The Development of Secretory Cavities in Zanthoxylum nitidum Leaves and the Pattern of Essential Oil Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Semi-Thin Sectioning
2.3. Extraction and Quantification of EO
2.4. GC-MS Analysis
2.5. FTIR Analysis
2.6. Hierarchical Cluster Analysis (HCA)
2.7. Principal Component Analysis (PCA)
2.8. Data Analysis
3. Results and Discussion
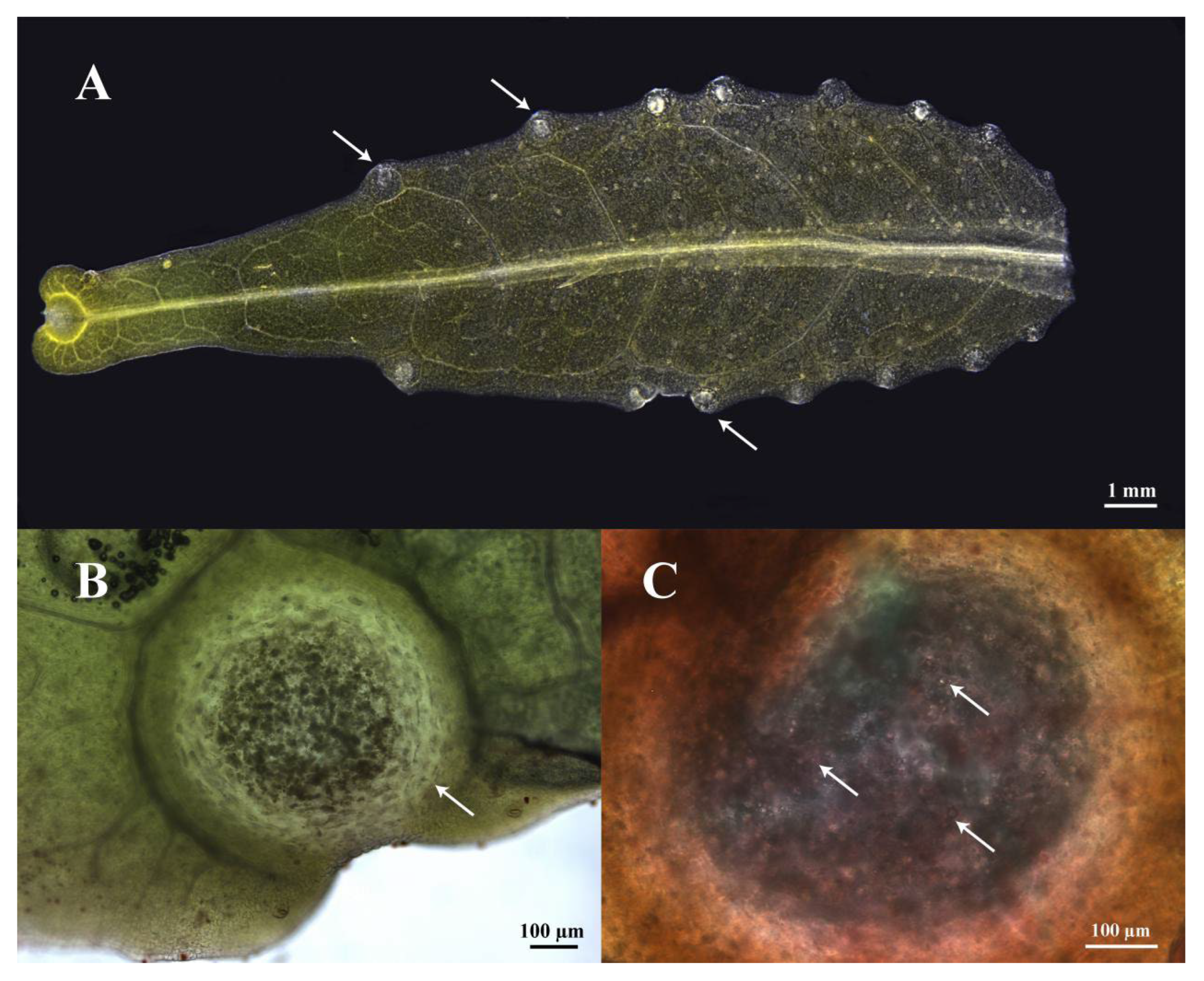
3.1. Distribution of Secretory Cavities
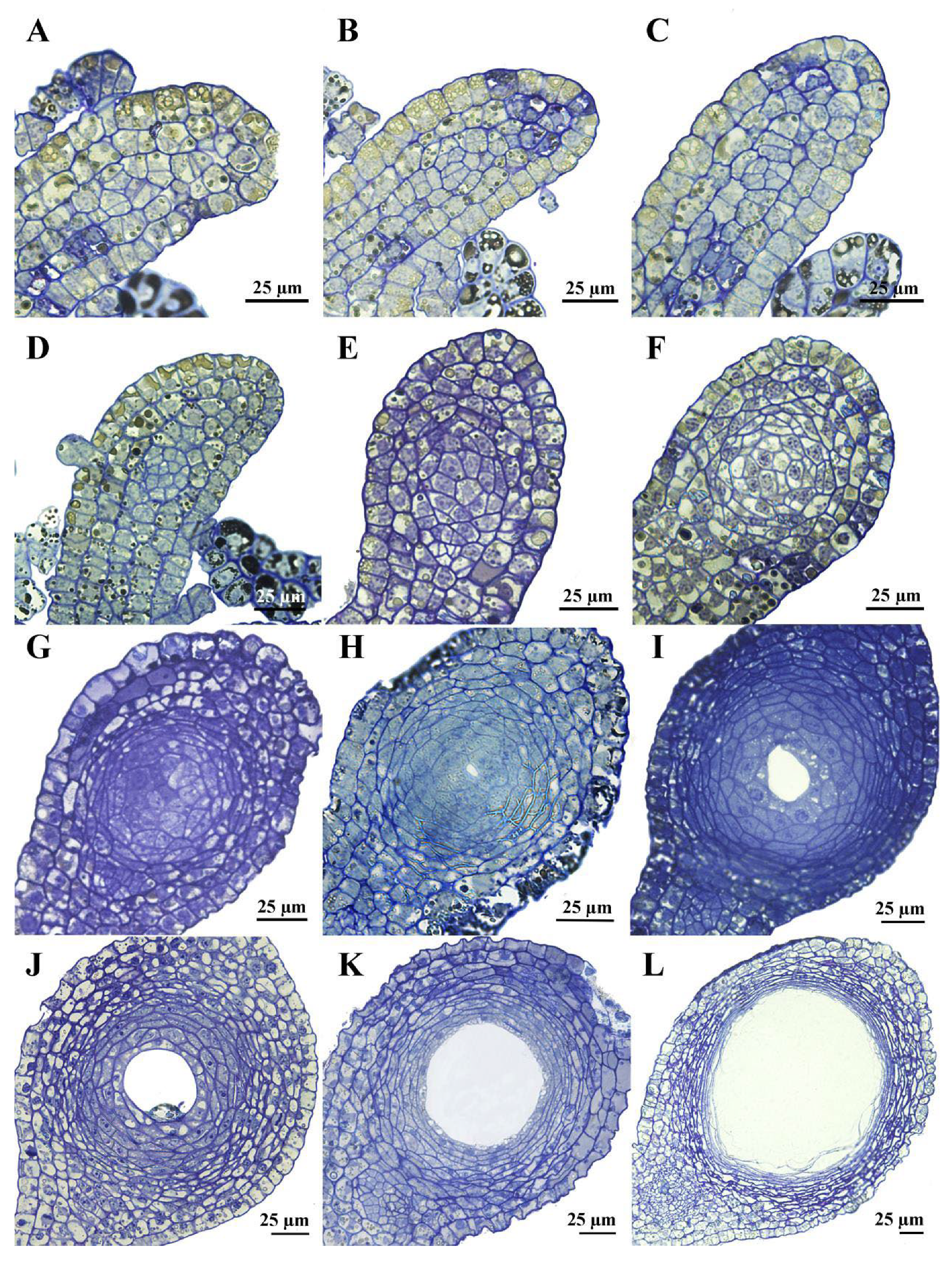
3.2. Ontogenetic Mode of Secretory Cavities
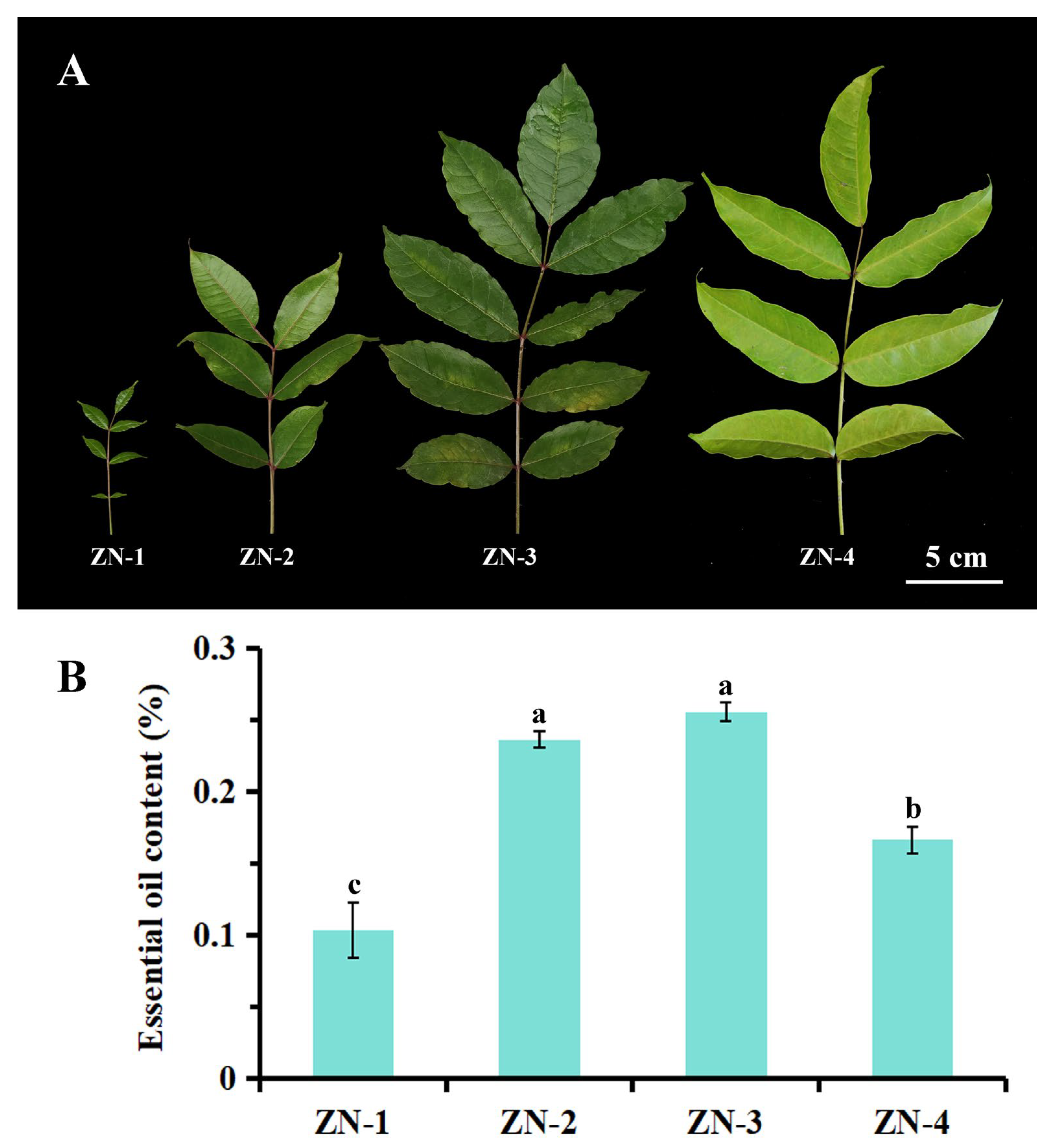
3.3. Comparison of EO Contents
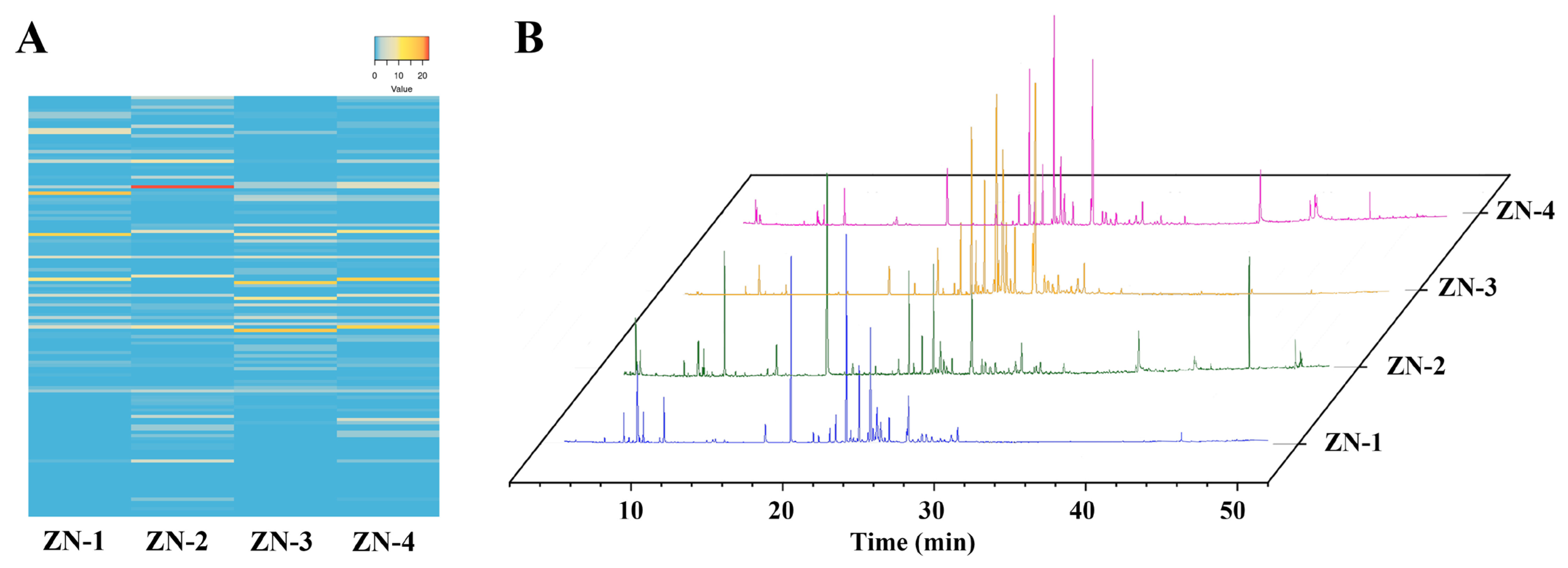
3.4. Chemical Composition of the EO
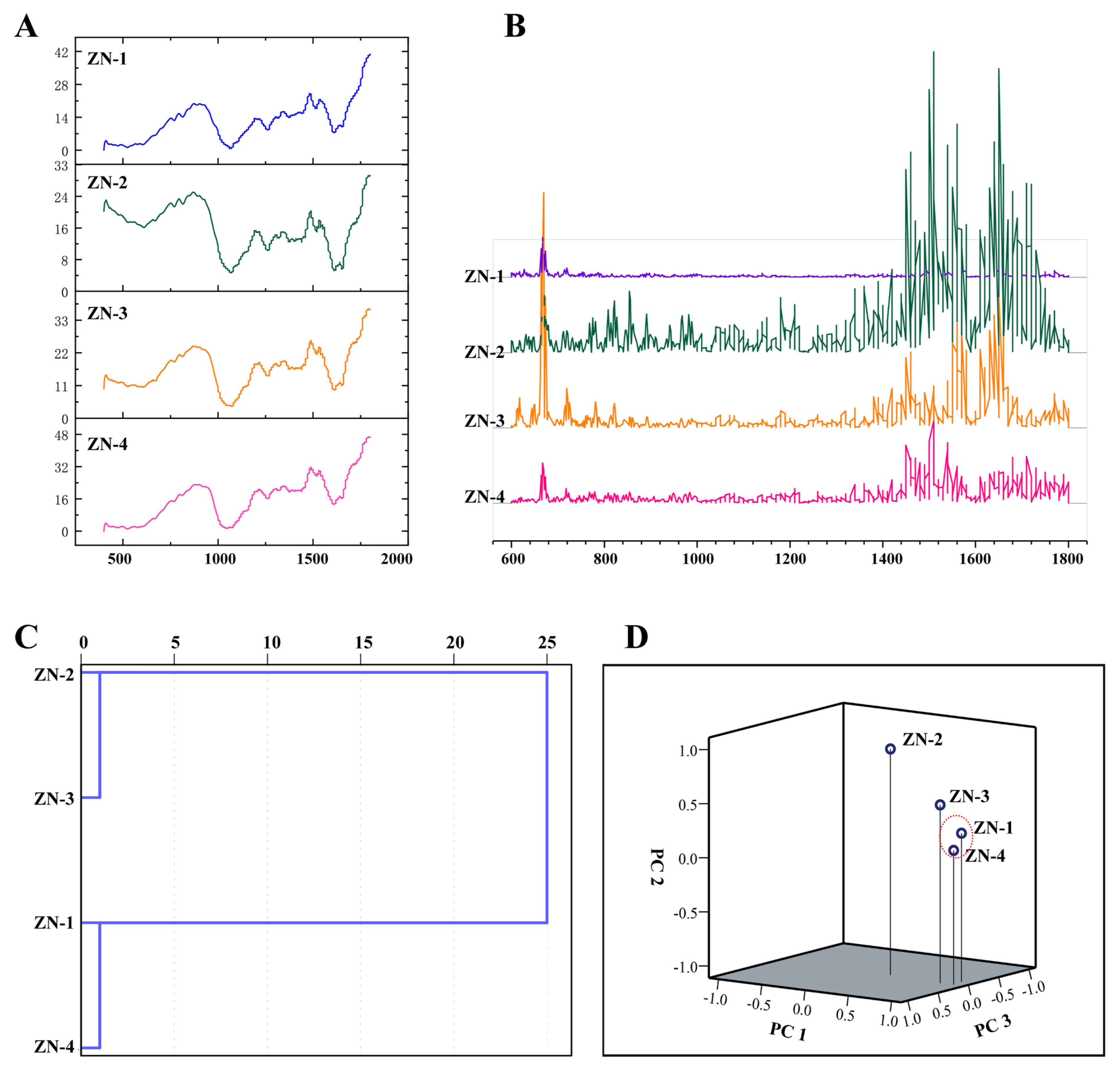
3.5. FTIR Fingerprints, HCA, and PCA of Z. nitidum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- China Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2025; pp. 181–182. [Google Scholar]
- Yang, Y.; Liu, Y.; Yang, Y.; Jia, H.; Tan, P.; Zeng, J.; Zeng, L.; Xie, T.; Geng, X.; He, H.; et al. A comparative analysis of the composition of the primary bioactive molecules, antioxidant activities, and anti-inflammation abilities of the roots of Zanthoxylum nitidum harvested at different years of cultivation. Ind. Crop Prod. 2024, 221, 119426. [Google Scholar] [CrossRef]
- Lu, Q.; Ma, R.; Yang, Y.; Mo, Z.; Pu, X.; Li, C. Zanthoxylum nitidum (Roxb.) DC: Traditional uses, phytochemistry, pharmacological activities and toxicology. J. Ethnopharmacol. 2020, 260, 112946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, J.; Liu, Y.; Zeng, J.; Zeng, L.; He, R.; Guiang, M.M.; Li, Y.; Wu, H. Assessment of Chinese suitable habitats of Zanthoxylum nitidum in different climatic conditions by Maxent model, HPLC, and chemometric methods. Ind. Crop Prod. 2023, 196, 116515. [Google Scholar] [CrossRef]
- Qin, F.; Wang, F.F.; Wang, C.G.; Chen, Y.; Li, M.S.; Zhu, Y.K.; Huang, X.C.; Fan, C.W.; Wang, H.S. The neurotrophic and antineuroinflammatory effects of phenylpropanoids from Zanthoxylum nitidum var. tomentosum (Rutaceae). Fitoterapia 2021, 153, 104990. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zeng, J.; Zeng, L.; Yang, L.; Ma, Q.; Wu, H.; Yang, Y. Comparison of the bioactive components and antioxidant activities of wild-type Zanthoxylum nitidum roots from various regions of Southern China. Nat. Prod. Res. 2024, 38, 4399–4410. [Google Scholar] [CrossRef]
- Peng, Z.H.; Wu, M.H.; Xie, Z.J.; Yang, X.B.; Lai, M.X. Investigation of wild resource of Zanthoxylum nitidum. Pharm. Today 2018, 28, 500–504. [Google Scholar]
- Yang, Y.; Li, Y.; Amoroso, V.; Acma, F.; Guiang, M.M.; Wu, H. Comparison of production of bioactive components in Zanthoxylum nitidum taproots from different regions in southern China. Biomed. Chromatogr. 2023, 37, e5602. [Google Scholar] [CrossRef]
- Tan, Y. Scientific and Reaconable Exploitation and Utilization of Zanthoxylum nitidum, the Essential Raw Materials of Sanjiu Weitai Granules. Ph.D. Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2011. [Google Scholar]
- Yang, Y.; Li, Y.; He, H.; Yang, L.; Zeng, J.; Bai, M.; Wu, H. Comparison of essential oil components and in vitro antioxidant activity of Zanthoxylum nitidum from different parts. Plants 2025, 14, 1194. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; He, H.; Yang, L.; Zeng, J.; Bai, M.; Wu, H. UPLC specific chromatogram study and pharmacodynamic composition analysis of roots and stems of Zanthoxylum nitidum. J. Chin. Med. Mater. 2020, 43, 2959–2965. [Google Scholar]
- He, L.L.; Lin, Z.H.; Hu, Y.Z.; Qin, Q.Y.; Huang, G.W.; Hu, D.N. Dynamic accumulation research of active ingredient in different part and growth phase of caltivative Zanthoxylum nitidum by UPLC-DAD. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 165–168. [Google Scholar]
- Vilaysack, M. Studies on the Anti-Inflammatory and Analgesic Active of Different Portions and Low-Polarity Portion Chemical Constituents in the Leaves of Zanthoxylum nitidum DC. Master’s Thesis, Guangxi Medical University, Nanning, China, 2013. [Google Scholar]
- Lee, J.H.; Chang, K.M.; Kim, G.H. Composition and antin-flammatory activities of Zanthoxylum schinifolium essential oil: Suppression of inducible nitric oxidesynthase, cyclooxy-genase-2, cytokines and cellular adhesion. J. Sci. Food Agric. 2009, 89, 1762–1769. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.J.; Shi, Y.X.; Zhao, M.; Ji, K.L.; Zhang, P.; Xu, Y.K.; Hu, H.B. Chemical composition, antimicrobial and anti-inflammatory activities of the essential oil from Maqian (Zanthoxylum myriacanthum var. pubescens) in Xishuangbanna, southwest China. J. Ethnopharmacol. 2014, 158, 43–48. [Google Scholar] [CrossRef]
- Ji, K.L.; Gan, X.Q.; Xu, Y.K.; Li, X.F.; Guo, J.; Dahab, M.M.; Zhang, P. Protective effect of the essential oil of Zanthoxylum myriacanthum var. pubescens against dextran sulfate sodium-induced intestinal inflammation in mice. Pytomedicine 2016, 23, 883–890. [Google Scholar] [CrossRef]
- Guo, R.H.; Park, J.U.; Jo, S.J.; Ahn, J.H.; Park, J.H.; Yang, J.Y.; Lee, S.S.; Park, M.J.; Kim, Y.R. Anti-allergic inflammatory effects of the essential oil from fruits of Zanthoxylum coreanum Nakai. Front. Pharmacol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Tuyen, T.T.; Quan, P.M.; Thu Le, V.T.; Toan, T.Q.; Nghi, D.H.; Bach, P.C.; Inh, C.T.; Hanh, N.P.; Vien, T.A.; Hong Minh, P.T.; et al. Chemical composition, antimicrobial, and cytotoxic activities of leaf, fruit, and branch essential oils obtained from Zanthoxylum nitidum grown in Vietnam. Nat. Prod. Commun. 2021, 16, 1934578X20985649. [Google Scholar] [CrossRef]
- Hu, Z.; Tian, W.; Lv, H. Anatomy of Plant Secretory Structures, 1st ed.; Shanghai Science and Technology Press: Shanghai, China, 2012; pp. 12–18. [Google Scholar]
- Li, Y.; Kong, D.; Hong, W. Analysis and evaluation of essential oil components of Cinnamon barks using GC-MS and FTIR spectroscopy. Ind. Crop Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Huang, R.S.; Liang, H.L.; Xu, C.G.; Wu, H. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind. Crop Prods 2013, 47, 92–101. [Google Scholar] [CrossRef]
- Platt-Aloia, K.A.; Oross, J.W.; Thomson, W.W. Ultrastructural study of the development of oil cells in the mesocarp of avocado fruit. Bot. Gaz. 1983, 144, 49–55. [Google Scholar] [CrossRef]
- Liu, P.; Liang, S.; Yao, N.; Wu, H. Programmed cell death of secretory cavity cells in fruits of Citrus grandis cv. tomentosa is associated with activation of caspase 3-like protease. Trees 2012, 26, 1821–1835. [Google Scholar] [CrossRef]
- Liang, S.J.; Wu, H.; Lun, X.; Lu, D.W. Secretory cavity development and its relationship with the accumulation of essential oil in fruits of Citrus medica L. var. sarcodactylis (Noot.) swingle. J. Integr. Plant Biol. 2006, 48, 573–583. [Google Scholar] [CrossRef]
- Cai, X.; Hu, Z.; He, Y. The relationship between ultrastructure changesand produce of the oil during the oil celldevelopment in Liriodendron chinense. Acta Bot. Boreali-Occident. Sin. 2002, 22, 327–332. [Google Scholar]
- Liu, W.; Hu, Z. Comparative anatomy of secretory cavities in leaves of the Rutaceae in China. J. Syst. Evol. 1998, 36, 119–127. [Google Scholar]
- Heinrich, G. Licht -und elektronenmikroskopische untersuchungen zur genese der exkrete in den lysigenen exkreträumen von Citrus medica. Flora Allg Bot. Zeitung. Abt. A Physiol. Biochem. 1966, 156, 451–456. [Google Scholar] [CrossRef]
- Heinrich, G. Elektronenmikroskopische beobachtungen zur entstehungsweise der exkretbehälter von Ruta graveolens, Citrus limon und Poncirus trifoliata. Osterr. Bot. Z.—Plant Syst. Evol. 1969, 117, 397–403. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W. Anatomical studies on the secretory cavity development in the stem and leaf of Evodia rutaecarpa. J. Northwest Sci-Tech Univ. Agric. For. 2000, 28, 27–31. [Google Scholar]
- Thomson, W.W.; Platt-Aloia, K.A.; Endress, A.G. Ultrastructure of oil gland development in leaf of Citrus sinensis L. Bot. Gaz. 1976, 137, 330–340. [Google Scholar] [CrossRef]
- Yang, Y.; Medecilo-Guiang, M.M.P.; Yang, L.; Huang, B.; He, J.; Chen, P. Structure and characteristics of foliar variegation in four species of medicinal Zingiberaceae. Braz. J. Bot. 2023, 46, 423–432. [Google Scholar] [CrossRef]
- Bai, M.; Jin, X.; Cen, Z.; Yu, K.; Yu, H.; Xiao, R.; Deng, J.; Lai, Z.; Wu, H.; Li, Y. GC–MS and FTIR spectroscopy for the identification and assessment of essential oil components of five Cinnamon leaves. Braz. J. Bot. 2021, 44, 525–535. [Google Scholar] [CrossRef]
- Buchgraber, M.; Ulberth, F.; Anklam, E. Cluster analysis for the systematic grouping of genuine cocoa butter and cocoa butter equivalent samples based on triglyceride patterns. J. Agric. Food Chem. 2004, 52, 3855–3860. [Google Scholar] [CrossRef]
- de Andrade, C.R.B.; Martins, F.M.; Brandão, H.N.; Alves, C.Q.; de Freitas-Silva, L. Leaf anatomy and histochemistry of secretory structures of Zanthoxylum caribaeum Lam. (Rutaceae). Braz. J. Bot. 2020, 43, 961–968. [Google Scholar] [CrossRef]
- Shunmugam, S.; Roslia, N.S.; Manickamb, S.; Yusoffa, N.F.M.; Alitheena, N.B.; Namasivayama, P. Morphology and anatomy of leaf, stem and petiole of Luvunga crassifolia Tanaka (Rutaceae). Malays. J. Fund. Appl. Sci. 2021, 17, 818–828. [Google Scholar] [CrossRef]
- Heinrich, G. Elektronenmikroskopische beobachtungen an den Drüsenzellen von Poncirus trifoliata; zugleich ein bBeitrag zur wirkung ätherischer Öle auf pflanzenzellen und eine methode zur unterscheidung flüchtiger von nichtflüchtigen lipophilen komponenten. Protoplasma 1970, 69, 15–36. [Google Scholar] [CrossRef]
- Liu, W.; Hu, Z. Ultrastructure of the secretory cavity development in the fruit of Zanthoxylum bungeanum. J. Integr. Plant Biol. 1997, 39, 200–204. [Google Scholar]
- Turner, G.W. Comparative development of secretory cavities in the tribes amorpheae and psoraleeae (Leguminosae: Papilionoideae). Am. J. Bot. 1986, 73, 1178–1192. [Google Scholar] [CrossRef]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Secretory structures. In Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants; Springer International Publishing: Cham, Switzerland, 2018; pp. 443–476. [Google Scholar]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I.; Hutton, I. Composition of the leaf oils of the Australian and lord howe Island species of Zanthoxylum (Rutaceae). J. Essent. Oil Res. 2000, 12, 285–291. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Zaman, M.K. Essential oil composition of fruits and leaves of Zanthoxylum nitidum grown in upper Assam region of India. Pharmacogn. Res. 2009, 1, 148–151. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour. Frag. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Kong, D.X.; Li, Y.Q.; Bai, M.; He, H.J.; Liang, G.X.; Wu, H. Correlation between the dynamic accumulation of the main effective components and their associated regulatory enzyme activities at different growth stages in Lonicera japonica Thunb. Ind. Crop Prod. 2017, 96, 16–22. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Secondary metabolites. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023; pp. 765–808. [Google Scholar]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Weng, S.F. Fourier Transform Infrared Spectrometer; Beijing Industry Press: Beijing, China, 2005; pp. 320–326. [Google Scholar]
- Zhang, Y.; Wang, Z. Comparative analysis of essential oil components of three Phlomis species in Qinling Mountains of China. J. Pharmaceut Biomed. 2008, 47, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Yang, W.; Liu, W.; Jiang, T. Study on establishment of RP-HPLC and GC-MS fifingerprints for wild germplasm resource of Ophiopogon japonicus in Sichuan and hierarchical clustering analysis. China J. Chin. Mater. Med. 2010, 35, 2726–2730. [Google Scholar]
- Li, Y.; Yang, Y.; Gu, D.; Cheng, Y.; Lv, X.; Huang, Y.; Ye, P.; Zhang, X.; Zhang, J.; Jian, W.; et al. Investigation of the impact of diverse climate conditions on the cultivation suitability of Cinnamomum cassia using the MaxEnt model, HPLC and chemometric methods in China. Sci. Rep. 2024, 14, 25686. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.; Feng, L.; Li, H.; Dai, Z.; Cheng, T.; Liu, S.; Ma, L.; Luo, X.; Wang, Y.; et al. The combined effects of climate, soil, and rhizospheric microorganisms determine the quality and suitable production zones of Stellaria dichotoma L. var. lanceolata Bge. in China. Chem. Biol. Technol. Agric. 2024, 11, 185. [Google Scholar] [CrossRef]
| No. | Compound Name | RI | Relative Content (%) | Identification | |||
|---|---|---|---|---|---|---|---|
| ZN-1 | ZN-2 | ZN-3 | ZN-4 | ||||
| 1 | 2-Hexenal | 850 | — | 2.95 ± 0.059 | — | 1.25 ± 0.072 | GC-MS, RI |
| 2 | 3-Hexen-1-ol | 859 | — | 0.49 ± 0.047 | — | 0.91 ± 0.054 | GC-MS, RI |
| 3 | trans-2-Hexenol | 861 | — | 1.56 ± 0.064 | — | 0.72 ± 0.030 | GC-MS, RI |
| 4 | β-Thujene | 936 | 0.26 ± 0.010 | — | — | — | GC-MS, RI |
| 5 | α-Pinene | 948 | 2.18 ± 2.18 | — | — | — | GC-MS, RI |
| 6 | (+)-Sabinene | 983 | 4.70 ± 2.35 | — | 1.37 ± 0.040 | — | GC-MS, RI |
| 7 | α-Myrcene | 991 | 1.14 ± 0.57 | 0.70 ± 0.019 | — | — | GC-MS, RI |
| 8 | Isocarvestrene | 1018 | — | 2.27 ± 0.023 | — | — | GC-MS, RI |
| 9 | Eucalyptol | 1031 | — | 1.76 ± 0.017 | — | 0.34 ± 0.019 | GC-MS, RI |
| 10 | α-Tolualdehyde | 1044 | — | 0.36 ± 0.027 | — | — | GC-MS, RI |
| 11 | (E)-β-Ocimene | 1048 | 1.78 ± 0.049 | 1.31 ± 0.0090 | — | 0.96 ± 0.037 | GC-MS, RI |
| 12 | Terpinolen | 1082 | 0.31 ± 0.0030 | — | — | — | GC-MS, RI |
| 13 | Linalool | 1101 | 3.38 ± 0.078 | 8.17 ± 0.043 | 0.39 ± 0.010 | 2.24 ± 0.089 | GC-MS, RI |
| 14 | Terpinen-4-ol | 1162 | — | 0.33 ± 0.055 | — | — | GC-MS, RI |
| 15 | (-)-α-Terpineol | 1197 | 0.32 ± 0.00 | 2.74 ± 0.035 | — | 0.62 ± 0.017 | GC-MS, RI |
| 16 | Decanal | 1208 | 0.21 ± 0.00 | — | — | — | GC-MS, RI |
| 17 | 2-Isopropyl-5-methyl-3-cyclohexen-1-one | 1251 | — | — | — | 3.76 ± 1.88 | GC-MS, RI |
| 18 | Piperitone | 1262 | 2.11 ± 0.020 | 22.47 ± 0.057 | 0.68 ± 0.68 | 2.00 ± 2.00 | GC-MS, RI |
| 19 | 2-Undecanone | 1294 | 18.00 ± 0.092 | 0.56 ± 0.012 | 0.57 ± 0.0090 | — | GC-MS, RI |
| 20 | δ-EIemene | 1347 | — | 0.21 ± 0.11 | 0.70 ± 0.70 | 0.91 ± 0.46 | GC-MS, RI |
| 21 | (-)-α-Cubebene | 1361 | 0.45 ± 0.00 | — | 0.25 ± 0.0030 | 0.23 ± 0.0030 | GC-MS, RI |
| 22 | α-Copaene | 1375 | 0.31 ± 0.31 | — | — | — | GC-MS, RI |
| 23 | α-Ylangene | 1377 | 0.61 ± 0.31 | — | 0.47 ± 0.0030 | — | GC-MS, RI |
| 24 | Elemene | 1402 | — | 0.20 ± 0.20 | 3.03 ± 0.077 | — | GC-MS, RI |
| 25 | β-Caryophyllene | 1430 | 15.37 ± 0.013 | 6.51 ± 0.046 | 8.78 ± 0.11 | 9.80 ± 0.12 | GC-MS, RI |
| 26 | β-Copaene | 1437 | 9.93 ± 0.095 | — | 15.92 ± 0.057 | 15.87 ± 0.035 | GC-MS, RI |
| 27 | γ-Elemene | 1444 | 0.90 ± 0.040 | 0.45 ± 0.028 | 2.27 ± 0.069 | 1.41 ± 0.12 | GC-MS, RI |
| 28 | Isogermacrene D | 1448 | — | — | 0.45 ± 0.00 | — | GC-MS, RI |
| 29 | (+)-Aromadendrene | 1456 | — | — | 0.38 ± 0.00 | — | GC-MS, RI |
| 30 | α-Humulene | 1471 | 6.01 ± 0.023 | 2.52 ± 0.0090 | 5.61 ± 0.012 | 4.00 ± 0.030 | GC-MS, RI |
| 31 | γ-Muurolene | 1489 | 0.99 ± 0.015 | — | 0.96 ± 0.0030 | — | GC-MS, RI |
| 32 | Germacrene D | 1497 | — | 8.35 ± 0.093 | — | — | GC-MS, RI |
| 33 | 2-Tridecanone | 1456 | 0.43 ± 0.015 | — | — | — | GC-MS, RI |
| 34 | Bicyclogermacrene | 1495 | 4.52 ± 0.022 | 2.65 ± 0.056 | 9.61 ± 0.042 | 5.87 ± 0.055 | GC-MS, RI |
| 35 | α-Farnesene | 1505 | 1.83 ± 0.059 | 0.51 ± 0.25 | 4.01 ± 0.14 | 2.29 ± 0.069 | GC-MS, RI |
| 36 | α-Amorphene | 1512 | 0.33 ± 0.0070 | — | — | — | GC-MS, RI |
| 37 | γ-Amorphene | 1518 | 0.37 ± 0.0070 | — | 0.89 ± 0.012 | 0.20 ± 0.017 | GC-MS, RI |
| 38 | δ-Amorphene | 1521 | 2.43 ± 0.024 | 1.07 ± 0.032 | 3.87 ± 0.038 | 1.78 ± 0.013 | GC-MS, RI |
| 39 | Germacrene B | 1561 | 1.17 ± 0.064 | 0.72 ± 0.047 | 2.80 ± 0.12 | 1.99 ± 0.10 | GC-MS, RI |
| 40 | trans-Nerolidol | 1564 | 4.84 ± 0.084 | 8.55 ± 0.082 | 17.39 ± 0.035 | 15.55 ± 0.039 | GC-MS, RI |
| 41 | (-)-Spathulenol | 1572 | 0.25 ± 0.006 | 1.06 ± 0.035 | 1.31 ± 0.015 | 1.13 ± 0.022 | GC-MS, RI |
| 42 | Caryophyllene oxide | 1578 | — | — | 0.40 ± 0.40 | 1.26 ± 0.029 | GC-MS, RI |
| 43 | (-)-Globulol | 1580 | 0.94 ± 0.023 | 0.88 ± 0.050 | 0.78 ± 0.39 | — | GC-MS, RI |
| 44 | (-)-Epicedrol | 1585 | 0.70 ± 0.024 | 0.65 ± 0.019 | — | 1.14 ± 0.012 | GC-MS, RI |
| 45 | Cedrol | 1589 | — | — | 1.40 ± 0.021 | — | GC-MS, RI |
| 46 | Humulene epoxide II | 1606 | — | — | 0.22 ± 0.0070 | — | GC-MS, RI |
| 47 | Junenol | 1618 | 0.35 ± 0.012 | — | 0.24 ± 0.00 | — | GC-MS, RI |
| 48 | Isospathulenol | 1630 | — | — | 0.29 ± 0.0060 | 0.31 ± 0.0090 | GC-MS, RI |
| 49 | T-Muurolol | 1640 | 0.89 ± 0.012 | 0.75 ± 0.019 | 1.24 ± 0.020 | 0.92 ± 0.028 | GC-MS, RI |
| 50 | α-Cadinol | 1653 | 1.59 ± 0.037 | 2.39 ± 0.078 | 2.05 ± 0.047 | 2.02 ± 0.027 | GC-MS, RI |
| 51 | β-Sinensal | 1693 | — | — | — | 0.64 ± 0.035 | GC-MS, RI |
| 52 | Mintsulfide | 1742 | — | — | 0.32 ± 0.010 | — | GC-MS, RI |
| 53 | α-Sinensal | 1765 | — | 0.46 ± 0.023 | — | 0.49 ± 0.015 | GC-MS, RI |
| 54 | m-Camphorene | 1944 | — | 0.23 ± 0.023 | — | — | GC-MS, RI |
| 55 | n-Hexadecanoic acid | 1959 | — | 3.06 ± 0.041 | — | 4.81 ± 0.26 | GC-MS, RI |
| 56 | Conjugated linoleic acid | 2060 | — | 0.56 ± 0.56 | — | — | GC-MS, RI |
| 57 | Linoelaidic acid | 2065 | — | 1.04 ± 0.52 | — | — | GC-MS, RI |
| 58 | Phytol | 2111 | — | — | 0.31 ± 0.020 | 1.37 ± 0.050 | GC-MS, RI |
| 59 | Linolenic acid | 2159 | — | 1.18 ± 0.052 | — | 1.73 ± 0.024 | GC-MS, RI |
| 60 | 11,14-Eicosadienoic acid | 2250 | — | — | — | 1.84 ± 0.065 | GC-MS, RI |
| Total | 89.60 | 89.67 | 88.96 | 90.36 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Hou, J.; Zeng, J.; Fang, Y.; Tian, T.; Wang, X.; Kai, R.; Zhang, S.; Liao, W.; Chang, T.; et al. The Development of Secretory Cavities in Zanthoxylum nitidum Leaves and the Pattern of Essential Oil Accumulation. Plants 2025, 14, 3449. https://doi.org/10.3390/plants14223449
Yang Y, Hou J, Zeng J, Fang Y, Tian T, Wang X, Kai R, Zhang S, Liao W, Chang T, et al. The Development of Secretory Cavities in Zanthoxylum nitidum Leaves and the Pattern of Essential Oil Accumulation. Plants. 2025; 14(22):3449. https://doi.org/10.3390/plants14223449
Chicago/Turabian StyleYang, Yang, Jiating Hou, Jiaxin Zeng, Yue Fang, Tao Tian, Xin Wang, Rui Kai, Sisheng Zhang, Weiyao Liao, Tao Chang, and et al. 2025. "The Development of Secretory Cavities in Zanthoxylum nitidum Leaves and the Pattern of Essential Oil Accumulation" Plants 14, no. 22: 3449. https://doi.org/10.3390/plants14223449
APA StyleYang, Y., Hou, J., Zeng, J., Fang, Y., Tian, T., Wang, X., Kai, R., Zhang, S., Liao, W., Chang, T., Zheng, R., Chen, Y., Li, Y., Bai, M., & Wu, H. (2025). The Development of Secretory Cavities in Zanthoxylum nitidum Leaves and the Pattern of Essential Oil Accumulation. Plants, 14(22), 3449. https://doi.org/10.3390/plants14223449








