Microclimate Modification, Evapotranspiration, Growth and Essential Oil Yield of Six Medicinal Plants Cultivated Beneath a Dynamic Agrivoltaic System in Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Field Experiment Design
2.2. Cultural Practices
2.3. The Climate
2.4. Microclimate Measurements
2.5. Water Consumption: Theoretical Calculation of the ET0 and Etc
- ET0 represents the reference evapotranspiration, expressed in mm/day, averaged over monthly periods;
- T = monthly mean daytime air temperature in °C in °C;
- Rad = monthly mean daytime solar radiation in Wm−2;
- (c) = local correction factor = 0.80.
- ET0 is the reference evapotranspiration, expressed in mm/day, averaged over monthly periods;
- Kc is the crop coefficient, which varies depending on crop type and growth stage;
- ETc is the crop evapotranspiration expressed in mm/day, averaged over monthly periods.
2.6. Plant Growth Measurements
2.7. Statistical Analysis
3. Results
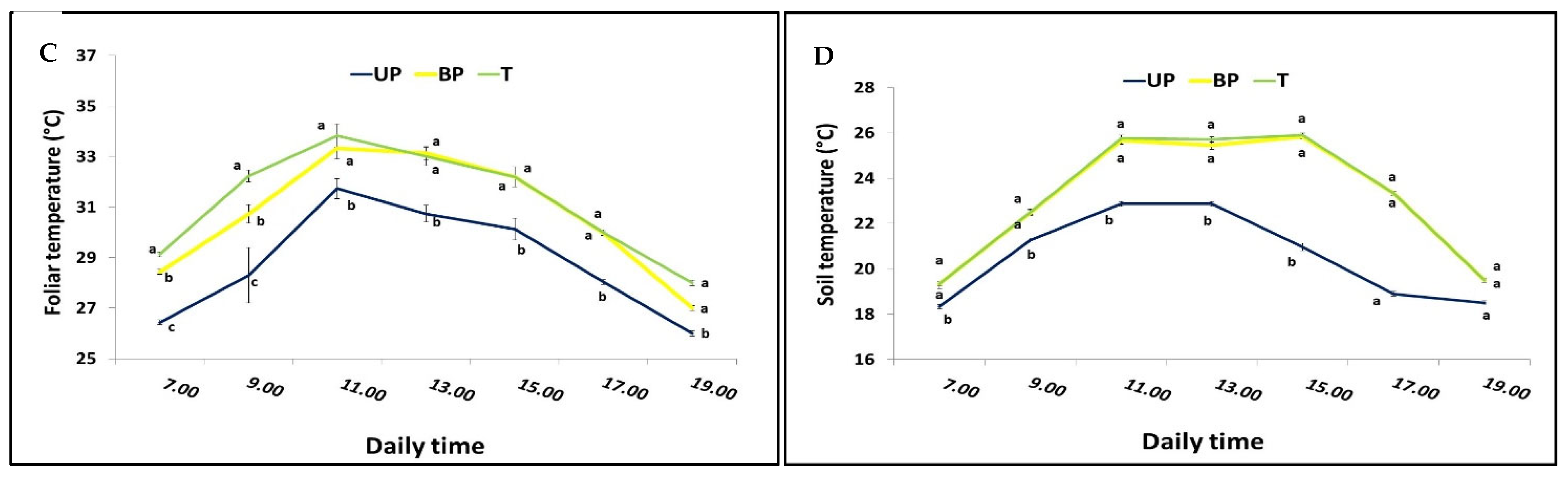
3.1. Effect of AV System on Microclimate
3.2. Effect of AV System on Crop Water Use
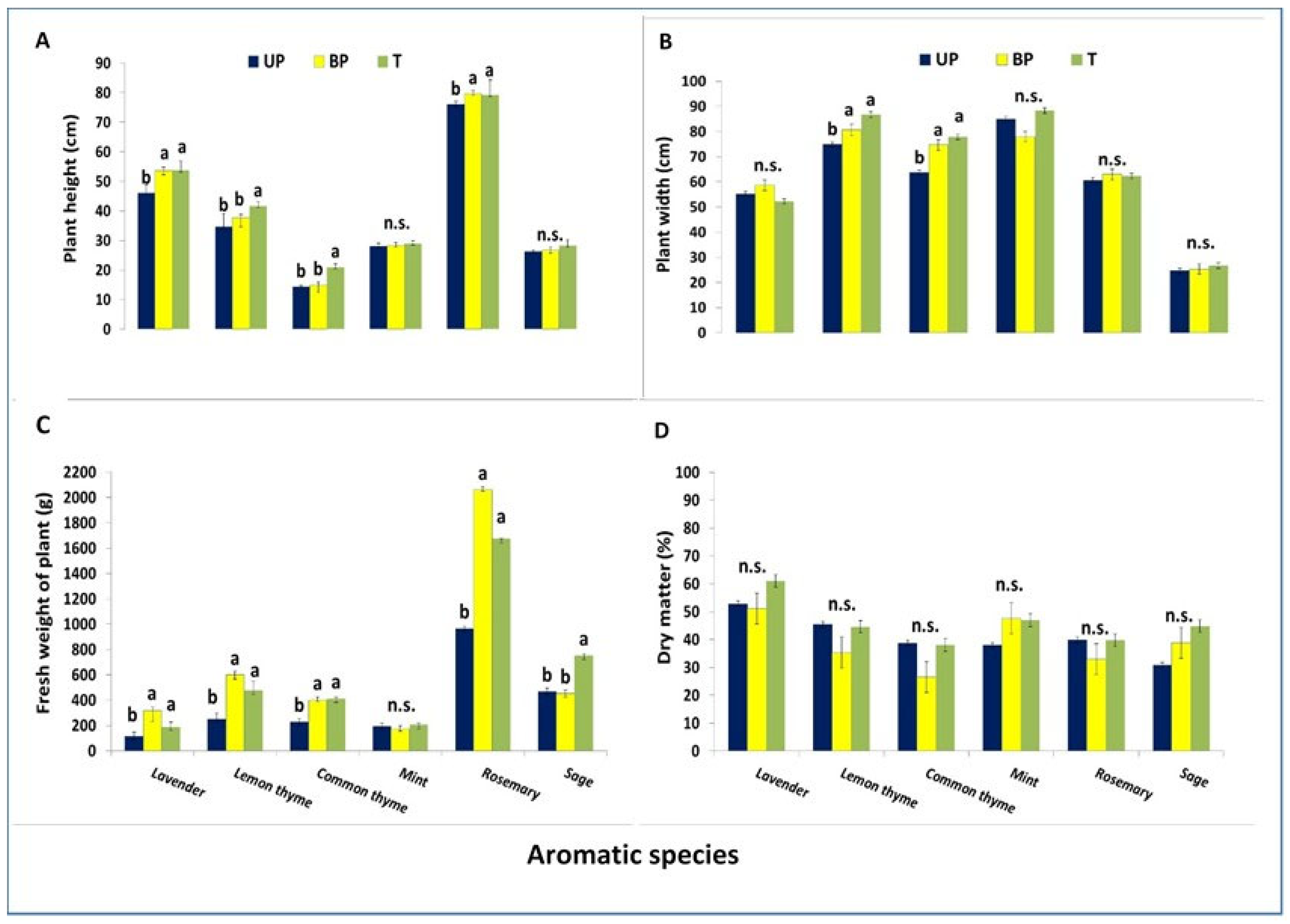
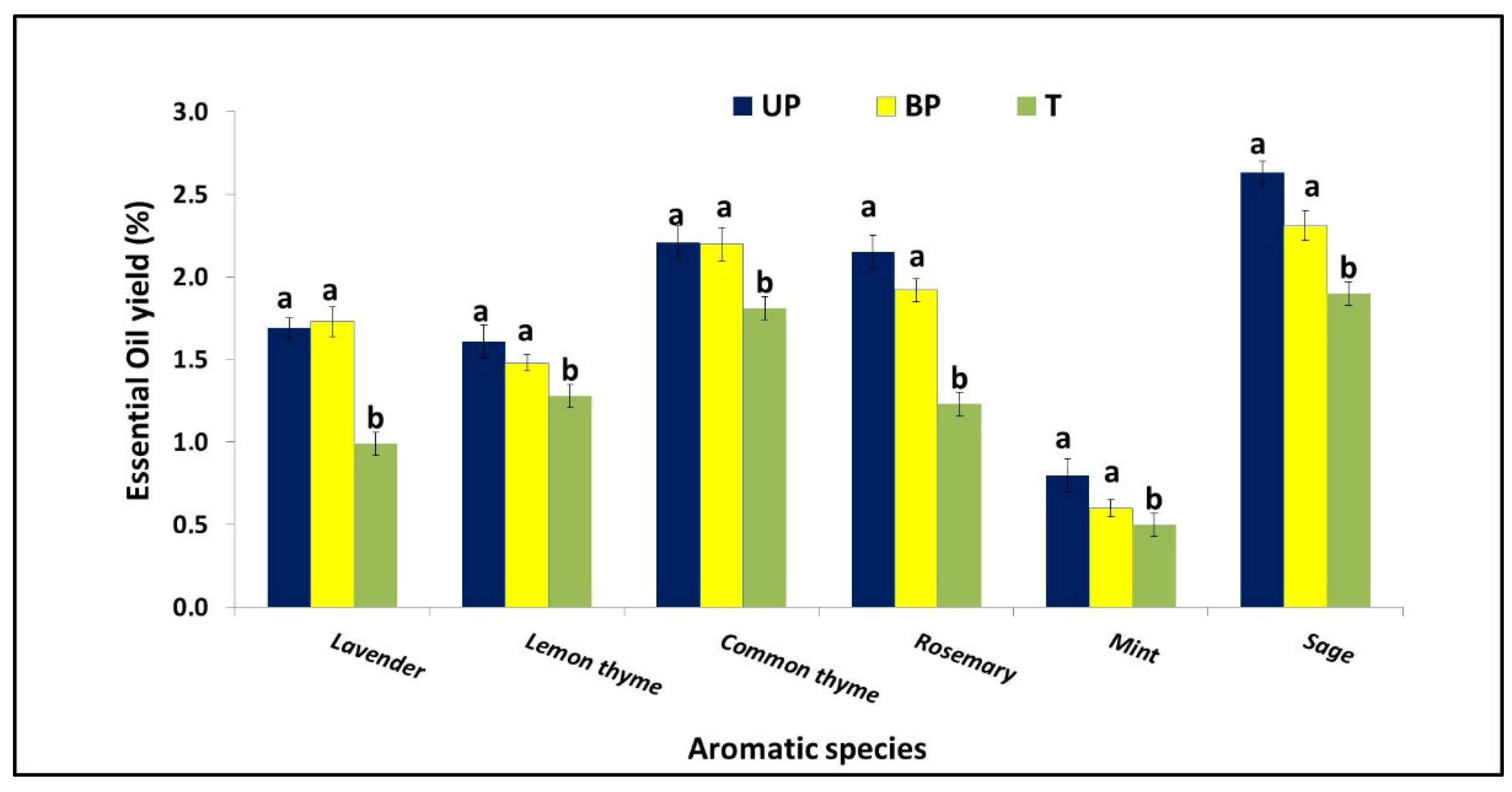
3.3. Effect of AV System on Plant Growth
4. Discussion
4.1. Microclimatic Parameter and Plant Growth Variations
4.1.1. Solar Radiation
4.1.2. Daytime Air Temperature
4.1.3. Infrared Plant Temperature
4.1.4. Soil Temperature
4.1.5. Crop Evapotranspiration
4.1.6. Plant Growth
4.1.7. Essential Oil Yield Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Othman, N.F.; Yaacob, M.E.; Su, A.S.M.; Jaafar, J.N.; Hizam, H.; Shahidan, M.F.; Jamaluddin, A.H.; Chen, G.; Jalaludin, A. Modeling of stochastic temperature and heat stress directly underneath agrivoltaic conditions with Orthosiphon stamineus crop cultivation. Agronomy 2020, 10, 1472. [Google Scholar] [CrossRef]
- Weselek, A.; Bauerle, A.; Hartung, J.; Zikeli, S.; Lewandowski, I.; Högy, P. Agrivoltaic system impacts on microclimate and yield of different crops within an organic crop rotation in a temperate climate. Agron. Sustain. Dev. 2021, 41, 59. [Google Scholar] [CrossRef]
- Guerriero, V.; Scorzini, A.R.; Di Lena, B.; Iulianella, S.; Di Bacco, M.; Tallini, M. Impact of climate change on crop yields: Insights from the abruzzo region, central Italy. Sustainability 2023, 15, 14235. [Google Scholar] [CrossRef]
- VanDerZanden, A.M. Environmental factors affecting plant growth. Extension Service OSU. 2024, pp. 1–16. Available online: https://extension.oregonstate.edu/gardening/techniques/environmental-factors-affecting-plant-growth (accessed on 3 April 2025).
- Ma Lu, S.; Zainali, S.; Stridh, B.; Avelin, A.; Amaducci, S.; Colauzzi, M.; Campana, P.E. Photosynthetically active radiation decomposition models for agrivoltaic systems applications. Sol. Energy 2022, 244, 536–549. [Google Scholar] [CrossRef]
- Firmansyah, A.N.; Argosubekti, N.A. review of heat stress signaling in plants. IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012041. [Google Scholar] [CrossRef]
- Mishra, S.; Spaccarotella, K.; Gido, J.; Samanta, I.; Chowdhary, G. Effects of heat stress on plant-nutrient relations: An update on nutrient uptake, transport, and assimilation. Int. J. Mol. Sci. 2023, 24, 15670. [Google Scholar] [CrossRef] [PubMed]
- Baille, A.; Kittas, C.; Katsoulas, N. Influence of whitening on greenhouse microclimate and crop energy partitioning. Agric. For. Meteorol. 2001, 107, 293–306. [Google Scholar] [CrossRef]
- Ahemd, H.A.; Al-Faraj, A.A.; Abdel-Ghany, A.M. Shading greenhouses to improve the microclimate, energy and water saving in hot regions: A review. Sci. Hortic. 2016, 201, 36–45. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Broekhuijsen, A.G.M.; Meinen, E.; Nijs, E.M.F.M.; Raaphorst, M.G.M. Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. 2006, 711, 97–104. [Google Scholar] [CrossRef]
- Baudoin, W.; Nersisyan, A.; Shamilov, A.; Hodder, A.; Gutierrez, D.; de Pascale, S.; Nicola, S.; Gruda, N.; Urban, L.; Tanny, J. Good Agricultural Practices for Greenhouse Vegetable Production in the South East European Countries—Principles for Sustainable Intensification of Smallholder Farms; FAO Plant Production and Protection Paper 230; FAO: Rome, Italy, 2017; p. 428. Available online: www.researchgate.net/publication/317042928 (accessed on 10 April 2025)ISBN 978-92-5-109622-2.
- Elamri, Y.B.; Cheviron, B.; Lopez, J.-M.; Dejean, C.; Belaud, G. Water budget and crop modelling for agrivoltaic systems: Application to irrigated lettuces. Agric. Water Manag. 2018, 208, 440–453. [Google Scholar] [CrossRef]
- Schindele, S.; Trommsdorff, M.; Schlaak, A.; Obergfell, T.; Bopp, G.; Reise, C.; Braun, C.; Weselek, A.; Bauerle, A.; Högy, P.; et al. Implementation of agrophotovoltaics: Techno-economic analysis of the price-performance ratio and its policy implications. Appl. Energy 2020, 265, 114737. [Google Scholar] [CrossRef]
- Cossu, M.; Yano, A.; Solinas, S.; Deligios, P.A.; Tiloca, M.T.; Cossu, A.; Ledda, L. Agricultural sustainability estimation of the European photovoltaic greenhouses. Eur. J. Agron. 2020, 118, 126074. [Google Scholar] [CrossRef]
- Friman-Peretz, M.; Ozer, S.; Geoola, F.; Magadley, E.; Yehia, I.; Levi, A.; Brikman, R.; Gantz, S.; Levy, A.; Kacira, M. Microclimate and crop performance in a tunnel greenhouse shaded by organic photovoltaic modules—Comparison with conventional shaded and unshaded tunnels. Biosyst. Eng. 2020, 197, 12–31. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Pavao-Zuckerman, M.A.; Minor, R.L.; Sutter, L.F.; Barnett-Moreno, I.; Blackett, D.T.; Thompson, M.; Dimond, K.; Gerlak, A.K.; Nabhan, G.P.; et al. Agrivoltaics provide mutual benefits across the food–energy–water nexus in drylands. Nat. Sustain. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Lee, S.I.; Lee, J.H.; Seo, B.H.; Kim, D.S.; Lee, J.M.; Choi, W. simulation of solar irradiance distribution under agrivoltaic facilities. J. Korean Soc. Agric. Eng. 2022, 64, 1–13. [Google Scholar] [CrossRef]
- Weselek, A.; Ehmann, A.; Zikeli, S.; Lewandowski, I.; Schindele, S.; Högy, P. Agrophotovoltaic systems: Applications, challenges, and opportunities. A review. Agron. Sustain. Dev. 2019, 39, 35. [Google Scholar] [CrossRef]
- Feifel, M.; Durner, W.; Hohenbrink, T.L.; Peters, A. Effects of improved water retention by increased soil organic matter on the water balance of arable soils: A numerical analysis. Vadose Zone J. 2023, 23, e20302. [Google Scholar] [CrossRef]
- Marrou, H.; Wery, J.; Dufour, L.; Dupraz, C. Productivity and radiation use efficiency of lettuces grown in the partial shade of photovoltaic panels. Eur. J. Agron. 2013, 44, 54–66. [Google Scholar] [CrossRef]
- Dinesh, H.; Pearce, J.M. The potential of agrivoltaic systems. Renew. Sustain. Energy Rev. 2016, 54, 299–308. [Google Scholar] [CrossRef]
- Valle, B.; Simonneau, T.; Sourd, F.; Pechier, P.; Hamard, P.; Frisson, T.; Ryckewaert, M.; Christophe, A. Increasing the total productivity of a land by combining mobile photovoltaic panels and food crops. Appl. Energy 2017, 206, 1495–1507. [Google Scholar] [CrossRef]
- Sekiyama, T.; Nagashima, A. Solar sharing for both food and clean energy production: Performance of agrivoltaic systems for corn, a typical shade-intolerant crop. Environments 2019, 6, 65. [Google Scholar] [CrossRef]
- Chae, S.H.; Kim, H.J.; Moon, H.W.; Kim, Y.H.; Ku, K.M. Agrivoltaic systems enhance farmers’ profits through broccoli visual quality and electricity production without dramatic changes in yield, antioxidant capacity, and glucosinolates. Agronomy 2022, 12, 1415. [Google Scholar] [CrossRef]
- Schweiger, A.H.; Pataczek, L. How to reconcile renewable energy and agricultural production in a drying world. Plants People Planet 2023, 5, 650–661. [Google Scholar] [CrossRef]
- Semeraro, T.; Scarano, A.; Curci, L.M.; Leggieri, A.; Lenucci, M.; Basset, A.; Santino, A.; Piro, G.; De Caroli, M. Shading effects in agrivoltaic systems can make the difference in boosting food security in climate change. Appl. Energy 2024, 358, 122565. [Google Scholar] [CrossRef]
- Scarano, A.; Semeraro, T.; Calisi, A.; Aretano, R.; Rotolo, C.; Lenucci, M.S.; Santino, A.; Piro, G.; De Caroli, M. Effects of the agrivoltaic system on crop production: The case of tomato (Solanum lycopersicum L.). Appl. Sci. 2024, 14, 3095. [Google Scholar] [CrossRef]
- Touil, S.; Richa, A.; Fizir, M.; B Bingwa, B. Shading effect of photovoltaic panels on horticulture crops production: A mini review. Environ. Sci. 2021, 20, 281–296. [Google Scholar] [CrossRef]
- Zahrawi, A.A.; Aly, A.M. A Review of agrivoltaic systems: Addressing challenges and enhancing sustainability. Sustainability 2024, 16, 8271. [Google Scholar] [CrossRef]
- Macaluso, D.; Licciardo, F.; Carbone, K. Farming of medicinal and aromatic plants in italy: Structural features and economic results. Agriculture 2024, 14, 151. [Google Scholar] [CrossRef]
- Disciglio, G.; Frabboni, L.; Tarantino, A.; Stasi, A. Association between dynamic agrivoltaic system and cultivation: Viability, yields and qualitative assessment of medical plants. Sustainability 2023, 15, 16252. [Google Scholar] [CrossRef]
- UNESCO/FAO. Bioclimatic Map of the Mediterranean Zone; Explanatory Notes, Arid Zone Research; UNESCO/FAO: Rome, Italy, 1963; Volume 2217, p. 26. [Google Scholar]
- Ventrella, D.; Charfeddine, M.; Moriondo, M.; Rinaldi, M.; Bindi, M. Agronomic adaptation strategies under climate change for winter durum wheat and tomato in southern Italy: Irrigation and nitrogen fertilization. Reg. Environ. Chang. 2012, 12, 407–412. [Google Scholar] [CrossRef]
- Centro Funzionale Decentrato Regione Puglia—Protezione Civile Puglia. Available online: https://protezionecivile.regione.puglia.it (accessed on 2 April 2025).
- Rubino, P.; Tarantino, E.; Mstrorilli, M.; Lonigro, A.; Mastro, M.A.; Gatta, G.; Disciglio, G.; Campi, P.; Stellacci, A.M.; Navarro, A.; et al. Impatti agronomici ed ambientali, 203–248. In Progetto PON in.Te.R.R.A. Linee Guida per il Riuso Delle Acque Reflue Depurate; Rubino, P., Lonigro, A., Eds.; Edizioni di Pagina: Bari, Italy, 2015; Volume 271, ISBN 978-88-7470-405-7. (In Italian) [Google Scholar]
- Doorenbos, J.; Pruitt, W.O. Crop Water Requirements. In FAO Irrigation and Drainage; Paper 24; FAO: Rome, Italy, 1977; p. 144. [Google Scholar]
- Castrignanò, A.; De Caro, A.; Tarantino, E. Verifica sulla validità di alcuni metodi empirici di stima dell’evapotraspirazione potenziale nel Metapontino. Riv. L’Irrigazione 1985, 32, 23–28. (In Italian) [Google Scholar]
- Calcagno, G. Analisi dei Flussi Energetici per la Stima Dell’evapotraspirazione Attraverso Tecniche di Telerilevamento Satellitare. Ph.D. Thesis, University of Calabria, Arcavacata, Italy, 2007; p. 187. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-guidelines for computing crop water requirements-FAO. In Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Noorollahi, M.; Hassanli, A.M.; Ghanbarian, G.; Taghvaei, M. Determination of crop coefficient (Kc) for Rosmarinus officinalis L., Lavandula angustifolia Mill. and Silybum marianum (L.) gaertnas medicinal plants using water balance approach. Iran J. Irrig Drain. 2016, 10, 117–127. Available online: https://idj.iaid.ir/article_55274_en.html (accessed on 5 May 2025).
- Marino, S.; Ahmad, U.; Ferreira, M.I.; Alvino, A. Evaluation of the effect of irrigation on biometric growth, physiological response, and essential oil of Mentha spicata (L.). Water 2019, 11, 2264. [Google Scholar] [CrossRef]
- Ghamarnia, H.; Palash, M.; Amiri, S. Deficit irrigation effects on rosemary plant (Rosmarinus officinalis L.) parameters in a semi-arid climate. Agrotech. Indust. Crops 2022, 2, 57–64. [Google Scholar] [CrossRef]
- Orte, T. Evaluación de dos Materiales de Orégano Europeo (Origanum vulgare), Romero (Rosmarinus officinalis), Ajenjo (Artemisia absinthium) y Melisa (Melissa officinalis), en el Marco de la Red de Cultivos Aromáticos del Sudoeste Bonaerense, Sitio Cabildo, Ciclo 2018–2019; Trabajo Final de Intensificación; Universidad Nacional del Sur: Bahia Blanca, Argentina, 2022; pp. 12–29. Available online: https://repositoriodigital.uns.edu.ar/handle/123456789/6280 (accessed on 10 April 2025).
- Pereira, L.S.; Mota, M.; Raziei, T.; Paredes, P. Water requirements and crop coefficients of edible, spicy and medicinal herbs and vegetables; a review aimed at supporting plant and water management. Irrig. Sci. 2024, 42, 1199–1228. [Google Scholar] [CrossRef]
- Frabboni, L.; Tarantino, A.; Petruzzi, F.; Disciglio, G. Bio-herbicidal effects of oregano and rosemary essential oils on chamomile (Matricaria chamomilla L.) crop in organic farming system. Agronomy 2019, 9, 475. [Google Scholar] [CrossRef]
- Armstrong, A.; Ostle, N.J.; Whitaker, J. Solar Park Microclimate and Vegetation Management Effects on Grassland Carbon Cycling. Environ. Res. Lett. 2016, 11, 074016. [Google Scholar] [CrossRef]
- Choi, C.S.; Macknick, J.; Li, Y.; Bloom, D.; McCall, J.; Ravi, S. Environmental co-benefits of maintaining native vegetation with solar photovoltaic infrastructure. Earth’s Future 2023, 11, e2023EF00354. [Google Scholar] [CrossRef]
- Gomez–Casanovas, N.; Mwebaze, P.; Khanna, M.; Branham, B.; Time, A.; De Lucia, E.H.; Bernacchi, C.J.; Knapp, A.K.; Hoque, M.J.; Du, X.; et al. Knowns, uncertainties, and challenges in agrivoltaics to sustainably intensify energy and food production. Cell Rep. Phys. Sci. 2023, 4, 101518. [Google Scholar] [CrossRef]
- Marrou, H.; Guilioni, L.; Dufour, L.; Dupraz, C.; Wery, J. Microclimate under agrivoltaic systems: Is crop growthrate affected in the partial shade of solar panels? Agric. For. Meteorol. 2013, 177, 117–132. [Google Scholar] [CrossRef]
- Omer, A.A.A.; Li, M.; Zhang, F.; Hassaan, M.M.E.; Kolaly, W.E.; Zhang, X.; Lan, H.; Liu, J.; Liu, W. Impacts of agrivoltaic systems on microclimate, water use efficiency, and crop yield: A systematic review. Renew. Sustain. Energy Rev. 2025, 221, 115930. [Google Scholar] [CrossRef]
- Pang, K.; van Sambeek, J.W.; Navarrete-Tindall, N.E.; Lin, C.-H.; Jose, S.; Garrett, H.E. Responses of legumes and grasses to non, moderate, and dense shade in Missouri, USA. I. Forage yield and its species-level plasticity. Agrofor. Syst. 2017, 93, 11–24. [Google Scholar] [CrossRef]
- Magarelli, A.; Mazzeo, A.; Ferrara, G. Fruit crop species with agrivoltaic systems: A critical Review. Agronomy 2024, 14, 722. [Google Scholar] [CrossRef]
- Willockx, B.; Reher, T.; Lavaert, C.; Herteleer, B.; Van De Poel, B.; Cappelle, J. Design and evaluation of an agrivoltaic system for a pear orchard. Appl. Energy 2024, 353, 122166. [Google Scholar] [CrossRef]
- Al Mamun, M.A.; Dargusch, P.; Wadley, D.; Zulkarnain, N.A.; Aziz, A.A. review of research on agrivoltaic systems. Renew. Sustain. Energy Rev. 2022, 161, 112351. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Jeong, Y.; Kim, D.; Seo, B.; Seo, Y.; Kim, T.; Cho, W. Agrivoltaic system designing for sustainability and smart farming: Agronomic aspects and design criteria with safety assessment. Appl. Energy 2024, 365, 123258. [Google Scholar] [CrossRef]
- Gates, D.M. Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Gambín, J.M.B.; Tortosa, P.A.N.; Cabañero, J.J.A.; Nicolás, E. Determination of Crop Water Stress Index by Infrared Thermometry in Grapefruit Trees Irrigated with Saline Reclaimed Water Combined with Deficit Irrigation. Remote Sens. 2019, 11, 757. [Google Scholar] [CrossRef]
- Katimbo, A.; Rudnick, D.R.; DeJonge, K.C.; Lo, T.H.; Qiao, X.; Franz, T.E.; Nakabuye, H.N.; Duan, J. Crop water stress index computation approaches and their sensitivity to soil water dynamics. Agric. Water Manag. 2022, 266, 107575. [Google Scholar] [CrossRef]
- Still, C.J.; Rastogi, B.; Gerald, F.M.; Page, G.F.M.; Griffith, D.M.; Sibley, A.; Schulze, M.; Hawkins, L.; Pau, S.; Detto, M. Imaging canopy temperature: Shedding (thermal) light on ecosystem processes. New Phytol. 2021, 230, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Migicovsky, Z.; Swift, J.F.; Helget, Z.; Klein, L.L.; Ly, A.; Maimaitiyiming, M.; Woodhouse, K.; Fennell, A.; Kwasniewski, M.; Miller, A.J.; et al. Grapevine leaf size influences canopy temperature. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Kearney, M.R.; Leigh, A. Fast, accurate and accessible calculations of leaf temperature and its physiological consequences with NicheMapR. Methods Ecol. Evol. 2024, 15, 1516–1531. [Google Scholar] [CrossRef]
- Al-Shammary, A.A.G.; Al-Shihmani, L.S.S.; Fernández-Gálvez, J.; Caballero-Calvo, A. A comprehensive review of impacts of soil management practices and climate adaptation strategies on soil thermal conductivity in agricultural soils. Rev. Environ. Sci. Biotechnol. 2025, 24, 513–543. [Google Scholar] [CrossRef]
- Warmann, E.; Jenerette, G.D.; Barron-Gafford, G.A. Agrivoltaic system design tools for managing trade-offs between energy production, crop productivity and water consumption. Environ. Res. Lett. 2024, 19, 034046. [Google Scholar] [CrossRef]
- Reeza, A.A.; Noor, N.F.M.; Ahmed, O.H.; Masuri, M.A. Shading effect of photovoltaic panels on growth of selected tropical vegetable crops. Sci. Hortic. 2024, 324, 112574. [Google Scholar] [CrossRef]
- Sarr, A.; Soro, Y.M.; Tossa, A.K.; Diop, L. Agrivoltaic, a Synergistic co-location of agricultural and energy production in perpetual mutation: A comprehensive review. Processes 2023, 11, 948. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Zubay, P.; Gosztola, B.; Radácsi, P.; Ladányi, M.; Szabó, K. Shade induced changes in the volatile profiles of some selected essential oil bearing medicinal and aromatic plants. Agrofor. Syst. 2025, 99, 128. [Google Scholar] [CrossRef]



| Month | Tmax | Tmin | RHmax | RHmin | Rad | Ws | P |
|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (%) | (%) | (W·m−2) | (m·s−1) | (mm) | |
| April | 19.6 | 6.3 | 95.8 | 49.6 | 194 | 3.5 | 20.8 |
| May | 28.5 | 12.7 | 93.7 | 43.4 | 207 | 3.2 | 22.2 |
| June | 33.9 | 18.6 | 83.8 | 36.0 | 280 | 3.7 | 51.3 |
| July | 34.7 | 19.0 | 82.5 | 33.5 | 300 | 3.4 | 49.8 |
| August | 32.3 | 19.1 | 85.3 | 36.7 | 267 | 3.7 | 20.0 |
| Mean | 29.8 | 15.4 | 88.2 | 39.8 | 249.6 | 3.5 | 32.8 |
| Total | 164.1 |
| Month | TUP | TBP | TT | RadUP | RadBP | RadT | EToUP | EToBP | EToT | Kc | ETcUP | ETcBP | ETcT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (Wm−2) | (Wm−2) | (Wm−2) | (mm) | (mm) | (mm) | (mm) | (mm) | (mm) | ||
| April | 14.8 | 14.9 | 15.0 | 50 | 450 | 517 | 12.1 | 59.3 | 67.5 | 0.40 | 4.8 | 23.7 | 27.0 |
| May | 22.4 | 22.6 | 22.7 | 70 | 478 | 549 | 17.1 | 75.4 | 85.5 | 0.60 | 10.3 | 45.2 | 51.0 |
| June | 28.9 | 29.1 | 29.3 | 110 | 644 | 740 | 25.1 | 109.0 | 124.1 | 0.80 | 20.1 | 87.2 | 99.2 |
| July | 29.5 | 29.8 | 29.9 | 115 | 740 | 796 | 25.9 | 125.0 | 134.3 | 0.95 | 24.6 | 118.7 | 127.6 |
| August | 28.0 | 28.4 | 28.4 | 80 | 616 | 708 | 20.1 | 92.9 | 118.0 | 0.95 | 19.1 | 87.4 | 112.1 |
| Mean | 24.7 | 25.0 | 25.1 | 85 | 586 | 662 | 0.74 | ||||||
| Total | 281.2 | 461.6 | 529.4 | 78.9 | 362.2 | 416.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Disciglio, G.; Stasi, A.; Tarantino, A.; Frabboni, L. Microclimate Modification, Evapotranspiration, Growth and Essential Oil Yield of Six Medicinal Plants Cultivated Beneath a Dynamic Agrivoltaic System in Southern Italy. Plants 2025, 14, 2428. https://doi.org/10.3390/plants14152428
Disciglio G, Stasi A, Tarantino A, Frabboni L. Microclimate Modification, Evapotranspiration, Growth and Essential Oil Yield of Six Medicinal Plants Cultivated Beneath a Dynamic Agrivoltaic System in Southern Italy. Plants. 2025; 14(15):2428. https://doi.org/10.3390/plants14152428
Chicago/Turabian StyleDisciglio, Grazia, Antonio Stasi, Annalisa Tarantino, and Laura Frabboni. 2025. "Microclimate Modification, Evapotranspiration, Growth and Essential Oil Yield of Six Medicinal Plants Cultivated Beneath a Dynamic Agrivoltaic System in Southern Italy" Plants 14, no. 15: 2428. https://doi.org/10.3390/plants14152428
APA StyleDisciglio, G., Stasi, A., Tarantino, A., & Frabboni, L. (2025). Microclimate Modification, Evapotranspiration, Growth and Essential Oil Yield of Six Medicinal Plants Cultivated Beneath a Dynamic Agrivoltaic System in Southern Italy. Plants, 14(15), 2428. https://doi.org/10.3390/plants14152428








