Insecticidal Potential of Aniba canelilla (H.B.K.) Mez Essential Oil Against Aedes aegypti: Larvicidal and Adulticidal Activities, Mechanism of Action, and Formulation Development
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Essential Oil from Aniba canelilla (EOANIB) and Isolation of 1-Nitro-2-Phenylethane (NFTANE)
2.2. Evaluation of Polymeric Micelles Loaded with EOANIB, NFTANE and NFTENE
2.2.1. Zeta Potential (ZP) and Particle Size
2.2.2. Accelerated Stability Assay of the Formulations
2.2.3. Room Temperature Stability Assay of PEOANIB, PNFTANE and PFTENO Formulations
2.3. Larvicidal Activity
2.4. Adulticidal Activity
2.5. Toxicity to the Non-Target Insect (Artemia salina)
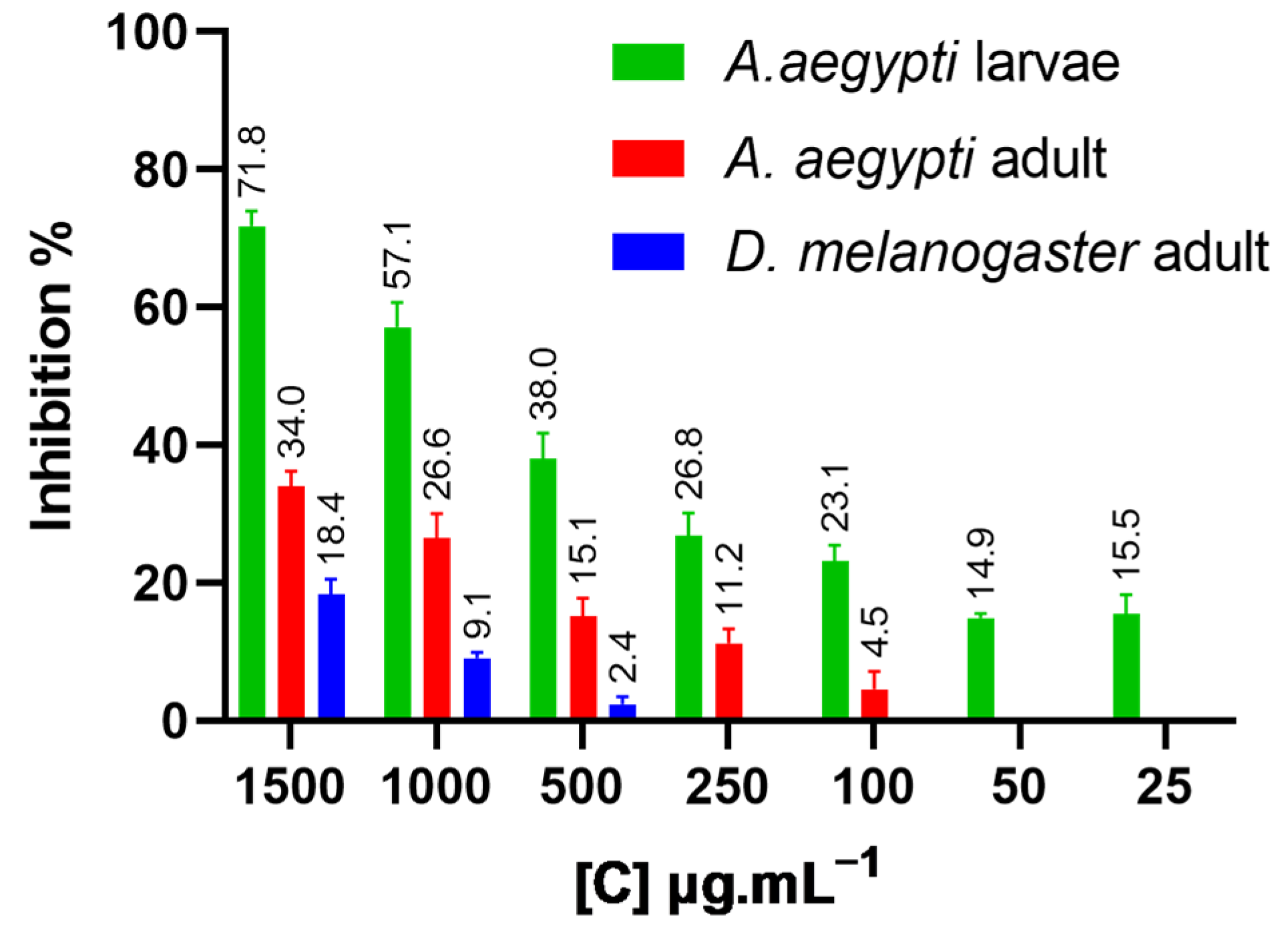
2.6. Toxicity to the Non-Target Insect (Drosophila melanogaster)
2.7. Acetylcholinesterase (AChE) Inhibition Activity
2.7.1. Inhibitory Activity on Commercial Enzyme (Electrophorus electricus)
2.7.2. Inhibitory Activity in Insect Homogenates
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Plant Material
3.3. Obtention of the Essential Oil from Aniba canelilla (EOANIB) and Isolation of 1-Nitro-2-Phenylethane (NFTANE)
3.4. GC–MS Analysis of Samples
3.5. Preparation of Micelles with Pluronic® F-127 (PF-127)
3.5.1. Micelle Evaluation
3.5.2. Stability Evaluation Under Thermal Stress and at Room Temperature
3.6. Larvicidal Activity Against Aedes aegypti
3.7. Adulticidal Activity Against Aedes aegypti
3.8. Non-Target Organism Assay
3.8.1. Non-Target Toxicity (Artemia salina)
3.8.2. Non-Target Organism Toxicity (Drosophila melanogaster)
3.9. Enzymatic Assays
3.9.1. Preparation of Aedes aegypti (Adults and L3 Larvae) and Drosophila melanogaster Adult Homogenates
3.9.2. Total Protein, Catalytic Units (µmol∙min−1), and Specific Activity
3.9.3. Acetylcholinesterase (AChE) Inhibition Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOANIB | Essential oil leaves of Aniba canelilla |
| NFTANE | 1-Nitro-2-phenylethane |
| NFTENE | 1-Nitro-2-phenylethene |
| PEOANIB | Formulation Essential oil leaves of Aniba canelilla |
| PNFTANE | Formulation 1-Nitro-2-phenylethane |
| PNFTENE | Formulation 1-Nitro-2-phenylethene |
| AChE | Acetylcholinesterase |
| NTDs | Neglected tropical diseases |
| WHO | World Health Organization |
| DMSO | Dimethyl sulfoxide |
| BCA | Bicinchoninic acid |
References
- World Health Organization. Global Diffusion of EHealth: Making Universal Health Coverage Achievable: Report of the Third Global Survey on EHealth; WHO: Geneva, Switzerland, 2017; Volume 1, ISBN 978-92-4-151178-0. [Google Scholar]
- Branda, F.; Ali, A.Y.; Ceccarelli, G.; Albanese, M.; Binetti, E.; Giovanetti, M.; Ciccozzi, M.; Scarpa, F. Assessing the Burden of Neglected Tropical Diseases in Low-Income Communities: Challenges and Solutions. Viruses 2024, 17, 29. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-NG, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C. Alternative Strategies for Mosquito-Borne Arbovirus Control. PLoS Neglected Trop. Dis. 2019, 13, e0006822. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A.; Ignacimuthu, S. Efficacy of Plant Products in Controlling Disease Vector Mosquitoes, a Review. Entomol. Exp. Appl. 2024, 172, 195–214. [Google Scholar] [CrossRef]
- Pollett, S.; Melendrez, M.C.; Maljkovic Berry, I.; Duchêne, S.; Salje, H.; Cummings, D.A.T.; Jarman, R.G. Understanding Dengue Virus Evolution to Support Epidemic Surveillance and Counter-Measure Development. Infect. Genet. Evol. 2018, 62, 279–295. [Google Scholar] [CrossRef]
- Burt, F.J.; Chen, W.; Miner, J.J.; Lenschow, D.J.; Merits, A.; Schnettler, E.; Kohl, A.; Rudd, P.A.; Taylor, A.; Herrero, L.J. Chikungunya Virus: An Update on the Biology and Pathogenesis of This Emerging Pathogen. Lancet Infect. Dis. 2017, 17, e107–e117. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.S.; Costa Vasconcelos, P.F. da Microcephaly and Zika Virus. J. Pediatr. 2016, 92, 103–105. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO Alerta Epidemiológico Aumento de Casos de Dengue Na Região Das Américas 7 de Outubro de 2024. 2024, pp. 1–17. Available online: https://www.paho.org/en/documents/epidemiological-alert-increase-dengue-cases-americas-region-7-october-2024 (accessed on 30 July 2025).
- Brem, J.; Elankeswaran, B.; Erne, D.; Hedrich, N.; Lovey, T.; Marzetta, V.; Salvado, L.T.; Züger, C.; Schlagenhauf, P. Dengue “Homegrown” in Europe (2022 to 2023). New Microbes New Infect. 2024, 56, 101205. [Google Scholar] [CrossRef] [PubMed]
- Zatta, M.; Brichler, S.; Vindrios, W.; Melica, G.; Gallien, S. Autochthonous Dengue Outbreak, Paris Region, France, September–October 2023. Emerg. Infect. Dis. 2023, 29, 2538–2540. [Google Scholar] [CrossRef]
- PAHO/WHO Epidemiological Update Dengue, Chikungunya and Zika. 2023. Available online: https://www.paho.org/en/documents/epidemiological-update-dengue-chikungunya-and-zika-25-january-2023 (accessed on 30 July 2025).
- Frasca, F.; Sorrentino, L.; Fracella, M.; D’Auria, A.; Coratti, E.; Maddaloni, L.; Bugani, G.; Gentile, M.; Pierangeli, A.; d’Ettorre, G. An Update on the Entomology, Virology, Pathogenesis, and Epidemiology Status of West Nile and Dengue Viruses in Europe (2018–2023). Trop. Med. Infect. Dis. 2024, 9, 166. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Luz, T.R.S.A.; de Mesquita, L.S.S.; do Amaral, F.M.M.; Coutinho, D.F. Essential Oils and Their Chemical Constituents against Aedes aegypti L. (Diptera: Culicidae) Larvae. Acta Trop. 2020, 212, 105705. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P. Contemporary Status of Insecticide Resistance in the Major Aedes Vectors of Arboviruses Infecting Humans. PLoS Neglected Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Cubeddu, L.X.; Gill, E.J. Harnessing Nanotechnology to Enhance Essential Oil Applications. Molecules 2025, 30, 520. [Google Scholar] [CrossRef]
- Silva, J.K.R.; Sousa, P.J.C.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant Capacity and Cytotoxicity of Essential Oil and Methanol Extract of Aniba canelilla (H.B.K.) Mez. J. Agric. Food Chem. 2007, 55, 9422–9426. [Google Scholar] [CrossRef]
- Kreutz, T.; Carneiro, S.B.; Soares, K.D.; Limberger, R.P.; Apel, M.A.; Veiga-Junior, V.F.; Koester, L.S. Aniba canelilla (Kunth) Mez Essential Oil-Loaded Nanoemulsion: Improved Stability of the Main Constituents and in Vitro Antichemotactic Activity. Ind. Crops Prod. 2021, 171, 113949. [Google Scholar] [CrossRef]
- da Trindade, R.C.S.; Xavier, J.K.A.M.; Setzer, W.N.; Maia, J.G.S.; da Silva, J.K.R. Chemical Diversity and Therapeutic Effects of Essential Oils of Aniba Species from the Amazon: A Review. Plants 2021, 10, 1854. [Google Scholar] [CrossRef]
- Silva, N.N.S.; Silva, J.R.A.; Alves, C.N.; Andrade, E.H.A.; Silva, J.K.R.; Maia, J.G.S. Acetylcholinesterase Inhibitory Activity and Molecular Docking Study of 1-Nitro-2-Phenylethane, the Main Constituent of Aniba canelilla Essential Oil. Chem. Biol. Drug Des. 2014, 84, 192–198. [Google Scholar] [CrossRef] [PubMed]
- de Campos, D.L.; Queiroz, L.Y.; Fontes-Junior, E.A.; Pinheiro, B.G.; da Silva, J.K.R.; Maia, C.S.F.; Maia, J.G.S. Aniba canelilla (Kunth) Mez Essential Oil and Its Primary Constituent, 1-Nitro-2-Phenylethane, Inhibits Acetylcholinesterase and Reverse Memory Impairment in Rodents. J. Ethnopharmacol. 2023, 303, 116036. [Google Scholar] [CrossRef]
- Sugimoto, M.; Amazonas, J.; Brito, L.F.; Borges, S.; Amaral, F.; De, A.; Ordoñez, M.; Tavares, J.C.; Sousa, L.P.; Lima, E. Anti-Inflammatory Potential of 1-Nitro-2-Phenylethylene. Molecules 2017, 22, 1977. [Google Scholar] [CrossRef]
- Vale, J.; Lima, A.; Pinheiro, B.; Cardoso, A.; Silva, J.; Maia, J.; de Sousa, G.; da Silva, A.; Sousa, P.; Borges, R. Evaluation and Theoretical Study on the Anti-Inflammatory Mechanism of 1-Nitro-2-Phenylethane. Planta Medica 2013, 79, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Bruns, R.F.; Watson, I.A. Rules for Identifying Potentially Reactive or Promiscuous Compounds. J. Med. Chem. 2012, 55, 9763–9772. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, M.L.; Tidor, B. Specificity in Molecular Design: A Physical Framework for Probing the Determinants of Binding Specificity and Promiscuity in a Biological Environment. J. Phys. Chem. B 2007, 111, 13419–13435. [Google Scholar] [CrossRef] [PubMed]
- Tarcsay, Á.; Keserű, G.M. Contributions of Molecular Properties to Drug Promiscuity. J. Med. Chem. 2013, 56, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Barbosa, L.; Rodrigues, K.M.S.; Souza-Neto, F.C.V.; Duarte, G.P.; Borges, R.S.; Magalhães, P.J.C.; Lahlou, S. Vasorelaxant Effects of 1-Nitro-2-Phenylethene in Rat Isolated Aortic Rings. Vasc. Pharmacol. 2014, 63, 55–62. [Google Scholar] [CrossRef]
- Viana, V.C.R.; Machado, F.P.; Esteves, R.; Duarte, J.A.D.; Enríquez, J.J.S.; Campaz, M.L.M.; Oliveira, E.E.; Santos, M.G.; Ricci-Junior, E.; Ruppelt, B.M. Green Nanobioinsecticide of a Brazilian Endemic Plant for the Aedes aegypti Control. Sustain. Chem. Pharm. 2023, 32, 100992. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, X.; Sun, Y.; Ma, L.; Shen, B.; Zhu, C. Genomic Analysis of Detoxification Supergene Families in the Mosquito Anopheles sinensis. PLoS ONE 2015, 10, e0143387. [Google Scholar] [CrossRef]
- Maldaner, J.; Oliveira, M.N.; Santos, D.D.A.; Silva, S.Y.S.; Silva, S.D.C.; Lima, T.D.C.; Silva, M.L.D.; Silva, S.H.T.L.; Siqueira-Silva, D.H.; Steffen, G.P.K. Bioherbicide and Anesthetic Potential of Aniba canelilla Essential Oil, a Contribution to the Demands of the Agricultural Sector. Biocatal. Agric. Biotechnol. 2022, 42, 102353. [Google Scholar] [CrossRef]
- Taveira, F.S.N.; de Lima, W.N.; Andrade, E.H.A.; Maia, J.G.S. Seasonal Essential Oil Variation of Aniba canelilla. Biochem. Syst. Ecol. 2003, 31, 69–75. [Google Scholar] [CrossRef]
- Manhães, A.P.; da Veiga-Júnior, V.F.; Wiedemann, L.S.M.; Fernandes, K.S.; Sampaio, P.d.T.B. Biomass Production and Essential Oil Yield from Leaves, Fine Stems and Resprouts Using Pruning the Crown of Aniba canelilla (H.B.K.) (Lauraceae) in the Central Amazon. Acta Amaz. 2012, 42, 355–362. [Google Scholar] [CrossRef]
- Barbosa, P.C.S.; Fernandes, K.S.; Manhães, A.P.; Carneiro, S.B.; Sampaio, P.d.T.B.; Wiedemann, L.S.M.; Junior, V.F.D.V. New and Sustainable Essential Oils Obtained from the Long-Term Explored Cinnamomum-like Aniba canelilla. J. Appl. Res. Med. Aromat. Plants 2017, 5, 60–71. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Owolabi, M.S.; Adewale, O.R.; Dosoky, N.S.; Setzer, W.N. 1-Nitro-2-Phenylethane Dominates the Chemical Composition of the Leaf Essential Oil of Uvaria chamae from Badagry, Nigeria. AJEONP 2013, 1, 48–50. [Google Scholar]
- Gottlieb, O.; Magalhaes, M. Communications Occurrence of 1-Nitro-2-Phenylethane in Octea pretiosa and Aniba canelilla. The J. Org. Chem. 1959, 24, 2070–2071. [Google Scholar] [CrossRef]
- Ugheighele, S.E.; Imafidon, K.E.; Choudhary, M.I.; Shakil, A.; Khan, M.; Sherwani, Z.A. Zaheer Ul-Haq Anti-Urease and Cytotoxic Activity of 1-Nitro-2-Phenylethane and Nerolidol; Two Major Compounds Isolated from the Seeds of Dennettia tripetala. Med. Chem. Res. 2020, 29, 1874–1881. [Google Scholar] [CrossRef]
- Giongo, J.L.; Vaucher, R.A.; Da Silva, A.S.; Oliveira, C.B.; de Mattos, C.B.; Baldissera, M.D.; Sagrillo, M.R.; Monteiro, S.G.; Custódio, D.L.; Matos, M.S. Trypanocidal Activity of the Compounds Present in Aniba canelilla Oil against Trypanosoma evansi and Its Effects on Viability of Lymphocytes. Microb. Pathog. 2017, 103, 13–18. [Google Scholar] [CrossRef]
- Silva, J.R.d.A.; do Carmo, D.F.M.; Reis, É.M.; Machado, G.M.C.; Leon, L.L.; da Silva, B.O.; Ferreira, J.L.P.; Amaral, A.C.F. Chemical and Biological Evaluation of Essential Oils with Economic Value from Lauraceae Species. J. Braz. Chem. Soc. 2009, 20, 1071–1076. [Google Scholar] [CrossRef]
- Siqueira, R.J.B.; Macedo, F.I.B.; Interaminense, L.F.L.; Duarte, G.P.; Magalhães, P.J.C.; Brito, T.S.; Silva, J.K.R.; Maia, J.G.S.; Sousa, P.J.C.; Leal-Cardoso, J.H. 1-Nitro-2-Phenylethane, the Main Constituent of the Essential Oil of Aniba canelilla, Elicits a Vago-Vagal Bradycardiac and Depressor Reflex in Normotensive Rats. Eur. J. Pharmacol. 2010, 638, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Souza-Junior, F.J.C.; Luz-Moraes, D.; Pereira, F.S.; Barros, M.A.; Fernandes, L.M.P.; Queiroz, L.Y.; Maia, C.F.; Maia, J.G.S.; Fontes-Junior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae): A Review of Ethnobotany, Phytochemical, Antioxidant, Anti-Inflammatory, Cardiovascular, and Neurological Properties. Front. Pharmacol. 2020, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.M.; Rocha, R.L.; Brandão, C.M.; Xavier, J.K.A.M.; Camara, M.B.P.; Mendonça, C.d.J.S.; de Lima, R.B.; Souza, M.P.; Costa, E.V.; Gonçalves, R.S. Development of an Eco-Friendly Nanogel Incorporating Pectis brevipedunculata Essential Oil as a Larvicidal Agent against Aedes aegypti. Pharmaceutics 2024, 16, 1337. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.L.; Rodrigues, A.B.L.; Rabelo, É.M.; Santos, L.L.; Brandão, E.B.; Faustino, C.G.; Farias, A.L.F.; Sá, D.M.C.; Cantuária, P.C.; Galardo, A.K.R. Development of Larvicide Nanoemulsion from the Essential Oil of Aeollanthus suaveolens Mart. Ex Spreng against Aedes aegypti, and Its Toxicity in Non-Target Organism. Arab. J. Chem. 2021, 14, 103148. [Google Scholar] [CrossRef]
- Santos, D.D.L.; Besegato, J.F.; Melo, P.B.G.; Junior, J.A.O.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.d.S. Curcumin—Loaded Pluronic® F—127 Micelles as a Drug Delivery System for Curcumin—Mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]
- Sarmah, K.; Anbalagan, T.; Marimuthu, M.; Mariappan, P.; Angappan, S.; Vaithiyanathan, S. Innovative Formulation Strategies for Botanical- and Essential Oil-Based Insecticides. J. Pest Sci. 2024, 98, 1–30. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Florence, A.T. Non-Ionic Surfactant Vesicles (Niosomes): Physical and Pharmaceutical Chemistry. Adv. Colloid Interface Sci. 1995, 58, 1–55. [Google Scholar] [CrossRef]
- Vicente, F.A.; Cardoso, I.S.; Sintra, T.E.; Lemus, J.; Marques, E.F.; Ventura, M.; Coutinho, P. Impact of Surface Active Ionic Liquids on the Cloud Points of Nonionic Surfactants and the Formation of Aqueous Micellar Two-Phase Systems. J. Phys. Chem. B 2017, 121, 8742–8755. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.P.; Folly, D.; Salas, J.; Mello, C.B.; Esteves, R.; Araújo, R.S.; Pedro, F.S.T.; Mantilla-Afanador, J.G.; Santos, M.G.; Oliveira, E.E. Nanoemulsion of Ocotea indecora (Shott) Mez Essential Oil: Larvicidal Effects against Aedes aegypti. Ind. Crops Prod. 2023, 192, 116031. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric Micelles as Drug Delivery Vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Bora, L.; Burkard, T.; Herrero, M.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F. Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Kumar, S.; Tripathi, A.; Basu, M.; Verma, G.; Sarma, H.D.; Chaudhari, D.P.; Aswal, V.K.; Melo, J.S. Structural and Therapeutic Properties of Pluronic® P123/F127 Micellar Systems and Their Modulation by Salt and Essential Oil. J. Mol. Liq. 2020, 310, 113231. [Google Scholar] [CrossRef]
- Thapa, R.K.; Cazzador, F.; Grønlien, K.G.; Tønnesen, H.H. Effect of Curcumin and Cosolvents on the Micellization of Pluronic F127 in Aqueous Solution. Colloids and Surfaces B. Biointerfaces 2020, 195, 111250. [Google Scholar] [CrossRef]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric Micelle Stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Esmaili, F.; Sanei-Dehkordi, A.; Osanloo, M. A Review on the Use of Essential Oil-Based Nanoformulations in Control of Mosquitoes. Biointerface Res. Appl. Chem. 2021, 11, 12516–12529. [Google Scholar] [CrossRef]
- Menossi, M.; Ollier, R.P.; Casalongué, C.A.; Alvarez, V.A. Essential Oil—Loaded Bio—Nanomaterials for Sustainable Agricultural Applications. J. Chem. Technol. Biotechnol. 2021, 96, 2109–2122. [Google Scholar] [CrossRef]
- Truzzi, E.; Vanti, G.; Grifoni, L.; Maretti, E.; Leo, E.; Bilia, A.R. Plant Resin Delivery by Nanovectors as an Emerging Approach to Boost Solubility, Permeability and Bioavailability. Pharmaceutics 2025, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C. Nano-Enabled Pesticides for Sustainable Agriculture and Global Food Security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.Q.d.S.; Rodrigues, G.d.S.; Barroso, A.d.S.; Figueiredo, P.L.B.; Machado, F.P.; Ferreira, M.A.; Fernandes, C.P.; dos Santos, G.B.; Mourão, R.H.V. Essential Oil of Lippia origanoides Kunth: Nanoformulation, Anticholinesterase Activity, and Molecular Docking. Molecules 2025, 30, 1554. [Google Scholar] [CrossRef]
- Zhang, W.; Gilstrap, K.; Wu, L.; Bahadur, R.; Moss, M.A.; Wang, Q.; Lu, X.; He, X. Synthesis and Characterization of Thermally Responsive Pluronic F127−Chitosan Nanocapsules for Controlled Release and Intracellular Delivery of Small Molecules. ACS Nano 2010, 4, 6747–6759. [Google Scholar] [CrossRef]
- Jaquilin, R.P.J.; Oluwafemi, O.S.; Thomas, S.; Oyedeji, A.O. Recent Advances in Drug Delivery Nanocarriers Incorporated in Temperature-Sensitive Pluronic F-127–a Critical Review. J. Drug Deliv. Sci. Technol. 2022, 72, 103390. [Google Scholar] [CrossRef]
- Singla, P.; Singh, O.; Sharma, S.; Betlem, K.; Aswal, V.K.; Peeters, M.; Mahajan, R.K. Temperature-Dependent Solubilization of the Hydrophobic Antiepileptic Drug Lamotrigine in Different Pluronic Micelles a Spectroscopic, Heat Transfer Method, Small-Angle Neutron Scattering, Dynamic Light Scattering, and in Vitro Release Study. ACS Omega 2019, 4, 11251–11262. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-Loaded Pluronic P123/F127 Mixed Polymeric Micelles: Formulation, Optimization and in Vitro Characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.H.; Kim, S.H.; Park, T.G. Thermally Sensitive Cationic Polymer Nanocapsules for Specific Cytosolic Delivery and Efficient Gene Silencing of SiRNA: Swelling Induced Physical Disruption of Endosome by Cold Shock. J. Control. Release 2008, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, J.-H.; Choi, S.-M.; Park, T.G. Thermally Reversible Pluronic/Heparin Nanocapsules Exhibiting 1000-Fold Volume Transition. Langmuir 2006, 22, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, S.H.; Park, T.G. Temperature-Sensitive Pluronic/Poly(Ethylenimine) Nanocapsules for Thermally Triggered Disruption of Intracellular Endosomal Compartment. Biomacromolecules 2006, 7, 1864–1870. [Google Scholar] [CrossRef]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green Micro-and Nanoemulsions for Managing Parasites, Vectors and Pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef]
- Lucia, A.; Girard, C.; Fanucce, M.; Coviella, C.; Rubio, R.G.; Ortega, F.; Guzmán, E. Development of an Environmentally Friendly Larvicidal Formulation Based on Essential Oil Compound Blend to Control Aedes aegypti Larvae: Correlations between Physicochemical Properties and Insecticidal Activity. ACS Sustain. Chem. Eng. 2020, 8, 10995–11006. [Google Scholar] [CrossRef]
- Valenzuela-Oses, J.K.; García, M.C.; Feitosa, V.A.; Pachioni-Vasconcelos, J.A.; Gomes-Filho, S.M.; Lourenço, F.R.; Cerize, N.N.P.; Bassères, D.S.; Rangel-Yagui, C.O. Development and Characterization of Miltefosine-Loaded Polymeric Micelles for Cancer Treatment. Mater. Sci. Eng. C 2017, 81, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Essential Oils for the Development of Eco-Friendly Mosquito Larvicides: A Review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Santos, D.R.; Chaves, L.L.; Pires, V.C.; Rodrigues, J.S.; Assunção, M.A.S.; Faierstein, G.B.; Neto, A.G.B.; Rebouças, J.S.; Albuquerque, E.C.M.; Melo, S.A.B.V. New Weapons against the Disease Vector Aedes aegypti: From Natural Products to Nanoparticles. Int. J. Pharm. 2023, 643, 123221. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.A.; Almeida, V.C.; Malheiros, B.; Castro, R.D.; Barbosa, L.R.S.; Cerize, N.N.P.; Oliveira Rangel-Yagui, C. Polymeric Micelles of Pluronic F127 Reduce Hemolytic Potential of Amphiphilic Drugs. Colloids Surf. B Biointerfaces 2019, 180, 177–185. [Google Scholar] [CrossRef]
- Sungkhaphan, P.; Risangud, N.; Hankamolsiri, W.; Sonthithai, P.; Janvikul, W. Pluronic-F127 and Click Chemistry-Based Injectable Biodegradable Hydrogels with Controlled Mechanical Properties for Cell Encapsulation. React. Funct. Polym. 2022, 181, 105439. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Sun, H.; Chen, S. Sustained Delivery of IL-1Ra from PF127-Gel Reduces Hyperglycemia in Diabetic GK-Rats. PLoS ONE 2013, 8, e55925. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.N.; Moraes, D.F.C. Essential Oils and their Compounds as Aedes aegypti L. (Diptera: Culicidae) Larvicides: Review. Parasitol. Res. 2013, 113, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Luz, T.R.S.A.; Leite, J.A.C.; Mesquita, L.S.S.; Bezerra, S.A.; Ribeiro, E.C.G.; Silveira, D.P.B.; Mesquita, J.W.C.; Amaral, F.M.M.; Coutinho, D.F. Seasonal Variation in the Chemical Composition and Larvicidal Activity against Aedes aegypti L. of Essential Oils from Brazilian Amazon. Exp. Parasitol. 2022, 243, 108405. [Google Scholar] [CrossRef] [PubMed]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant Natural Products for the Control of Aedes aegypti: The Main Vector of Important Arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef]
- Brogdon, W.; Chan, A. Guidelines for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay/Methods in Anopheles Research; CDC Technical Report. P 343; CDC: Atlanta, GA, USA, 2010; pp. 1–28. [Google Scholar]
- França, L.P.; Amaral, A.C.F.; Ramos, A.d.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.T.; Araujo, E.S.; Branches, A.D.S.; Silva, J.N.; Silva, N.G. Piper capitarianum Essential Oil: A Promising Insecticidal Agent for the Management of Aedes aegypti and Aedes albopictus. Environ. Sci. Pollut. Res. 2020, 28, 9760–9776. [Google Scholar] [CrossRef]
- de Almeida, R.R.P.; Souto, R.N.P.; Bastos, C.N.; da Silva, M.H.L.; Maia, J.G.S. Chemical Variation in Piper aduncum and Biological Properties of its Dillapiole-Rich Essential Oil. Chem. Biodivers. 2009, 6, 1427–1434. [Google Scholar] [CrossRef]
- García-Díaz, J.; Souto, R.N.P.; Escalona-Arranz, J.C.; Ferreira, R.M.D.A.; Costa, T.S.D.; González-Fernández, R.; Yamile, H.-D.; Idelsy, C.-N.; de la Vega, J.; Monzote, L. Larvicidal and Adulticidal Activity of Essential Oils from Four Cuban Plants against Three Mosquito Vector Species. Plants 2023, 12, 4009. [Google Scholar] [CrossRef]
- Hutcheson, R.P.; Ebrahimi, B.; Njiru, B.N.; Foster, W.A.; Jany, W. Attraction of the Mosquitoes Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to a 3-Part Phytochemical Blend in a Mesocosm. J. Med. Entomol. 2021, 59, 440–445. [Google Scholar] [CrossRef]
- Corzo-Gómez, J.C.; Espinosa-Juárez, J.V.; Ovando-Zambrano, J.C.; Briones-Aranda, A.; Cruz-Salomón, A.; Esquinca-Avilés, H.A. A Review of Botanical Extracts with Repellent and Insecticidal Activity and Their Suitability for Managing Mosquito-Borne Disease Risk in Mexico. Pathogens 2024, 13, 737. [Google Scholar] [CrossRef]
- Souza, M.A.; da Silva, L.; Macêdo, M.J.F.; Lacerda-Neto, L.J.; dos Santos, M.A.C.; Coutinho, H.D.M.; Cunha, F.A.B. Adulticide and Repellent Activity of Essential Oils against Aedes aegypti (Diptera: Culicidae) a Review. South Afr. J. Bot. 2019, 124, 160–165. [Google Scholar] [CrossRef]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Medica 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Rao, V.J.; Kavitha, P.; Jakka, N.M.; Sridhar, V.; Usman, P.K. Toxicity of Organophosphates on Morphology and Locomotor Behavior in Brine Shrimp, Artemia Salina. Arch. Environ. Contam. Toxicol. 2007, 53, 227–232. [Google Scholar] [CrossRef]
- Mok, Z.H. The Effect of Particle Size on Drug Bioavailability in Various Parts of the Body. Pharm. Sci. Adv. 2024, 2, 100031. [Google Scholar] [CrossRef]
- Lee, D.-H.; Eom, H.-J.; Kim, M.; Jung, J.-H.; Rhee, J.-S. Non-Target Effects of Antifouling Agents on Mortality, Hatching Success, and Acetylcholinesterase Activity in the Brine Shrimp Artemia salina. Toxicol. Environ. Health Sci. 2017, 9, 237–243. [Google Scholar] [CrossRef]
- Ferreira, O.O.; Cruz, J.N.; de Moraes, Â.A.B.; Franco, C.d.J.P.; Lima, R.R.; dos Anjos, T.O.; Siqueira, G.M.; do Nascimento, L.D.; Cascaes, M.M.; Oliveira, M.S. Essential Oil of the Plants Growing in the Brazilian Amazon: Chemical Composition, Antioxidants, and Biological Applications. Molecules 2022, 27, 4373. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraju, A.V.; Rao, T.V.N.; Sundararaju, D.; Vanisree, M.; Tsay, H.-S.; Subbaraju, G.V. Assessment of Bioactivity of Indian Medicinal Plants Using Brine Shrimp (Artemia salina) Lethality Assay. Int. J. Appl. Sci. Eng. 2005, 3, 125–134. [Google Scholar]
- Panini, M.; Manicardi, G.C.; Moores, G.D.; Mazzoni, E. An Overview of the Main Pathways of Metabolic Resistance in Insects. ISJ 2001, 13, 326–335. [Google Scholar] [CrossRef]
- Gnagey, A.L.; Forte, M.; Rosenberry, T.L. Isolation and Characterization of Acetylcholinesterase from Drosophila. J. Biol. Chem. 1987, 262, 13290–13298. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.T.; de Souza, M.T.; Morais, M.C.; Oliveira, D.d.C.; de Melo, D.J.; Figueiredo, L.; Zarbin, P.H.G.; Zawadneak, M.A.C.; Bernardi, D. Essential Oils as a Source of Ecofriendly Insecticides for Drosophila suzukii (Diptera: Drosophilidae) and Their Potential Non-Target Effects. Mol./Mol. Online/Mol. Annu. 2022, 27, 6215. [Google Scholar] [CrossRef] [PubMed]
- Demir, E. Drosophila as a Model for Assessing Nanopesticide Toxicity. Nanotoxicology 2020, 14, 1271–1279. [Google Scholar] [CrossRef]
- Emameh, R.Z.; Syrjänen, L.; Barker, H.; Supuran, C.T.; Parkkila, S. Drosophila melanogaster: A Model Organism for Controlling Diptera vectors and Pests. J. Enzym. Inhib. Med. Chem. 2014, 30, 505–513. [Google Scholar] [CrossRef]
- Staats, S.; Lüersen, K.; Wagner, A.E.; Rimbach, G. Drosophila Melanogaster as a Versatile Model Organism in Food and Nutrition Research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef]
- Duque, J.E.; Urbina, D.L.; Vesga, L.C.; Ortiz-Rodríguez, L.A.; Vanegas, T.S.; Stashenko, E.E.; Mendez-Sanchez, S.C. Insecticidal Activity of Essential Oils from American Native Plants against Aedes Aegypti (Diptera: Culicidae): An Introduction to Their Possible Mechanism of Action. Sci. Rep. 2023, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Bride, J.M.; Hoffmann, F.; Karch, F. Acetylcholinesterase. Two Types of Modifications Confer Resistance to Insecticide. J. Biol. Chem. 1992, 267, 14270–14274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, W.; Jian, R.; Ren, X.; Chen, X.; Hong, W.D.; Wu, M.; Cai, J.; Lao, C.; Xu, X. Larvicidal, Acetylcholinesterase Inhibitory Activities of Four Essential Oils and Their Constituents against Aedes albopictus, and Nanoemulsion Preparation. J. Pest Sci. 2022, 96, 961–971. [Google Scholar] [CrossRef]
- Kreutz, T.; Lucca, L.G.; Carneiro, S.B.; Limberger, R.P.; Veiga-Junior, V.F.; Araújo, B.V.; Teixeira, H.F.; Koester, L.S. Hydrogel-Thickened Nanoemulsion Containing Amazonian Aniba canelilla (Kunth) Mez Essential Oil: Skin Permeation and in Vivo Anti-Inflammatory Efficacy. J. Drug Deliv. Sci. Technol. 2023, 87, 104771. [Google Scholar] [CrossRef]
- Abrantes, D.C.; Rogério, C.B.; Ramos, V.; Germano-Costa, T.; Vigato, A.A.; Machado, I.P.; Sepulveda, A.F.; de Lima, R.; Ribeiro, D.; Fraceto, L.F. Repellent Active Ingredients Encapsulated in Polymeric Nanoparticles: Potential Alternative Formulations to Control Arboviruses. J. Nanobiotechnol. 2022, 20, 520. [Google Scholar] [CrossRef]
- Gomes, B.; Ogélio, H.; Brant, F.; Pereira-Pinto, C.J.; Workman, M.J.; Costa, M.M.; Bento, J.; Martins, A.J.; Ramalho-Ortigão, M.; Durvasula, R. High Larvicidal Efficacy of Yeast-Encapsulated Orange Oil against Aedes Aegypti Strains from Brazil. Parasites Vectors 2021, 14, 272. [Google Scholar] [CrossRef]
- Gomes, P.R.B.; Silva, A.L.S.; Pinheiro, H.A.; Carvalho, L.L.; Lima, H.S.; Silva, E.F.; Silva, R.P.; LouzeirO, C.H.; Oliveira, M.B.; Filho, V.E.M. Avaliação Da Atividade Larvicida Do Óleo Essencial Do Zingiber Officinale Roscoe (Gengibre) Frente Ao Mosquito Aedes aegypti. Rev. Bras. Plantas Med. 2016, 18, 597–604. [Google Scholar] [CrossRef]
- Corbel, V.; Kont, M.D.; Ahumada, M.L.; Andréo, L.; Bayili, B.; Bayili, K.; Brooke, B.; Pinto Caballero, J.A.; Lambert, B.; Churcher, T.S. A New WHO Bottle Bioassay Method to Assess the Susceptibility of Mosquito Vectors to Public Health Insecticides: Results from a WHO-Coordinated Multi-Centre Study. Parasites Vectors 2023, 16, 21. [Google Scholar] [CrossRef]
- Denlinger, D.S.; Creswell, J.A.; Anderson, J.L.; Reese, C.K.; Bernhardt, S.A. Diagnostic Doses and Times for Phlebotomus papatasi and Lutzomyia longipalpis Sand Flies (Diptera: Psychodidae: Phlebotominae) Using the CDC Bottle Bioassay to Assess Insecticide Resistance. Parasites Vectors 2016, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Valle, D.; Montella, I.R.; Ribeiro, R.A.; Viana-Medeiros, P.F.; Martins-Júnior, A.J.; Lima, J.B.P. Quantification Methodology for Enzyme Activity Related to Insecticide Resistance in Aedes aegypti; Fundação Oswaldo Cruz, Secretaria de Vigilância em Saúde/Ministério da Saúde: Rio de Janeiro, Brasília, 2006; pp. 1–129. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, E.N.; Díaz-González, E.E.; Marina, C.F.; Bond, J.G.; Rodríguez-Rojas, J.J.; Ponce-García, G.; Sánchez-Casas, R.M.; Fernández-Salas, I. Temporal Viability of Aedes aegypti and Aedes Albopictus Eggs Using Two Hygroscopic Substances as Preservatives under a Sterile Insect Technique (SIT) Program in Southern Mexico. Insects 2021, 13, 15. [Google Scholar] [CrossRef] [PubMed]
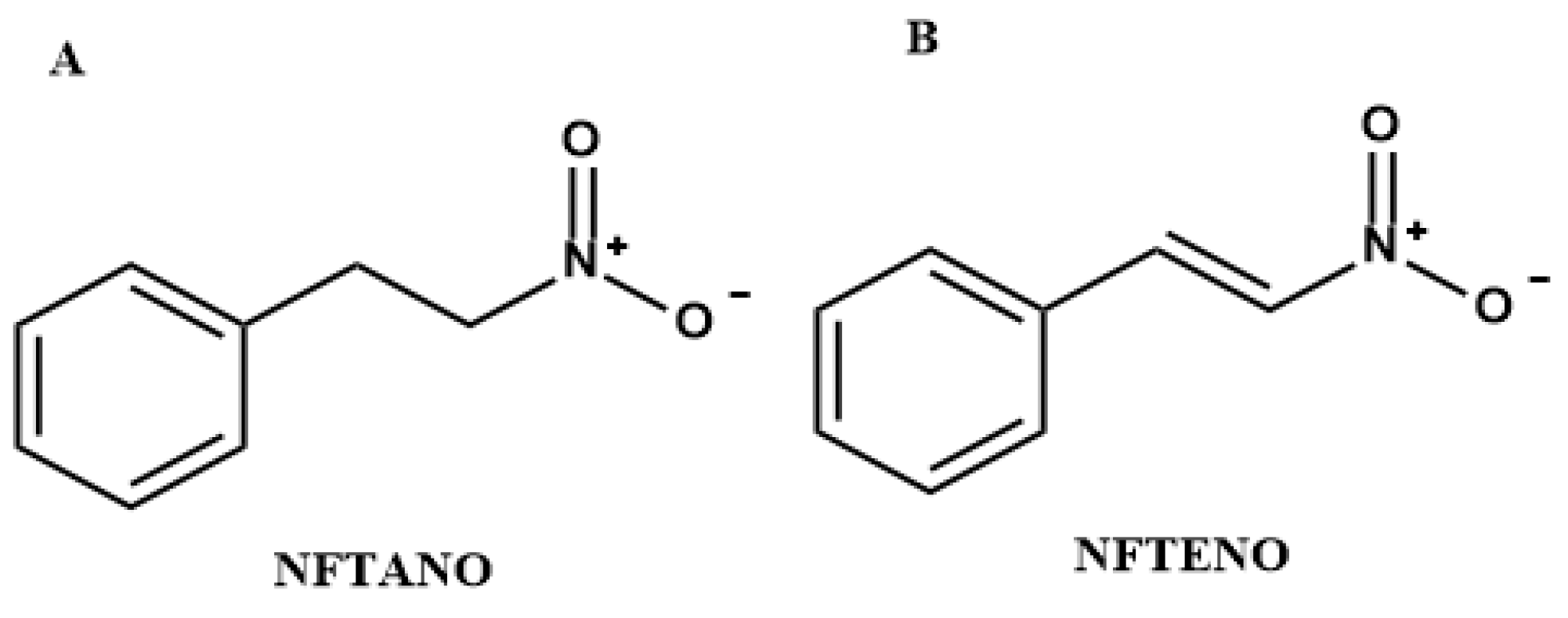

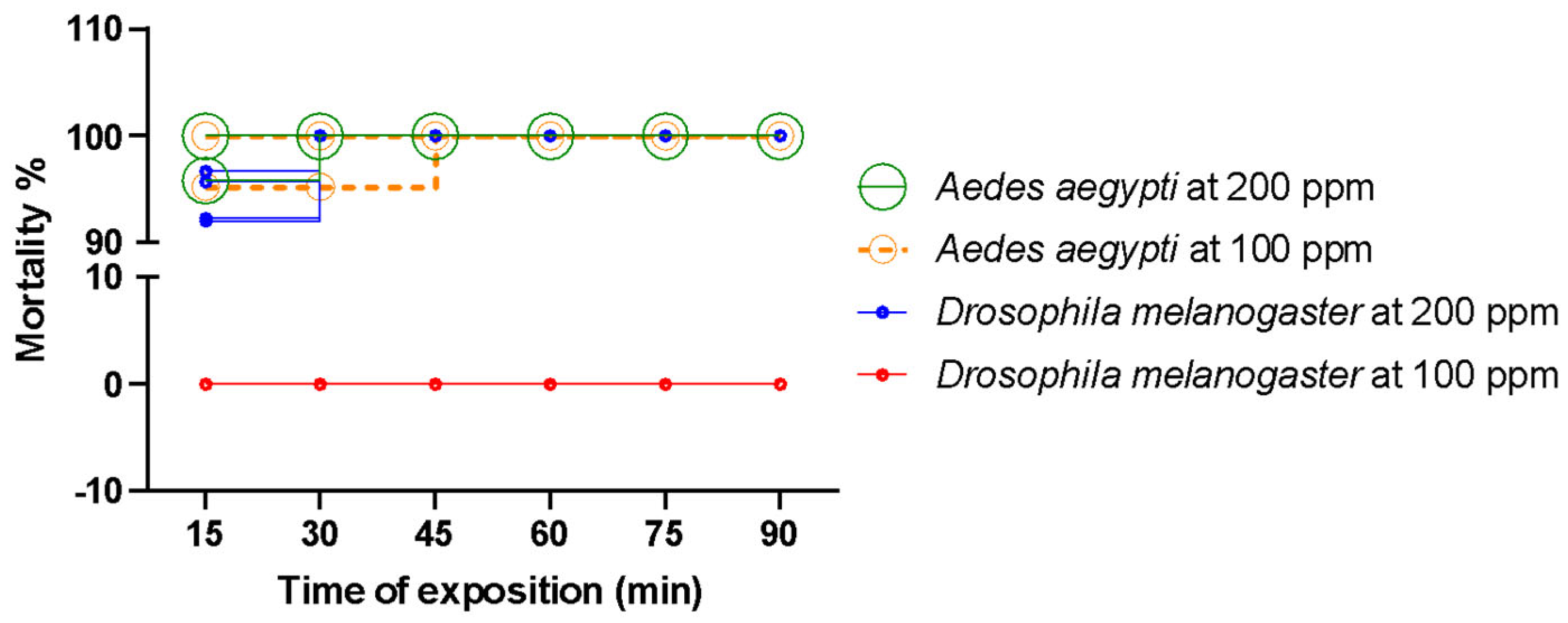
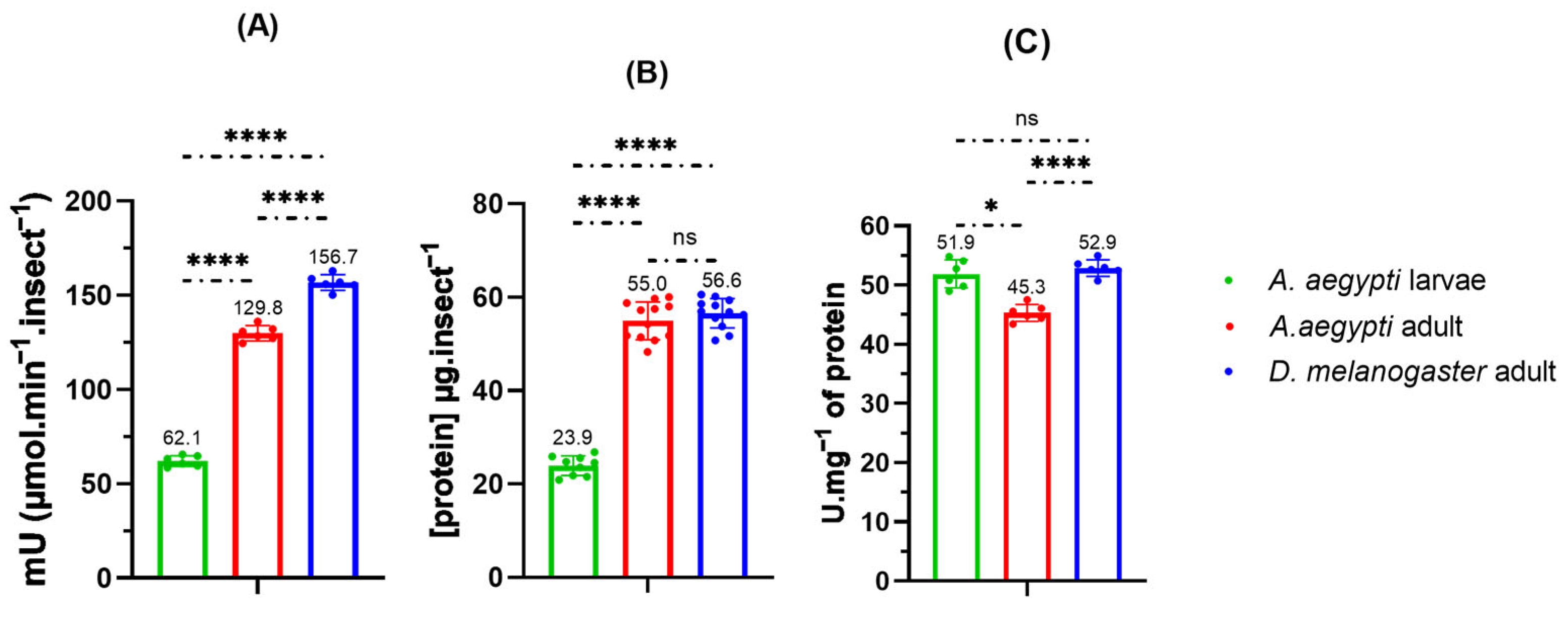
| Compounds * | RT (min) | RIL | EOANIB (% Area) |
|---|---|---|---|
| α-pinene | 12.9 | 932 | 1.4 |
| benzene acetaldehyde | 17.8 | 1036 | 4.2 |
| benzene acetonitrile | 21.9 | 1134 | 0.6 |
| 1-Nitro-2-phenylethane | 28.0 | 1350 | 70.0 |
| β-caryophyllene | 31.0 | 1417 | 2.2 |
| α-caryophyllene | 31.5 | 1452 | 1.7 |
| β-bisabolene | 34.2 | 1505 | 1.1 |
| (E)-nerolidol | 38.7 | 1561 | 3.2 |
| % Total | 84.4 |
| Sample | PS (nm) | Area % | PDI | T% | IZP (mV) ± SD | FZP (mV) ± SD |
|---|---|---|---|---|---|---|
| PEOANIB | 33.4 ± 12.8 | 100 | 0.20 | 87.2 | −11.4 ± 0.9 | −6.3 ± 1.6 * |
| PNFTANE | 32.4 ± 3.0 | 100 | 0.15 | 86.3 | −23.1 ± 1.1 | −14.4 ± 2.4 * |
| PNFTENE | 370 ± 63 | 95.1 | 0.13 | 77.5 | −17.8 ± 0.6 | −16.6 ± 0.1 ** |
| Sample | Exposition Time | LC50 ± SEM (LCL-UCL) ppm | Slope ± SEM | LC99 ± SEM (LCL-UCL) ppm | Slope ± SEM | χ2 ppm |
|---|---|---|---|---|---|---|
| EOANIB | 24 h | 86.9 ± 0.8 (78.2–94.7) * | 14.8 ± 2.0 | 133.1 ± 4.0 (117.5–175.7) | 19.7 ± 3.4 | 62.5 |
| 48 h | 84.3 ± 0.6 (77.2–88.7) | 22.9 ± 3.0 | 108.2 ± 2.3 (100.1–133.4) | 29.6 ± 5.4 | 76.9 | |
| NFTANE | 24 h | 84.8 ± 0.5 (75.6–92.4) * | 18.2 ± 2.0 | 131.4 ± 2.7 (116.1–172.5) | 22.2 ± 3.1 | 62.8 |
| 48 h | 83.6 ± 0.6 (78.3–87.1) * | 31.0 ± 3.0 | 100.1 ± 1.9 (94.6–114.9) | 36.9 ± 8.0 | 85.4 | |
| NFTENE | 24 h | 10.9 ± 0.2 (8.0–14.0) | 2.5 ± 0.1 | 24.8 ± 6.7 (19.9–35.7) | 2.4 ± 0.1 | 66.8 |
| 48 h | 7.7 ± 0.1 (5.2–10.3) | 3.7 ± 0.1 | 16.0 ± 1.2 (12.5–26.7) | 3.7 ± 0.1 | 54.8 | |
| PEOANIB | 24 h | 67.6 ± 1.1 (59.4–76.0) | 7.6 ± 0.9 | 106.3 ± 9.7 (92.5–140.9) | 8.3 ± 1.3 | 66.5 |
| 48 h | 50.5 ± 0.8 (42.0–60.7) | 6.8 ± 0.5 | 79.1 ± 7.0 (66.9–110.2) | 5.8 ± 0.5 | 59.2 | |
| PNFTANE | 24 h | 63.9 ± 0.6 (57.9–70.6) | 17.9 ± 2.0 | 93.9 ± 1.9 (83.1–119.3) | 23.4 ± 3.0 | 96.4 |
| 48 h | 47.2 ± 0.6 (41.7–55.2) | 6.7 ± 0.6 | 69.6 ± 7.5 (59.6–101.9) | 6.2 ± 0.7 | 85.3 | |
| PNFTENE | 24 h | 6.5 ± 0.1 (4.9–8.3) | 3.8 ± 0.3 | 13.6 ± 2.4 (10.8–21.2) | 2.4 ± 0.2 | 51.3 |
| 48 h | 4.4 ± 0.1 (3.1–5.7) | 5.2 ± 0.8 | 7.8 ± 2.0 (6.3–14.0) | 3.7 ± 0.1 | 36.6 |
| Time (min) | LC50 ppm (LCL-UCL) | LC90 ppm (LCL-UCL) | LC99 ppm (LCL-UCL) | LC99.9 ppm (LCL-UCL) | χ2 ppm |
|---|---|---|---|---|---|
| 15 | 75.2 (65.6–83.9) | 119.2 (108.1–135.9) | 155.0 (137.8–183.1) | 181.1 (159.0–218.1) | 144.3 |
| 30 | 52.7 (43.3–60.0) | 80.8 (72.4–94.5) | 103.7 (91.0–127.8) | 120.4 (103.9–152.8) | 149.9 |
| 45 | 43.5 (38.0–48.3) | 74.8 (69.2–81.9) | 100.4 (91.9–112.0) | 119.0 (108.1–134.4) | 390.5 |
| 60 | 38.7 (28.4–47.2) | 70.6 (61.2–84.4) | 96.5 (83.0–119.6) | 115.5 (98.3–146.1) | 127.1 |
| 75 | 38.0 (27.7–46.5) | 69.9 (60.4–83.7) | 95.8 (82.3–118.9) | 114.9 (97.5–145.4) | 126.2 |
| 90 | 33.9 (23.5–42.6) | 65.5 (55.9–79.6) | 91.3 (77.7–114.5) | 110.2 (92.8–140.7) | 122.3 |
| 1440 | 42.2 (32.0–50.5) | 74.1 (64.9–87.9) | 100.2 (86.7–123.4) | 119.2 (101.9–150.1) | 129.1 |
| Sample | LC50 ± SEM (CI) ppm | Slope ± SEM |
|---|---|---|
| EOANIB | * 77.2 ± 1.8 (73.8–81.2) | 9.22 ± 1.9 |
| NFTANE | * 98.15 ± 3.4 (75.6–92.4) | 5.8 ± 0.9 |
| NFTENE | 1.9 ± 0.1 (1.8–2.1) | 4.5 ± 0.6 |
| PEOANIB | * 82.0 ± 1.7 (78.5–85.8) | 9.15 ± 1.5 |
| PNFTANE | * 91.2 ± 3.3 (82.2–98.7) | 4.3 ± 0,7 |
| Sample | IC50-SEM | CI 95% (LCL–UCL) | Slope ± SEM | R2 |
|---|---|---|---|---|
| EOANIB | 163.2 ± 7.03 a | 149.2–178.1 | 1.214 ± 0.062 | 0.969 |
| NFTANE | 189.7 ± 9.30 b | 171.6–209.4 | 1.175 ± 0.066 | 0.9579 |
| NFTENE | 148.9 ± 8.56 a | 131.7–167.4 | 1.283 ± 0.094 | 0.9401 |
| Galantamine | 0.72 ± 0.03 | 0.66–0.78 | 1.053 ± 0.053 | 0.9870 |
| Homogenate | Compound | Inhibition Rate (%) ± SD at 1.5 mg∙mL−1 | IC50-SEM | CI 95% (LCL–UCL) | Slope ± SEM | R2 |
|---|---|---|---|---|---|---|
| A. aegypti adults | NFTENE | 34.0 ± 2.18 | 3264 ± 370.9 | 2630 ± 4354 | 0.86 ± 0.07 | 0.95 |
| Galantamine | 95.01 ± 1.91 * | 0.36 ± 0.06 | 0.22–0.48 | 0.68 ± 0.08 | 0.97 | |
| A. aegypti larvae | NFTENE | 71.8 ± 2.21 | 672.3 ± 67.45 | 551.5–837.8 | 0.72 ± 0.06 | 0.92 |
| Galantamine | 98.3 ± 0.05 * | 0.30 ± 0.04 | 0.21–0.40 | 0.57 ± 0.05 | 0.97 | |
| D. melanogaster adults | NFTENE | 18.4 ± 2.11 | 3135 ± 281.31 | 2615–4039 | 2.02 ± 0.20 | 0.97 |
| Galantamine | 99.8 ± 0.41 * | 1.2 ± 0.17 | 0.87–1.59 | 0.73 ± 0.09 | 0.93 |
| Sample | SM (mg) | PM (mg) | [C] mg∙mL−1 |
|---|---|---|---|
| PEOANIB | 59.0 | 202.7 | 14.75 |
| PNFTANE | 57.7 | 201.8 | 14.43 |
| PNFTENE | 13.5 | 200.5 | 3.37 |
| PBlank | 0 | 200.3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.D.d.; Almeida, M.M.H.; Silva, M.A.M.; Silva, J.R.A.; Genta, F.A.; Amaral, A.C.F. Insecticidal Potential of Aniba canelilla (H.B.K.) Mez Essential Oil Against Aedes aegypti: Larvicidal and Adulticidal Activities, Mechanism of Action, and Formulation Development. Plants 2025, 14, 3348. https://doi.org/10.3390/plants14213348
Cruz JDd, Almeida MMH, Silva MAM, Silva JRA, Genta FA, Amaral ACF. Insecticidal Potential of Aniba canelilla (H.B.K.) Mez Essential Oil Against Aedes aegypti: Larvicidal and Adulticidal Activities, Mechanism of Action, and Formulation Development. Plants. 2025; 14(21):3348. https://doi.org/10.3390/plants14213348
Chicago/Turabian StyleCruz, Jefferson D. da, Maíra M. H. Almeida, Maria Athana M. Silva, Jefferson R. A. Silva, Fernando A. Genta, and Ana Claudia F. Amaral. 2025. "Insecticidal Potential of Aniba canelilla (H.B.K.) Mez Essential Oil Against Aedes aegypti: Larvicidal and Adulticidal Activities, Mechanism of Action, and Formulation Development" Plants 14, no. 21: 3348. https://doi.org/10.3390/plants14213348
APA StyleCruz, J. D. d., Almeida, M. M. H., Silva, M. A. M., Silva, J. R. A., Genta, F. A., & Amaral, A. C. F. (2025). Insecticidal Potential of Aniba canelilla (H.B.K.) Mez Essential Oil Against Aedes aegypti: Larvicidal and Adulticidal Activities, Mechanism of Action, and Formulation Development. Plants, 14(21), 3348. https://doi.org/10.3390/plants14213348








