Nutrient Diagnosis and Precise Fertilization Model Construction of ‘87-1’ Grape (Vitis vinifera L.) Cultivated in a Facility
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Material
2.3. Experimental Design
2.3.1. Mineral Nutrient Requirements in Each Period
2.3.2. ‘5416’ Field Fertilization Scheme
2.4. Determination of Nutrition and Fruit Quality
2.5. Determination of the Plant and Soil Nutritional Diagnostic Factor
2.6. Statistical Analysis
3. Results
3.1. Comprehensive Analysis of the Effect of Formula Fertilization on Grape Fruit Quality
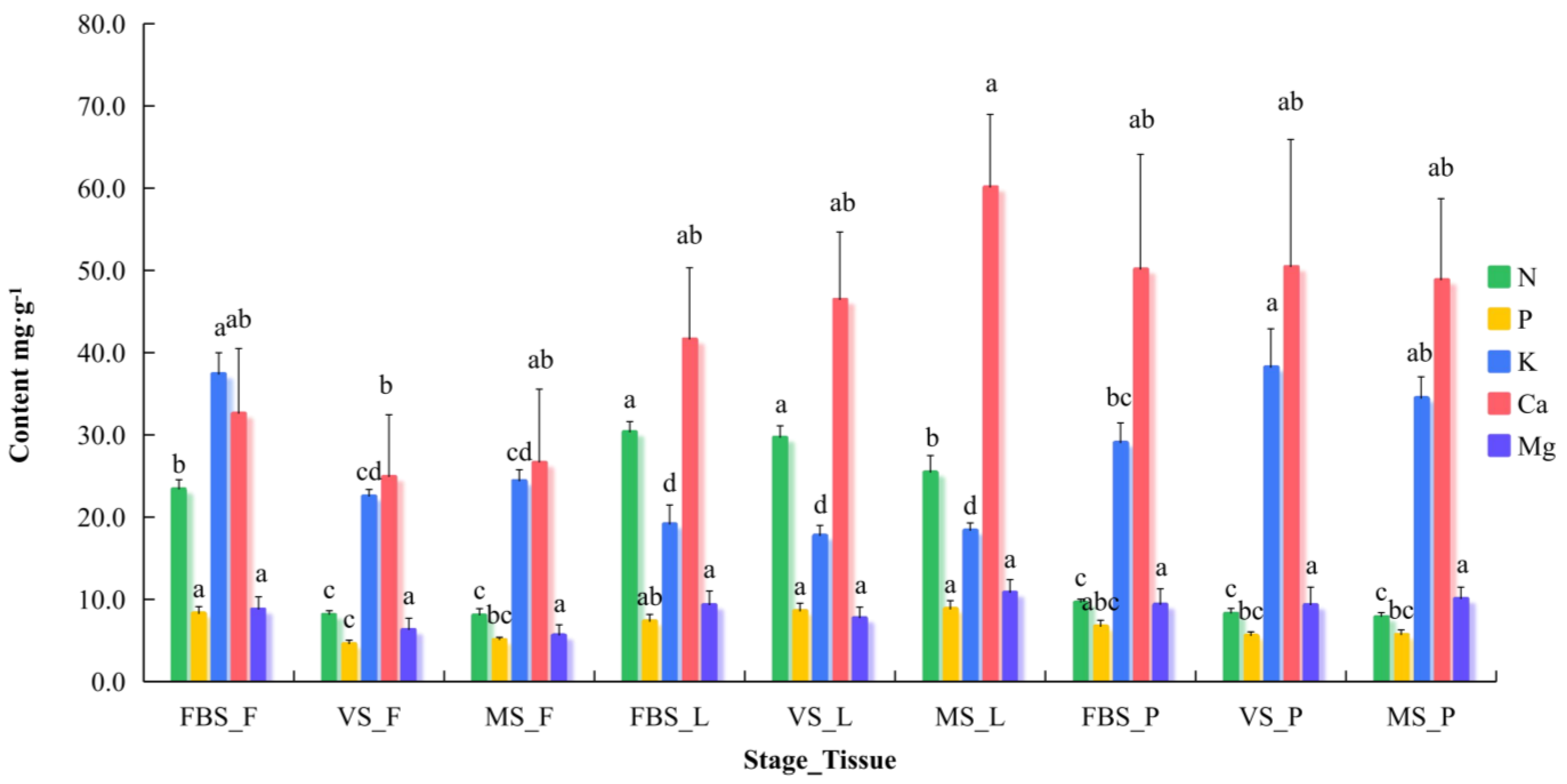
3.2. Plant and Soil Nutrient Diagnosis
3.3. Dynamic Analysis of Plant Nutrition in High FQI Sub-Populations
3.4. Dynamic Analysis of Soil Nutrition in High FQI Sub-Populations
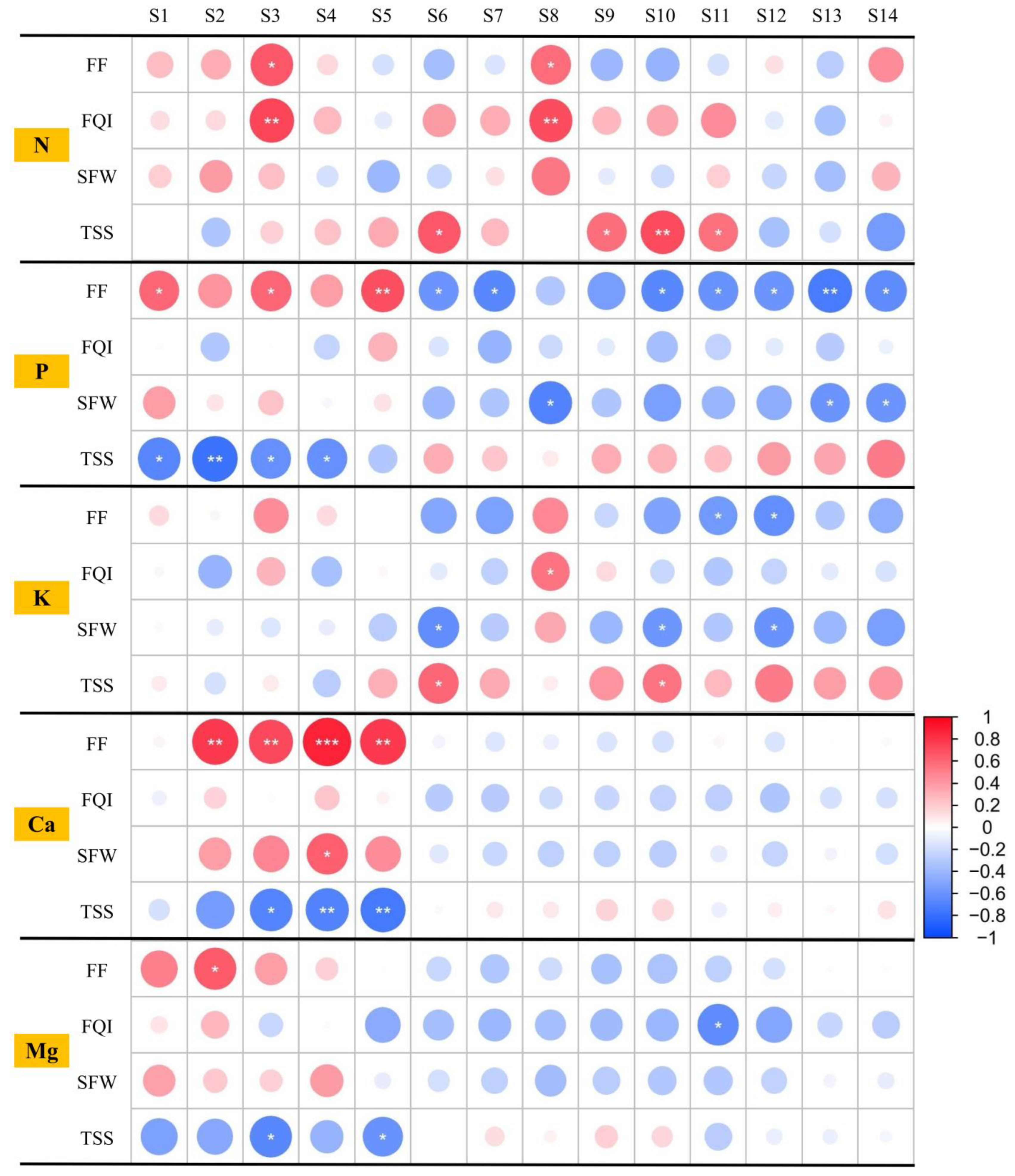
3.5. Correlations Among the Mineral Element Contents and Quality Traits
3.6. Construction and Verification of the Precision Fertilization Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, F.Z. The current status and high-quality development direction of China’s grape industry. Agric. Knowl. 2023, 10, 10–14. (In Chinese) [Google Scholar]
- Prabaharan, E.S.N.; Surendar, K. Role of mineral nutrition on root growth of crop plants—A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2810–2837. [Google Scholar] [CrossRef]
- Musa, Y.S.; Ahmad, M.A.; Mustapha, A.; Abdurrashid, H. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: A review. Pedosphere 2023, 33, 385–406. [Google Scholar]
- Wang, H.Y.; Yang, Y.; Yao, C.G.; Feng, Y.H.; Wang, H.J.; Kong, Y.X.; Riaz, U.; Zaman, Q.U.; Sultan, K.; Fahad, S.; et al. The correct combination and balance of macronutrients nitrogen, phosphorus and potassium promote plant yield and quality through enzymatic and antioxidant activities in potato. J. Plant Growth Regul. 2024, 43, 4716–4734. [Google Scholar] [CrossRef]
- Li, Z.G.; Zhang, R.H.; Xia, S.J.; Wang, L.; Liu, C.; Zhang, R.Q.; Fan, Z.H.; Chen, F.; Liu, Y. Interactions between N, P and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Rashmi, I.; Trisha, R.; Kartika, K.S.; Rama, P.; Vassanda, C.; Kala, S.; Shinoji, K.C. Organic and inorganic fertilizer contaminants in agriculture: Impact on Soil and Water Resources. In Contaminants in Agriculture: Sources, Impacts and Management; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Salt stress alleviation through fertilization in fruit crops. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 465–480. [Google Scholar]
- Panhwar, Q.A.; Ali, A.; Naher, U.A.; Memon, M.Y. Chapter 2—Fertilizer management strategies for enhancing nutrient use efficiency and sustainable wheat production. In Organic Farming; Woodhead Publishing: Cambridge, UK, 2019; pp. 17–39. [Google Scholar]
- Zhang, X.Y.; Li, S.S.; An, X.L.; Song, Z.J.; Zhu, Y.Z.; Tan, Y.; Guo, X.; Wang, D. Effects of nitrogen, phosphorus and potassium formula fertilization on the yield and berry quality of blueberry. PLoS ONE 2023, 18, e0283137. [Google Scholar] [CrossRef]
- Verdenal, T.; Dienes, Á.; Spangenberg, J.E.; Zufferey, V.; Spring, J.L.; Viret, O.; Marin-Carbonne, J.; van Leeuwen, C. Understanding and managing nitrogen nutrition in grapevine: A review. OENO One 2021, 55, 1–43. [Google Scholar] [CrossRef]
- Debnath, S.; Paul, M.; Rahaman, D.M.M.; Debnath, T.; Zheng, L.; Baby, T.; Schmidtke, L.M.; Rogiers, S.Y. Identifying individual nutrient deficiencies of grapevine leaves using hyperspectral imaging. Remote Sens. 2021, 13, 3317. [Google Scholar] [CrossRef]
- Asad, A.; Sikandar, A.; Mujtaba, H.; Muhammad, S.M.M.; Ali, S.; Mukhtaj, K. Detection of deficiency of nutrients in grape leaves using deep network. Math. Probl. Eng. 2022, 2022, 3114525. [Google Scholar] [CrossRef]
- Rozane, D.E.; Vahl, P.B.; Wellington, B.M.G.; Haitzmann, S.E.M.; Trentin, E.; Marchezan, C.; Stefanello, S.L.O.; Tassinari, A.; Dotto, L.; Nunes, O.F.; et al. Compositional nutrient diagnosis (CND) applied to grapevines grown in subtropical climate region. Horticulturae 2020, 6, 56. [Google Scholar] [CrossRef]
- Bendaly, L.M.; Serra, A.P.; Ben, M.M. Establishment of nutrients optimal range for nutritional diagnosis of mandarins based on DRIS and CND methods. Commun. Soil Sci. Plan 2018, 49, 2557–2570. [Google Scholar] [CrossRef]
- Xu, Q.N.; Zhang, G.T.; Liu, Z.M.; Liu, J.B.; Yu, F.Y. Combined application of nitrogen and phosphorus promotes the nutrient element accumulation of Phoebe bournei container seedlings. Trees 2025, 39, 18. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, Z.W.; Zhong, X.M.; Ji, X.H.; Shi, X.B.; Liu, C.; Zhang, Y.C.; Wang, Z.Q.; Wang, H.B. Precise fertilization technology of fruit trees based on quality analysis in China. Technol. Hortic. 2023, 3. [Google Scholar] [CrossRef]
- Shi, X.B.; Wang, X.D.; Wang, B.L.; Wang, Z.Q.; Ji, X.H.; Wang, X.L.; Liu, F.Z.; Wang, H.B. Requirement Rule of nitrogen, phosphorus, potassium, calcium and magnesium of ‘Red Globe’ grapevine. Acta Hortic. Sin. 2021, 48, 2146–2160. (In Chinese) [Google Scholar]
- Wang, X.L.; Shao, X.D.; Zhang, Z.W.; Zhong, X.M.; Ji, X.H.; Shi, X.B.; Liu, C.; Wang, Z.Q.; Liu, F.Z.; Wang, H.B. Multi-nutrient fertilization-based analysis of fruit quality and mineral element composition during fruit development in Merlot wine grapevines. J. Integr. Agric. 2025, 24, 1503–1514. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, P.; Zhou, X.; Yan, M.; Jia, Y.; Ding, X.; Zhang, J.; Huang, A.; Deng, H.; Zhu, Z.; et al. Quality Analysis of Different Kiwifruit Cultivars and Their Relationship with Soil Elements. J. Soil Sci. Plant Nutr. 2025, 25, 1–12. [Google Scholar] [CrossRef]
- Zhou, H.J.; Yu, Z.F.; Ye, Z.W.; Su, M.S. Multiplex analyses of the changes of aromatic compounds during the development of peach fruit using GC–MS and iTRAQ proteomic techniques. Sci. Hortic. 2018, 236, 96–105. [Google Scholar] [CrossRef]
- Zulqarnain, R.M.; Abdal, S.; Maalik, A.; Ali, B.; Zafar, Z.; Ahamad, M.I.; Younas, S.; Mariam, A.; Dayan, F. Application of TOPSIS method in decision making via soft set. Biomed. J. Sci. Tech. Res. 2020, 24, 18208–18215. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, C.; Ji, X.H.; Shi, X.B.; Wang, Z.Q.; Wang, B.L.; Liu, F.Z.; Wang, H.B. An efficient irrigation method for facility-cultivated grape trees at various stages of development. Hortic. Plant J. 2024. [Google Scholar] [CrossRef]
- Ferrández, M.; Martínez, J.J.; Alfosea, M.; Cámara, J.M.; Melgarejo, P.; García, F. Estimation of diagnosis and recommendation integrated system (DRIS), compositional nutrient diagnosis (CND) and range of normality (RN) norms for mineral diagnosis of almonds trees in Spain. Horticulturae 2021, 7, 481. [Google Scholar] [CrossRef]
- Tadayon, M.S.; Saghafi, K.; Sadeghi, S. Applying the compositional nutrient diagnosis (CND) to pomegranate (Punica granatum cv. ‘Rabab’) under saline and calcareous soil condition. J. Plant Nutr. 2022, 46, 1–16. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Plants; Academic Press: Cambridge, MA, USA, 2023; pp. 499–522. [Google Scholar]
- Hernandes, A.; Parent, S.É.; Natale, W.; Parent, L.É. Balancing guava nutrition with liming and fertilization. Rev. Bras. Frutic. 2012, 34, 1224–1234. [Google Scholar] [CrossRef]
- Parent, S.É.; Parent, L.E.; Egozcue, J.J.; Rozane, D.E.; Hernandes, A.; Lapointe, L.; Hébert, V.; Naess, K.; Marchand, S.; Lafond, J. The plant ionome revisited by the nutrient balance concept. Front. Plant Sci. 2013, 4, 39. [Google Scholar] [CrossRef]
- Parent, S.É.; Parent, L.E.; Rozane, D.E.; Natale, W. Plant ionome diagnosis using sound balances: Case study with mango (Mangifera indica). Front. Plant Sci. 2013, 4, 449. [Google Scholar] [CrossRef]
- Albadawy, H. Implication of using potassium and magnesium fertilization to improve growth, yield and quality of Crimson seedless grapes (Vitis vinifera L.). J. Plant Prod. 2019, 10, 133–141. [Google Scholar] [CrossRef]
- Ollrady, A.; Hegab, S.; Abd-Eaal, H.A.; Khalafalla, M. Effect of nitrogen fertilization on growth, yield and fruit quality of superior grapevines. J. Soil Sci. Agric. Eng. 2019, 10, 485–489. [Google Scholar] [CrossRef]
- Schreiner, R.P.; Scagel, C.F.; Lee, J. N, P, and K supply to Pinot Noir grapevines: Impact on berry phenolics and free amino acids. Am. J. Enol. Viticult. 2014, 65, 43–49. [Google Scholar] [CrossRef]
- Arrobas, M.; Ferreira, I.Q.; Freitas, S.; Verdial, J.; Rodrigues, M.Â. Guidelines for fertilizer use in vineyards based on nutrient content of grapevine parts. Sci. Hortic. 2014, 172, 191–198. [Google Scholar] [CrossRef]
- Armachius, J.; Athuman, M.; Andekelile, M.; William, R.E.; Emmanuel, M.; Kobusinge, A.; Elirehema, S.; Joseph, M.F.; Cornel, M. A review on the influence of fertilizers application on grape yield and quality in the tropics. J. Plant Nutr. 2023, 46, 2936–2957. [Google Scholar]
- Jin, L.F.; Guo, D.Y.; Ning, D.Y.; Hussain, S.B.; Liu, Y.Z. Covering the trees of Kinokuni tangerine with plastic film during fruit ripening improves sweetness and alters the metabolism of cell wall components. Acta Physiol. Plant 2018, 40, 182. [Google Scholar] [CrossRef]
- Xu, F.J.; An, H.S.; Zhang, J.Y.; Xu, Z.H.; Jiang, F. Effects of fruit load on sugar/acid quality and puffiness of delayed-harvest citrus. Horticulturae 2021, 7, 189. [Google Scholar] [CrossRef]
- Edema, H.; Ashraf, M.F.; Samkumar, A.; Jaakola, L.; Karppinen, K. Characterization of cellulases from softening fruit for enzymatic depolymerization of cellulose. Carbohyd. Poly. 2024, 343, 122493. [Google Scholar] [CrossRef]
- Maria, M.; Carla, D.M.; Fabio, S.; Stefano, S.; Michele, P.; Dino, M. Influence of phosphorus management on melon (Cucumis melo L.) fruit quality. J. Sci. Food Agric. 2016, 96, 2715–2722. [Google Scholar]
- Gulbagca, F.; Burhan, H.; Elmusa, F.; Sen, F. Calcium nutrition in fruit crops: Agronomic and physiological implications—ScienceDirect. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–190. [Google Scholar]
- Dong, J.L.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z.Q. Effects of Elevated CO2 on Nutritional Quality of Vegetables: A Review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Wang, R.; Qi, Y.B.; Wu, J.; Shukla, M.K.; Sun, Q. Influence of the application of irrigated water-soluble calcium fertilizer on wine grape properties. PLoS ONE 2019, 14, e0222104. [Google Scholar] [CrossRef]
- Ma, T.H.; Hui, Y.R.; Zhang, L.; Su, B.F.; Wang, R. Foliar application of chelated sugar alcohol calcium fertilizer for regulating the growth and quality of wine grapes. Int. J. Agric. Biol. Eng. 2022, 15, 153–158. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.F.; Yin, M.Q.; Li, L.L.; Zheng, J.G.; Yuan, X.Y.; Wen, Y.Y. Optimized potassium application rate increases foxtail millet grain yield by improving photosynthetic carbohydrate metabolism. Front. Plant Sci. 2022, 13, 1044065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.J.; Li, G.H.; Lu, W.P.; Lu, D.L. Interactive Effects of Nitrogen and Potassium on Grain Yield and Quality of Waxy Maize. Plants 2022, 11, 2528. [Google Scholar] [CrossRef]
- Nèjia, F.; Amine, E.; Walid, Z.; Abderrazak, S.; Chedly, A.; Mokded, R. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Vassilis, L.; Athanasia, M.; Loannis, V.; Nikolaos, T.; Menelaos, S. Sustainable Viticulture: First Determination of the Environmental Footprint of Grapes. Sustainability 2020, 12, 8812. [Google Scholar] [CrossRef]
- Tutus, A.; Sensoy, R.I.G. Pruning and Fertilization Impact on Leaf-Mineral Composition in High-Altitude Cultivation of Grapevines (Vitis vinifera L.). Appl. Fruit Sci. 2024, 66, 1867–1876. [Google Scholar] [CrossRef]
- Prado, R.D.M.; Rozane, D.E. Leaf analysis as diagnostic tool for balanced fertilization in tropical fruits—ScienceDirect. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–143. [Google Scholar]
- Zhao, Z.; Chu, C.B.; Zhou, D.P.; Sha, Z.M.; Wu, S.H. Soil nutrient status and the relation with planting area, planting age and grape varieties in urban vineyards in Shanghai. Heliyon 2019, 5, e02362. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.L.; Raíssa, S.; Augusto, S.R.; Laerson, D.G.; Lessandro, D.C.; Pierre, P.L.; Adriele, T.; Souza, K.M.S.; Bicalho, S.I.C.; Gustavo, B. Ideal nitrogen concentration in leaves for the production of high-quality grapes cv ‘Alicante Bouschet’ (Vitis vinifera L.) subjected to modes of application and nitrogen doses. Eur. J. Agron. 2021, 123, 126200. [Google Scholar] [CrossRef]
- Cameron, W.; Petrie, P.R.; Barlow, E.W.R. The effect of temperature on grapevine phenological intervals: Sensitivity of budburst to flowering. Agric. For. Meteorol. 2022, 315, 108841. [Google Scholar] [CrossRef]

| Figure | Factor A | Factor B | Factor C | Factor D | Factor E |
|---|---|---|---|---|---|
| Level | N (kg·hm−2) | P2O5 (kg·hm−2) | K2O (kg·hm−2) | CaO (kg·hm−2) | MgO (kg·hm−2) |
| 1 (0) | 0 | 0 | 0 | 0 | 0 |
| 2 (1/2) | 187.5 | 70.5 | 168.8 | 168.8 | 70.5 |
| 3 (1) | 375.0 | 141.0 | 337.5 | 337.5 | 141.0 |
| 4 (3/2) | 562.5 | 211.5 | 506.25 | 506.25 | 211.5 |
| Code | Treatment | N (kg·hm−2) | P2O5 (kg·hm−2) | K2O (kg·hm−2) | CaO (kg·hm−2) | MgO (kg·hm−2) |
|---|---|---|---|---|---|---|
| T1 | N1P1K1Ca1Mg1 | 0 | 0 | 0 | 0 | 0 |
| T2 | N1P2K2Ca2Mg2 | 0 | 70.5 | 168.8 | 168.8 | 70.5 |
| T3 | N1P3K3Ca3Mg3 | 0 | 141.0 | 337.5 | 337.5 | 141.0 |
| T4 | N1P4K4Ca4Mg4 | 0 | 211.5 | 506.3 | 506.3 | 211.5 |
| T5 | N2P1K2Ca3Mg4 | 187.5 | 0 | 168.8 | 337.5 | 211.5 |
| T6 | N2P2K1Ca4Mg3 | 187.5 | 70.5 | 0 | 506.3 | 141.0 |
| T7 | N2P3K4Ca1Mg2 | 187.5 | 141.0 | 506.3 | 0 | 70.5 |
| T8 | N2P4K3Ca2Mg1 | 187.5 | 211.5 | 337.5 | 168.8 | 0 |
| T9 | N3P1K3Ca4Mg2 | 375.0 | 0 | 337.5 | 506.3 | 70.5 |
| T10 | N3P2K4Ca3Mg1 | 375.0 | 70.5 | 506.3 | 337.5 | 0 |
| T11 | N3P3K1Ca2Mg4 | 375.0 | 141.0 | 0 | 168.8 | 211.5 |
| T12 | N3P4K2Ca1Mg3 | 375.0 | 211.5 | 168.8 | 0 | 141.0 |
| T13 | N4P1K4Ca2Mg3 | 562.5 | 0 | 506.3 | 168.8 | 141.0 |
| T14 | N4P2K3Ca1Mg4 | 562.5 | 70.5 | 337.5 | 0 | 211.5 |
| T15 | N4P3K2Ca4Mg1 | 562.5 | 141.0 | 168.8 | 506.3 | 0 |
| T16 | N4P4K1Ca3Mg2 | 562.5 | 211.5 | 0 | 337.5 | 70.5 |
| Quality | Treatment | Fertilizer Level | SFW (g) | TSS (%) | FF (g) | FQI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | CaO | MgO | ||||||
| T1 | 1 | 1 | 1 | 1 | 1 | 4.77 ± 0.50a | 18.11 ± 1.10a | 412.62 ± 25.71ab | 0.4123 ± 0.1131a | |
| T2 | 1 | 2 | 2 | 2 | 2 | 4.62 ± 0.26a | 17.85 ± 1.74a | 420.17 ± 49.90ab | 0.4265 ± 0.2218a | |
| T3 | 1 | 3 | 3 | 3 | 3 | 4.64 ± 0.49a | 18.41 ± 1.01a | 399.10 ± 52.60ab | 0.4064 ± 0.1478a | |
| T4 | 1 | 4 | 4 | 4 | 4 | 4.73 ± 0.34a | 17.89 ± 1.97a | 427.92 ± 62.74ab | 0.4491 ± 0.1480a | |
| T5 | 2 | 1 | 2 | 3 | 4 | 5.09 ± 0.35a | 18.63 ± 0.65a | 436.88 ± 55.16ab | 0.5181 ± 0.1065a | |
| T6 | 2 | 2 | 1 | 4 | 3 | 4.99 ± 0.51a | 18.47 ± 1.11a | 381.79 ± 15.14ab | 0.3620 ± 0.1234a | |
| T7 | 2 | 3 | 4 | 1 | 2 | 4.75 ± 0.43a | 19.31 ± 1.39a | 419.92 ± 61.57ab | 0.5221 ± 0.1584a | |
| T8 | 2 | 4 | 3 | 2 | 1 | 4.86 ± 0.42a | 19.26 ± 1.16a | 402.00 ± 15.51ab | 0.4569 ± 0.1332a | |
| T9 | 3 | 1 | 3 | 4 | 2 | 4.73 ± 0.78a | 18.67 ± 1.23a | 455.11 ± 95.64a | 0.6003 ± 0.2408a | |
| T10 | 3 | 2 | 4 | 3 | 1 | 4.83 ± 0.75a | 18.79 ± 1.42a | 380.28 ± 46.30b | 0.3889 ± 0.2002a | |
| T11 | 3 | 3 | 1 | 2 | 4 | 4.83 ± 0.67a | 19.20 ± 1.41a | 430.42 ± 44.80ab | 0.5435 ± 0.1867a | |
| T12 | 3 | 4 | 2 | 1 | 3 | 4.54 ± 0.63a | 19.90 ± 2.26a | 416.74 ± 21.86ab | 0.5336 ± 0.1396a | |
| T13 | 4 | 1 | 4 | 2 | 3 | 4.85 ± 0.48a | 18.50 ± 1.60a | 414.53 ± 23.05ab | 0.4359 ± 0.1383a | |
| T14 | 4 | 2 | 3 | 1 | 4 | 4.47 ± 0.54a | 20.10 ± 2.17a | 380.42 ± 66.33b | 0.4356 ± 0.1644a | |
| T15 | 4 | 3 | 2 | 4 | 1 | 4.60 ± 0.48a | 19.36 ± 1.46a | 404.23 ± 27.20ab | 0.4694 ± 0.1614a | |
| T16 | 4 | 4 | 1 | 3 | 2 | 4.98 ± 0.33a | 18.22 ± 1.33a | 408.18 ± 34.71ab | 0.4176 ± 0.2265a | |
| SFW (g) | L1 | 4.68 ± 0.10b | 4.85 ± 0.17a | 4.90 ± 0.12a | 4.65 ± 0.17a | 4.78 ± 0.13a | Ca > N > K > P > Mg N2P1K1Ca3Mg2 | |||
| L2 | 4.95 ± 0.13a | 4.73 ± 0.22a | 4.70 ± 0.27a | 4.78 ± 0.13a | 4.78 ± 0.17a | |||||
| L3 | 4.70 ± 0.14b | 4.70 ± 0.12a | 4.68 ± 0.17a | 4.88 ± 0.22a | 4.73 ± 0.22a | |||||
| L4 | 4.73 ± 0.22ab | 4.78 ± 0.22a | 4.78 ± 0.05a | 4.75 ± 0.17a | 4.78 ± 0.25a | |||||
| Best level | 2 | 1 | 1 | 3 | 1/2/4 | |||||
| F value | 0.79 | 0.33 | 0.64 | 0.88 | 0.01 | |||||
| TSS (%) | L1 | 18.06 ± 0.26b | 18.48 ± 0.26a | 18.50 ± 0.49a | 19.35 ± 0.90a | 18.88 ± 0.57a | N > Ca > K > P > Mg N3P3K3Ca1Mg4 | |||
| L2 | 18.92 ± 0.43ab | 18.80 ± 0.95a | 18.94 ± 0.89a | 18.70 ± 0.66a | 18.51 ± 0.63a | |||||
| L3 | 19.14 ± 0.55a | 19.07 ± 0.45a | 19.11 ± 0.75a | 18.51 ± 0.25a | 18.82 ± 0.72a | |||||
| L4 | 19.04 ± 0.85a | 18.81 ± 0.93a | 18.62 ± 0.59a | 18.60 ± 0.61a | 18.95 ± 0.93a | |||||
| Best level | 3 | 3 | 3 | 1 | 4 | |||||
| F value | 2.14 | 0.52 | 0.70 | 1.27 | 0.32 | |||||
| FF (g) | L1 | 414.95 ± 12.27a | 429.79 ± 20.16a | 408.25 ± 20.09a | 407.43 ± 18.25a | 399.78 ± 13.78a | P > Mg > N > Ca > K N3P1K2Ca4Mg2 | |||
| L2 | 410.15 ± 23.67a | 390.66 ± 19.68b | 419.51 ± 13.46a | 416.78 ± 11.85a | 425.84 ± 20.30a | |||||
| L3 | 420.64 ± 31.24a | 413.42 ± 14.39ab | 409.16 ± 32.09a | 406.11 ± 23.58a | 403.04 ± 16.20a | |||||
| L4 | 401.84 ± 14.90a | 413.71 ± 11.24ab | 410.66 ± 20.99a | 417.26 ± 31.49a | 418.91 ± 25.94a | |||||
| Best level | 3 | 1 | 2 | 4 | 2 | |||||
| F value | 0.54 | 2.19 | 0.23 | 0.3 | 1.32 | |||||
| FQI | L1 | 0.4236 ± 0.0190a | 0.4916 ± 0.0855a | 0.4339 ± 0.0773a | 0.4759 ± 0.0609a | 0.4319 ± 0.0377a | N > P > Mg > K > Ca N3P1K2Ca1Mg2 | |||
| L2 | 0.4648 ± 0.0748a | 0.4032 ± 0.0341a | 0.4869 ± 0.0487a | 0.4657 ± 0.0534a | 0.4916 ± 0.0865a | |||||
| L3 | 0.5166 ± 0.0900a | 0.4854 ± 0.0612a | 0.4748 ± 0.0862a | 0.4328 ± 0.0581a | 0.4345 ± 0.0727a | |||||
| L4 | 0.4396 ± 0.0216a | 0.4643 ± 0.0492a | 0.4490 ± 0.0552a | 0.4702 ± 0.0984a | 0.4866 ± 0.0524a | |||||
| Best level | 3 | 1 | 2 | 1 | 2 | |||||
| F value | 1.17 | 1.15 | 0.41 | 0.27 | 0.74 | |||||
| Type | Stage_(Tissue) _Element | Functional Relationship | R2 | A | B | Y = −B/3A | Optimum Range 1 (mg·g−1) | Optimum Range 2 (mg·g−1) |
|---|---|---|---|---|---|---|---|---|
| Plant | FBS_L_N | Fic (VN) = −61.412X3 + 150.17X2 − 191.27X + 105.24 | 0.9918 | −61.412 | 150.170 | 0.8151 | 7.192–12.516 | 7.192–12.516 |
| VS_P_P | Fic (VP) = −269.76X3 + 533.91X2 − 368.98X + 101.4 | 0.9504 | −269.760 | 533.910 | 0.6597 | 3.097–7.122 | 3.097–7.122 | |
| FBS_F_K | Fic (VK) = 123.71X3 − 204.39X2 − 8.7805X + 99.531 | 0.9960 | 123.710 | −204.390 | 0.5507 | 18.375–27.350 | 18.375–27.350 | |
| FBS_F_Ca | Fic (VCa) = 43.685X3 − 50.586X2 − 94.536X + 104.11 | 0.9967 | 43.685 | −50.586 | 0.3860 | 23.801–107.960 | 23.801–107.960 | |
| FBS_F_Mg | Fic (VMg) = 110.3X3 − 154.03X2 − 54.098X + 101.26 | 0.9975 | 110.300 | −154.030 | 0.4655 | 5.166–21.232 | 5.166–21.232 | |
| R | Fic (VR) = −49.511X3 + 107.22X2 − 157.32X + 101.89 | 0.9920 | −49.511 | 107.220 | 0.7219 | |||
| Soil | GS_N | Fic (VN) = 28.046X3 − 20.587X2 − 96.761X + 91.247 | 0.9941 | 28.046 | −20.587 | 0.2447 | 0.017–0.417 | 0.017–0.336 |
| GS_P | Fic (VP) =36.006X3 − 23.91X2 − 79.894X + 70.59 | 0.9550 | 36.006 | −23.910 | 0.2214 | 0.036–1.542 | 0.040–1.394 | |
| GS_K | Fic (VK) = 8.6264X3 + 10.172X2 − 118.34X + 102.38 | 0.9943 | 8.626 | 10.172 | −0.3931 | 0.126–1.746 | 0.126–1.746 | |
| GS_Ca | Fic (VCa) = 62.153X3 − 96.819X2 − 59.121X + 94.235 | 0.9978 | 62.153 | −96.819 | 0.5193 | 3.252–9.250 | 3.530–9.208 | |
| GS_Mg | Fic (VMg) = 59.112X3 − 38.225X2 − 121.36X + 104.55 | 0.9938 | 59.112 | −38.225 | 0.2156 | 0.339–2.382 | 0.414–1.164 | |
| GS_R | Fic (VR) = −36.161X3 + 96.896X2 − 120.17X + 60.185 | 0.8850 | −36.161 | 96.896 | 0.8932 | |||
| IFS_N | Fic (VN) = 164.03X3 − 228.51X2 − 33.682X + 101.45 | 0.9979 | 164.030 | −228.510 | 0.4644 | 0.012–1.034 | 0.012–0.590 | |
| IFS_P | Fic (VP) =3.9675X3 + 61.777X2 − 166.95X + 107 | 0.9920 | 3.968 | 61.777 | −5.1903 | 0.215–2.111 | 0.249–2.111 | |
| IFS_K | Fic (VK) = 40.813X3 − 40.888X2 − 93.662X + 95.866 | 0.9965 | 40.813 | −40.888 | 0.3339 | 0.105–1.696 | 0.134–1.592 | |
| IFS_Ca | Fic (VCa) = 242.91X3 − 373X2 + 35.339X + 98.462 | 0.9966 | 242.910 | −373.000 | 0.5118 | 2.702–11.000 | 2.702–7.418 | |
| IFS_Mg | Fic (VMg) = 123.4X3 − 174.27X2 − 48.309X + 101.6 | 0.9980 | 123.400 | −174.270 | 0.4707 | 0.299–1.886 | 0.302–1.208 | |
| IFS_R | Fic (VR) = 78.171X3 − 88.892X2 − 77.024X + 91.917 | 0.9934 | 78.171 | −88.892 | 0.3790 | |||
| EBS_N | Fic (VN) = 147.11X3 − 231.04X2 − 8.6896X + 98.115 | 0.9973 | 147.110 | −231.040 | 0.5235 | 0.017–1.160 | 0.017–1.160 | |
| EBS_P | Fic (VP) =109.39X3 − 170.67X2 − 33.997X + 100.97 | 0.9962 | 109.390 | −170.670 | 0.5201 | 0.112–1.591 | 0.112–1.591 | |
| EBS_K | Fic (VK) = −18.397X3 + 27.472X2 − 84.172X + 76.546 | 0.9736 | −18.397 | 27.472 | 0.4978 | 0.148–1.210 | 0.148–1.210 | |
| EBS_Ca | Fic (VCa) = 73.097X3 − 111.06X2 − 56.813X + 101.48 | 0.9960 | 73.097 | −111.060 | 0.5065 | 2.686–8.473 | 2.686–8.231 | |
| EBS_Mg | Fic (VMg) = −44.971X3 + 100.51X2 − 146.64X + 95.082 | 0.9956 | −44.971 | 100.510 | 0.7450 | 0.428–1.277 | 0.428–1.266 | |
| EBS_R | Fic (VR) = 137.64X3 − 205.95X2 − 22.919X + 97.99 | 0.9972 | 137.640 | −205.950 | 0.4988 | |||
| VS_N | Fic (VN) = 26.465X3 + 4.702X2 − 111.76X + 82.782 | 0.9866 | 26.465 | 4.702 | −0.0592 | 0.024–0.966 | 0.024–0.735 | |
| VS_P | Fic (VP) =44.319X3 − 37.04X2 − 94.919X + 94.1 | 0.9952 | 44.319 | −37.040 | 0.2786 | 0.119–4.143 | 0.119–2.828 | |
| VS_K | Fic (VK) = 14.899X3 + 10.976X2 − 126.11X + 105.48 | 0.9919 | 14.899 | 10.976 | −0.2456 | 0.254–1.212 | 0.254–1.212 | |
| VS_Ca | Fic (VCa) = 133.93X3 − 209.96X2 − 24.111X + 101.95 | 0.9974 | 133.930 | −209.960 | 0.5226 | 3.043–7.661 | 3.043–7.095 | |
| VS_Mg | Fic (VMg) = −213.05X3 + 404.96X2 − 266.02X + 71.346 | 0.8856 | −213.050 | 404.960 | 0.6336 | 0.313–1.197 | 0.477–1.197 | |
| VS_R | Fic (VR) = −32.596X3 + 88.467X2 − 123.67X + 70.449 | 0.9479 | −32.596 | 88.467 | 0.9047 | |||
| MS_N | Fic (VN) = 70.275X3 − 104.08X2 − 51.727X + 85.866 | 0.9905 | 70.275 | −104.080 | 0.4937 | 0.021–0.691 | 0.021–0.691 | |
| MS_P | Fic (VP) = 73.229X3 − 94.688X2 − 69.975X + 101 | 0.9953 | 73.229 | −94.688 | 0.4310 | 0.078–1.258 | 0.155–1.258 | |
| MS_K | Fic (VK) = 51.873X3 − 44.305X2 − 98.387X + 101.85 | 0.9945 | 51.873 | −44.305 | 0.2847 | 0.127–1.448 | 0.249–1.114 | |
| MS_Ca | Fic (VCa) = 31.18X3 − 52.216X2 − 63.023X + 89.425 | 0.9942 | 31.180 | −52.216 | 0.5582 | 2.659–8.777 | 2.659–8.777 | |
| MS_Mg | Fic (VMg) = −47.682X3 + 128.5X2 − 165.6X + 86.876 | 0.9881 | −47.682 | 128.500 | 0.8983 | 0.326–1.186 | 0.391–1.186 | |
| MS_R | Fic (VR) = −138.77X3 + 261.57X2 − 166.14X + 41.788 | 0.5508 | −138.770 | 261.570 | 0.6283 |
| Stage_Element | Proportion of Annual Absorption (%) | Precise Fertilization Model (y = ax + b) |
|---|---|---|
| GS-IFS_N | 21.8 ± 2.4b | y = −10.5800x + 3.5549 |
| IFS-EBS_N | 20.1 ± 2.2b | y = −9.0830x + 5.3590 |
| EBS-VS_N | 31.6 ± 5.7a | y = −5.9055x + 6.8504 |
| VS-MS_N | 8.8 ± 3.7c | y = −27.426x + 20.158 |
| MS-DS_N | 17.7 ± 3.4b | y = −3.9179x + 2.7073 |
| GS-IFS_P | 18.2 ± 0.4c | y = −1.8744x + 2.6130 |
| IFS-EBS_P | 8.3 ± 2.0d | y = −0.3029x + 0.6394 |
| EBS-VS_P | 27.1 ± 7.8a | y = −0.6673x + 1.0617 |
| VS-MS_P | 21.6 ± 2.3b | y = −2.8106x + 7.9485 |
| MS-DS_P | 24.8 ± 0.7a | y = −2.1732x + 2.7338 |
| GS-IFS_K | 21.9 ± 0.8b | y = −1.4583x + 2.5463 |
| IFS-EBS_K | 6.7 ± 1.3c | y = −3.0093x + 4.7907 |
| EBS-VS_K | 48.4 ± 6.0a | y = −2.8602x + 3.4608 |
| VS-MS_K | 7.1 ± 4.1c | y = −21.138x + 25.619 |
| MS-DS_K | 15.9 ± 2.6b | y = −4.2919x + 4.7812 |
| GS-IFS_Ca | 19.7 ± 3.1b | y = −0.8322x + 7.6625 |
| IFS-EBS_Ca | 2.9 ± 1.3d | y = −0.5010x + 3.7161 |
| EBS-VS_Ca | 10.3 ± 2.1c | y = −0.4261x + 3.5069 |
| VS-MS_Ca | 46.7 ± 3.2a | y = −5.3307x + 37.821 |
| MS-DS_Ca | 20.4 ± 2.9b | y = −0.4413x + 3.8735 |
| GS-IFS_Mg | 25.2 ± 1.6b | y = −1.1280x + 1.3130 |
| IFS-EBS_Mg | 17.7 ± 3.3c | y = −1.2450x + 1.5040 |
| EBS-VS_Mg | 15.7 ± 4.2c | y = −1.0095x + 1.2781 |
| VS-MS_Mg | 3.8 ± 2.3d | y = −12.533x + 15.002 |
| MS-DS_Mg | 37.6 ± 1.4a | y = −2.8377x + 3.3656 |
| Treatment | SFW (g) | TSS (%) | FF (g) | FQI | Ranking | Normalized FQI |
|---|---|---|---|---|---|---|
| T1 | 3.3 ± 0.0cde | 17.8 ± 0.1b | 493.5 ± 49.4bcd | 0.4718 | 11 | 0.3925 |
| T2 | 3.3 ± 0.0cd | 17.7 ± 0.1bc | 507.3 ± 63.7bcd | 0.5080 | 8 | 0.4517 |
| T3 | 3.8 ± 0.1ab | 17.1 ± 0.1e | 488.2 ± 2.4cd | 0.4087 | 14 | 0.2894 |
| T4 | 3.3 ± 0.2de | 17.1 ± 0.1de | 505.6 ± 35.2bcd | 0.4434 | 12 | 0.3461 |
| T5 | 3.3 ± 0.1cd | 16.1 ± 0.1g | 482.3 ± 49.6cd | 0.2527 | 17 | 0.0343 |
| T6 | 3.9 ± 0.3a | 17.7 ± 0.7bc | 503.9 ± 35.8bcd | 0.5182 | 7 | 0.4684 |
| T7 | 3.2 ± 0.3de | 16.8 ± 0.1ef | 500.3 ± 50.2bcd | 0.3882 | 16 | 0.2558 |
| T8 | 3.6 ± 0.1abcd | 16.1 ± 0.1g | 536.4 ± 36.6abcd | 0.4737 | 10 | 0.3956 |
| T9 | 3.2 ± 0.1de | 17.7 ± 0.1bc | 527.7 ± 44.7abcd | 0.5784 | 5 | 0.5668 |
| T10 | 3.6 ± 0.4abcd | 17.0 ± 0.1e | 606.4 ± 38.3a | 0.7611 | 3 | 0.8655 |
| T11 | 2.8 ± 0.3e | 16.7 ± 0.1f | 447.2 ± 14.4d | 0.2317 | 18 | 0.0000 |
| T12 | 3.6 ± 0.1abcd | 15.2 ± 0.1h | 541.4 ± 41.9abcd | 0.4245 | 13 | 0.3152 |
| T13 | 3.3 ± 0.2cde | 16.5 ± 0.1f | 562.9 ± 78.6abc | 0.5923 | 4 | 0.5895 |
| T14 | 3.4 ± 0.2bcd | 17.4 ± 0.1cd | 507.9 ± 49.3bcd | 0.4860 | 9 | 0.4157 |
| T15 | 3.2 ± 0.5de | 18.4 ± 0.1a | 512.0 ± 74.5bcd | 0.5728 | 6 | 0.5576 |
| T16 | 3.6 ± 0.3abcd | 17.4 ± 0.1cd | 478.3 ± 72.5cd | 0.4015 | 15 | 0.2776 |
| T17 | 3.7 ± 0.1abc | 18.5 ± 0.1a | 572.0 ± 36.4abc | 0.8322 | 2 | 0.9817 |
| T18 | 3.6 ± 0.0abcd | 17.9 ± 0.2b | 586.0 ± 42.2ab | 0.8434 | 1 | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, X.; Liu, C.; Shi, X.; Ji, X.; Wang, S.; Li, T. Nutrient Diagnosis and Precise Fertilization Model Construction of ‘87-1’ Grape (Vitis vinifera L.) Cultivated in a Facility. Plants 2025, 14, 3345. https://doi.org/10.3390/plants14213345
Wang H, Wang X, Liu C, Shi X, Ji X, Wang S, Li T. Nutrient Diagnosis and Precise Fertilization Model Construction of ‘87-1’ Grape (Vitis vinifera L.) Cultivated in a Facility. Plants. 2025; 14(21):3345. https://doi.org/10.3390/plants14213345
Chicago/Turabian StyleWang, Haibo, Xiaolong Wang, Chang Liu, Xiangbin Shi, Xiaohao Ji, Shengyuan Wang, and Tianzhong Li. 2025. "Nutrient Diagnosis and Precise Fertilization Model Construction of ‘87-1’ Grape (Vitis vinifera L.) Cultivated in a Facility" Plants 14, no. 21: 3345. https://doi.org/10.3390/plants14213345
APA StyleWang, H., Wang, X., Liu, C., Shi, X., Ji, X., Wang, S., & Li, T. (2025). Nutrient Diagnosis and Precise Fertilization Model Construction of ‘87-1’ Grape (Vitis vinifera L.) Cultivated in a Facility. Plants, 14(21), 3345. https://doi.org/10.3390/plants14213345





