Manipulation of StPTST1 Affects Starch Content and Physicochemical Properties of Potato (Solanum tuberosum L.)
Abstract
1. Introduction
2. Results
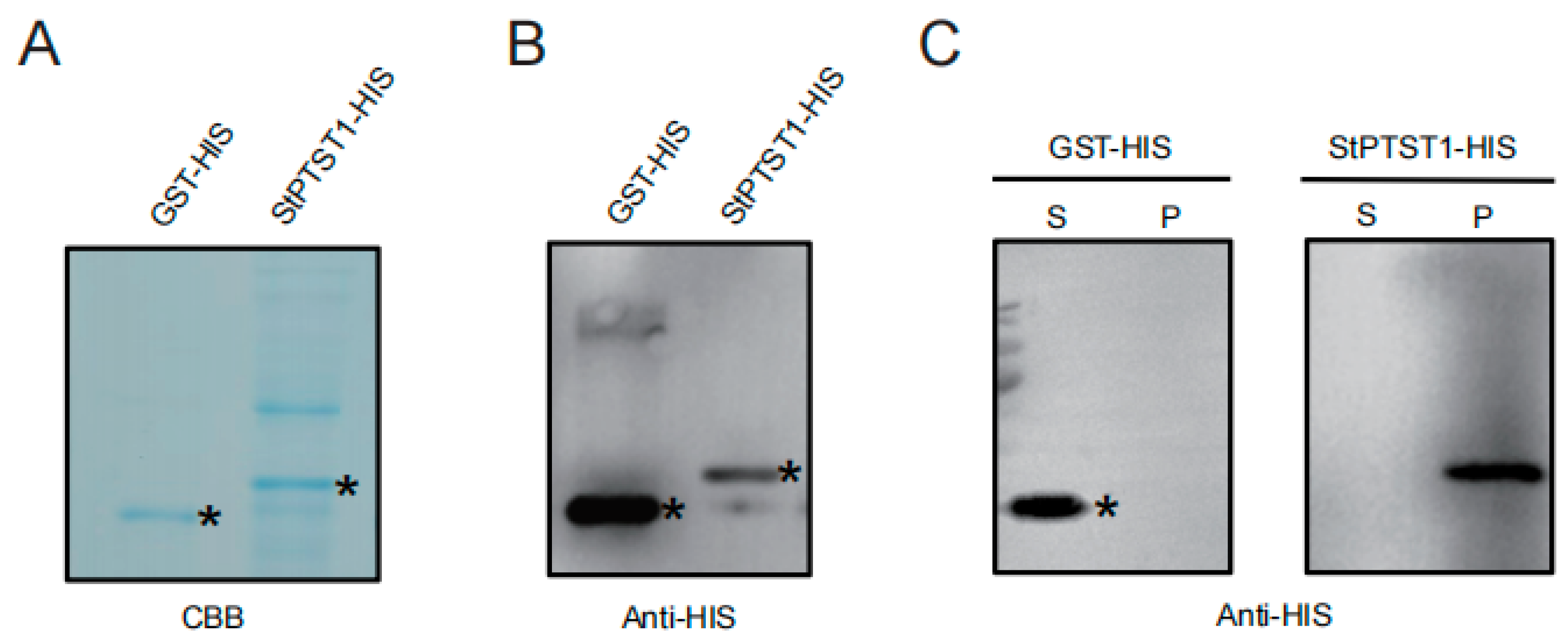
2.1. StPTST1 Binding to Starch
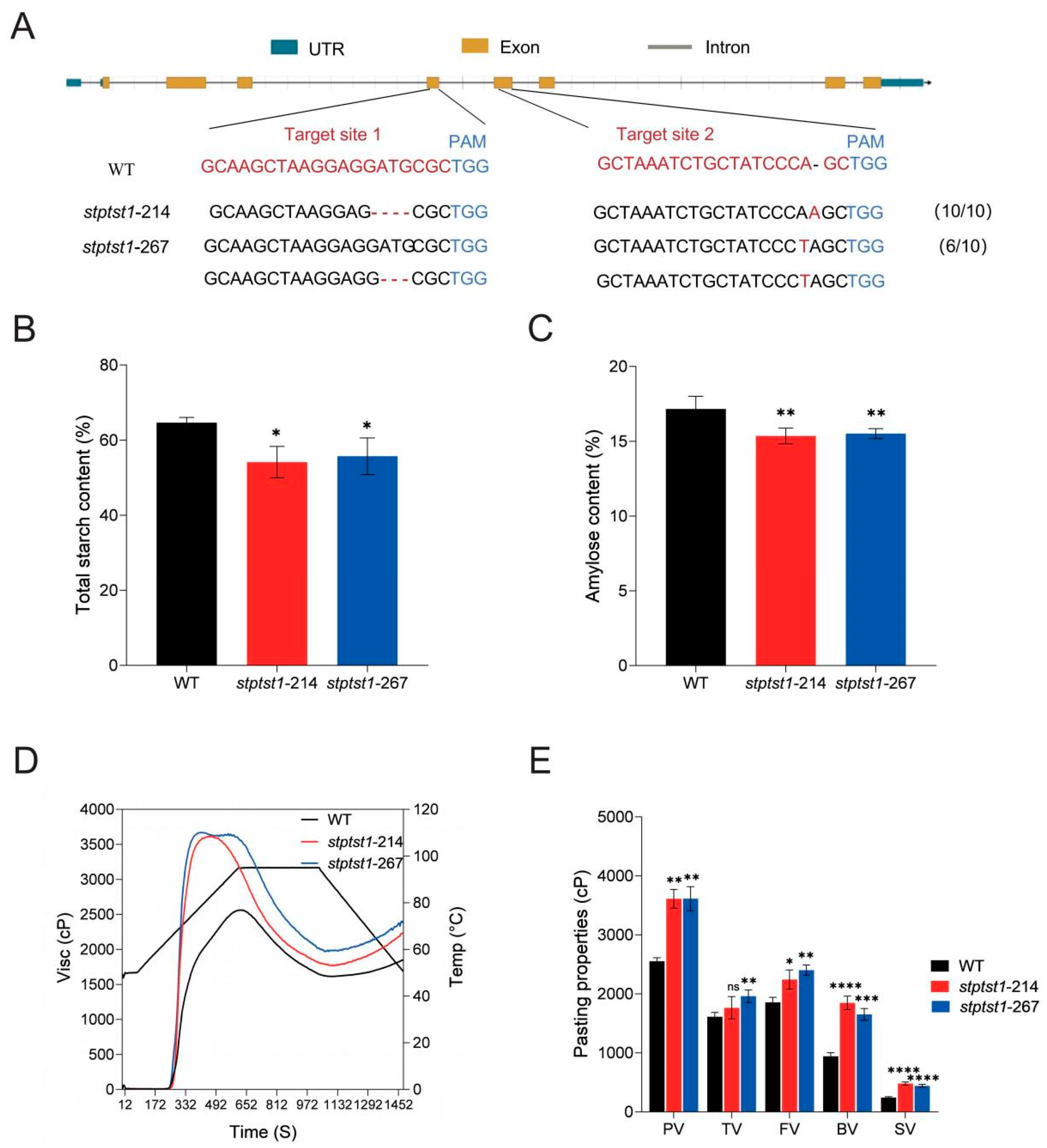
2.2. Knockout of StPTST1 Affected Starch Content and Starch Physicochemical Properties
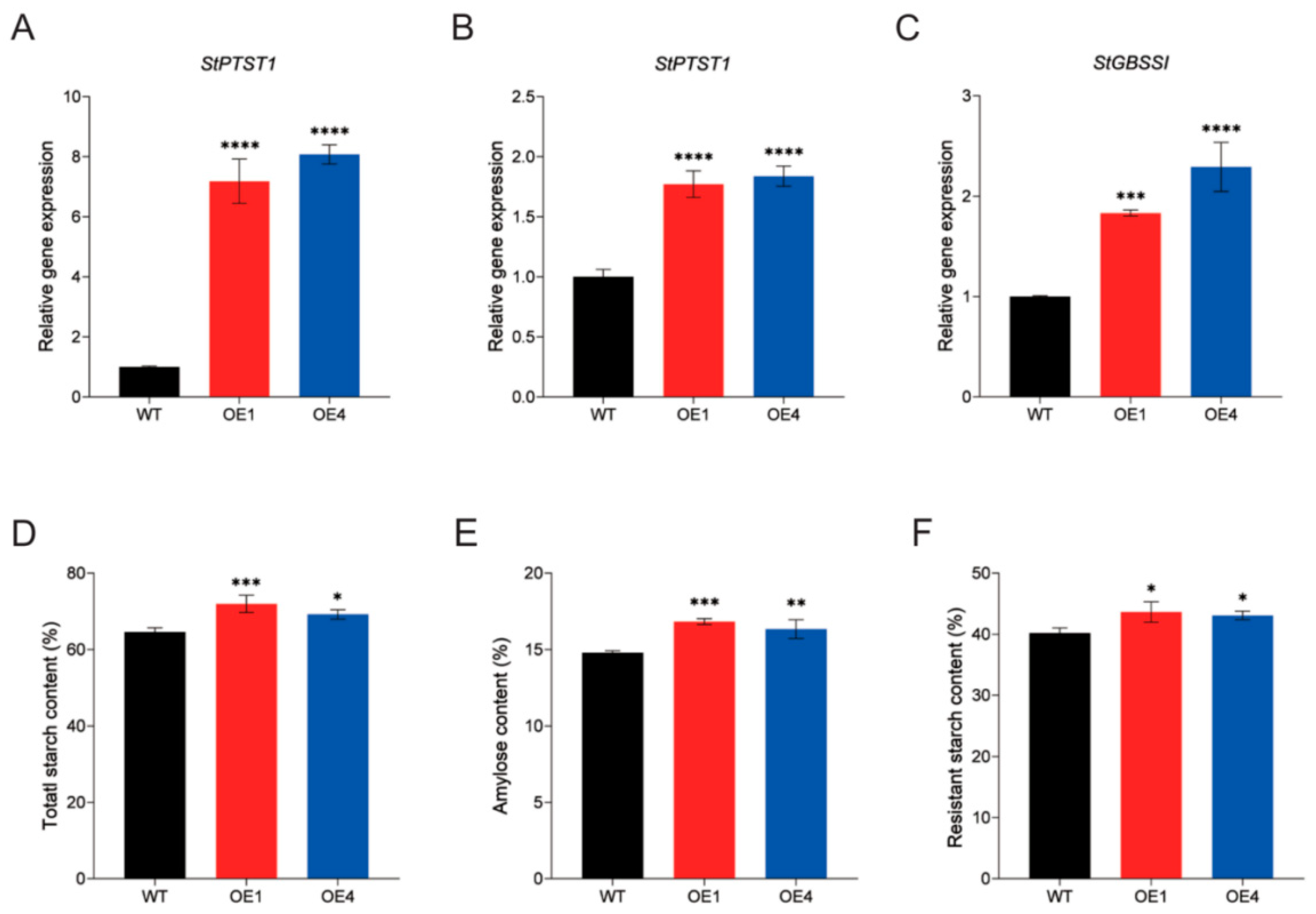
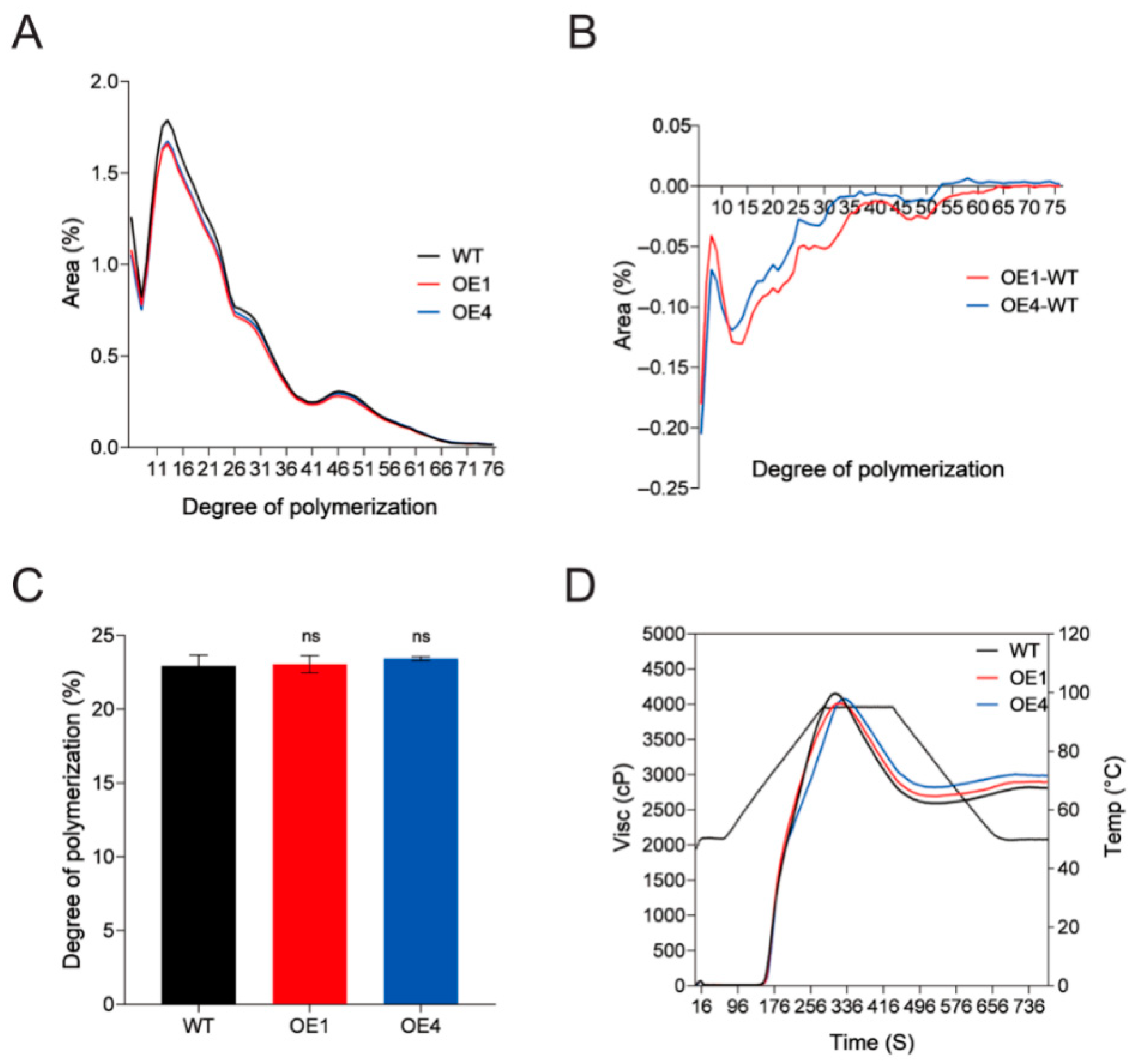
2.3. Overexpression of StPTST1 Led to Enhanced RS Content and Reduced Proportion of Short Chains
3. Discussion
4. Materials and Methods
4.1. Starch-Binding Assay
4.2. Engineering of Vectors and Transformation of Potato
4.3. Plant Growth and Molecular Analysis
4.4. Quantitative Real-Time PCR
4.5. Starch Extraction
4.6. Measurement of Total Starch, Amylose, and RS Contents
4.7. Chain Length Distribution Analysis
4.8. Pasting Property Analysis
4.9. Staining of Starch in Leaves
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, J.; Kaur, L.; McCarthy, Q.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Wang, S.J.; Li, C.L.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2022, 19, 2769–2789. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Zhen, X.; Che, Y.; Yu, H.; Ge, Y.; Wang, X.; Li, R.; Geng, M.; Zhou, B.; et al. Editing of the soluble starch synthase gene MeSSIII-1 enhanced the amylose and resistant starch contents in cassava. Carbohydr. Polym. 2025, 348, 122903. [Google Scholar] [CrossRef] [PubMed]
- Bojarczuk, A.; Skapska, S.; Khaneghah, A.M.; Marszatek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods. 2022, 93, 105094. [Google Scholar] [CrossRef]
- Liang, D.; Li, N.; Dai, X.; Zhang, H.; Hu, H.H. Effects of different types of potato resistant starches on intestinal microbiota and short-chain fatty acids under in vitro fermentation. Int. J. Food Sci. Technol. 2020, 56, 2432–2442. [Google Scholar] [CrossRef]
- Kweon, M.R.; Shin, M.S. Comparison of enzyme resistant starches formed during heat-moisture treatment and retrogradation of high amylose corn starches. Appl. Biol. Chem. 1997, 40, 508–513. [Google Scholar]
- Huang, X.J.; Zhang, C.H.; Zhao, Z.Y. Effect of amylose starch to the formation of resistant starch. Food Sci. Technol. 2006, 31, 18–20. [Google Scholar]
- Wang, Z. Research progress on influencing factors of resistant starch formation. Sci. Technol. Food Ind. 2007, 7, 216–220. [Google Scholar]
- Leloir, L.F.; De Fekete, M.A.; Cardini, C.E. Starch and oligosaccharide synthesis from uridine diphosphate glucose. J. Biol. Chem. 1961, 36, 636–641. [Google Scholar] [CrossRef]
- Myers, A.M.; Morell, M.K.; James, M.G.; Ball, S.G. Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol. 2000, 122, 989–997. [Google Scholar] [CrossRef]
- Sánchez, T.; Dufour, D.; Moreno, I.X.; Ceballos, H. Comparison of pasting and gel stabilities of waxy and normal starches from potato, maize, and rice with those of a novel waxy cassava starch under thermal, chemical, and mechanical stress. J. Agric. Food Chem. 2010, 58, 5093–5099. [Google Scholar] [CrossRef]
- Nakamura, T.; Vrinten, P.; Hayakawa, K.; Ikeda, J. Characterization of a granule-bound starch synthase isoform found in the pericarp of wheat. Plant Physiol. 1998, 118, 451–459. [Google Scholar] [CrossRef]
- Hovenkamp-Hermelink, J.H.M.; Jacobsen, E.; Ponstein, A.S.; Visser, R.G.F.; Vos-Scheperkeuter, G.H.; Bijmolt, E.W.; De Vries, J.N.; Witholt, B.; Feenstra, W.J. Isolation of an amylose-free starch mutant of the potato (Solanum tuberosum L.). Theor. Appl. Genet. 1987, 75, 217–221. [Google Scholar] [CrossRef]
- Martin, C.; Smith, A.M. Starch biosynthesis. Plant Cell. 1995, 7, 971–985. [Google Scholar]
- Cheng, J.; Khan, M.A.; Qiu, W.M.; Li, J.; Zhou, H.; Zhang, Q.; Guo, W.; Zhu, T.; Peng, J.; Sun, F.; et al. Diversification of genes encoding granule-bound starch synthase in monocots and dicots is marked by multiple genome-wide duplication events. PLoS ONE 2012, 7, e30088. [Google Scholar] [CrossRef]
- Zhong, Y.; Qu, J.Z.; Liu, X.; Ding, L.; Liu, Y.; Bertoft, E.; Petersen, B.L.; Hamaker, B.R.; Hebelstrup, K.H.; Blennow, A. Different genetic strategies to generate high amylose starch mutants by engineering the starch biosynthetic pathways. Carbohydr. Polym. 2022, 287, 119327. [Google Scholar] [CrossRef] [PubMed]
- Seung, D.; Soyk, S.; Coiro, M.; Maier, B.A.; Eicke, S.; Zeeman, S.C. PROTEIN TARGETING TO STARCH is required for localising GRANULE-BOUND STARCH SYNTHASE to starch granules and for normal amylose synthesis in Arabidopsis. PLoS Biol. 2015, 13, e1002080. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Blennow, A.; Kofoed-Enevoldsen, O.; Jiang, D.; Hebelstrup, K.H. Protein Targeting to Starch 1 is essential for starchy endosperm development in barley. J. Exp. Bot. 2019, 70, 485–496. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.; Jiao, G.; Chen, W.; Wu, Y.; Sheng, Z.; Hu, S.; Xie, L.; Wang, J.; Tang, S.; et al. GBSS-BINDING PROTEIN, encoding a CBM48 domain-containing protein, affects rice quality and yield. J. Integr. Plant Biol. 2020, 62, 948–966. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Liu, F.; Ren, Y.; Zhou, K.; Lv, J.; Zheng, M.; Zhao, S.; Zhang, L.; Wang, C.; et al. FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 2014, 77, 917–930. [Google Scholar] [CrossRef]
- Shi, Z.M.; Yang, H.Q. Cloning of PTST1 and analysis of its expression and interaction in potato. Plant Physio. J. 2023, 59, 1575–1578. [Google Scholar]
- Cai, J.; Man, J.; Huang, J.; Liu, Q.; Wei, W.; Wei, C. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohydr. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef]
- You, H.; Liang, C.; Zhang, O.; Xu, H.; Xu, L.; Chen, Y.; Xiang, X. Variation of resistant starch content in different processing types and their starch granules properties in rice. Carbohydr. Polym. 2022, 276, 118742. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Liu, G.; Meng, X.; Jing, Y.; Shu, X.; Kong, X.; Sun, J.; Yu, H.; Smith, S.M.; et al. Critical roles of soluble starch synthase SSIIIa and granule-bound starch synthase Waxy in synthesizing resistant starch in rice. Proc. Natl. Acad. Sci. USA 2016, 113, 12844–12849. [Google Scholar] [CrossRef] [PubMed]
- Burlingame, B.; Mouille, B.; Charrondiere, R. Nutrients, bioactive non-nutrients and anti-nutrients in potatoes. J. Food Compos. Anal. 2009, 22, 494–502. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and nutrition–A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef]
- Navarre, D.A.; Pillai, S.S.; Shakya, R. HPLC profiling of phenolics in diverse potato genotypes. Food Chem. 2011, 127, 34–41. [Google Scholar] [CrossRef]
- Baldwin, P.M. Starch granule-associated proteins and polypeptides: A review. Starch-Stärke 2001, 53, 475–503. [Google Scholar] [CrossRef]
- Ahmad, D.; Ying, Y.; Bao, J. Understanding starch biosynthesis in potatoes for metabolic engineering to improve starch quality: A detailed review. Carbohydr. Polym. 2024, 346, 122592. [Google Scholar] [CrossRef]
- Helle, S.; Bray, F.; Verbeke, J.; Devassine, S.; Courseaux, A.; Facon, M.; Tokarski, C.; Rolando, C.; Szydlowski, N. Proteome analysis of potato starch reveals the presence of new starch metabolic proteins as well as multiple protease inhibitors. Front. Plant Sci. 2018, 9, 746. [Google Scholar] [CrossRef]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W.; et al. Accelerated ex situ breeding of GBSS—And PTST1—Edited cassava for modified starch. Sci. Adv. 2018, 4, eaat6086. [Google Scholar] [CrossRef]
- Katayama, K.; Nishinaka, M.; Nakamura, Y.; Kuranouchi, T.; Ohara-Takada, A.; Fujita, K.; Kitahara, K. New sweetpotato lines have high amylose and resistant starch contents. Starch-Stärke 2018, 71, 1800180. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miura, H.; Fukushima, M.; Ito, M.; Nishio, Z.; Kim, S.-J.; Hashimoto, N.; Noda, T.; Takigawa, S.; Matsuura-Endo, C.; et al. Physical properties of yellow alkaline noodles from near-isogenic wheat lines with different Wx protein deficiency. Starch-Stärke 2006, 58, 186–195. [Google Scholar] [CrossRef]
- Sharma, R.; Sissons, M.J.; Rathjen, A.J.; Jenner, C.F. The Null-4A allele at the waxy locus in durum wheat affects pasta cooking quality. J. Cereal Sci. 2002, 35, 287–297. [Google Scholar] [CrossRef]
- Chen, Z.; Schols, H.A.; Voragen, A.G.J. Starch granule size strongly determines starch noodle processing and noodle quality. J. Food Sci. 2003, 68, 1584–1589. [Google Scholar] [CrossRef]
- Guo, L.; Chen, H.; Zhang, Y.; Yan, S.; Chen, X.; Gao, X. Starch granules and their size distribution in wheat: Biosynthesis, physicochemical properties and their effect on flour-based food systems. Comput. Struct. Biotechnol. 2023, 21, 4172–4186. [Google Scholar] [CrossRef]
- Huang, X.F.; Nazarian-Firouzabadi, F.; Vincken, J.P.; Ji, Q.; Visser, R.G.; Trindade, L.M. Expression of an amylosucrase gene in potato results in larger starch granules with novel properties. Planta 2014, 240, 409–421. [Google Scholar] [CrossRef]
- Kumar, R.; Khatkar, B.S. Thermal, pasting and morphological properties of starch granules of wheat (Triticum aestivum L.) varieties. J. Food Sci. Technol. 2017, 54, 2403–2410. [Google Scholar] [CrossRef]
- Yu Chi, M.; Wu, J.M.; Gao, L.; Liu, D.Y.; Qu, L. Hypoglycemic and lipid lowering effects of potato resistant starch on hyperlipidemia rats. Mod. Food Sci. Technol. 2022, 38, 8–14. [Google Scholar]
- Xu, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. A more pronounced effect of type III resistant starch vs. type II resistant starch on ameliorating hyperlipidemia in high fat diet-fed mice is associated with its supramolecular structural characteristics. Food Funct. 2020, 11, 1982–1995. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, C.; Lin, S.; Zheng, M.; Chen, C.; Zheng, B.; Zhang, Y. Lotus seed resistant starch regulates gut microbiota and increases short-chain fatty acids production and mineral absorption in mice. J. Agric. Food Chem. 2017, 65, 9217–9225. [Google Scholar] [CrossRef]
- Zhu, X.R.; Ji, J.; Wang, G.; Ma, Z.G.; Yang, D.; Jin, C.; Li, C. Influence on the conversion efficiency of induced differentiation of various potato tissues. China Biotech. 2016, 36, 53–59. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Luo, S.; Ma, Q.; Zhong, Y.; Jing, J.; Wei, Z.; Zhou, W.; Lu, X.; Tian, Y.; Zhang, P. Editing of the starch branching enzyme gene SBE2 generates high-amylose storage roots in cassava. Plant Mol. Biol. 2022, 108, 429–442. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Cao, X.; Zhou, H.; Yu, T.; Shang, Y.; Xu, J.; Gao, D. Manipulation of StPTST1 Affects Starch Content and Physicochemical Properties of Potato (Solanum tuberosum L.). Plants 2025, 14, 3351. https://doi.org/10.3390/plants14213351
Shi Z, Cao X, Zhou H, Yu T, Shang Y, Xu J, Gao D. Manipulation of StPTST1 Affects Starch Content and Physicochemical Properties of Potato (Solanum tuberosum L.). Plants. 2025; 14(21):3351. https://doi.org/10.3390/plants14213351
Chicago/Turabian StyleShi, Zhenming, Xiaoyi Cao, Hongyuan Zhou, Ting Yu, Yi Shang, Jianfei Xu, and Dongli Gao. 2025. "Manipulation of StPTST1 Affects Starch Content and Physicochemical Properties of Potato (Solanum tuberosum L.)" Plants 14, no. 21: 3351. https://doi.org/10.3390/plants14213351
APA StyleShi, Z., Cao, X., Zhou, H., Yu, T., Shang, Y., Xu, J., & Gao, D. (2025). Manipulation of StPTST1 Affects Starch Content and Physicochemical Properties of Potato (Solanum tuberosum L.). Plants, 14(21), 3351. https://doi.org/10.3390/plants14213351





