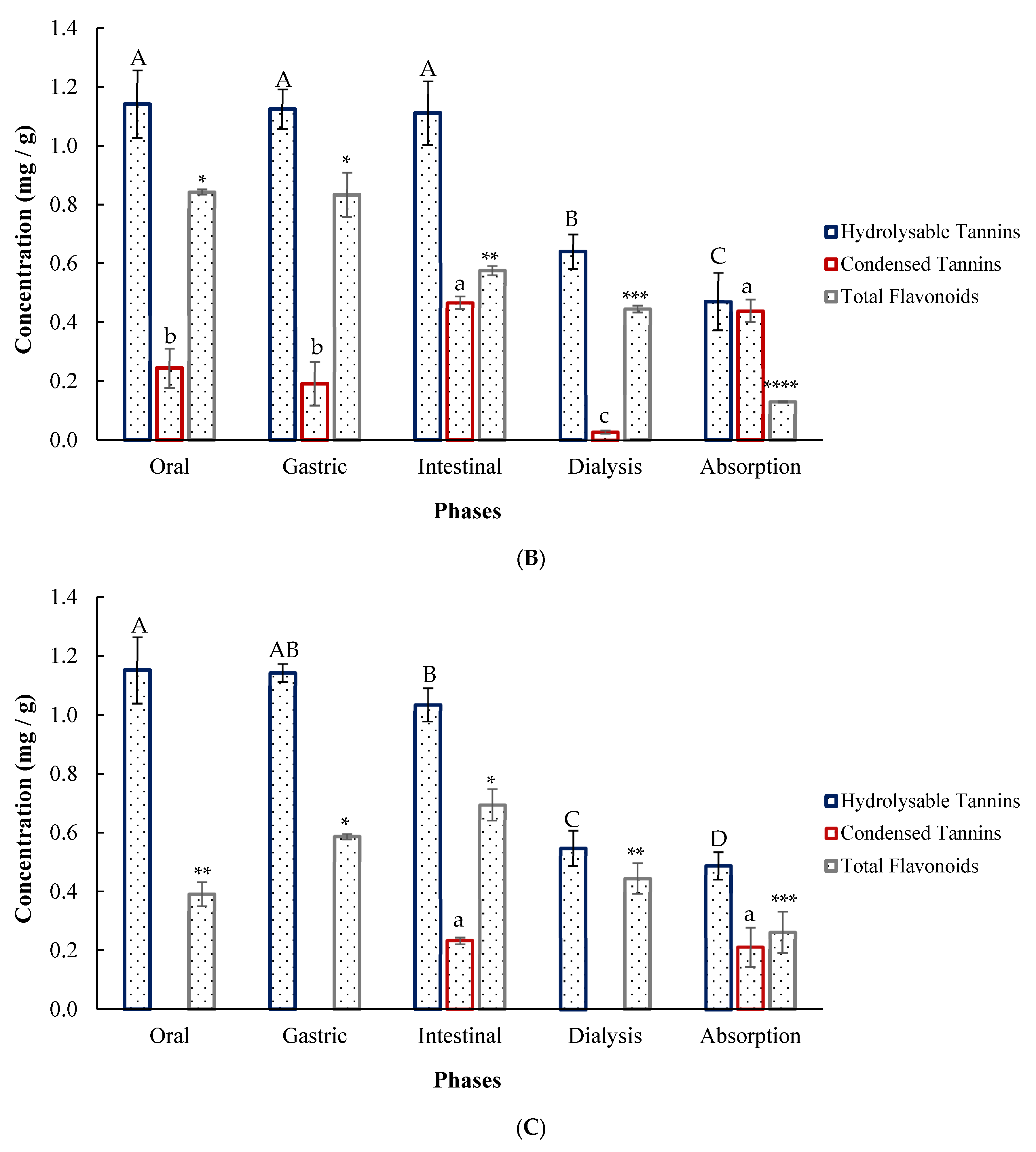
2.8. In Vitro Digestion of Agar-Agar Encapsulates
3. Discussion
3.1. Proximal Composition
3.2. Phytochemical Content
3.3. Antioxidant Activity
3.4. Parasiticidal Activity
3.5. Bactericidal Effect
3.6. Identification of Phytochemicals
3.7. Simulated Digestion of Encapsulates
4. Materials and Methods
4.1. Plant Material Pretreatment and Extraction
4.2. Proximal Composition of Medicinal Plants
4.3. Phytochemicals Quantification
4.3.1. Hydrolysable Tannins
4.3.2. Condensed Tannins
4.3.3. Total Flavonoids
4.4. Antioxidant Activity
4.4.1. DPPH Assay
4.4.2. ABTS Assay
4.5. Antileishmanial Activity
4.6. Antibacterial Activity
Spectrophotometric Broth Microdilution Method
4.7. HPLC-MS Analysis
4.8. Encapsulation
4.9. In Vitro Simulated Gastrointestinal Digestion
4.9.1. Simulated Oral Digestion
4.9.2. Simulated Gastric Digestion
4.9.3. Simulated Intestinal Digestion
4.9.4. Small Intestine Absorption—Dialysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IC50 | Media Inhibitory Concentration |
| CFU | Colony-Forming Unit |
| MIC | Minimum Inhibitory Concentration |
| OD | Optical Density |
| HPLC-MS | High-Performance Liquid Chromatography–Mass Spectrometry |
References
- Chamorro-Cevallos, G.; Mojica-Villegas, M.A.; García-Martínez, Y.; Pérez-Gutiérrez, S.; Madrigal-Santillán, E.; Vargas-Mendoza, N.; Morales-González, J.A.; Cristóbal-Luna, J.M. A Complete Review of Mexican Plants with Teratogenic Effects. Plants 2022, 11, 1675. [Google Scholar] [CrossRef]
- De La Cruz-Jiménez, L.; Hernández-Torres, M.A.; Monroy-García, I.N.; Rivas-Morales, C.; Verde-Star, M.J.; Gonzalez-Villasana, V.; Viveros-Valdez, E. Biological Activities of Seven Medicinal Plants Used in Chiapas, Mexico. Plants 2022, 11, 1790. [Google Scholar] [CrossRef]
- Julián-Flores, A.; Aguilar-Zárate, P.; Michel, M.R.; Sepúlveda-Torre, L.; Torres-León, C.; Aguilar, C.N.; Chávez-González, M.L. Exploring the Therapeutic Potential of Medicinal Plants in the Context of Gastrointestinal Health: A Review. Plants 2025, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Rebolledo Ramírez, F.; Aguirre-Joya, J.A.; Ramírez-Moreno, A.; Chávez-González, M.L.; Aguillón-Gutierrez, D.R.; Camacho-Guerra, L.; Ramírez-Guzmán, N.; Hernández Vélez, S.; Aguilar, C.N. Medicinal plants used by rural communities in the arid zone of Viesca and Parras Coahuila in northeast Mexico. Saudi Pharm. J. 2023, 31, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Palma-Wong, M.; Ascacio-Valdés, J.A.; Ramírez-Guzmán, N.; Aguirre-Joya, J.A.; Flores-Loyola, E.; Ramírez-Moreno, A.; Torres-León, C. Exploration of phenolic content and antioxidant potential from plants used in traditional medicine in Viesca, Mexico. Horticulturae 2023, 9, 1252. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Salas-Méndez, E.D.J.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.L.V.; Sáenz-Galindo, A.; Carrillo-Lomelí, D.A. Antifungal activity in vitro of ethanol and aqueous extracts of leaves and branches of Flourensia spp. against postharvest fungi. Ind. Crops Prod. 2017, 105, 41–49. [Google Scholar] [CrossRef]
- Assanga, S.B.I.; Luján, L.M.L.; Ruiz, J.C.G.; McCarty, M.F.; Cota-Arce, J.M.; Espinoza, C.L.L.; Ángulo, D.F. Comparative analysis of phenolic content and antioxidant power between parasitic Phoradendron californicum (toji) and their hosts from Sonoran Desert. Results Chem. 2020, 2, 100079. [Google Scholar] [CrossRef]
- Kamarauskaite, J.; Baniene, R.; Raudone, L.; Vilkickyte, G.; Vainoriene, R.; Motiekaityte, V.; Trumbeckaite, S. Antioxidant and mitochondrion-targeted activity of caffeoylquinic-acid-rich fractions of wormwood (Artemisia absinthium L.) and silver wormwood (Artemisia ludoviciana Nutt.). Antioxidants 2021, 10, 1405. [Google Scholar] [CrossRef]
- Moura, H.F.S.; Dias, F.D.S.; e Souza, L.B.S.; de Magalhães, B.E.A.; Tannus, C.D.A.; de Carvalho, W.C.; Brandão, G.C.; dos Santos, W.N.L.; Korn, M.G.A.; dos Santos, D.C.M.B.; et al. Evaluation of multielement/proximate composition and bioactive phenolics contents of unconventional edible plants from Brazil using multivariate analysis techniques. Food Chem. 2021, 363, 129995. [Google Scholar] [CrossRef]
- Ferreira, C.P.; de Lima, M.D.C.; da Silva, J.G.; Araujo, N.M.P. Nutritional composition, phenolic compounds and biological activities of selected unconventional food plants. Food Res. Int. 2024, 191, 114643. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Olivera-Montenegro, L. Encapsulation of bioactive compounds for food and agricultural applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of bioactive compounds from fruit and vegetable byproducts for food application—A review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Nagar, E.E.; Okun, Z.; Shpigelman, A. In vitro bioaccessibility of polyphenolic compounds: The effect of dissolved oxygen and bile. Food Chem. 2023, 404, 134490. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Interactions between blackcurrant polyphenols and food macronutrients in model systems: In vitro digestion studies. Foods 2021, 10, 847. [Google Scholar] [CrossRef]
- Gómez-García, R.; Vilas-Boas, A.A.; Machado, M.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Impact of simulated in vitro gastrointestinal digestion on bioactive compounds, bioactivity and cytotoxicity of melon (Cucumis melo L. inodorus) peel juice powder. Food Biosci. 2022, 47, 101726. [Google Scholar] [CrossRef]
- Aranda-Ledezma, N.E. Caracterización Avanzada de Candelilla y Otras Plantas de Semidesierto Mexicano. Master’s Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 2022. [Google Scholar]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef]
- González-González, G. Revalorización de Plantas Medicinales a Través del Conocimiento, Creencias y Prácticas de las Mujeres de la Localidad Benito Juárez, Estado de México y por su Actividad Antioxidante. Master’s Thesis, Universidad Autónoma del Estado de México, Toluca, Mexico, 2021. [Google Scholar]
- Hernández-García, A. Evaluación de Actividad Antimicrobiana (In Vitro) de Extractos Vegetales de Artemisa ludoviciana Nutt., Lepidium virginicum L. y Brickellia veronicifolia (kunth) A. Gray Sobre Bacterias y Hongos Fitopatógenos. Master’s Thesis, Universidad Autónoma del Estado de Querétaro, Santiago de Querétaro, Mexico, 2023. [Google Scholar]
- Alvarez-Perez, O.B.; Ventura-Sobrevilla, J.M.; Ascacio-Valdes, J.A.; Rojas, R.; Verma, D.K.; Aguilar, C.N. Valorization of Flourensia cernua DC as source of antioxidants and antifungal bioactives. Ind. Crops Prod. 2020, 152, 112422. [Google Scholar] [CrossRef]
- Aguirre-García, Y.L.; Castillo-Manzanares, A.; Palomo-Ligas, L.; Ascacio-Valdés, J.A.; Campos-Múzquiz, L.G.; Esparza-González, S.C.; Nery-Flores, S.D. Toxicity evaluation of a polyphenolic extract from Flourensia cernua DC through Artemia lethality assay, hemolytic activity, and acute oral test. J. Toxicol. 2024, 2024, 2970470. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Ballesteros-Monrreal, M.G.; Leyva, M.; Montaño-Leyva, B.; Aguilar-Martinez, M.; Rivera, D.E.V. Antioxidant, antiproliferative and antibacterial activity of Phoradendron californicum extracts; a parasitic plant from northwestern Mexico. Biotecnia 2024, 26, e2286. [Google Scholar] [CrossRef]
- de Rodríguez, D.J.; Torres-Moreno, H.; López-Romero, J.C.; Vidal-Gutiérrez, M.; Villarreal-Quintanilla, J.Á.; Carrillo-Lomelí, D.A.; Vilegas, W. Antioxidant, anti-inflammatory, and antiproliferative activities of Flourensia spp. Biocatal. Agric. Biotechnol. 2023, 47, 102552. [Google Scholar] [CrossRef]
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential oil and phenolic compounds of Artemisia herba-alba (Asso.): Composition, antioxidant, antiacetylcholinesterase, and antibacterial activities. Int. J. Food Prop. 2016, 19, 1425–1438. [Google Scholar] [CrossRef]
- Wubuli, A.; Abdulla, R.; Zhao, J.; Wu, T.; Aisa, H.A. Exploring anti-inflammatory and antioxidant-related quality markers of Artemisia absinthium L. based on spectrum–effect relationship. Phytochem. Anal. 2024, 35, 1152–1173. [Google Scholar] [CrossRef] [PubMed]
- Baldemir, A.; Karaman, Ü.; İlgün, S.; Kacmaz, G.; Demirci, B. Antiparasitic efficacy of Artemisia ludoviciana Nutt. (Asteraceae) essential oil for Acanthamoeba castellanii, Leishmania infantum and Trichomonas vaginalis. Indian J. Pharm. Educ. Res. 2018, 52, 3. [Google Scholar] [CrossRef]
- Ezeta-Miranda, A.; Vera-Montenegro, Y.; Avila-Acevedo, J.G.; García-Bores, A.M.; Estrella-Parra, E.A.; Francisco-Marquez, G.; Ibarra-Velarde, F. Efficacy of purified fractions of Artemisia ludoviciana Nutt. mexicana and ultrastructural damage to newly excysted juveniles of Fasciola hepatica in vitro. Vet. Parasitol. 2020, 285, 109184. [Google Scholar] [CrossRef]
- de Rodríguez, D.J.; Victorino-Jasso, M.C.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Díaz-Jiménez, L.; Rodríguez-García, R.; Flores-López, M.L. Flourensia retinophylla: An outstanding plant from northern Mexico with antibacterial activity. Ind. Crops Prod. 2022, 185, 115120. [Google Scholar] [CrossRef]
- Palacios-Espinosa, J.F.; Núñez-Aragón, P.N.; Gomez-Chang, E.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of Artemisia ludoviciana subsp. mexicana and two of its bioactive components, Estafiatin and Eupatilin. Molecules 2021, 26, 3654. [Google Scholar] [CrossRef]
- Romero, J.L.G.; Sosa, C.M.P.; Burgoa, G.L.; Leal, A.C.L.; El Kassis, E.G.; Rodríguez, E.B.; Juárez, Z.N. Antimycobacterial, cytotoxic, and anti-inflammatory activities of Artemisia ludoviciana. J. Ethnopharmacol. 2022, 293, 115249. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Mandal, M.K.; Domb, A.J. Antimicrobial activities of natural bioactive polyphenols. Pharmaceutics 2024, 16, 718. [Google Scholar] [CrossRef]
- Usme-Duque, L.K.; Claudio-Rizo, J.A.; Nuncio-Esquivel, J.A.; León-Campos, M.I.; Cruz-Requena, M.; Ríos-González, L.J.; Ascacio-Valdés, J.A.; Medina-Morales, M.A. Optimization of fungal fermentation for the extraction of polyphenols from Flourensia cernua and its effect on cellular metabolism. J. Biotechnol. 2025, 401, 60–73. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Rahmani, A.H. Apigenin: A bioflavonoid with a promising role in disease prevention and treatment. Biomedicines 2024, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, H.; Mahant, S.; Sharma, A.K.; Kumar, D.; Dua, K.; Chellappan, D.K.; Kapoor, D.N. Exploring the therapeutic potential of naturally occurring piceatannol in noncommunicable diseases. Biofactors 2024, 50, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Moćko, P.; Śladowska, K.; Kawalec, P.; Babii, Y.; Pilc, A. The potential of scopolamine as an antidepressant in major depressive disorder: A systematic review of randomized controlled trials. Biomedicines 2023, 11, 2636. [Google Scholar] [CrossRef] [PubMed]
- Boulebd, H.; Carmena-Bargueño, M.; Pérez-Sánchez, H. Exploring the antioxidant properties of caffeoylquinic and feruloylquinic acids: A computational study on hydroperoxyl radical scavenging and xanthine oxidase inhibition. Antioxidants 2023, 12, 1669. [Google Scholar] [CrossRef]
- Da Silva, A.P.G.; Sganzerla, W.G.; John, O.D.; Marchiosi, R. A comprehensive review of the classification, sources, biosynthesis, and biological properties of hydroxybenzoic and hydroxycinnamic acids. Phytochem. Rev. 2025, 24, 1061–1090. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Almatroodi, S.A. Myricetin: A significant emphasis on its anticancer potential via the modulation of inflammation and signal transduction pathways. Int. J. Mol. Sci. 2023, 24, 9665. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Vazquez-Flores, A.A.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Medina Campos, O.N.; Ávila-Nava, A.; González-Reyes, S.; Pedraza-Chaverri, J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J. Funct. Foods 2014, 7, 219–228. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate quantification of total phenolic content from plant extracts obtained by conventional and ultrasound methods. Phytochem. Anal. 2014, 25, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Ledesma, N.E.; Aguilar-Zárate, P.; Bautista-Hernández, I.; Rojas, R.; Robledo-Jiménez, C.L.; Martínez-Ávila, G.C.G. The optimization of ultrasound-assisted extraction for bioactive compounds from Flourensia cernua and Jatropha dioica and the evaluation of their functional properties. Horticulturae 2024, 10, 709. [Google Scholar] [CrossRef]
- Pereira, P.S.; Oliveira, C.V.B.; Maia, A.J.; Vega-Gomez, M.C.; Rolón, M.; Coronel, C.; Silva, T.G. Evaluation of the in vitro antiparasitic effect of the essential oil of Cymbopogon winterianus and its chemical composition analysis. Molecules 2022, 27, 2753. [Google Scholar] [CrossRef]
- Haq, F.U.; Imran, M.; Saleem, S.; Aftab, U.; Ghazal, A. Investigation of Morchella esculenta and Morchella conica for their antibacterial potential against methicillin-susceptible Staphylococcus aureus, methicillin-resistant Staphylococcus aureus and Streptococcus pyogenes. Arch. Microbiol. 2022, 204, 391. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef]
| Component (%) | A. ludoviciana | F. cernua | P. californicum |
|---|---|---|---|
| Moisture | 14.00 ± 0.00 a | 0.80 ± 0.00 b | 0.40 ± 0.00 b |
| Fat | 6.14 ± 0.59 a | 6.99 ± 1.11 a | 3.22 ± 0.10 b |
| Fiber | 13.65 ± 0.20 b | 8.58 ± 0.20 c | 16.35 ± 0.15 a |
| Protein | 3.65 ± 0.33 ab | 1.17 ± 1.08 b | 5.84 ± 2.60 a |
| Ash | 9.7 ± 0.00 b | 9.19 ± 0.01 b | 13.46 ± 0.02 a |
| Carbohydrates | 52.86 ± 0.00 c | 73.27 ± 0.00 a | 60.73 ± 0.00 b |
| Assay | A. ludoviciana | F. cernua | P. californicum |
|---|---|---|---|
| DPPH | 2294.29 ± 307.83 c | 1134.56 ± 100.65 b | 74.18 ± 18.43 a |
| ABTS | 2973.36 ± 304.29 b | 2926.36 ± 193.85 b | 333.38 ± 56.36 a |
| Plant | Retention Time (min) | Mass [M−H]− | Compound | Family |
|---|---|---|---|---|
| Artemisia ludoviciana | 5.238 | 317 | Myricetin | Flavonols |
| 7.582 | 352.9 | 1-Caffeoylquinic acid | Hydroxycinnamic acids | |
| 32.039 | 515 | 1,3-Dicaffeoylquinic acid | Hydroxycinnamic acids | |
| 44.778 | 327.1 | p-Coumaroyl tyrosine | Hydroxycinnamic acids | |
| Flourensia cernua | 5.289 | 341 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids |
| 19.413 | 353 | 1-Caffeoylquinic acid | Hydroxycinnamic acids | |
| 21.569 | 623 | Isorhamnetin 3-O-glucoside 7-O-rhamnoside | Methoxyflavonols | |
| 24.263 | 563 | Apigenin arabinoside-glucoside | Flavones | |
| 43.875 | 243.1 | Piceatannol | Stilbenes | |
| Phoradendron californicum | 6.255 | 190.9 | Scopoletin | Hydroxycoumarins |
| 18.99 | 366.9 | 3-Feruloylquinic acid | Methoxycinnamic acids | |
| 24.31 | 366.9 | 4-Feruloylquinic acid | Methoxycinnamic acids |
| Plant | Phases | DPPH (μg TE/mL) | ABTS (μg TE/mL) |
|---|---|---|---|
| Artemisia ludoviciana | Oral | ND | ND |
| Gastric | ND | 60.77 ± 15.38 a | |
| Intestinal | ND | ND | |
| Dialysis | ND | ND | |
| Flourensia cernua | Oral | ND | ND |
| Gastric | ND | 99.23 ± 15.38 ab | |
| Intestinal | ND | 91.46 ± 3.85 a | |
| Dialysis | ND | 114.62 ± 0.8 b | |
| Phoradendron californicum | Oral | 81.53 ± 4.59 c | ND |
| Gastric | 109.03 ± 3.76 b | 75.15 ± 15.38 a | |
| Intestinal | 60.14 ± 9.66 a | 238.97 ± 42.89 b | |
| Dialysis | 55.69 ± 4.19 a | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julián-Flores, A.; Michel, M.R.; Aguilar, C.N.; da Silva, T.G.; Torres-León, C.; Ascacio-Valdés, J.A.; Sepúlveda, L.; Aguilar-Zárate, P.; Chávez-González, M.L. Encapsulation and Digestive Evaluation of Infusion Extracts from Semi-Desert Mexican Plants: Phytochemical Profiling and Bioactivities. Plants 2025, 14, 3448. https://doi.org/10.3390/plants14223448
Julián-Flores A, Michel MR, Aguilar CN, da Silva TG, Torres-León C, Ascacio-Valdés JA, Sepúlveda L, Aguilar-Zárate P, Chávez-González ML. Encapsulation and Digestive Evaluation of Infusion Extracts from Semi-Desert Mexican Plants: Phytochemical Profiling and Bioactivities. Plants. 2025; 14(22):3448. https://doi.org/10.3390/plants14223448
Chicago/Turabian StyleJulián-Flores, Antonio, Mariela R. Michel, Cristóbal N. Aguilar, Teresinha Gonçalves da Silva, Cristian Torres-León, Juan A. Ascacio-Valdés, Leonardo Sepúlveda, Pedro Aguilar-Zárate, and Mónica L. Chávez-González. 2025. "Encapsulation and Digestive Evaluation of Infusion Extracts from Semi-Desert Mexican Plants: Phytochemical Profiling and Bioactivities" Plants 14, no. 22: 3448. https://doi.org/10.3390/plants14223448
APA StyleJulián-Flores, A., Michel, M. R., Aguilar, C. N., da Silva, T. G., Torres-León, C., Ascacio-Valdés, J. A., Sepúlveda, L., Aguilar-Zárate, P., & Chávez-González, M. L. (2025). Encapsulation and Digestive Evaluation of Infusion Extracts from Semi-Desert Mexican Plants: Phytochemical Profiling and Bioactivities. Plants, 14(22), 3448. https://doi.org/10.3390/plants14223448











