Integrated Metabolomic and Transcriptomic Analysis Reveals the Roles of Cutin, Suberin, and Flavonoid Metabolism in Apple Peel Deterioration Under Non-Bagging Cultivation
Abstract
1. Introduction
2. Results
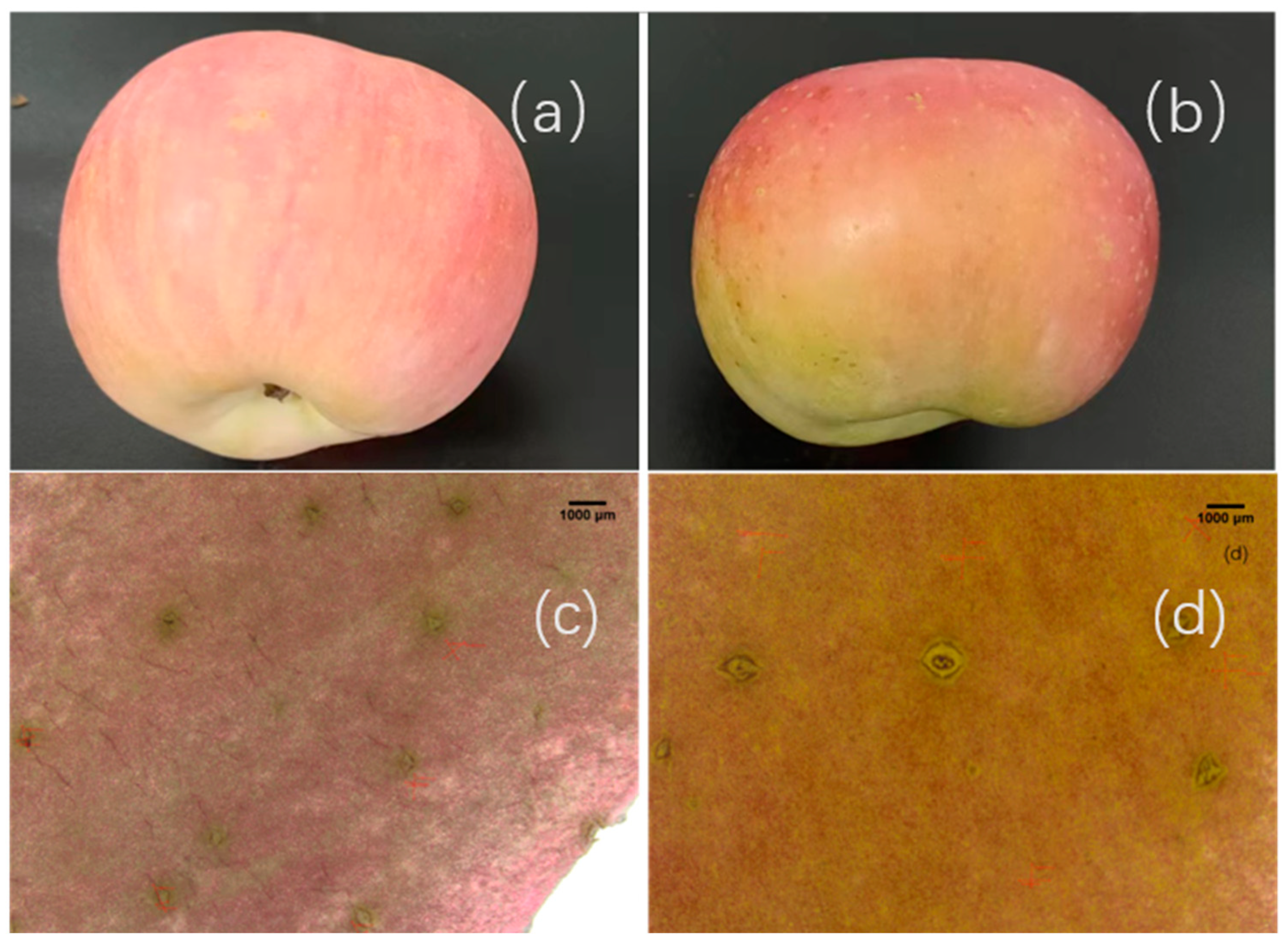
2.1. Effects of Non-Bagging and Bagging Cultivation on the Appearance Quality of Fuji Apples
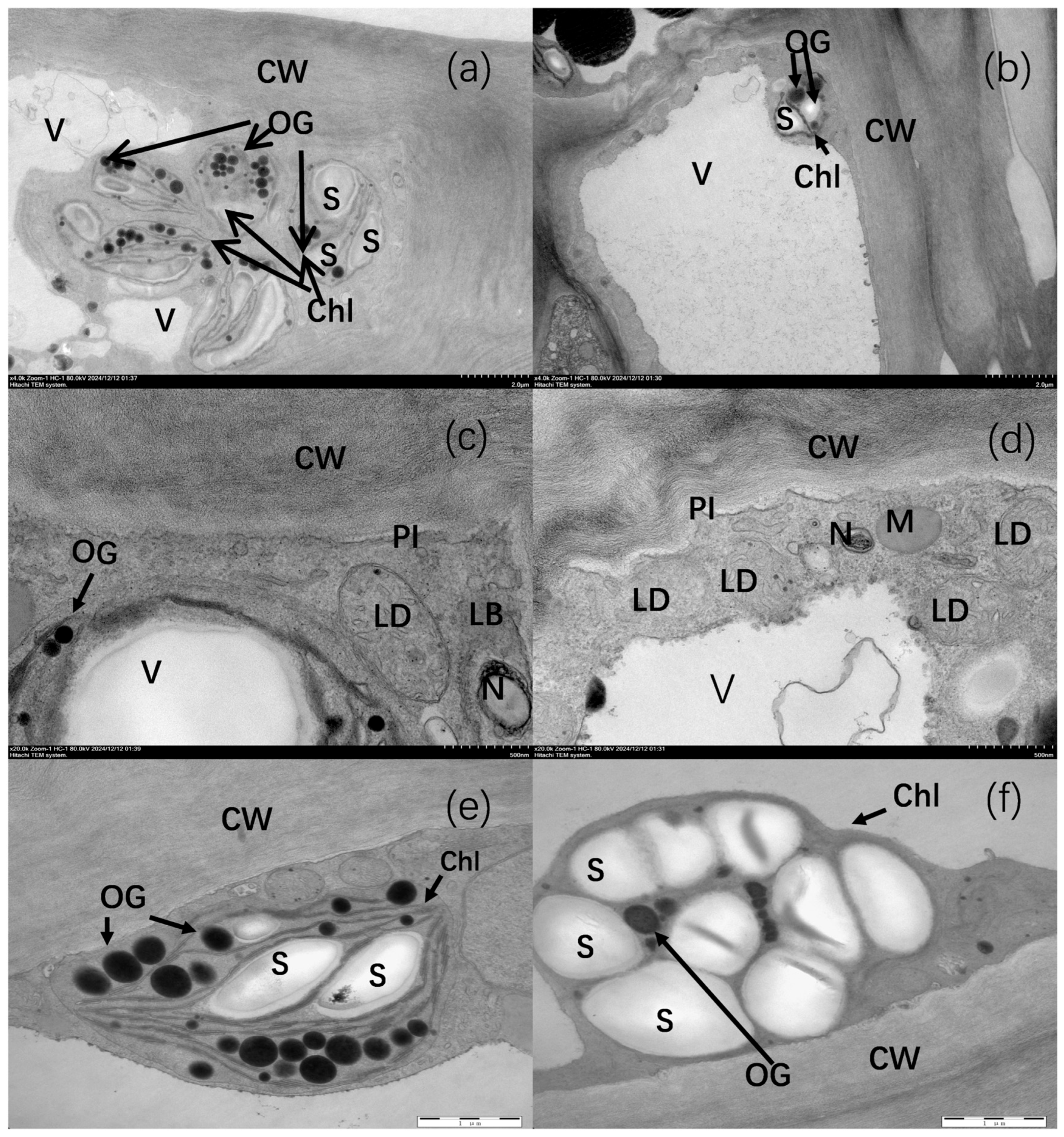
2.2. Effects of Non-Bagging and Bagging Cultivation on the Ultrastructure of Fuji Apple Peel
2.3. Effects of Non-Bagging and Bagging Cultivation on the Pigment Content of Fuji Apple Peel
2.3.1. Chlorophyll and Carotenoid Content in Fruit Peel
2.3.2. Total Anthocyanin and Total Flavonoid Content in Fuji Apple Fruit Peel
2.4. Transcriptomic Analysis of Non-Bagging and Bagging-Cultivated Fuji Apple Peels
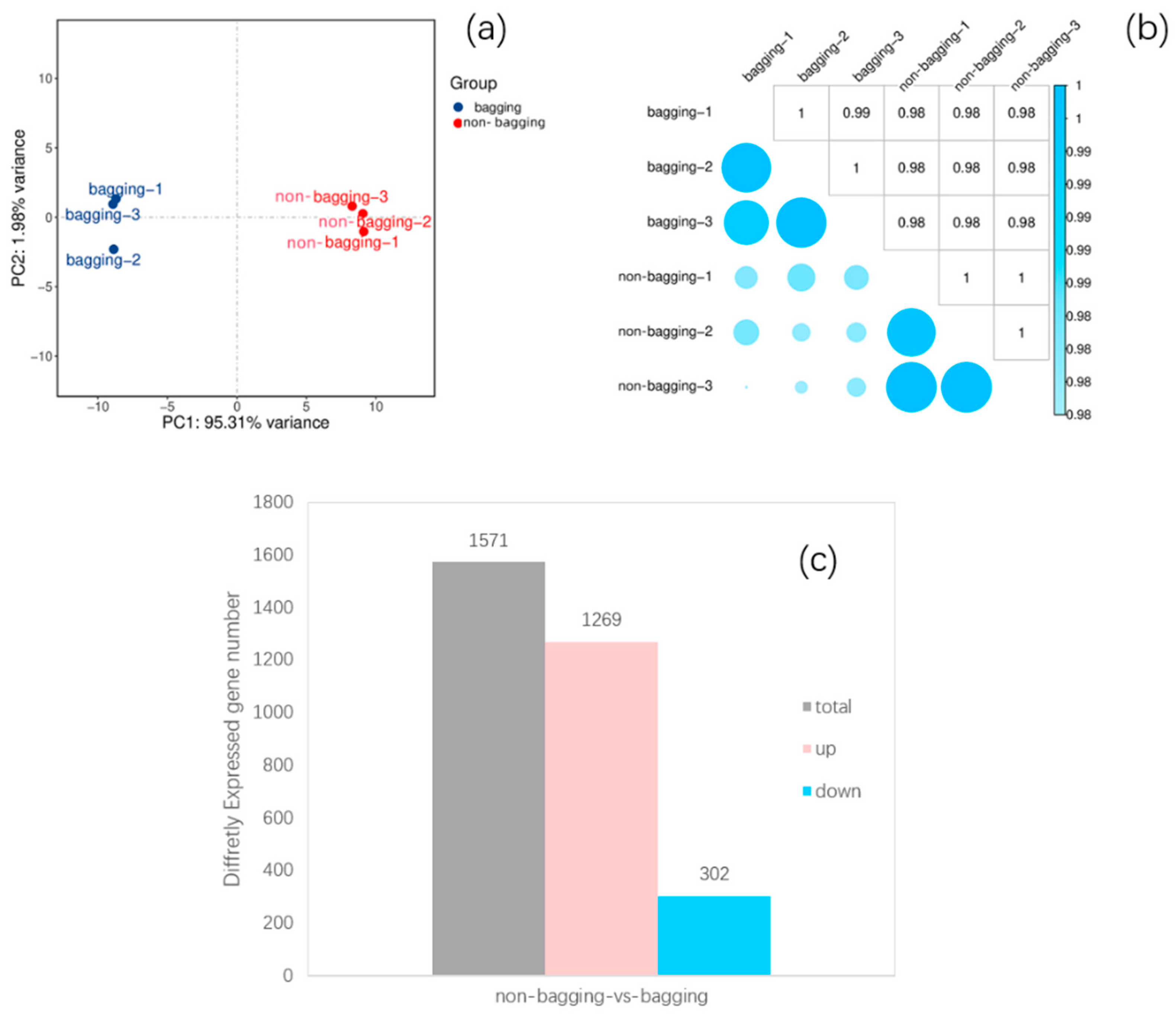
2.4.1. Transcriptome Sequencing and Quality Control of Fuji Apple Peel Samples
2.4.2. Identification of DEGs Between Non-Bagging and Bagging Fuji Apple Peels
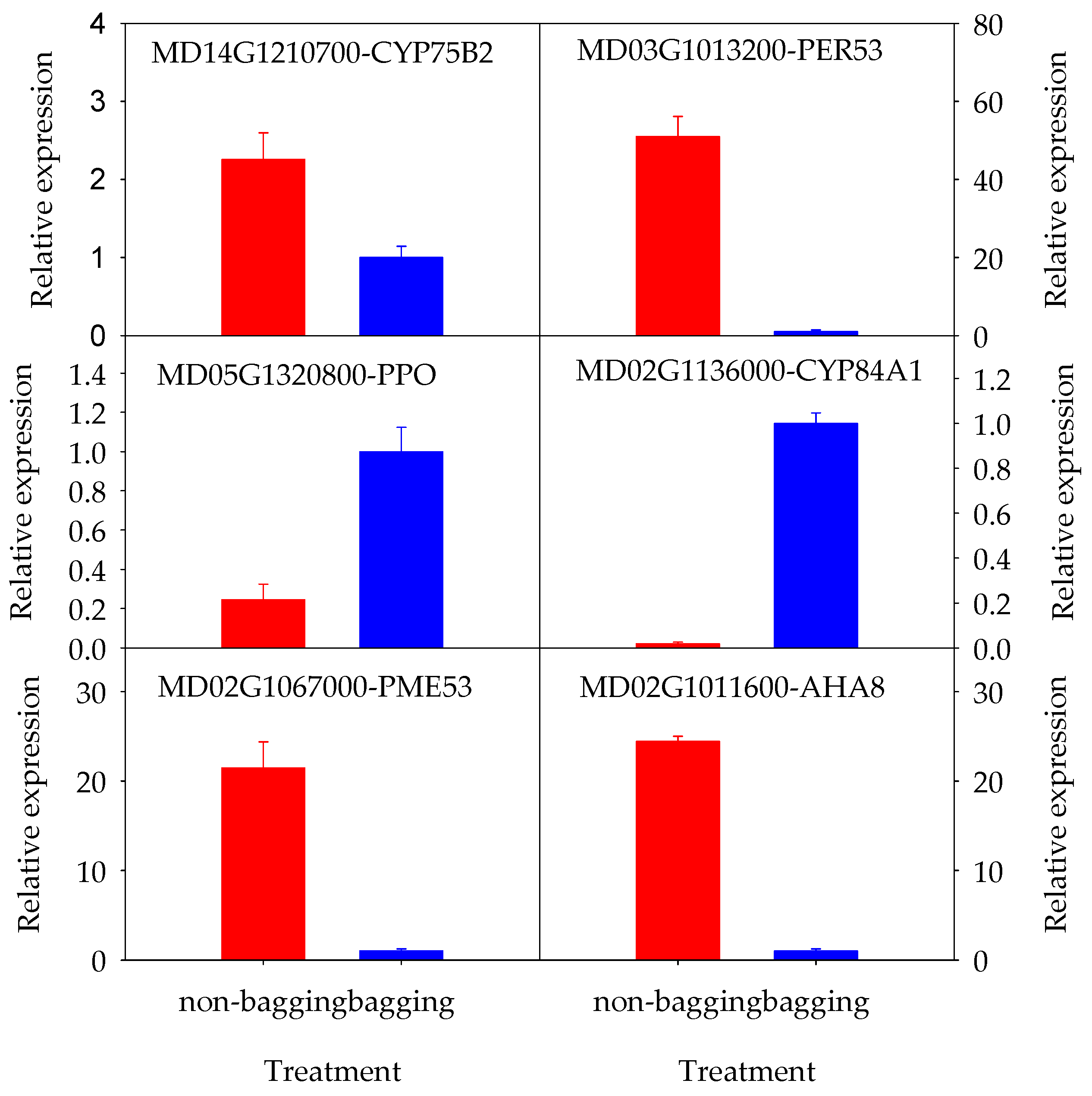
2.4.3. Screening of Candidate Genes Related to Appearance Quality Deterioration and qRT-PCR Validation
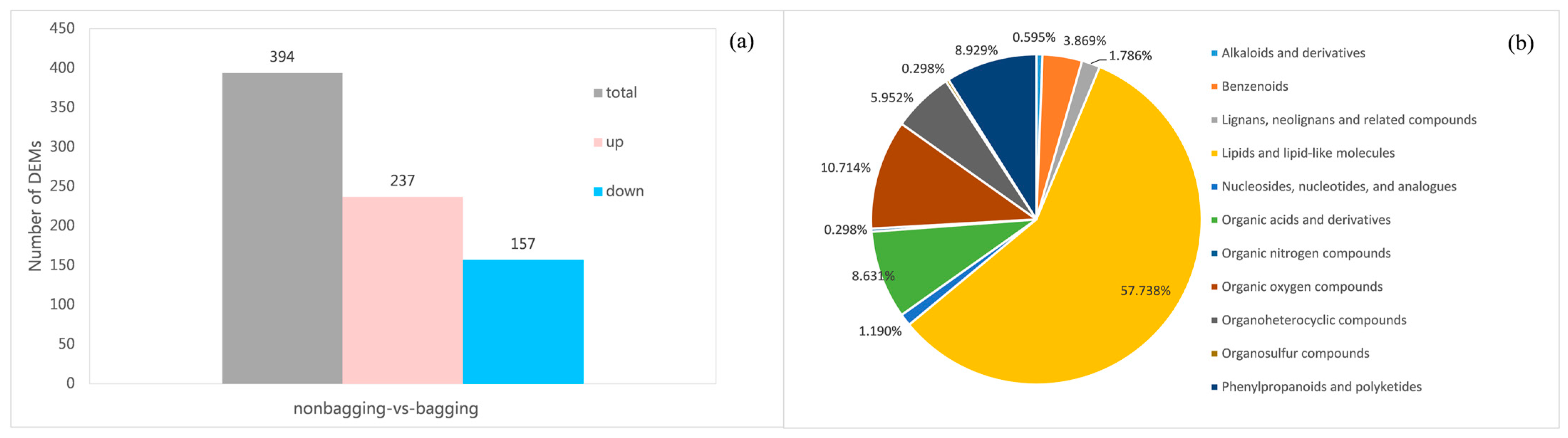
2.5. Metabolome Analysis of Non-Bagging and Bagging Fuji Apple Peels
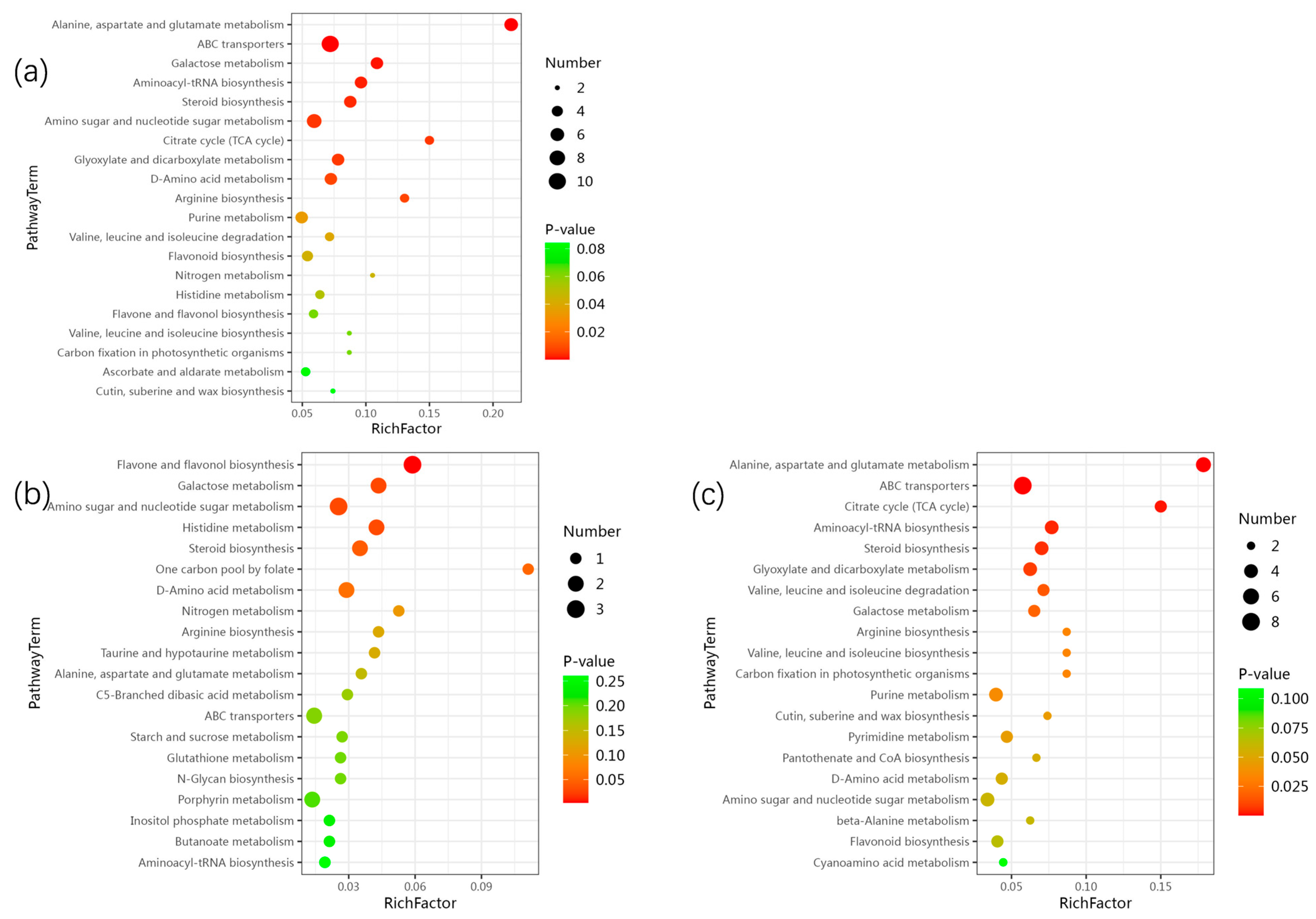
2.6. Joint Analysis of DEGs and DEMs
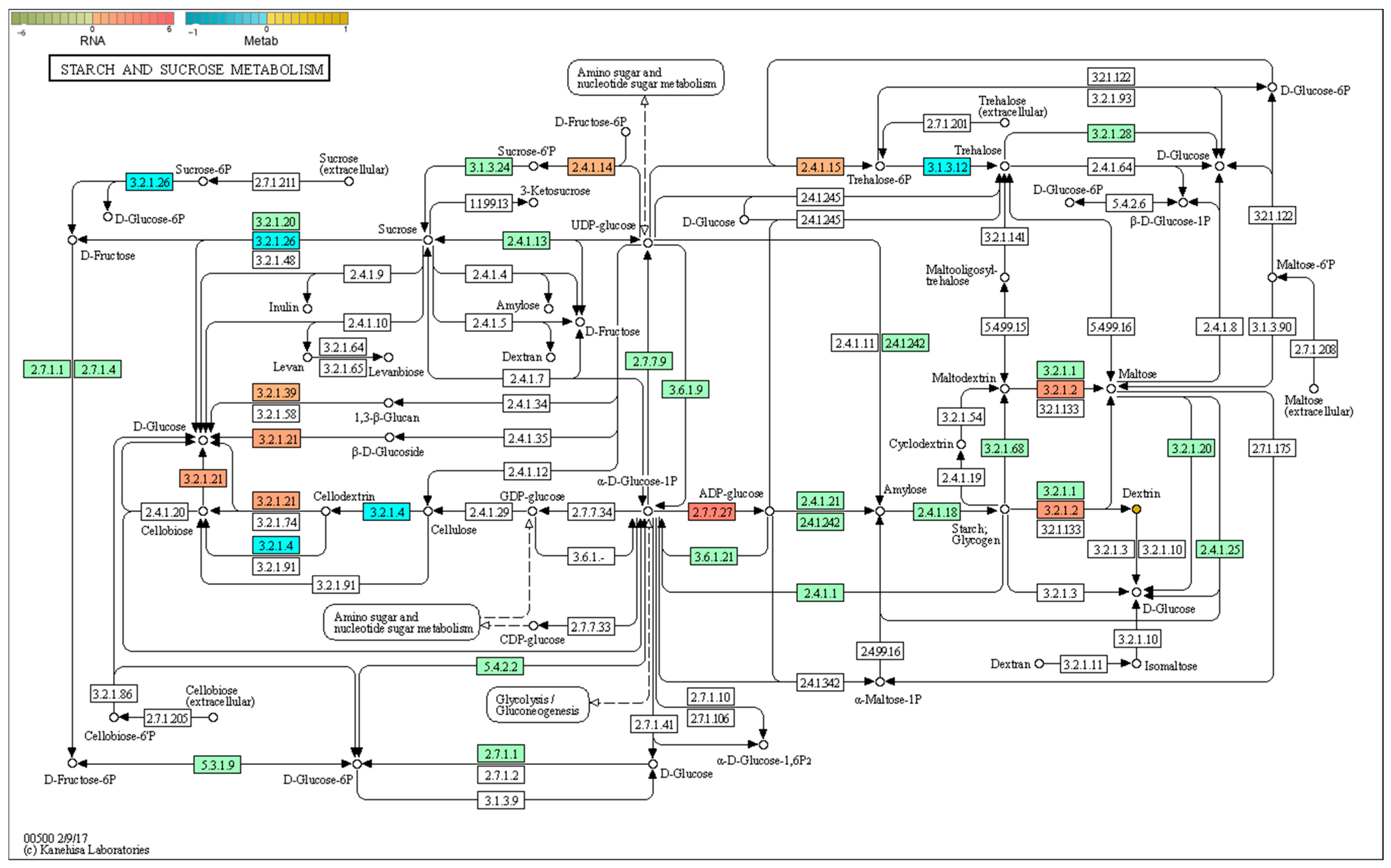
2.6.1. Starch and Sucrose Metabolism
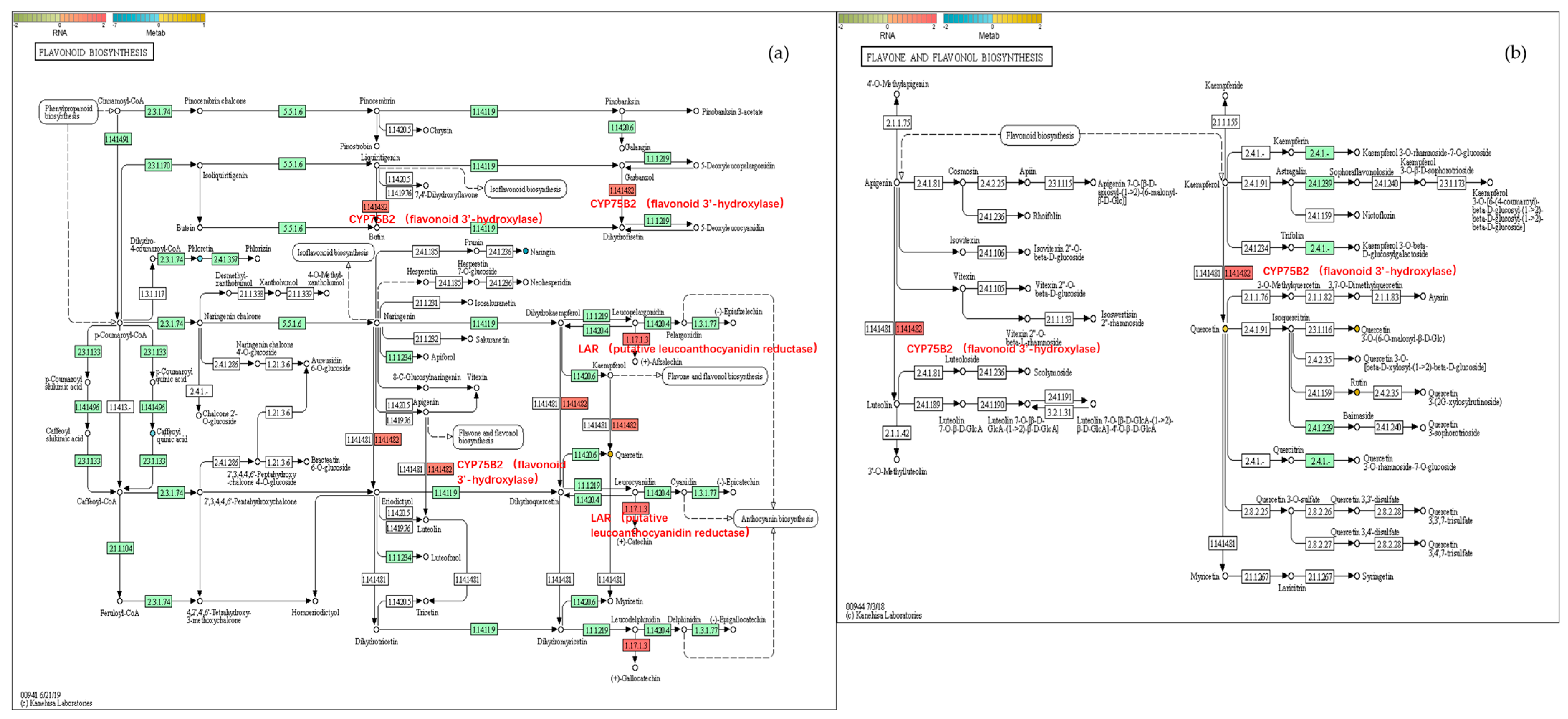
2.6.2. Flavonoid Biosynthesis
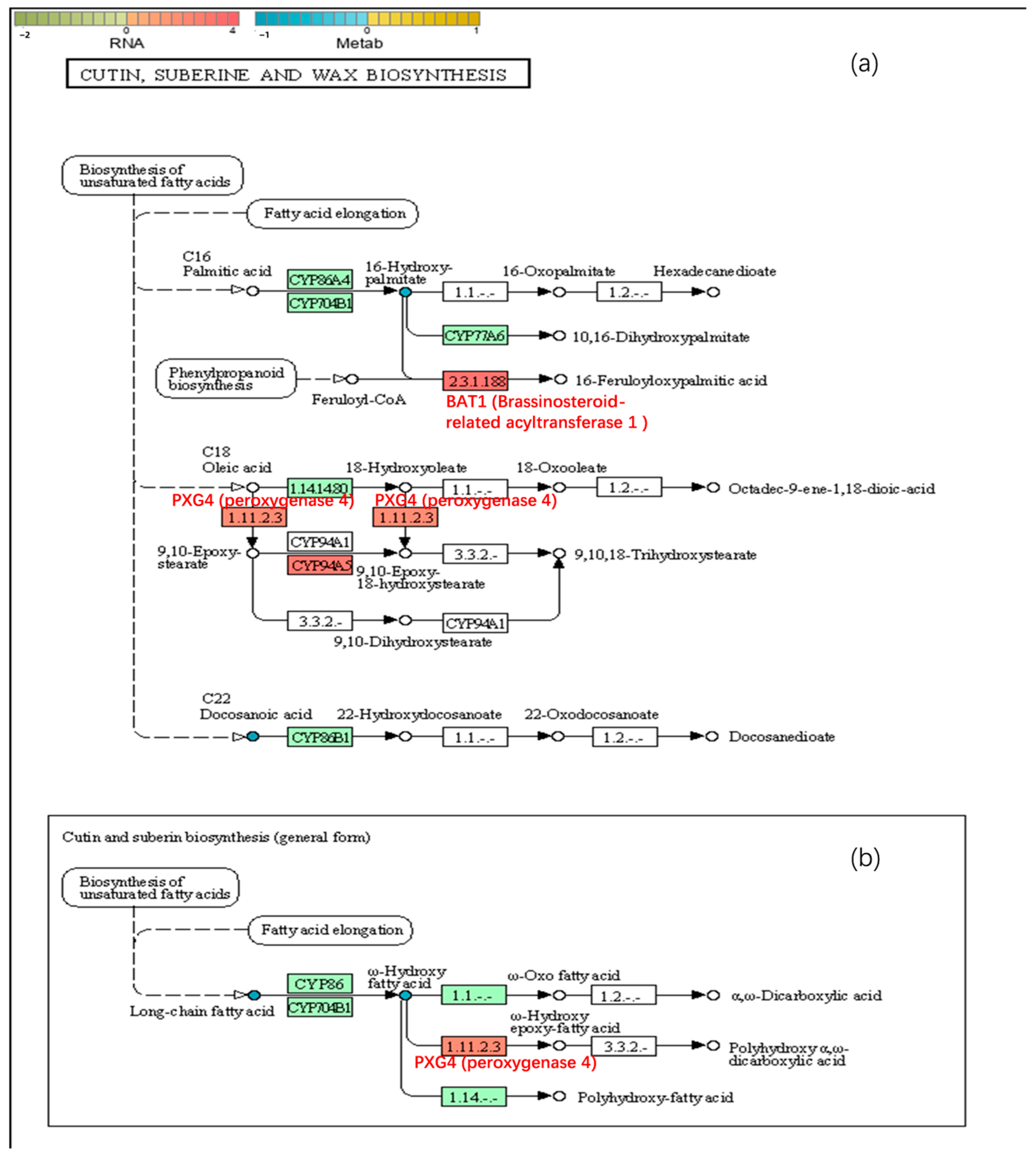
2.6.3. Cutin, Suberin and Wax Biosynthesis
3. Discussion
3.1. The Appearance Quality of Fuji Apples Under Non-Bagging Cultivation Is Significantly Lower than That Under Bagging Cultivation
3.2. The Reduction in Appearance Quality Is Related to Differences in the Expression of Genes Involved in the Synthesis of Peel Resistance-Related Substances
3.3. The Reduction in Appearance Quality Is Related to the Accumulation of Flavonoids in the Fruit Peel
3.4. Limitations and Future Perspectives
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Sampling Strategy
4.3. Measurement Methods
4.3.1. Determination of Fruit Appearance Quality
4.3.2. Observation of Peel’s Ultrastructure
4.3.3. Determination of Chlorophyll (Chl) and Carotenoid (Car) Contents
4.3.4. Determination of Total Anthocyanin Content
4.3.5. Determination of Total Flavonoid Content
4.3.6. The Total RNA Extraction and Transcriptome Sequencing
4.3.7. Functional Analysis of DEGs and qRT-PCR Validation
4.3.8. Extraction, Determination, and Analysis of Metabolites
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liu, T.J. Spatiotemporal evolution and suitability of apple production in China from climate change and land use transfer perspectives. Food Energy Secur. 2022, 11, 22. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, Y.; Lu, Y.H.; Li, M.Y. Competitiveness and sustainable development of Chinableapple industry. PLoS ONE 2022, 17, e0268476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, X.X. Theoretical explanation of China’s poverty reduction experience: Case of apple industry in Yanyuan County, Sichuan Province. Agric. Econ. Manag. 2024, 5, 92–103. [Google Scholar]
- Liu, Q. Should apples be bagged or not? Farm. Dail. 2022, 008, 1–5. [Google Scholar] [CrossRef]
- Chen, Z.J.; Yu, L.; Liu, W.J.; Zhang, J.; Wang, N.; Chen, X.S. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- Wang, G.P.; Xue, X.M.; Wang, J.Z. Research progress and development trend of apple bagging technology in China. J. Hebei Agric. Sci. 2021, 25, 44–48. [Google Scholar]
- Li, C.H.; Zhang, X.; Zhang, Y.; Yang, F.Y.; Lyu, D.G.; Qin, S. Analysis on the Problems and Strategies of Bag-Free Cultivation of ‘Hanfu’ Apple. North. Hortic. 2023, 2, 145–148. [Google Scholar]
- Yu, X.M.; Wang, J.Z.; Nie, P.X.; Xue, X.M.; Wang, G.P.; An, M. Control efficiency of Ca-containing foliar fertilizers on bitter pit in ‘Fuji’ apple and effects on the Ca and N contents of apple fruits and leaves. Acta Hortic. 2020, 1292, 181–188. [Google Scholar] [CrossRef]
- Ding, D.D.; Shao, Y.T.; Zhao, J.R.; Lin, J.S.; Zhang, X.Q.; Wang, X.K.; Xu, X.M.; Xu, C.N. Identification and pathogenicity of Alternaria and Fusarium species associated with bagged apple black spot disease in Shaanxi. China Front. Microbiol. 2024, 15, 1457315. [Google Scholar] [CrossRef]
- Sun, H.Y.; Li, L.; Li, M.S.; Wang, D.F.; Wang, D.X. Reflection on bagless cultivation of apple. J. Fruit Resour. 2020, 1, 63–65. [Google Scholar] [CrossRef]
- The ministry of agriculture and rural affairs released the top 10 leading technologies for 2020. Agric. Engin. 2020, 10, 3–4.
- Li, P.P.; Li, J.M.; Li, G.L.; Li, Y.F.; He, Y.; Li, Z.X. Preliminary report on the cultivation experiment of 12 apple varieties in Jingning, Gansu province, with low rootstock and close planting without bagging. China Fruits 2023, 78–82. [Google Scholar] [CrossRef]
- Sun, Y.X.; Song, L.Q.; Zhao, L.L.; Zhang, X.Y.; Liu, D.L.; Zhao, J.; Liu, X.Q.; Tang, Y. Breeding of a new early-ripening, yellow and bagging-free apple cultivar ‘Yanxiangyu’. J. Fruit Sci. 2022, 39, 499–501. [Google Scholar] [CrossRef]
- He, X.X. Quality Evaluation of Apples Cultivated without Bagging in Weibei Area of Shaanxi Province. Master’s Dissertation, Northwest A&F University, Xianyang, China, 2022. [Google Scholar] [CrossRef]
- Wang, Y.N. Apple Bag-Free Cultivation and Pest Control Technology. Anhui Agric. Sci. Bull. 2024, 30, 38–41. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, J.Z.; Wang, D.; Li, X.J.; Xue, X.M. Fungal pathogens identification of postharvest apples in bagging-free cultivation pattern and antifungal activity of essential oil. J. Fruit Sci. 2021, 38, 549–559. [Google Scholar] [CrossRef]
- Zeng, X. Study on Green Prevention and Control Technology of Non-Bagging Apple Diseases and Screening of Biocontrol Bacteria and Antifungal and Growth-Promoting Effects. Master’s Dissertation, Northwest A&F University, Xianyang, China, 2022. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.M.; Kai, J.R.; GAO, Y.Y.; Wang, C.Y.; Shi, X. Effects of Bag-free Biofilm Agent on Apple Fruit Quality and Mineral Elements. China Fruit Veg. 2024, 44, 77–83. [Google Scholar] [CrossRef]
- Gracia, A.; Cantin, C.M. Effects of Consumers’ Sensory Attributes Perception on Their Willingness to Pay for Apple Cultivars Grown at Different Altitudes: Are They Different. Foods 2022, 11, 3022. [Google Scholar] [CrossRef]
- Wang, Z.D.; Feng, Y.C.; Wang, H.; Liu, X.J.; Zhao, Z.Y. Effects of Different Pre-Harvest Bagging Times on Fruit Quality of Apple. Foods 2024, 13, 1243. [Google Scholar] [CrossRef]
- Jin, Q.; Fan, C.H.; Han, M.Y.; Liu, Y.Z. Observation of peel ultrastructure of the bagged ‘Hui Min’ Fuji Apple. J. Northwest A F Univ. (Nat. Sci. Ed.) 2004, S1, 87–90. [Google Scholar] [CrossRef]
- Yue, Z.Y. Effects of Non-Bagging Cultivation on Fruit Quality and Storage Tolerance of Apples. Master’s Thesis, Northwest A&F University, Xianyang, China, 2021. [Google Scholar] [CrossRef]
- Duan, R.W.; Wang, S.K.; Zhang, X.Z.; Yang, J.; Wang, L.; Su, Y.L.; Xue, H.B. Effect of different types of bag on fruit dots traits of ‘Zhongli No. 1’ pear. South China Fruits 2024, 53, 206–210. [Google Scholar] [CrossRef]
- Chi, X. Study on Coloring Factors, Methylation and Related Gene Expression of Fuji Apples with Different Hues After Bag Removal. Master’s Thesis, Yantai University, Yantai, China, 2020. [Google Scholar] [CrossRef]
- Cheng, C.G.; Liu, F.Z.; Wei, C.C.; Cong, P.H.; Yang, Z.F.; Dong, L.M. Effects of bagging on chlorophyll and anthocyanin contents in the peel of Fuji apples. China Fruits 2002, 4, 11–12. [Google Scholar] [CrossRef]
- Su, J.Y.; Li, W.S.; Lu, X.Z.; Zhang, Z.J.; Chen, G.A. Effects of bagging on peel color and activities of enzymes related to anthocyanin synthesis of Fuji bud mutation. Nonwood For. Res. 2022, 40, 153–162. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Du, Y.L.; Chen, C.Y.; Wang, D.F.; Zhong, Y.; Deng, Y. Integrative metabolomic and transcriptomic landscape during akebia trifoliata fruit ripening and cracking. Int. J. Mol. Sci. 2023, 24, 16732. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Wang, L.L.; Pi, Y.Z. Study on relieving lignification of ‘Jinling’ big jujube peel by decompression storage. Sci. Technol. Food Ind. 2010, 91, 307–309. [Google Scholar] [CrossRef]
- Heng, W.; Huang, H.N.; Li, F.; Hou, Z.Q.; Zhu, L.W. Comparative analysis of the structure, suberin and wax composition and key gene expression in the epidermis of ‘Dangshansuli’ pear and its russet mutant. Acta Physiol. Plant. 2017, 39, 150. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.F.; Zhang, P.F.; Bian, Y.H.; Liu, Z.Y.; Zhang, C.; Liu, X.; Wang, C.L. An integrated metabolic and transcriptomic analysis reveals the mechanism through which fruit bagging alleviates exocarp semi-russeting in pear fruit. Tree Physiol. 2021, 41, 1306–1318. [Google Scholar] [CrossRef]
- Yang, W.L.; Li, X.G.; Yang, Q.S.; Lin, J.; Sheng, B.L.; Chang, Y.H.; Wang, H. Comparative metabolic and transcriptomic analysis of the pericarp of Sucui 1, Cuiguan and Huasu pears. J. Fruit Sci. 2022, 39, 1989–2006. [Google Scholar] [CrossRef]
- Deuchande, T.; Carvalho, S.M.P.; Giné-Bordonaba, J.; Vasconcelos, M.W.; Larrigaudière, C. Transcriptional and biochemical regulation of internal browning disorder in ‘Rocha’ pear as affected by O2 and CO2 concentrations. Postharvest Biol. Technol. 2017, 132, 15–22. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Du, J.T.; Zhu, D.J.; Li, X.; Li, X.G. Metabolomic and transcriptomic analyses of anthocyanin biosynthesis mechanisms in the color mutant Ziziphus jujuba cv. ‘Tailihong’. J. Agric. Food Chem. 2020, 68, 13076–13085. [Google Scholar] [CrossRef]
- Eom, S.H. Pigmentation and Flavonoid Metabolite Diversity in Immature ‘Fuji’ Apple Fruits in Response to Lights and Methyl Jasmonate. Int. J. Mol. Sci. 2022, 23, 1722. [Google Scholar] [CrossRef]
- Wang, G.P.; Xue, X.M.; Zhao, H.J.; Zhai, H.; Wang, J.Z. Difference Analysis of Diseases and Insect Pests and Fruit Quality between Bagged and Bagless Fuji Apple. J. Anhui Agric. Sci. 2022, 50, 36–38. [Google Scholar] [CrossRef]
- Xie, J.; Cao, X.; Pan, W.; Du, L. Advances in plant flavonoid transport and accumulation mechanism. Chin. Bull. Bot. 2024, 3, 463–480. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis: A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Ni, G.Y.; Li, J.M.; Jiang, W.G.; Xu, Y. Effect of co-pigment on monomeric anthocyanin and color of wine. Food Ferment. Ind. 2019, 45, 54–59. [Google Scholar] [CrossRef]
- Lyu, J.; Li, J.; Jiang, W.; Liu, T.; Xu, Y.; Tang, K. Copigmentation effects of different phenolics on color stability of three basic anthocyanins in wines: Chromaticity, thermodynamics and molecular dynamics simulation. Food Chem. 2025, 476, 143499. [Google Scholar] [CrossRef]
- Wang, G.P.; Chen, R.; Han, X.P.; Xue, X.M. Effects and mechanism analysis of non-bagging and bagging cultivation on the growth and content change of specific substances of Fuji apple fruit. Plants 2023, 12, 3309. [Google Scholar] [CrossRef]
- Wu, B.H.; Cao, Y.G.; Guan, L.; Xin, H.P.; Li, J.H.; Li, S.H. Genome-wide transcriptional profiles of the berry skin of two red grape cultivars (Vitis vinifera) in which anthocyanin synthesis is sunlight-dependent or -independent. PLoS ONE 2014, 9, e105959. [Google Scholar] [CrossRef]
- Zhao, S.J.; Shi, G.A.; Dong, X.C. Techniques of Plant Physiological of Experiment, 1st ed.; Agricultural Science and Technology Press: Beijing, China, 2002; pp. 55–57. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Zheng, X.L.; Tian, S.P. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem. 2006, 96, 519–523. [Google Scholar] [CrossRef]
- Inácio, M.R.C.; de Lima, K.M.G.; Lopes, V.G.; Pessoa, J.D.C.; de Almeida Teixeira, G.H. Total anthocyanin content determination in intact acai (Euterpe oleracea Mart.) and palmitero-jucara (Euterpe edulis Mart.) fruit using near infrared spectroscopy (NIR) and multivariate calibration. Food Chem. 2013, 136, 1160–1164. [Google Scholar] [CrossRef]
- Koley, T.K.; Kaur, C.; Nagal, S.; Walia, S.; Jaggi, S. Antioxidant activity and phenolic content in genotypes of Indian jujube (Zizyphus mauritiana Lamk.). Arab. J. Chem. 2011, 9, S1044–S1052. [Google Scholar] [CrossRef]
- Xue, Z.P.; Feng, W.H.; Cao, J.K.; Cao, D.D.; Jiang, W.B. Antioxidant Activity and Total Phenolic Contents in Peel and Pulp of Chinese Jujube (Ziziphus Jujuba Mill.) Fruits. J. Food Biochem. 2009, 33, 613–629. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357. [Google Scholar] [CrossRef]
- Nicolas, D.; Jean-Marc, C.; Gareth, L.; Claude, B.; Nathalie, C.; Lio, S.; van de Henri, G.; Luca, B.; Diego, M.; Riccardo, V.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Chen, W.C.; Zhang, J.Q.; Zheng, S.; Wang, Z.Q.; Xu, C.M.; Zhang, Q.X.; Wu, J.S.; Lou, H.Q. Metabolite profiling and transcriptome analyses reveal novel regulatory mechanisms of melatonin biosynthesis in hickory. Hortic. Res. 2021, 8, 196. [Google Scholar] [CrossRef]
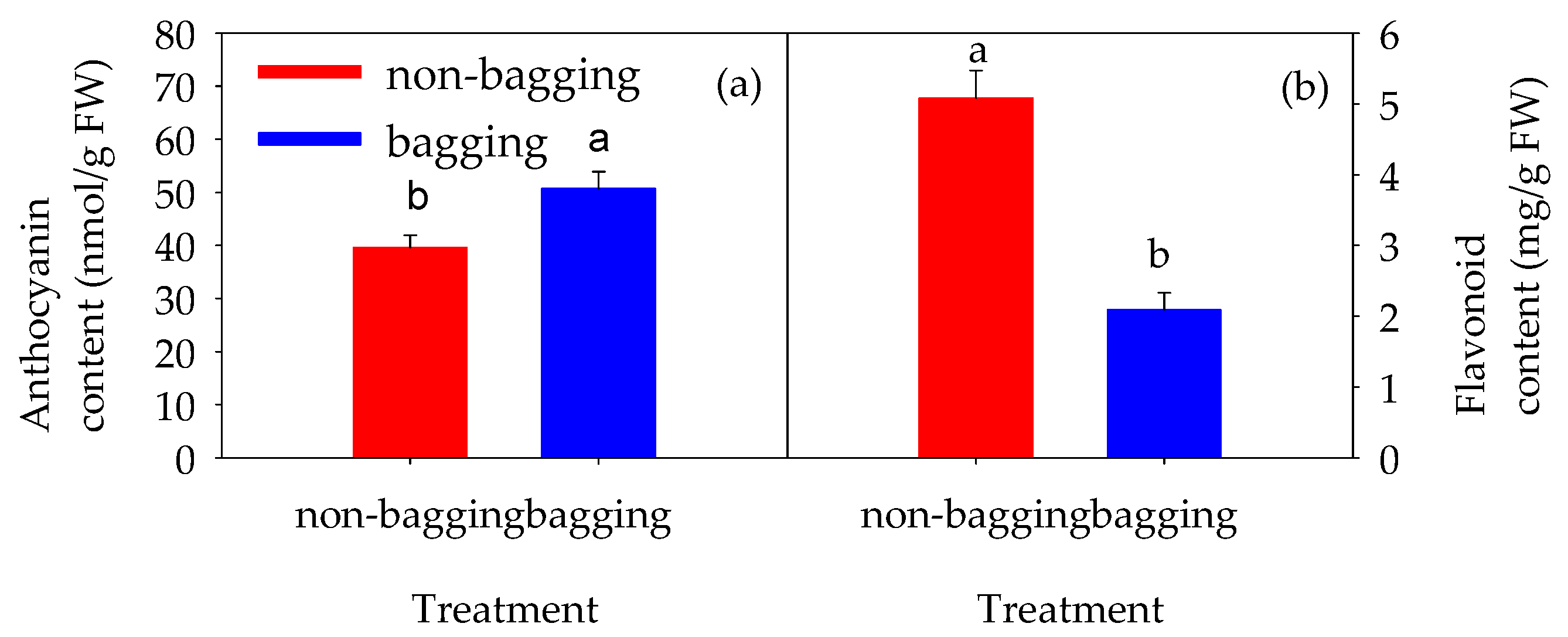

| Treatment | Fruit Dot Characteristics | ||
|---|---|---|---|
| Fruit Dot Density (No./cm2) | Length (μm) | Width (μm) | |
| non-bagging | 4.71 ± 0.37 b | 881.95 ± 2.75 b | 696.04 ± 1.72 a |
| bagging | 5.15 ± 0.19 a | 640.14 ± 3.26 a | 446.17 ± 0.70 a |
| Treatment | Coloring Index (%) | Finish Index (%) | Chromatic Aberration | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C | h° | |||
| non-bagging | 79.55 ± 3.21 b | 70.45 ± 2.89 b | 4.41 ± 3.71 b | 21.70 ± 2.75 b | 14.12 ± 1.72 a | 18.36 ± 1.69 b | 1.33 ± 0.33 b |
| bagging | 90.91 ± 2.15 a | 86.36 ± 2.54 a | 5.15 ± 1.93 a | 30.49 ± 3.26 a | 13.40 ± 0.70 a | 23.57 ± 2.03 a | 2.14 ± 0.35 a |
| Treatment | Chloroplast Pigment (mg/g FW) | |
|---|---|---|
| Chl | Car | |
| non-bagging | 0.019 ± 0.001 a | 0.016 ± 0.001 a |
| bagging | 0.002 ± 0.001 b | 0.008 ± 0.000 b |
| Sample | Non-Bagging-1 | Non-Bagging-2 | Non-Bagging-3 | Bagging-1 | Bagging-2 | Bagging-3 |
|---|---|---|---|---|---|---|
| Raw Reads (G) | 7.39 | 7.76 | 7.57 | 6.80 | 7.50 | 7.22 |
| Clean reads (G) | 6.53 | 6.92 | 6.77 | 6.08 | 6.68 | 6.56 |
| Q30 (%) | 95.00 | 94.17 | 94.72 | 94.50 | 93.80 | 94.39 |
| GC content (%) | 49.22 | 48.63 | 48.68 | 48.60 | 48.73 | 48.54 |
| Class | Gene ID | Gene Symbol | Description | Log2 Fold Change | p-Adjust | Regulate |
|---|---|---|---|---|---|---|
| secondary metabolites | MD14G1210700 | CYP75B2 | Flavonoid 3′-hydroxylase | 1.20 | 7.13 × 10−88 | Up |
| MD13G1046900 | LAR | Putative leucoanthocyanidin reductase | 1.45 | 3.67 × 10−4 | Up | |
| MD01G1162700 | PER52 | peroxidase P7-like | 2.40 | 5.14 × 10−46 | Up | |
| MD15G1252300 | PER72 | peroxidase 72 | 1.79 | 7.46 × 10−8 | Up | |
| MD03G1013200 | PER53 | peroxidase A2-like | 5.66 | 2.77 × 10−4 | Up | |
| MD05G1320800 | PPO | polyphenol oxidase, chloroplastic-like | −1.61 | 4.38 × 10−2 | Down | |
| MD14G1172500 | cao1 | amine oxidase 4 | −4.13 | 2.79 × 10−2 | Down | |
| MD02G1136000 | CYP84A1 | cytochrome P450 84A1-like | −5.64 | 7.48 × 10−13 | Down | |
| MD07G1300500 | COMT1 | caffeic acid 3-O-methyltransferase | 1.57 | 1.86 × 10−4 | Up | |
| MD13G1090300 | CCD4 | carotenoid cleavage dioxygenase 4 | −1.44 | 3.25 × 10−7 | Down | |
| MD10G1194200 | NCED1 | 9-cis-epoxycarotenoid dioxygenase NCED3, chloroplastic-like | 2.91 | 1.23 × 10−3 | Up | |
| Lipids | MD11G1023100 | LOX2.1 | linoleate 13S-lipoxygenase 2-1, chloroplastic-like | 1.62 | 3.98 × 10−108 | Up |
| MD16G1113200 | LOX6 | lipoxygenase | 1.15 | 4.93 × 10−2 | Up | |
| MD10G1190400 | CYP94A1 | cytochrome P450 94A1-like isoform X2 | 3.11 | 1.17 × 10−26 | Up | |
| MD08G1058900 | BAT1 | brassinosteroid-related acyltransferase 1-like | 3.30 | 6.62 × 10−5 | Up | |
| MD13G1029500 | PXG4 | probable peroxygenase 4 [Malus domestica] | 2.51 | 2.97 × 10−6 | Up | |
| Carbohydrate | MD16G1042700 | RBCS | ribulose bisphosphate carboxylase small chain, chloroplastic-like | −1.79 | 2.17 × 10−268 | Down |
| MD11G1295000 | rbcL | ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (chloroplast) | −1.18 | 2.52 × 10−17 | Down | |
| MD13G1093700 | GOLS1 | galactinol synthase 1-like | −2.12 | 4.51 × 10−142 | Down | |
| MD13G1186100 | ADG2 | glucose-1-phosphate adenylyltransferase large subunit 3 | 4.19 | 4.23 × 10−127 | Up | |
| MD04G1056200 | BAM3 | beta-amylase 3 | 2.81 | 1.85 × 10−14 | Up | |
| MD14G1167100 | At4g24780 | probable pectate lyase 18 | 3.28 | 1.76 × 10−108 | Up | |
| MD02G1067000 | PME53 | probable pectinesterase 53 | 4.42 | 2.39 × 10−2 | Up | |
| MD14G1128000 | At1g64390 | endoglucanase 6 | 1.21 | 7.52 × 10−22 | Up | |
| MD05G1006400 | SPS4 | probable sucrose-phosphate synthase 4 | 1.11 | 2.09 × 10−2 | Up | |
| Environmental adaptation | MD07G1292500 | CNGC1 | cyclic nucleotide-gated ion channel 1-like | 5.38 | 5.47 × 10−2 | Up |
| MD08G1037100 | CML41 | probable calcium-binding protein CML41 isoform X1 | 4.89 | 1.22 × 10−2 | Up | |
| MD02G1271900 | EIX2 | LRR receptor-like serine/threonine-protein kinase GSO2 | 3.98 | 2.00 × 10−2 | Up | |
| MD17G1257900 | CML27 | probable calcium-binding protein CML23 | 3.11 | 1.53 × 10−6 | Up | |
| Energy metabolism 00710 | MD15G1091000 | ME1 | NADP-dependent malic enzyme isoform X2 | 1.38 | 4.31 × 10−54 | Up |
| MD02G1011600 | AHA8 | ATPase 8, plasma membrane-type | 4.59 | 1.54 × 10−2 | Up | |
| MD13G1247200 | PMA4 | plasma membrane ATPase 4-like | 1.12 | 2.88 × 10−23 | Up | |
| Membrane transport-ABC | MD17G1042800 | ABCB15 | ABC transporter B family member 15-like | 4.98 | 9.74 × 10−38 | Up |
| MD10G1268400 | ABCB9 | ABC transporter B family member 9-like | 1.00 | 5.39 × 10−4 | Up |
| Class | Compound ID | Metabolite | VIP | FC | Type |
|---|---|---|---|---|---|
| Lipids and lipid-like molecules | HMDB0006928 | δ8,14-Sterol | 1.636 | 1.627 | Up |
| HMDB0006591 | Lactosamine | 2.724 | 4.278 | Up | |
| HMDB0029315 | Asparagoside B | 2.199 | 2.055 | Up | |
| LMST01010173 | cholesteryl α-D-glucoside | 3.816 | 2.626 | Up | |
| HMDB0006867 | S-(3-Methylbutanoyl)-dihydrolipoamide-E | 4.016 | 6.058 | Up | |
| LMPK12112185 | Quercetin 3-apiosyl-(1->2)-α-L-arabinopyranoside | 1.022 | 3.398 | Up | |
| LMPK12112087 | Quercetin 3-apiosyl-(1->2)-glucoside | 2.578 | 2.759 | Up | |
| LMPK12112155 | Quercetin 3-(2‴-p-coumarylsambubioside)-7-glucoside | 1.645 | 2.569 | Up | |
| LMFA01170020 | 5-methyl-tetradecanedioic acid | 1.635 | 6.452 | Up | |
| LMFA01050437 | 11-Hydroxy-9-tridecenoic acid | 1.227 | 6.255 | Up | |
| HMDB0031885 | 6-Hydroxypentadecanedioic acid | 2.440 | 5.986 | Up | |
| HMDB0006294 | 16-Hydroxyhexadecanoic acid | 2.242 | 0.730 | Down | |
| Organic acids and derivatives | HMDB00191 | L-Aspartic acid | 3.460 | 0.492 | Down |
| HMDB0000695 | Ketoleucine | 1.473 | 0.394 | Down | |
| HMDB0003705 | Phosphoguanidinoacetate | 1.014 | 0.304 | Down | |
| HMDB0012265 | N-Carbamoylsarcosine | 3.245 | 0.175 | Down | |
| HMDB0001325 | N6,N6,N6-Trimethyl-L-lysine | 1.43 | 0.170 | Down | |
| HMDB0000026 | Ureidopropionic acid | 3.919 | 0.320 | Down | |
| HMDB0000168 | L-Asparagine | 11.491 | 0.204 | Down | |
| HMDB0006483 | D-Aspartic acid | 6.139 | 0.350 | Down | |
| HMDB0000193 | Isocitric acid | 2.918 | 0.276 | Down | |
| HMDB00744 | Malic acid | 13.479 | 0.723 | Down | |
| HMDB0000094 | Citric acid | 4.323 | 0.322 | Down | |
| Organic oxygen compounds | HMDB0000143 | D-Galactose | 6.455 | 0.497 | Down |
| Phenylpropanoid and polyketide | HMDB03249 | Rutin | 11.034 | 2.807 | Up |
| LMPK12020046 | Catechin 7-O-β-D-xyloside | 1.020 | 3.886 | Up | |
| HMDB0255461 | Naringin dihydrochalcone | 1.212 | 0.014 | Down | |
| HMDB0000567 | Cinnamic acid | 2.489 | 0.372 | Down | |
| HMDB0038808 | Luteolin 3′-(3″-acetylglucuronide) | 1.620 | 3.039 | Up | |
| HMDB0037948 | Catechin 5-glucoside | 1.526 | 0.207 | Down | |
| HMDB0005794 | Quercetin | 4.680 | 1.494 | Up | |
| HMDB37368 | Quercetin 3-O-malonylglucoside | 2.244 | 1.994 | Up | |
| LMPK12112097 | Quercetin 3-neohesperidoside | 11.497 | 3.119 | Up | |
| HMDB32616 | Sinapic acid | 1.175 | 0.426 | Down | |
| Organoheterocyclic compounds | HMDB0001264 | Dehydroascorbic acid | 1.639 | 0.303 | Down |
| Nucleoside | HMDB0001202 | dCMP | 1.647 | 0.320 | Down |
| HMDB0001044 | 2′-Deoxyguanosine 5′-monophosphate | 1.018 | 0.358 | Down | |
| HMDB00302 | UDP-D-galactose | 1.001 | 1.735 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Li, H.; Chen, R.; Han, X.; Xue, X. Integrated Metabolomic and Transcriptomic Analysis Reveals the Roles of Cutin, Suberin, and Flavonoid Metabolism in Apple Peel Deterioration Under Non-Bagging Cultivation. Plants 2025, 14, 3339. https://doi.org/10.3390/plants14213339
Wang G, Li H, Chen R, Han X, Xue X. Integrated Metabolomic and Transcriptomic Analysis Reveals the Roles of Cutin, Suberin, and Flavonoid Metabolism in Apple Peel Deterioration Under Non-Bagging Cultivation. Plants. 2025; 14(21):3339. https://doi.org/10.3390/plants14213339
Chicago/Turabian StyleWang, Guiping, Huifeng Li, Ru Chen, Xueping Han, and Xiaomin Xue. 2025. "Integrated Metabolomic and Transcriptomic Analysis Reveals the Roles of Cutin, Suberin, and Flavonoid Metabolism in Apple Peel Deterioration Under Non-Bagging Cultivation" Plants 14, no. 21: 3339. https://doi.org/10.3390/plants14213339
APA StyleWang, G., Li, H., Chen, R., Han, X., & Xue, X. (2025). Integrated Metabolomic and Transcriptomic Analysis Reveals the Roles of Cutin, Suberin, and Flavonoid Metabolism in Apple Peel Deterioration Under Non-Bagging Cultivation. Plants, 14(21), 3339. https://doi.org/10.3390/plants14213339






