Influence of Nitrate and Light on Fucoxanthin Content and Key Gene Expression in the Marine Diatom Thalassiosira rotula
Abstract
1. Introduction
2. Results
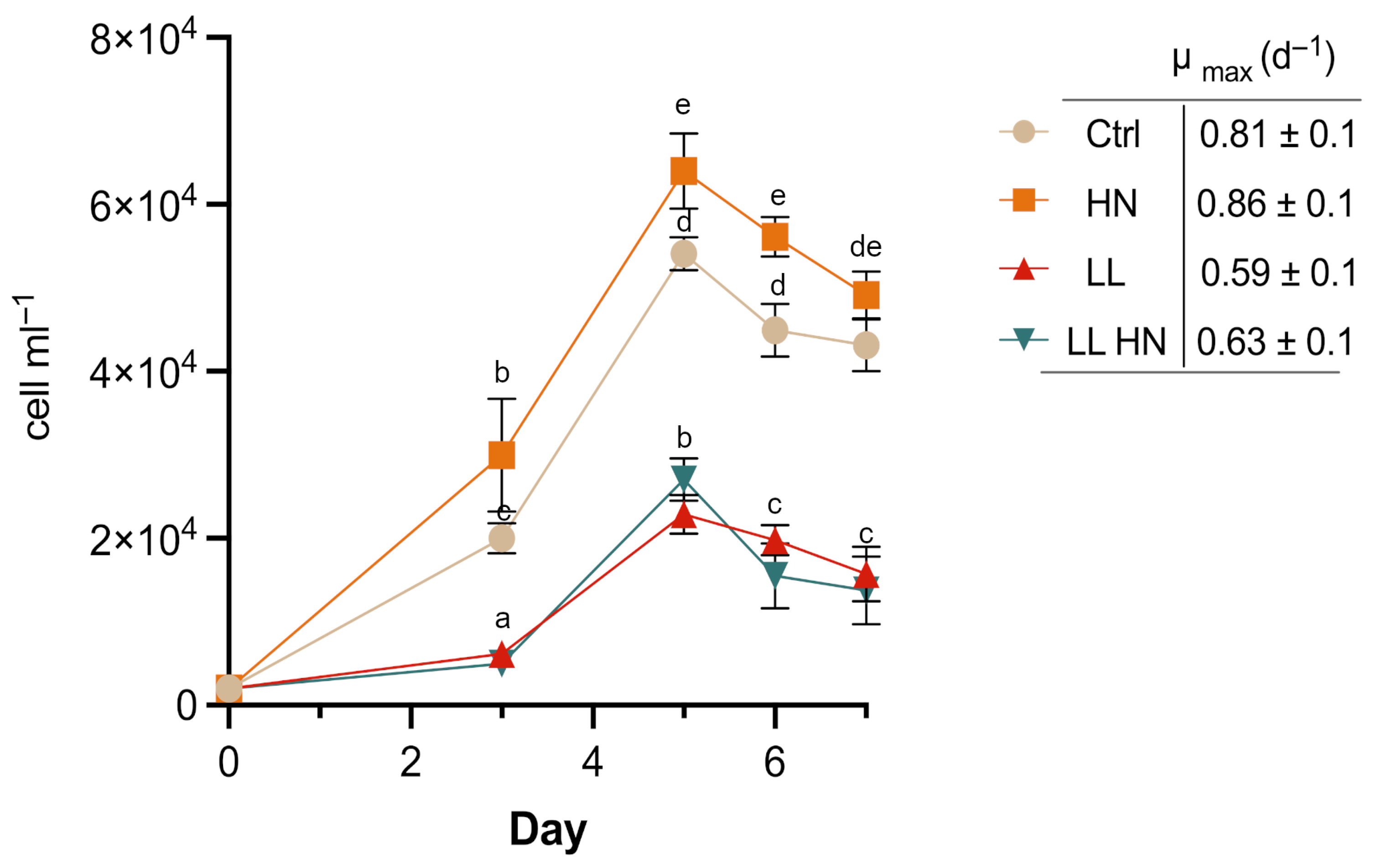
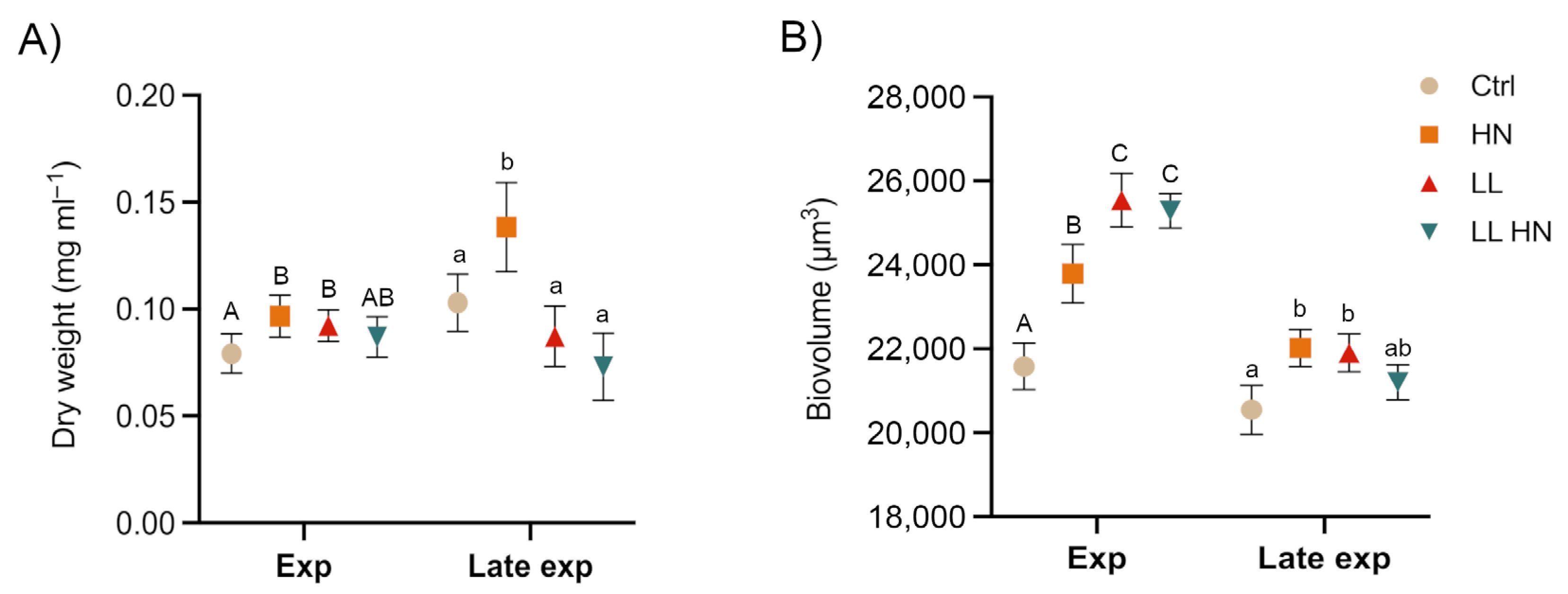
2.1. Growth Dynamic and Morphophysiological Analyses
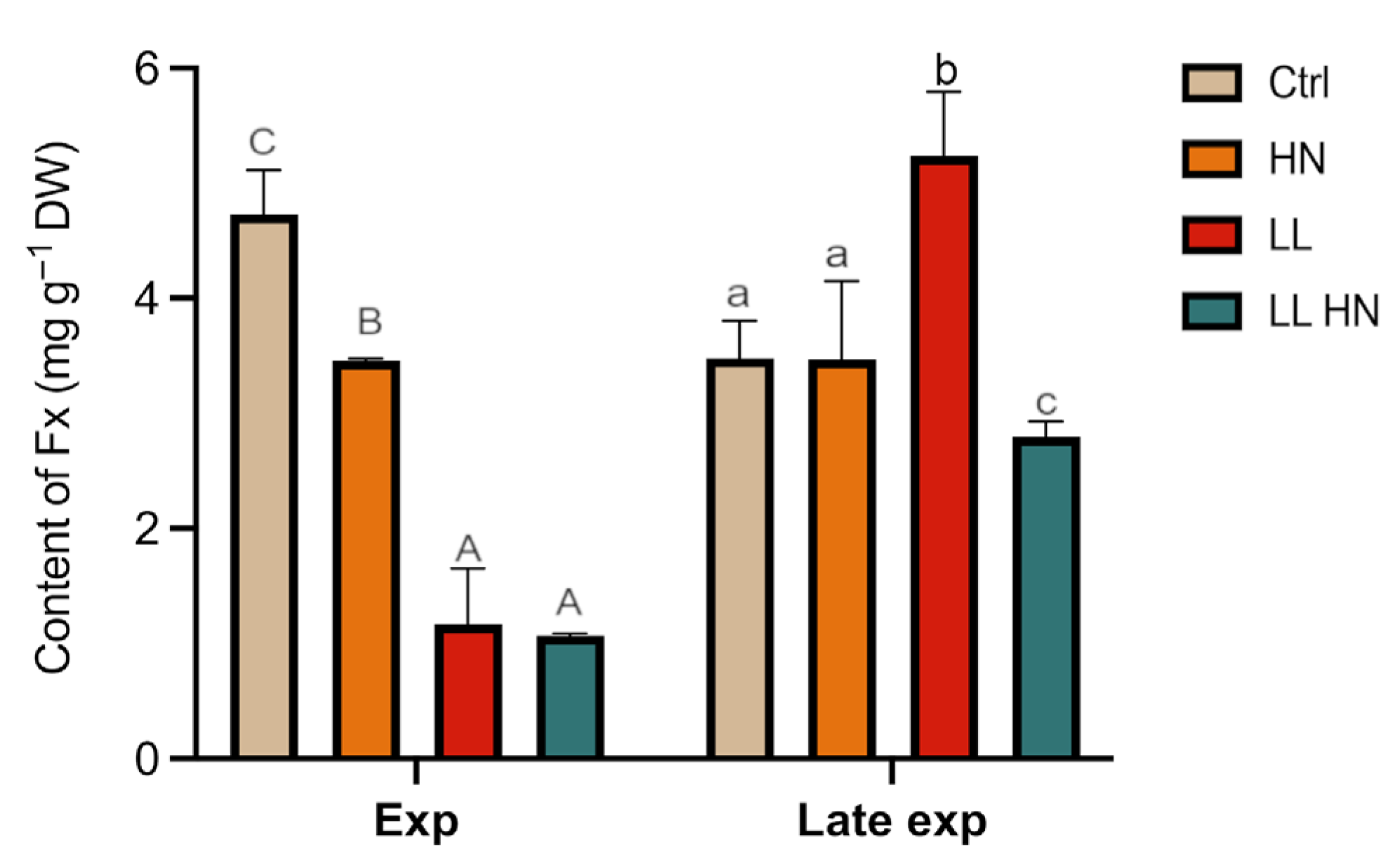
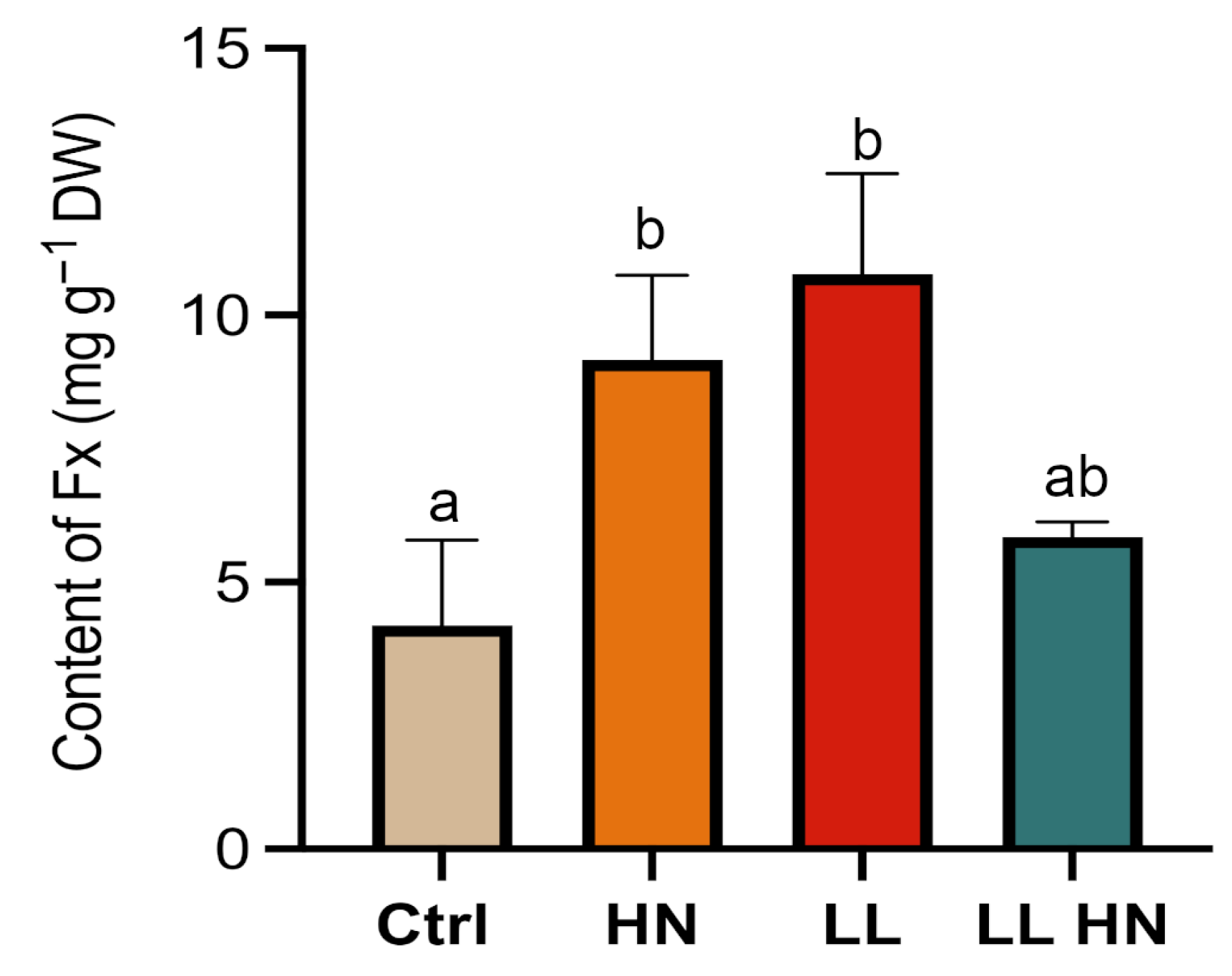
2.2. Effects of the Treatments on the Fucoxanthin Content
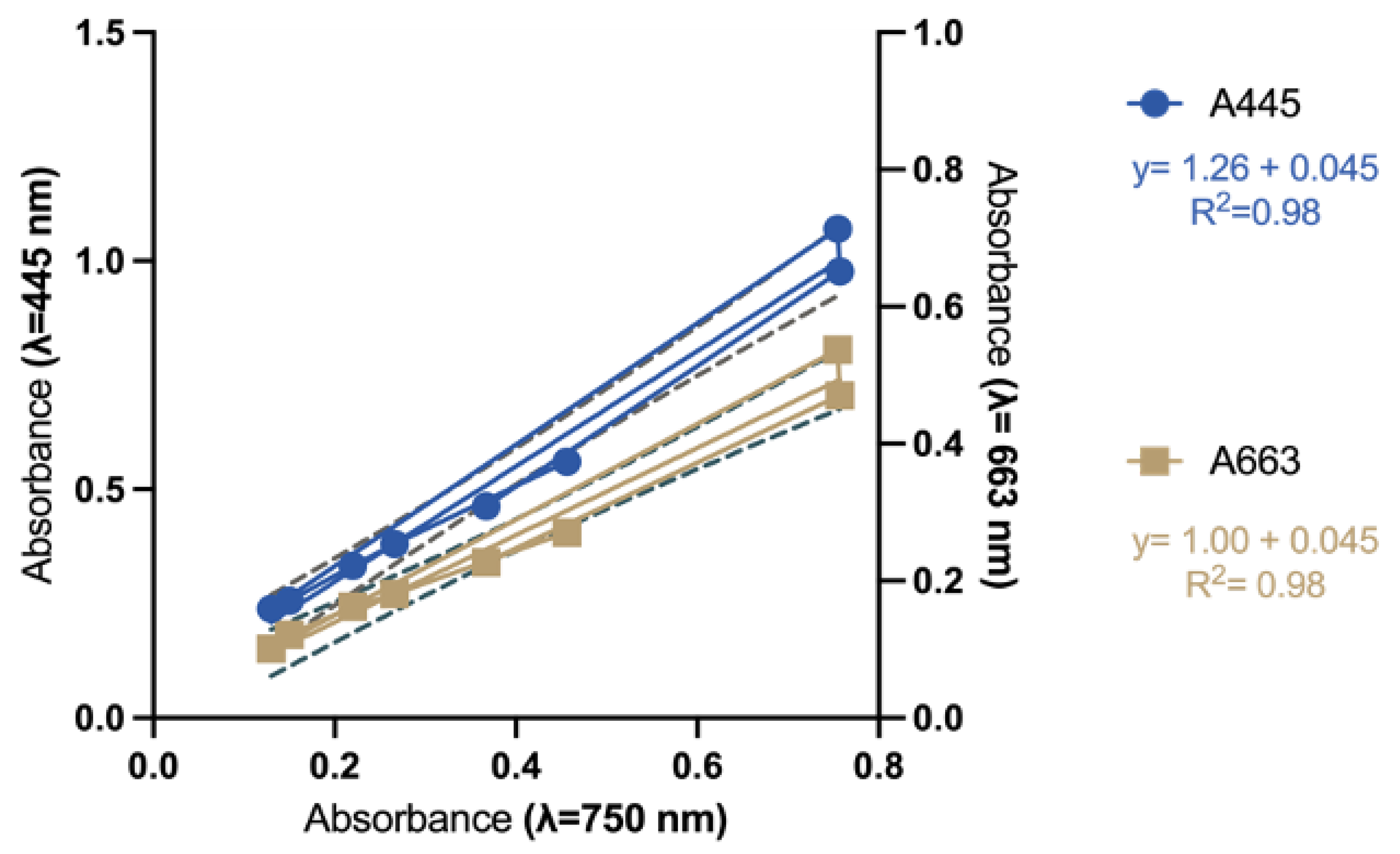
2.3. Rapid Spectrophotometric Assay to Determine Fx Content in Thalassiosira rotula
2.4. Fucoxanthin Quantification: HPLC Versus Spectrophotometric Analysis
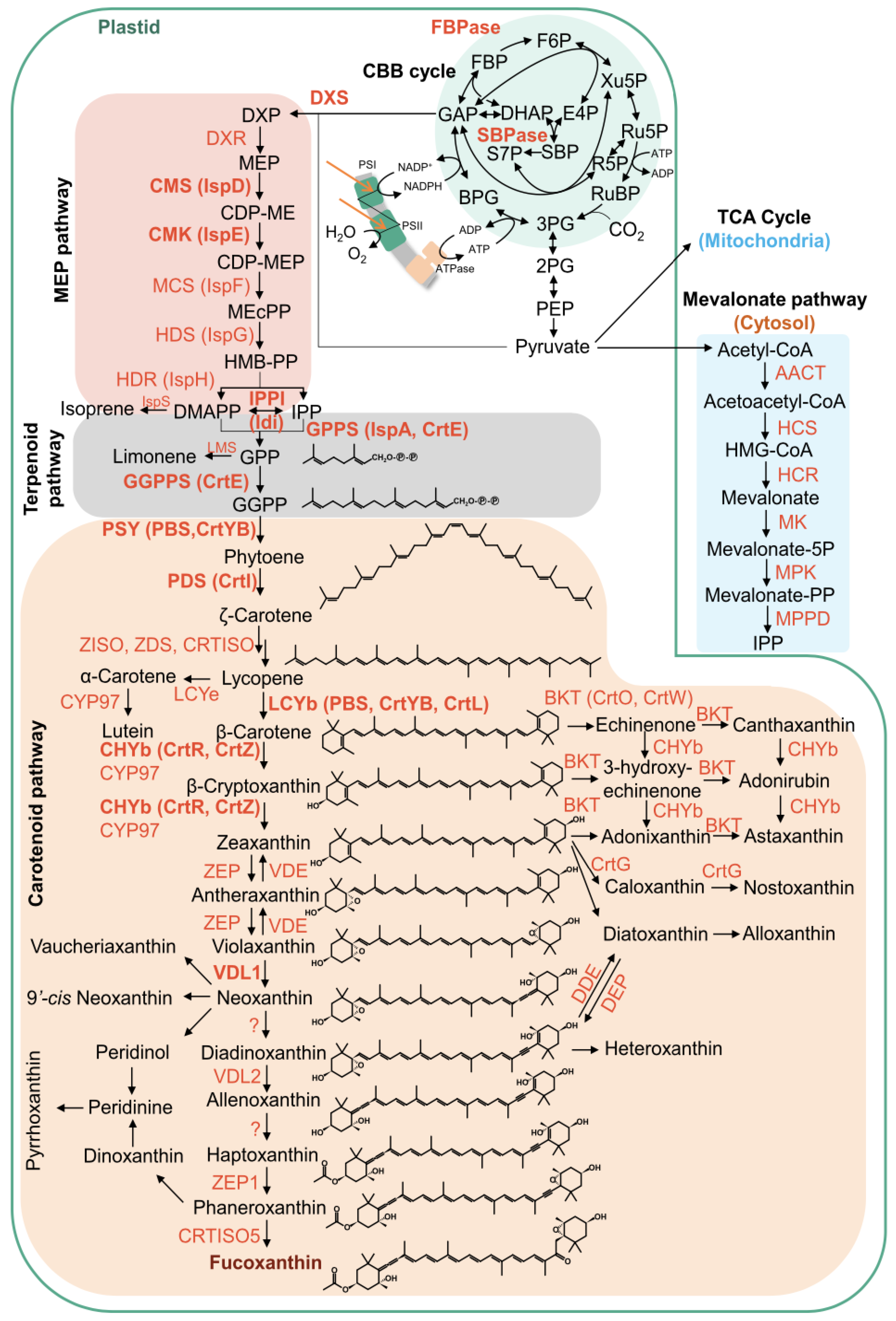
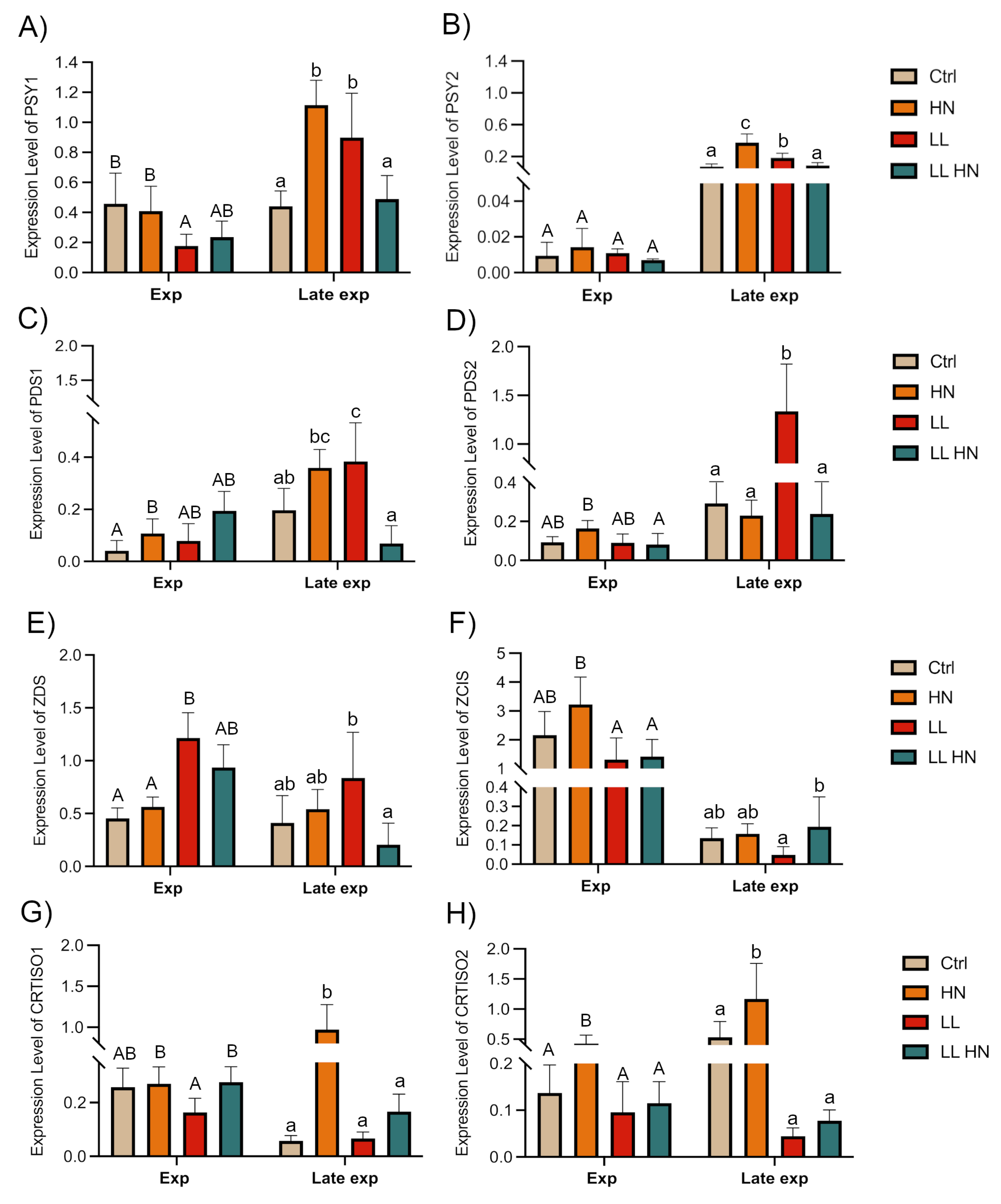
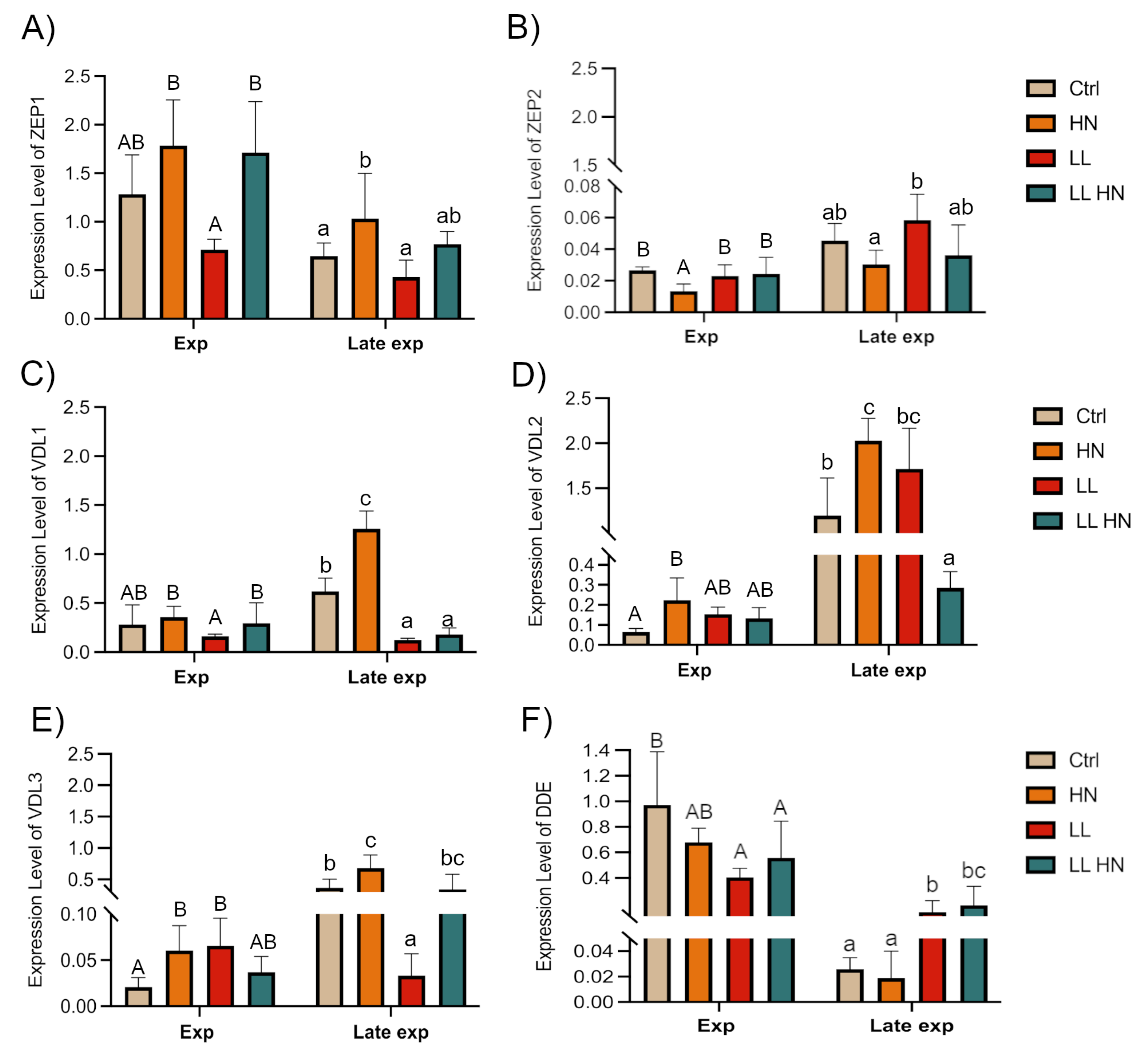
2.5. Expression Patterns of Key Genes Involved in the Fucoxanthin Biosynthetic Pathway
3. Discussion
4. Materials and Methods
4.1. Microalgal Strain and Growth Conditions
4.2. Growth Dynamic and Morphophysiological Analyses
4.3. Fucoxanthin Quantification by HPLC Analysis
- Area = area of picks (DAD);
- Coeff. = coefficient of pigment;
- V. Extr., Iniet., Filtr. = volume of samples extracted, injected and filtered, respectively.
4.4. Extraction and Spectrophotometric Determination of Fucoxanthin
4.5. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seth, K.; Kumar, A.; Rastogi, R.P.; Meena, M.; Vinayak, V. Harish Bioprospecting of Fucoxanthin from Diatoms—Challenges and Perspectives. Algal Res. 2021, 60, 102475. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid.-Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Sohag, A.A.M.; Hossain, M.T.; Zahan, M.S.; Uddin, M.J.; Hannan, M.A.; Moon, I.S.; Choi, J.S. A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Mar. Drugs 2022, 20, 279. [Google Scholar] [CrossRef]
- Mumu, M.; Das, A.; Emran, T.B.; Mitra, S.; Islam, F.; Roy, A.; Karim, M.M.; Das, R.; Park, M.N.; Chandran, D.; et al. Fucoxanthin: A Promising Phytochemical on Diverse Pharmacological Targets. Front. Pharmacol. 2022, 13, 929442. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, J.; Fang, C.; Cao, Q.; Xing, M.; Li, X.; Hou, J.; Ji, A.; Song, S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar. Drugs 2020, 18, 634. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin Content and Antioxidant Properties of Undaria Pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal Variations of Total Lipids, Fatty Acid Composition, and Fucoxanthin Contents of Sargassum Horneri (Turner) and Cystoseira Hakodatensis (Yendo) from the Northern Seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Fernandes, F.; Barbosa, M.; Oliveira, A.P.; Azevedo, I.C.; Sousa-Pinto, I.; Valentão, P.; Andrade, P.B. The Pigments of Kelps (Ochrophyta) as Part of the Flexible Response to Highly Variable Marine Environments. J. Appl. Phycol. 2016, 28, 3689–3696. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A Potential Commercial Source of Fucoxanthin Extracted from the Microalga Phaeodactylum Tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, G.; Pan, K.; Wang, L.; Hu, Z. A Review on the Progress, Challenges and Prospects in Commercializing Microalgal Fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R.; Takahashi, K. Fucoxanthin Production of Microalgae under Different Culture Factors: A Systematic Review. Mar. Drugs 2022, 20, 592. [Google Scholar] [CrossRef]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An Investigation into the Effect of Culture Conditions on Fucoxanthin Production Using the Marine Microalgae Phaeodactylum Tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Fu, J.; Xiong, J.; Zhang, C. Production of Fucoxanthin, Chrysolaminarin, and Eicosapentaenoic Acid by Odontella Aurita under Different Nitrogen Supply Regimes. J. Biosci. Bioeng. 2018, 126, 723–729. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D. Improving Fucoxanthin Production in Mixotrophic Culture of Marine Diatom Phaeodactylum Tricornutum by LED Light Shift and Nitrogen Supplementation. Front. Bioeng. Biotechnol. 2020, 8, 564273. [Google Scholar] [CrossRef]
- Wu, Z.; Qiu, S.; Abbew, A.W.; Chen, Z.; Liu, Y.; Zuo, J.; Ge, S. Evaluation of Nitrogen Source, Concentration and Feeding Mode for Co-Production of Fucoxanthin and Fatty Acids in Phaeodactylum Tricornutum. Algal Res. 2022, 63, 102655. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D.; Pohnert, G. Nitrogen Utilization Analysis Reveals the Synergetic Effect of Arginine and Urea in Promoting Fucoxanthin Biosynthesis in the Mixotrophic Marine Diatom Phaeodactylum Tricornutum. Front. Mar. Sci. 2022, 9, 947726. [Google Scholar] [CrossRef]
- Duarte, B.; Feijão, E.; Goessling, J.W.; Caçador, I.; Matos, A.R. Pigment and Fatty Acid Production under Different Light Qualities in the Diatom Phaeodactylum Tricornutum. Appl. Sci. 2021, 11, 2550. [Google Scholar] [CrossRef]
- Frick, K.; Ebbing, T.; Yeh, Y.C.; Schmid-Staiger, U.; Tovar, G.E.M. Influence of Light Conditions on the Production of Chrysolaminarin Using Phaeodactylum Tricornutum in Artificially Illuminated Photobioreactors. Microbiologyopen 2023, 12, e1378. [Google Scholar] [CrossRef]
- Truong, T.Q.; Park, Y.J.; Koo, S.Y.; Choi, J.H.; Enkhbayar, A.; Song, D.G.; Kim, S.M. Interdependence of Fucoxanthin Biosynthesis and Fucoxanthin-Chlorophyll a/c Binding Proteins in Phaeodactylum Tricornutum under Different Light Intensities. J. Appl. Phycol. 2023, 35, 25–42. [Google Scholar] [CrossRef]
- Ryabushko, V.I.; Zheleznova, S.N.; Nekhoroshev, M.V. Effect of Nitrogen on Fucoxanthin Accumulation in the Diatom Cylindrotheca closterium (Ehrenb.) Reimann et Lewin. Int. J. Algae 2017, 19, 79–84. [Google Scholar] [CrossRef]
- Afonso, C.; Bragança, A.R.; Rebelo, B.A.; Serra, T.S.; Abranches, R. Optimal Nitrate Supplementation in Phaeodactylum Tricornutum Culture Medium Increases Biomass and Fucoxanthin Production. Foods 2022, 11, 568. [Google Scholar] [CrossRef]
- Rui, X.; Amenorfenyo, D.K.; Peng, K.; Li, H.; Wang, L.; Huang, X.; Li, C.; Li, F. Effects of Different Nitrogen Concentrations on Co-Production of Fucoxanthin and Fatty Acids in Conticribra Weissflogii. Mar. Drugs 2023, 21, 106. [Google Scholar] [CrossRef]
- Nymark, M.; Valle, K.C.; Brembu, T.; Hancke, K.; Andresen, K.; Johnsen, G.; Bones, A.M. An Integrated Analysis of Molecular Acclimation to High Light in the Marine Diatom Phaeodactylum Tricornutum. PLoS ONE 2009, 4, 7743. [Google Scholar] [CrossRef]
- Rochaix, J.D. Regulation and Dynamics of the Light-Harvesting System. Annu. Rev. Plant Biol. 2014, 65, 287–309. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth Kinetics and Fucoxanthin Production of Phaeodactylum Tricornutum and Isochrysis Galbana Cultures at Different Light and Agitation Conditions. J. Appl. Phycol. 2016, 28, 849–860. [Google Scholar] [CrossRef]
- Chinnappan, S.; Cai, J.; Li, Y.; Yang, Z.; Sheng, Y.; Cheng, K.; Du, H.; Liu, W.; Li, P. Light and Nutrient Conditions Influence Fucoxanthin Production of the Microalgae Cyclotella Meneghiniana. Appl. Sci. 2024, 14, 5504. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Liana, F.M.; Putri, A.N.L.D.; Anasstasia, T.T.; Pawignya, H.; Djarot, I.N. Enhancing Fucoxanthin Production in Chaetoceros Calcitrans under Mixotrophic Condition and LED Wavelength Shift Strategy. Biocatal. Agric. Biotechnol. 2024, 57, 103120. [Google Scholar] [CrossRef]
- Seo, S.; Chang, K.S.; Choi, M.S.; Jin, E.S. Overexpression of PtVDL1 in Phaeodactylum Tricornutum Increases Fucoxanthin Content under Red Light. J. Microbiol. Biotechnol. 2023, 34, 198. [Google Scholar] [CrossRef]
- Tanaka, K.; Lan, J.C.W.; Kondo, A.; Hasunuma, T. Metabolic Engineering and Cultivation Strategies for Efficient Production of Fucoxanthin and Related Carotenoids. Appl. Microbiol. Biotechnol. 2025, 109, 57. [Google Scholar] [CrossRef]
- Manfellotto, F.; Stella, G.R.; Ferrante, M.I.; Falciatore, A.; Brunet, C. Engineering the Unicellular Alga Phaeodactylum Tricornutum for Enhancing Carotenoid Production. Antioxidants 2020, 9, 757. [Google Scholar] [CrossRef]
- Hao, T.B.; Lu, Y.; Zhang, Z.H.; Liu, S.F.; Wang, X.; Yang, W.D.; Balamurugan, S.; Li, H.Y. Hyperaccumulation of Fucoxanthin by Enhancing Methylerythritol Phosphate Pathway in Phaeodactylum Tricornutum. Appl. Microbiol. Biotechnol. 2021, 105, 8783–8793. [Google Scholar] [CrossRef]
- Li, C.; Pan, Y.; Yin, W.; Liu, J.; Hu, H. A Key Gene, Violaxanthin de-Epoxidase-like 1, Enhances Fucoxanthin Accumulation in Phaeodactylum Tricornutum. Biotechnol. Biofuels Bioprod. 2024, 17, 49. [Google Scholar] [CrossRef]
- Orefice, I.; Chandrasekaran, R.; Smerilli, A.; Corato, F.; Caruso, T.; Casillo, A.; Corsaro, M.M.; Piaz, F.D.; Ruban, A.V.; Brunet, C. Light-Induced Changes in the Photosynthetic Physiology and Biochemistry in the Diatom Skeletonema Marinoi. Algal Res. 2016, 17, 1–13. [Google Scholar] [CrossRef]
- Di Dato, V.; Di Costanzo, F.; Barbarinaldi, R.; Perna, A.; Ianora, A.; Romano, G. Unveiling the Presence of Biosynthetic Pathways for Bioactive Compounds in the Thalassiosira Rotula Transcriptome. Sci. Rep. 2019, 9, 9893. [Google Scholar] [CrossRef]
- Cutignano, A.; Conte, M.; Tirino, V.; Del Vecchio, V.; De Angelis, R.; Nebbioso, A.; Altucci, L.; Romano, G. Cytotoxic Potential of the Marine Diatom Thalassiosira Rotula: Insights into Bioactivity of 24-Methylene Cholesterol. Mar. Drugs 2022, 20, 595. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Freitas, M.A.V.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Biotechnological Potential of Phaeodactylum Tricornutum for Biorefinery Processes. Fuel 2020, 268, 117357. [Google Scholar] [CrossRef]
- Frleta Matas, R.; Popović, M.; Čagalj, M.; Šimat, V. The Marine Diatom Thalassiosira Rotula: Chemical Profile and Antioxidant Activity of Hydroalcoholic Extracts. Front. Mar. Sci. 2023, 10, 1221417. [Google Scholar] [CrossRef]
- Di Costanzo, F.; Di Marsico, M.; Orefice, I.; Kristoffersen, J.B.; Kasapidis, P.; Chaumier, T.; Ambrosino, L.; Miralto, M.; Aiese Cigliano, R.; Verret, F.; et al. High-Quality Genome Assembly and Annotation of Thalassiosira Rotula (Synonym of Thalassiosira Gravida). Sci. Data 2025, 12, 310. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Abo-Shanab, W.A.; Ende, S.S.W.; Alquraishi, M.; El-Shenody, R.A. Optimizing Phaeodactylum Tricornutum Cultivation: Integrated Strategies for Enhancing Biomass, Lipid, and Fucoxanthin Production. Biotechnol. Biofuels Bioprod. 2025, 18, 7. [Google Scholar] [CrossRef]
- Wang, L.J.; Fan, Y.; Parsons, R.L.; Hu, G.R.; Zhang, P.Y.; Li, F.-L. A Rapid Method for the Determination of Fucoxanthin in Diatom. Mar. Drugs 2018, 16, 33. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ni, W.M.; Zhu, Y.M.; Pan, Y.D. Effects of Different Nitrogen Species on Sensitivity and Photosynthetic Stress of Three Common Freshwater Diatoms. Aquat. Ecol. 2013, 47, 25–35. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Yoshiyama, K. Contrasting Size Evolution in Marine and Freshwater Diatoms. Proc. Natl. Acad. Sci. USA 2009, 106, 2665–2670. [Google Scholar] [CrossRef]
- Stolte, W.; Riegman, R. Effect of Phytoplankton Cell Size on Transient-State Nitrate and Ammonium Uptake Kinetics. Microbiology 1995, 141, 1221–1229. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of Diatom Strains and Characterization of Cyclotella Cryptica as a Potential Fucoxanthin Producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia Laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef]
- Derwenskus, F.; Metz, F.; Gille, A.; Schmid-Staiger, U.; Briviba, K.; Schließmann, U.; Hirth, T. Pressurized Extraction of Unsaturated Fatty Acids and Carotenoids from Wet Chlorella Vulgaris and Phaeodactylum Tricornutum Biomass Using Subcritical Liquids. GCB Bioenergy 2019, 11, 335–344. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Chan, K.W.; Khong, N.M.H.; Yau, S.K. Production of Fucoxanthin-Rich Fraction (FxRF) from a Diatom, Chaetoceros Calcitrans (Paulsen) Takano 1968. Algal Res. 2015, 12, 26–32. [Google Scholar] [CrossRef]
- Pajot, A.; Huynh, G.H.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef]
- Bhattacharjya, R.; Kiran Marella, T.; Tiwari, A.; Saxena, A.; Kumar Singh, P.; Mishra, B. Bioprospecting of Marine Diatoms Thalassiosira, Skeletonema and Chaetoceros for Lipids and Other Value-Added Products. Bioresour. Technol. 2020, 318, 124073. [Google Scholar] [CrossRef]
- Park, Y.H.; Han, S., II.; Oh, B.; Kim, H.S.; Jeon, M.S.; Kim, S.; Choi, Y.E. Microalgal Secondary Metabolite Productions as a Component of Biorefinery: A Review. Bioresour. Technol. 2022, 344, 126206. [Google Scholar] [CrossRef]
- Eilers, U.; Dietzel, L.; Breitenbach, J.; Büchel, C.; Sandmann, G. Identification of Genes Coding for Functional Zeaxanthin Epoxidases in the Diatom Phaeodactylum Tricornutum. J. Plant Physiol. 2016, 192, 64–70. [Google Scholar] [CrossRef]
- Græsholt, C.; Brembu, T.; Volpe, C.; Bartosova, Z.; Serif, M.; Winge, P.; Nymark, M. Zeaxanthin Epoxidase 3 Knockout Mutants of the Model Diatom Phaeodactylum Tricornutum Enable Commercial Production of the Bioactive Carotenoid Diatoxanthin. Mar. Drugs 2024, 22, 185. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric Models for Calculating Cell Biovolume and Surface Area for Phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and Modifications of Primer Design Program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Kõressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating Masking of Template Sequence with Primer Design Software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
| HPLC (mg g−1 DW) | Spectro (mg g−1 DW) | Error (%) | |
|---|---|---|---|
| Ctrl | 3.63 ± 0.5 | 4.14 ± 0.7 | 14 ± 0.3 |
| HN | 3.47 ± 0.8 * | 9.17 ± 0.9 | 164 ± 1.5 |
| LL | 5.2 ± 0.9 * | 10.77 ± 0.8 | 79 ± 0.5 |
| LL HN | 2.79 ± 0.5 * | 5.85 ± 0.6 | 52 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeo, M.L.; Orefice, I.; Ferrari, M.; Greca, T.; Bruno, L.; Romano, G.; Cozza, R. Influence of Nitrate and Light on Fucoxanthin Content and Key Gene Expression in the Marine Diatom Thalassiosira rotula. Plants 2025, 14, 3344. https://doi.org/10.3390/plants14213344
Madeo ML, Orefice I, Ferrari M, Greca T, Bruno L, Romano G, Cozza R. Influence of Nitrate and Light on Fucoxanthin Content and Key Gene Expression in the Marine Diatom Thalassiosira rotula. Plants. 2025; 14(21):3344. https://doi.org/10.3390/plants14213344
Chicago/Turabian StyleMadeo, Maria Letizia, Ida Orefice, Michele Ferrari, Teresa Greca, Leonardo Bruno, Giovanna Romano, and Radiana Cozza. 2025. "Influence of Nitrate and Light on Fucoxanthin Content and Key Gene Expression in the Marine Diatom Thalassiosira rotula" Plants 14, no. 21: 3344. https://doi.org/10.3390/plants14213344
APA StyleMadeo, M. L., Orefice, I., Ferrari, M., Greca, T., Bruno, L., Romano, G., & Cozza, R. (2025). Influence of Nitrate and Light on Fucoxanthin Content and Key Gene Expression in the Marine Diatom Thalassiosira rotula. Plants, 14(21), 3344. https://doi.org/10.3390/plants14213344









