Effects of Arbuscular Mycorrhizal Fungi and Metal-Tolerant Pseudomonas fluorescens on Mitigating Cadmium and Zinc Stress in Tomato
Abstract
1. Introduction
2. Results
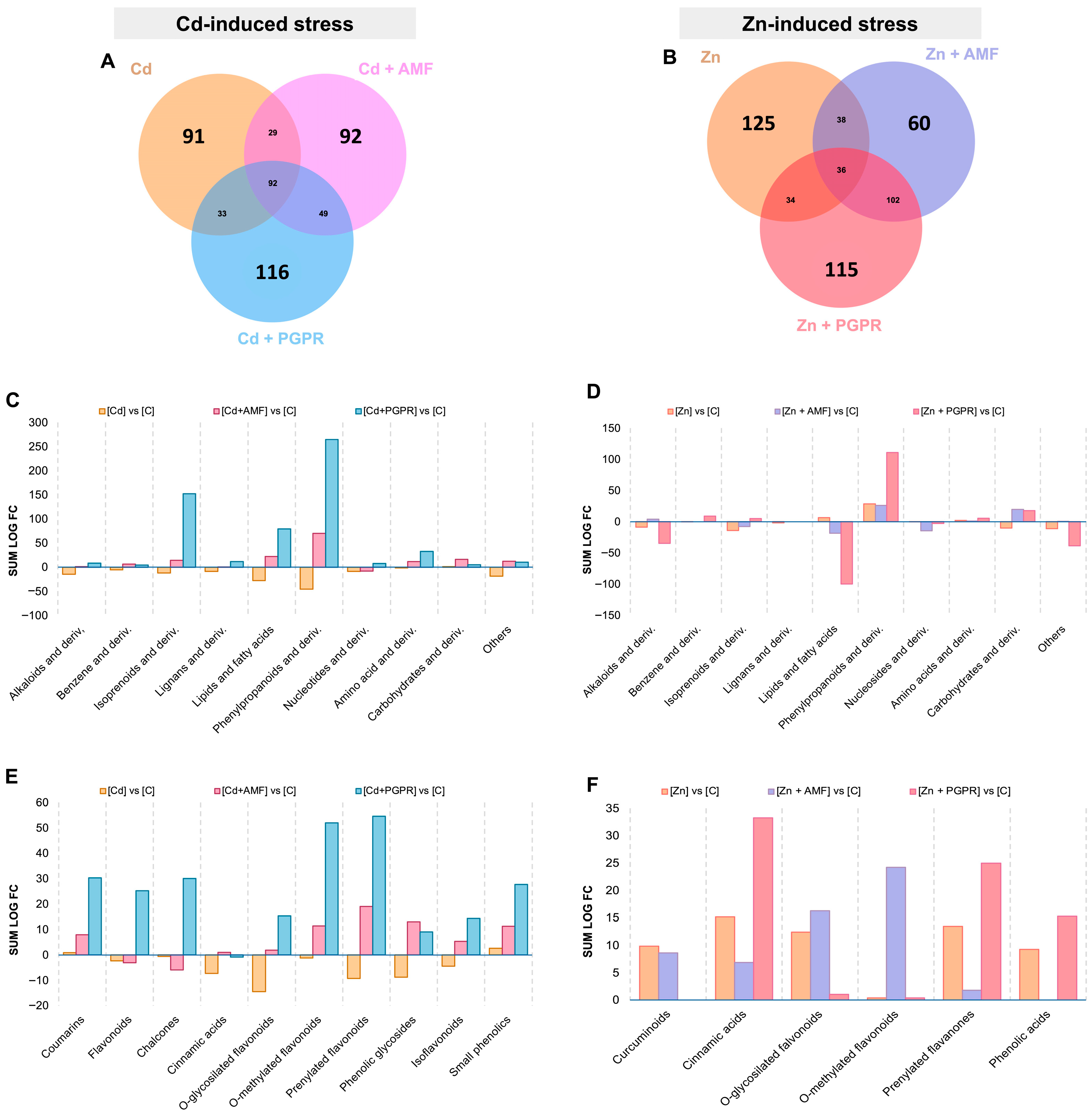
2.1. MBs Specifically Modulate the Root Exudates Profile Under Cd- and Zn-Induced Stress
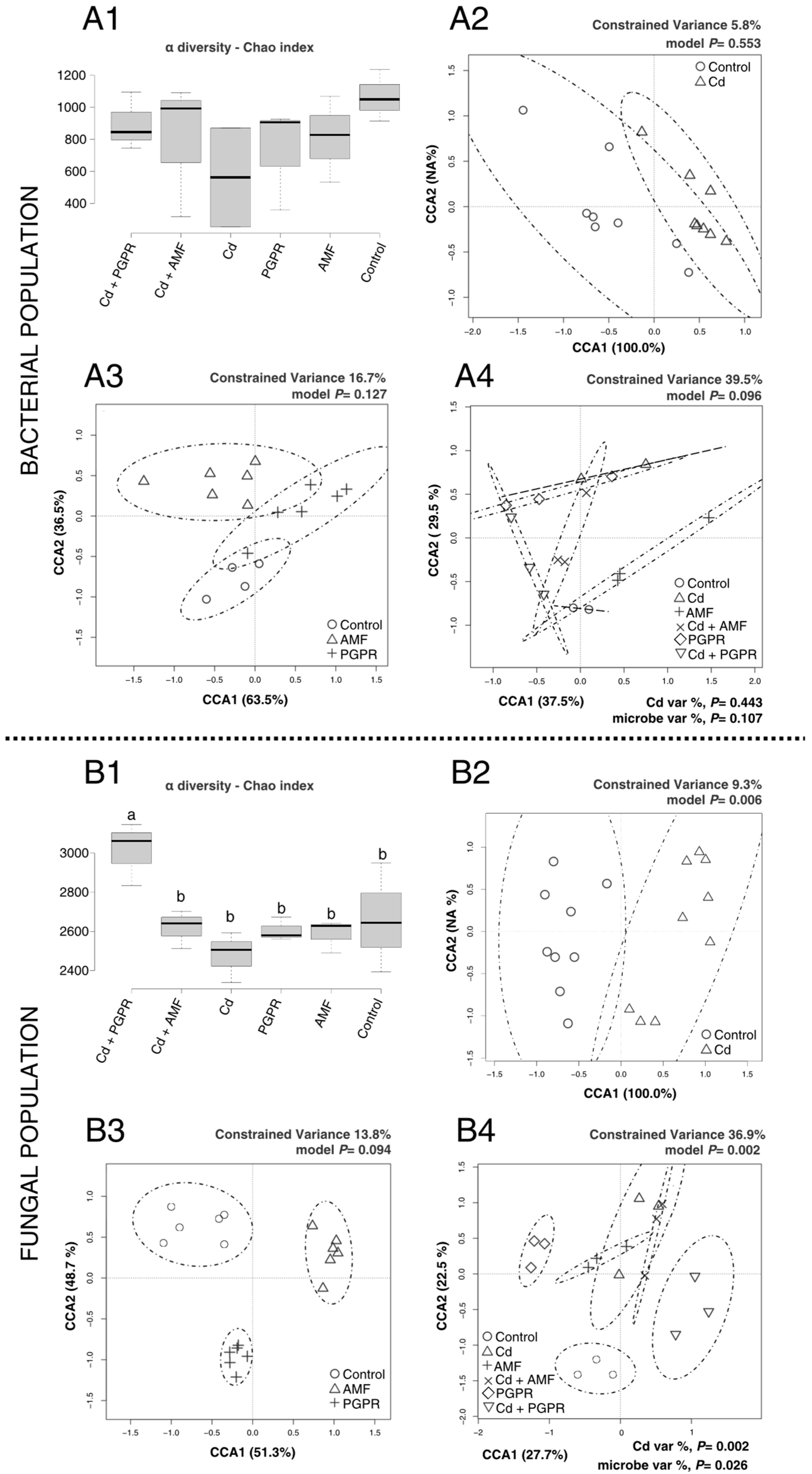
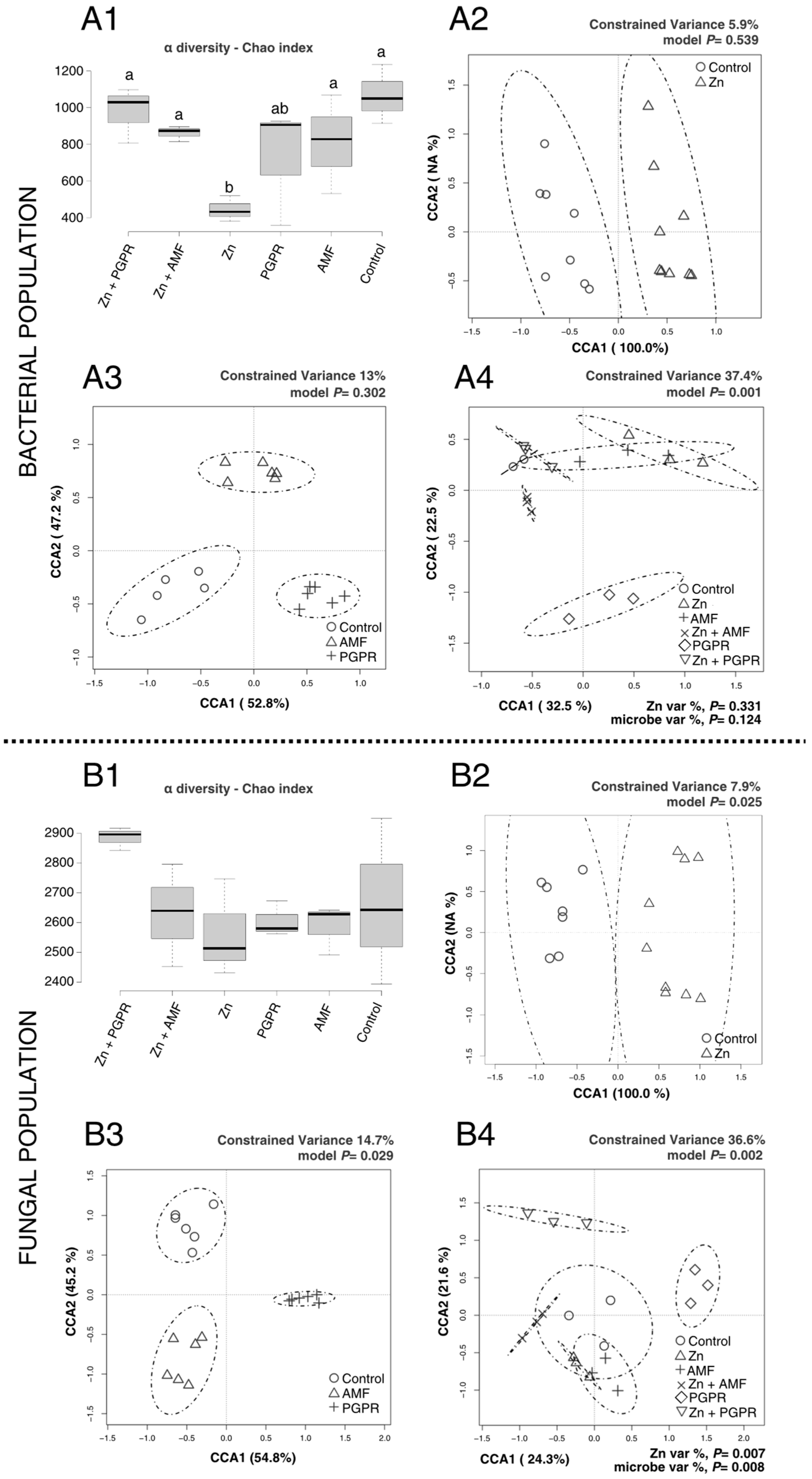
2.2. MBs Specifically Modulate the Rhizosphere Microbial Population Under Cd and Zn-Induced Stress
2.3. Multi-Omics Data Integration of Fungal, Bacterial, and Exudates
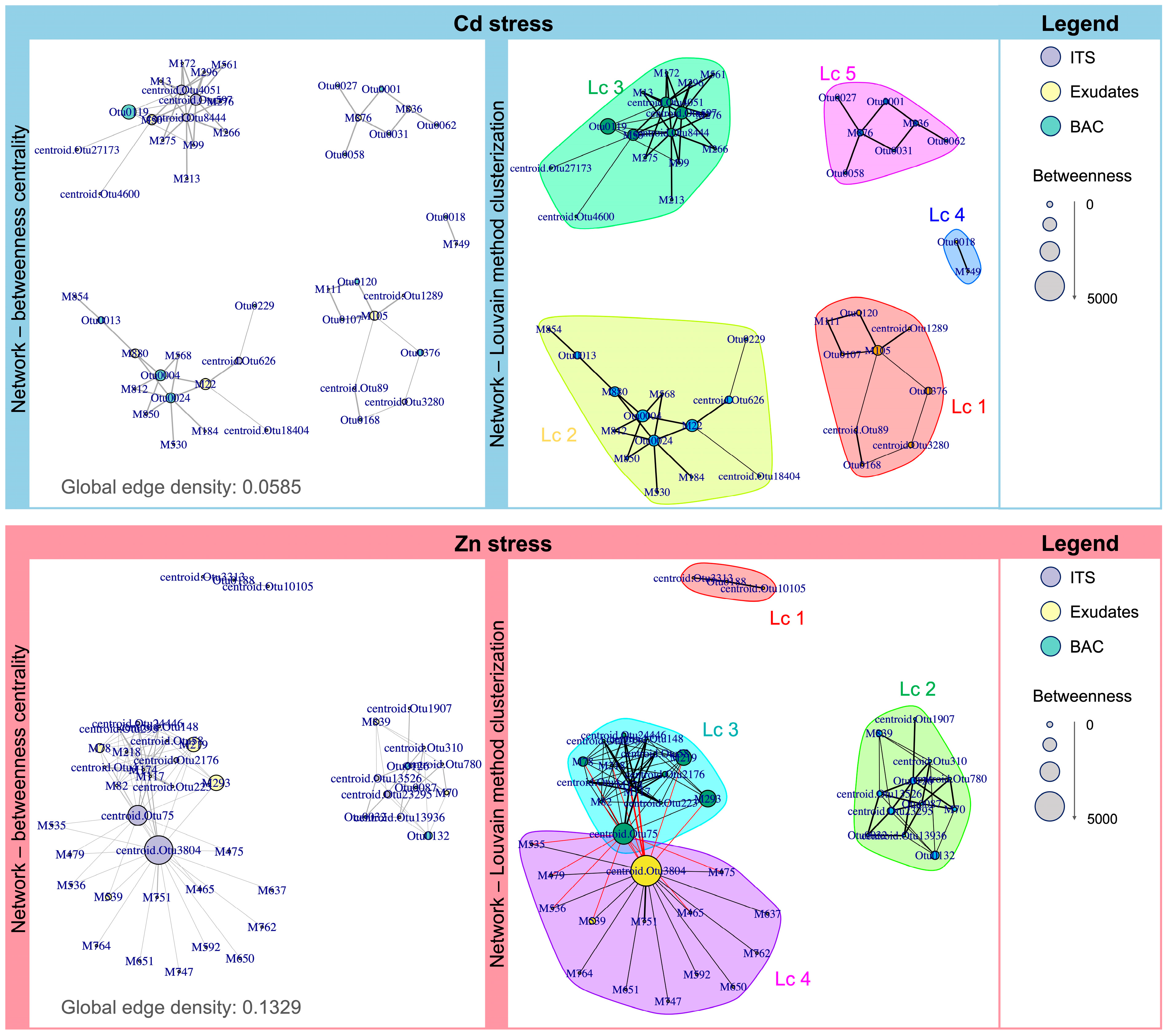
2.4. Network Analysis of Highly Discriminant Features Obtained from Multi-Omics Data Integration in Cd- and Zn-Stressed Models
3. Discussion
3.1. Plant–Microbial Communication Mechanism Under Cd and Zn Stress
3.2. Mitigation Mechanisms of Microbial Biostimulants
4. Materials and Methods
4.1. Plant Growth Condition
4.2. Exudate Profiling
4.3. Amplicon Sequencing
4.4. Multi-Omics Data Integration
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barra Caracciolo, A.; Terenzi, V. Rhizosphere Microbial Communities and Heavy Metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef]
- Hnini, M.; Rabeh, K.; Oubohssaine, M. Interactions between Beneficial Soil Microorganisms (PGPR and AMF) and Host Plants for Environmental Restoration: A Systematic Review. Plant Stress 2024, 11, 100391. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, S.; KC, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, Effects and Present Perspectives of Heavy Metals Contamination: Soil, Plants and Human Food Chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Cozma, P.; Roșca, M.; Minuț, M.; Gavrilescu, M. Phytoremediation: A Sustainable and Promising Bio-Based Approach to Heavy Metal Pollution Management. Sci. Total Environ. 2025, 1001, 180458. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; El Din Mahmoud, A.; Dutta, J.; Singh, R.; Mohammad Said Al-Tawaha, A.R.; Malik, T. Eco-Engineered Remediation: Microbial and Rhizosphere-Based Strategies for Heavy Metal Detoxification. Curr. Res. Biotechnol. 2025, 9, 100297. [Google Scholar] [CrossRef]
- de Carvalho Neta, S.J.; Araújo, V.L.V.P.; Fracetto, F.J.C.; da Silva, C.C.G.; de Souza, E.R.; Silva, W.R.; Lumini, E.; Fracetto, G.G.M. Growth-Promoting Bacteria and Arbuscular Mycorrhizal Fungus Enhance Maize Tolerance to Saline Stress. Microbiol. Res. 2024, 284, 127708. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, H.; Du, D.; Li, G.; Alam, O.; Cheng, Z.; Liu, X.; Jiang, S.; Li, J. Remediation of Heavy Metals Polluted Soil Environment: A Critical Review on Biological Approaches. Ecotoxicol. Environ. Saf. 2024, 284, 116883. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for Help with Root Exudates: Adaptive Mechanisms by Which Stressed Plants Assemble Health-Promoting Soil Microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef]
- Zhang, L.; Zuluaga, M.Y.A.; Pii, Y.; Barone, A.; Amaducci, S.; Miras-Moreno, B.; Martinelli, E.; Bellotti, G.; Trevisan, M.; Puglisi, E.; et al. A Pseudomonas Plant Growth Promoting Rhizobacterium and Arbuscular Mycorrhiza Differentially Modulate the Growth, Photosynthetic Performance, Nutrients Allocation, and Stress Response Mechanisms Triggered by a Mild Zinc and Cadmium Stress in Tomato. Plant Sci. 2023, 337, 111873. [Google Scholar] [CrossRef]
- Davidova, S.; Milushev, V.; Satchanska, G. The Mechanisms of Cadmium Toxicity in Living Organisms. Toxics 2024, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Cuajungco, M.P.; Ramirez, M.S.; Tolmasky, M.E. Zinc: Multidimensional Effects on Living Organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.L.; Moraes, R.M.; Esteves, G.; Bastos, R.G.; Goolsby, E.; Mason, C.; Azevedo, R.A.; Marques, D.N. Current Research on the Role of Plant Primary and Secondary Metabolites in Response to Cadmium Stress. In Approaches to the Remediation of Inorganic Pollutants; Hasanuzzaman, M., Ed.; Springer: Singapore, 2021; pp. 125–153. ISBN 978-981-15-6221-1. [Google Scholar]
- Lin, S.; He, Q.; Zhang, M.; Huang, Y.; Liu, H.; Mu, Q.; Wang, S.; Nie, J. Effects of Cadmium Stress on Root Exudates and Soil Rhizosphere Microorganisms of Rice (Oryza sativa L.) and Its Ecological Regulatory Mechanisms. Plants 2025, 14, 1695. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Yao, N. Sphingolipids in Plant Immunity. Phytopathol. Res. 2022, 4, 20. [Google Scholar] [CrossRef]
- Rogowska, A.; Pączkowski, C.; Szakiel, A. Modulation of Steroid and Triterpenoid Metabolism in Calendula officinalis Plants and Hairy Root Cultures Exposed to Cadmium Stress. Int. J. Mol. Sci. 2022, 23, 5640. [Google Scholar] [CrossRef]
- Berkey, R.; Bendigeri, D.; Xiao, S. Sphingolipids and Plant Defense/Disease: The “Death” Connection and Beyond. Front. Plant Sci. 2012, 3, 21731. [Google Scholar] [CrossRef]
- Stamatakis, K.; Tsimilli-Michael, M.; Papageorgiou, G.C. On the Question of the Light-Harvesting Role of β-Carotene in Photosystem II and Photosystem I Core Complexes. Plant Physiol. Biochem. 2014, 81, 121–127. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Loreto, F.; Centritto, M.; Ferrini, F.; Tattini, M. Isoprene Responses and Functions in Plants Challenged by Environmental Pressures Associated to Climate Change. Front. Plant Sci. 2017, 8, 1281. [Google Scholar] [CrossRef]
- Noor, W.; Majeed, G.; Lone, R.; Tyub, S.; Kamili, A.N.; Azeez, A. Interactive Role of Phenolics and PGPR in Alleviating Heavy Metal Toxicity in Wheat. In Plant Phenolics in Abiotic Stress Management; Lone, R., Khan, S., Mohammed Al-Sadi, A., Eds.; Springer Nature: Singapore, 2023; pp. 287–320. ISBN 978-981-19-6426-8. [Google Scholar]
- Henschel, J.M.; de Andrade, A.N.; dos Santos, J.B.L.; da Silva, R.R.; da Mata, D.A.; Souza, T.; Batista, D.S. Lipidomics in Plants Under Abiotic Stress Conditions: An Overview. Agronomy 2024, 14, 1670. [Google Scholar] [CrossRef]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere Interactions: Root Exudates, Microbes, and Microbial Communities1. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Afridi, M.S.; Kumar, A.; Javed, M.A.; Dubey, A.; de Medeiros, F.H.V.; Santoyo, G. Harnessing Root Exudates for Plant Microbiome Engineering and Stress Resistance in Plants. Microbiol. Res. 2024, 279, 127564. [Google Scholar] [CrossRef]
- Li, C.; Zhong, M.; Guo, E.; Xu, H.; Wen, C.; Zhu, S.; Li, Q.; Zhu, D.; Luo, X. Response of Bacterial and Fungal Communities in Natural Biofilms to Bioavailable Heavy Metals in a Mining-Affected River. Water Res. 2024, 267, 122470. [Google Scholar] [CrossRef]
- Sisó-Terraza, P.; Luis-Villarroya, A.; Fourcroy, P.; Briat, J.F.; Abadía, A.; Gaymard, F.; Abadía, J.; Álvarez-Fernández, A. Accumulation and Secretion of Coumarinolignans and Other Coumarins in Arabidopsis thaliana Roots in Response to Iron Deficiency at High PH. Front. Plant Sci. 2016, 7, 1711. [Google Scholar] [CrossRef] [PubMed]
- Falagán, C.; Johnson, D.B. Acidibacter ferrireducens Gen. Nov., Sp. Nov.: An Acidophilic Ferric Iron-Reducing Gammaproteobacterium. Extremophiles 2014, 18, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Khaswal, A.; Chaturvedi, N.; Mishra, S.K.; Kumar, P.R.; Paul, P.K. Current Status and Applications of Genus Geobacillus in the Production of Industrially Important Products—A Review. Folia Microbiol. 2022, 67, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Du, X.R.; Zhang, Z.Y.; Feng, F.J.; Zhang, J.M. Rhizosphere Interface Microbiome Reassembly by Arbuscular Mycorrhizal Fungi Weakens Cadmium Migration Dynamics. iMeta 2023, 2, e133. [Google Scholar] [CrossRef]
- Rikuan, Z.; Ruining, C.; Chong, W.; Rui, L.; Chaomin, S. Characterization of the First Cultured Representative of “Candidatus Thermofonsia” Clade 2 within Chloroflexi Reveals Its Phototrophic Lifestyle. mBio 2022, 13, e00287-22. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, Q.; She, Z.; Gao, M.; Zhao, Y.; Guo, L.; Jin, C. Nitrogen Removal Pathway and Dynamics of Microbial Community with the Increase of Salinity in Simultaneous Nitrification and Denitrification Process. Sci. Total Environ. 2019, 697, 134047. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The Role of Gibberellin Signalling in Plant Responses to Abiotic Stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Mishra, V.; Sarkar, A.K. Serotonin: A Frontline Player in Plant Growth and Stress Responses. Physiol. Plant. 2023, 175, e13968. [Google Scholar] [CrossRef]
- Ragonezi, C.; Teixeira, D.; Caldeira, A.T.; Martins, M.R.; Santos-Silva, C.; Ganhão, E.; Klimaszewska, K.; Zavattieri, M.A. O-Coumaric Acid Ester, a Potential Early Signaling Molecule in Pinus pinea and Pisolithus arhizus Symbiosis Established in Vitro. J. Plant Interact. 2014, 9, 297–305. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signaling Molecules in Plant-Microbe Symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gu, T.; Gao, Y.; Qu, J.; Zheng, H.; Guan, Y.; Peng, J. Transcriptomic Profiling Reveals the Involvement of the Phenylpropanoid–Lignin Pathway in the Response of Maize Roots to Zinc Stress. Plants 2025, 14, 1657. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of Phenolic Acids on Free Radical Scavenging and Heavy Metal Bioavailability in Kandelia Obovata under Cadmium and Zinc Stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
- Singh, R.; Soni, S.K.; Bajpai, A. Cooperative Interaction of Glomus Intraradices with Plant Growth-Promoting Rhizobacteria Promotes Plant Development and Essential Oil Yield of Pogostemon Cablin and Reduces Disease Occurrence under Organic Field Conditions. Australas. Plant Pathol. 2023, 52, 595–607. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J.; Dijkstra, F.A.; Sun, L.; Yin, L.; Wang, P.; Cheng, W. Rhizosphere Priming Effects and Trade-Offs among Root Traits, Exudation and Mycorrhizal Symbioses. Soil Biol. Biochem. 2025, 202, 109690. [Google Scholar] [CrossRef]
- Ferrarini, A.; Fracasso, A.; Spini, G.; Fornasier, F.; Taskin, E.; Fontanella, M.C.; Beone, G.M.; Amaducci, S.; Puglisi, E. Bioaugmented Phytoremediation of Metal-Contaminated Soils and Sediments by Hemp and Giant Reed. Front. Microbiol. 2021, 12, 645893. [Google Scholar] [CrossRef] [PubMed]
- Hothem, S.D.; Marley, K.A.; Larson, R.A. Photochemistry in Hoagland’s Nutrient Solution. J. Plant. Nutr. 2003, 26, 845–854. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Neumann, S.; Schober, D.; Hummel, J.; Billiau, K.; Kopka, J.; Correa, E.; Reijmers, T.; Rosato, A.; Tenori, L.; et al. COordination of Standards in MetabOlomicS (COSMOS): Facilitating Integrated Metabolomics Data Access. Metabolomics 2015, 11, 1587–1597. [Google Scholar] [CrossRef]
- Vasileiadis, S.; Puglisi, E.; Trevisan, M.; Scheckel, K.G.; Langdon, K.A.; McLaughlin, M.J.; Lombi, E.; Donner, E. Changes in Soil Bacterial Communities and Diversity in Response to Long-Term Silver Exposure. FEMS Microbiol. Ecol. 2015, 91, fiv114. [Google Scholar] [CrossRef]
- Bellotti, G.; Taskin, E.; Guerrieri, M.C.; Beone, G.M.; Menta, C.; Remelli, S.; Bandini, F.; Tabaglio, V.; Fiorini, A.; Capra, F.; et al. Agronomical valorization of eluates from the industrial production of microorganisms: Chemical, microbiological, and ecotoxicological assessment of a novel putative biostimulant. Front. Plant Sci. 2022, 13, 907349. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]

| Effect Name | RSS | RSS p-Value | R2Y p-Value | Tp1 | Tp2 | Tp3 | Tp4 | Tp5 | Tp6 | To1 |
|---|---|---|---|---|---|---|---|---|---|---|
| HM | 9.0% | 0.01 | 0.01 | 1.8% | 2.6% | 94.4% | 13.4% | 73.0% | 12.7% | 26.2% |
| MB | 23.2% | 0.01 | 0.01 | 94.3% | 1.7% | 1.3% | 57.7% | 6.4% | 8.5% | 17.6% |
| HM × MB | 14.4% | 0.01 | 0.01 | 1.7% | 92.5% | 1.9% | 12.8% | 9.1% | 63.7% | 25.0% |
| Residuals | 53.3% | - | - | 2.2% | 3.1% | 2.4% | 16.0% | 11.4% | 15.1% | 31.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Bellotti, G.; Salehi, H.; Puglisi, E.; Lucini, L. Effects of Arbuscular Mycorrhizal Fungi and Metal-Tolerant Pseudomonas fluorescens on Mitigating Cadmium and Zinc Stress in Tomato. Plants 2025, 14, 3353. https://doi.org/10.3390/plants14213353
Zhang L, Bellotti G, Salehi H, Puglisi E, Lucini L. Effects of Arbuscular Mycorrhizal Fungi and Metal-Tolerant Pseudomonas fluorescens on Mitigating Cadmium and Zinc Stress in Tomato. Plants. 2025; 14(21):3353. https://doi.org/10.3390/plants14213353
Chicago/Turabian StyleZhang, Leilei, Gabriele Bellotti, Hajar Salehi, Edoardo Puglisi, and Luigi Lucini. 2025. "Effects of Arbuscular Mycorrhizal Fungi and Metal-Tolerant Pseudomonas fluorescens on Mitigating Cadmium and Zinc Stress in Tomato" Plants 14, no. 21: 3353. https://doi.org/10.3390/plants14213353
APA StyleZhang, L., Bellotti, G., Salehi, H., Puglisi, E., & Lucini, L. (2025). Effects of Arbuscular Mycorrhizal Fungi and Metal-Tolerant Pseudomonas fluorescens on Mitigating Cadmium and Zinc Stress in Tomato. Plants, 14(21), 3353. https://doi.org/10.3390/plants14213353










