The Transmembrane Glutamate Serves as a pH Sensor for Tha4 Oligomerization During Twin Arginine Transport of Proteins
Abstract
1. Introduction

2. Results
2.1. Tha4 Complementation Assays
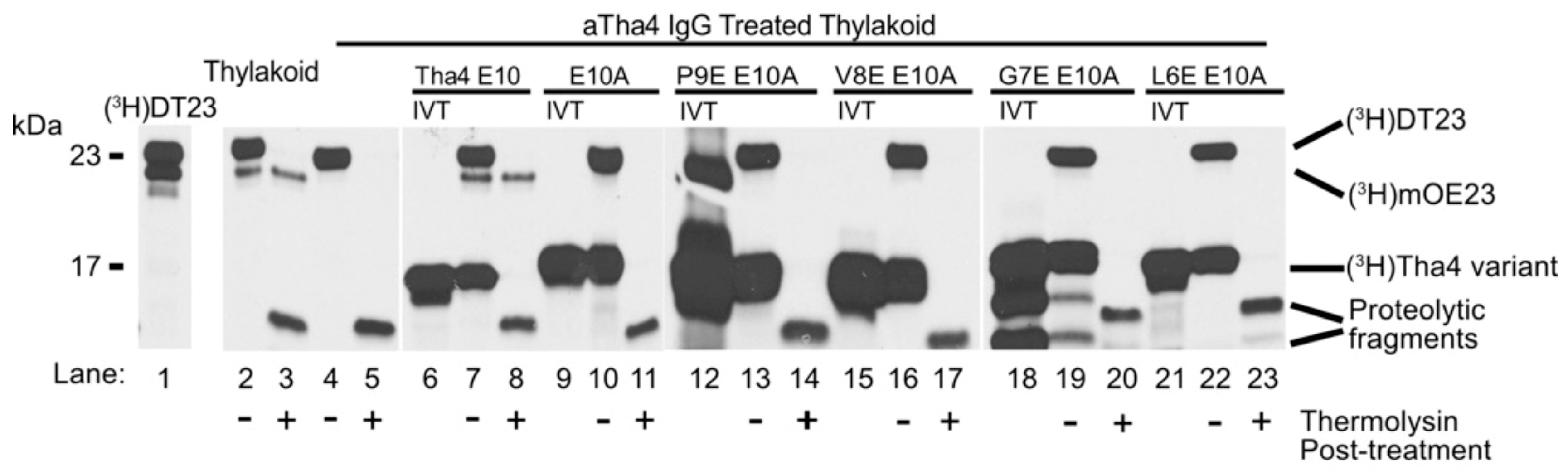
2.1.1. Sequential Glutamate Substitutions in the TMH of Tha4 E10A Failed to Complement the Loss of Function in Thylakoids Treated with αTha4 IgG
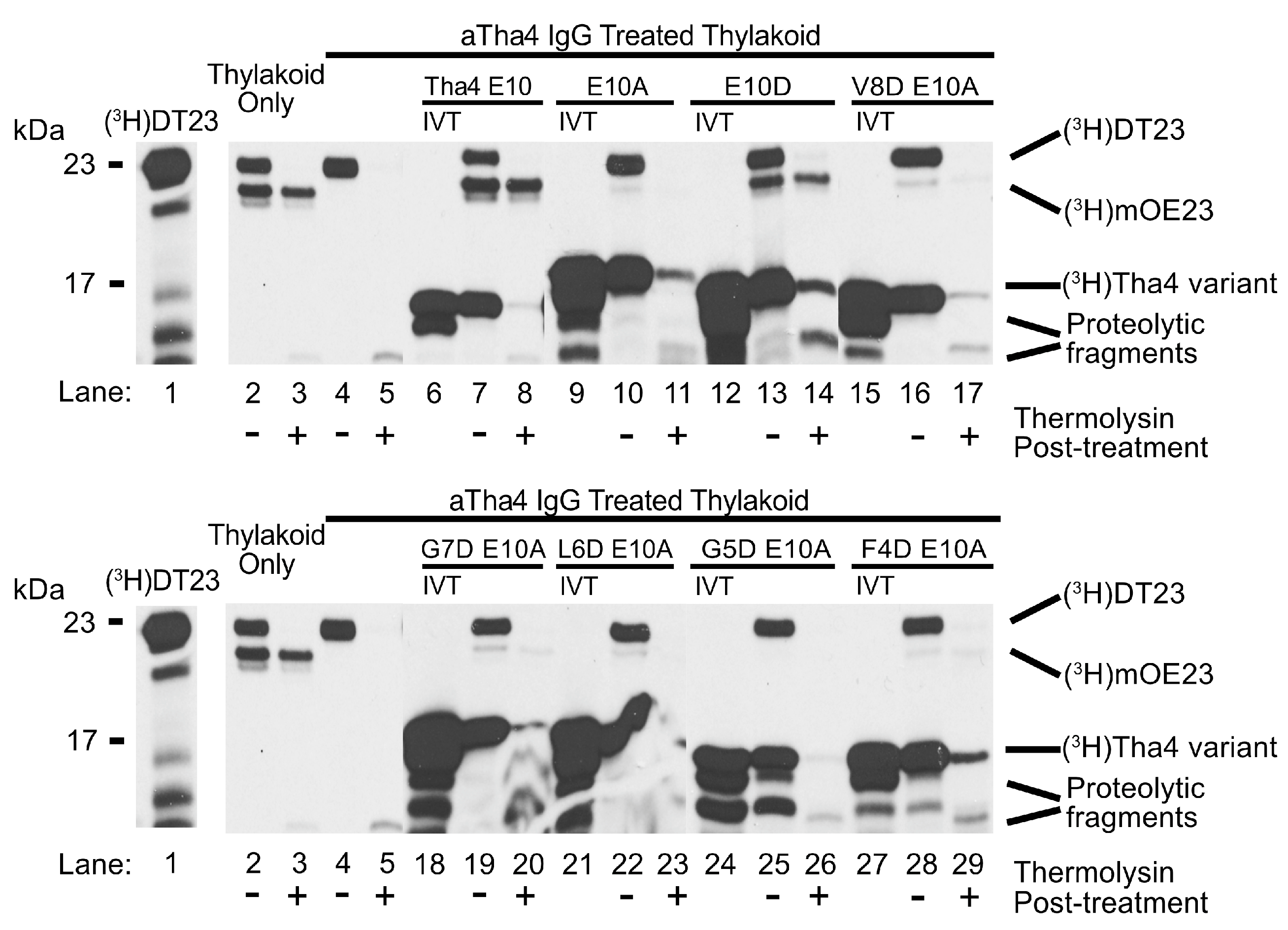
2.1.2. Aspartate Substitutions in the TMH of the Tha4 E10A Variant Weakly Complement the Loss of Function in αTha4 IgG-Treated Thylakoids
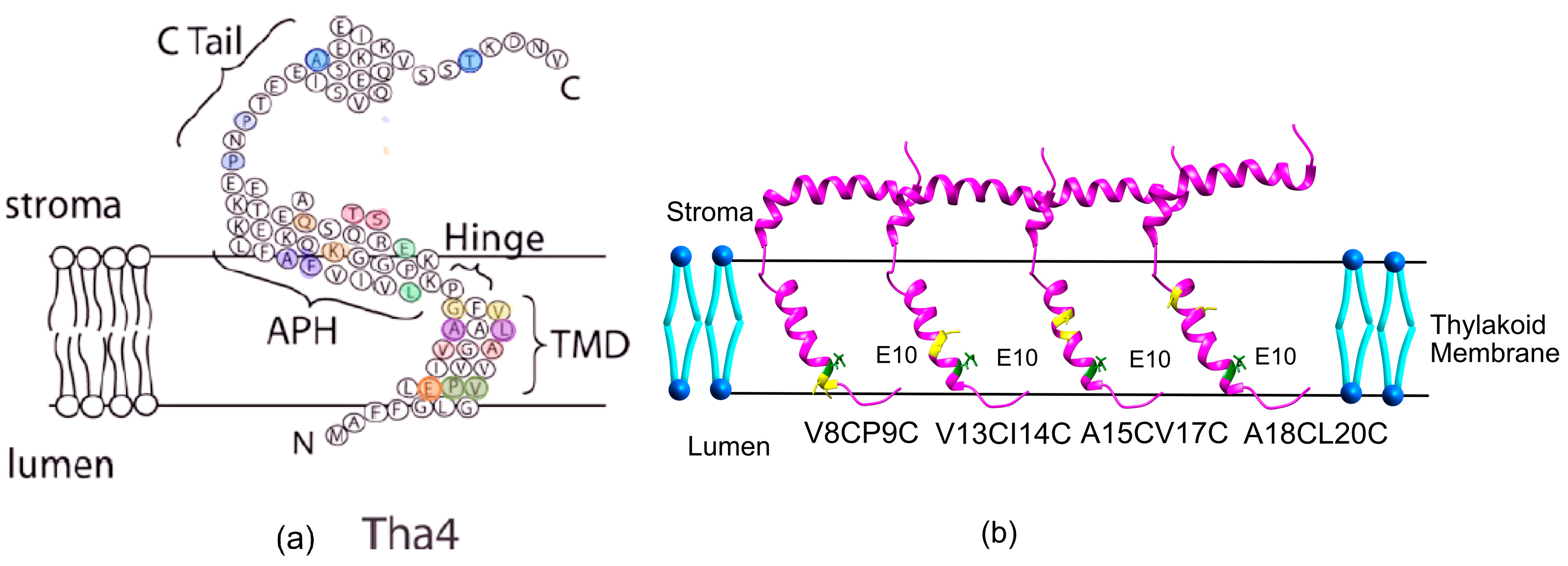
2.2. Tha4 Oligomerization
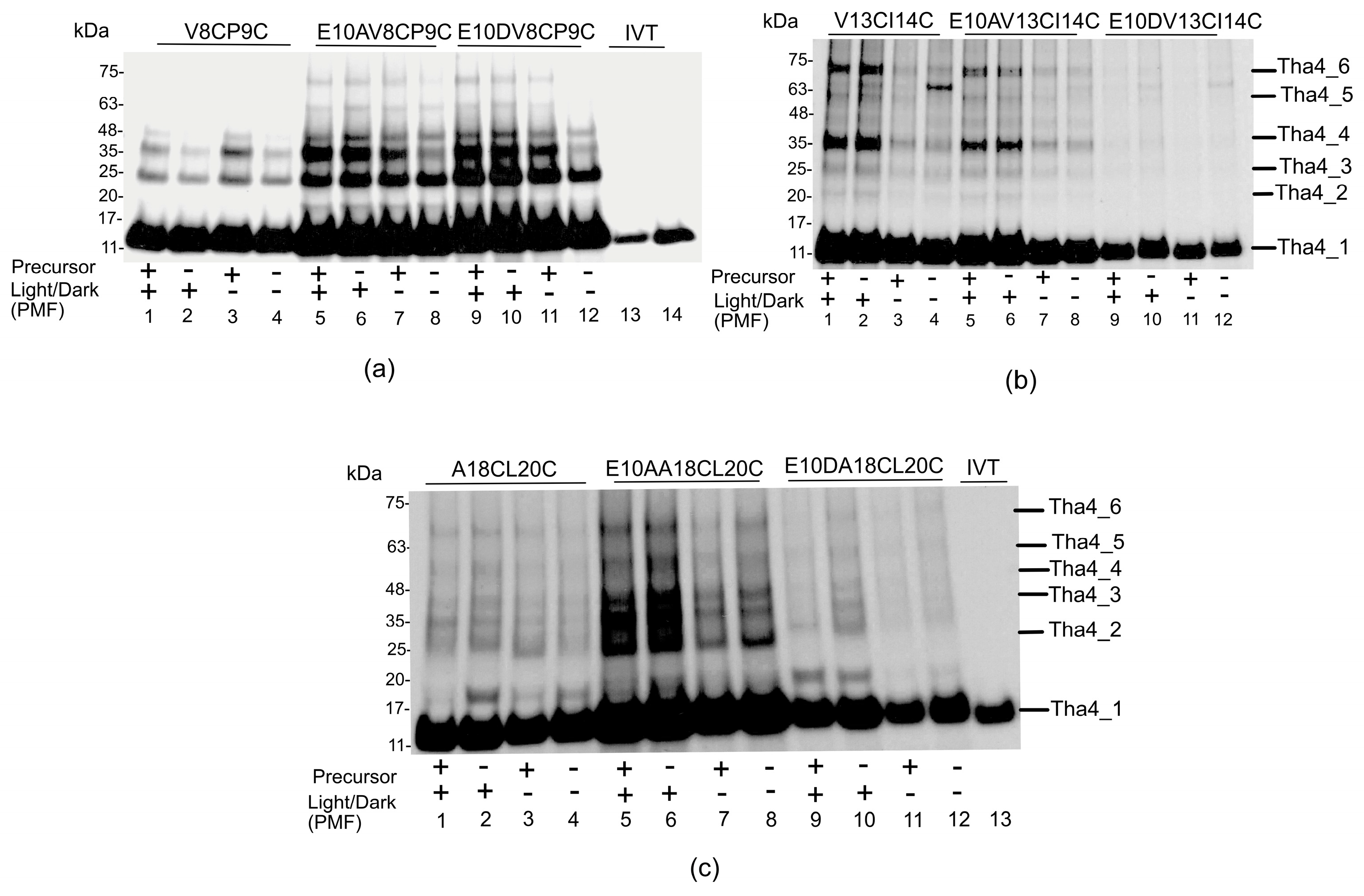
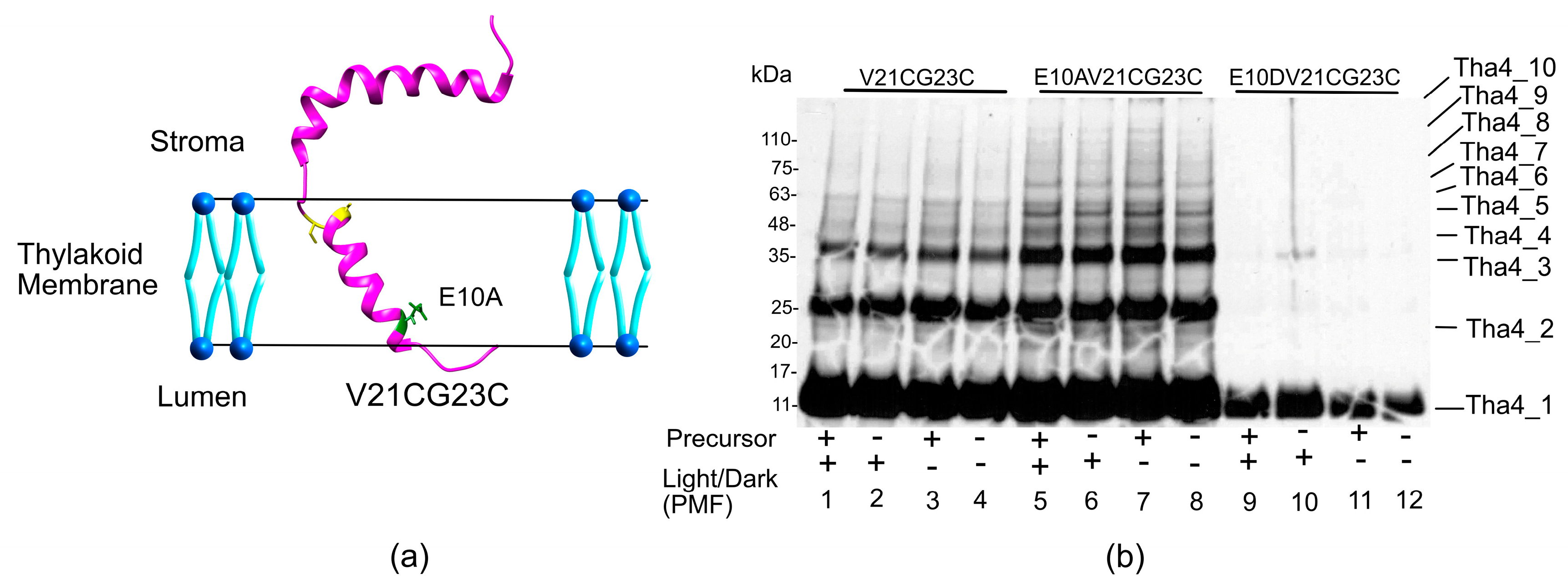
2.2.1. Crosslinking Formation in the TMH Region Depends on the Presence of a Functional Precursor, Not on the PMF
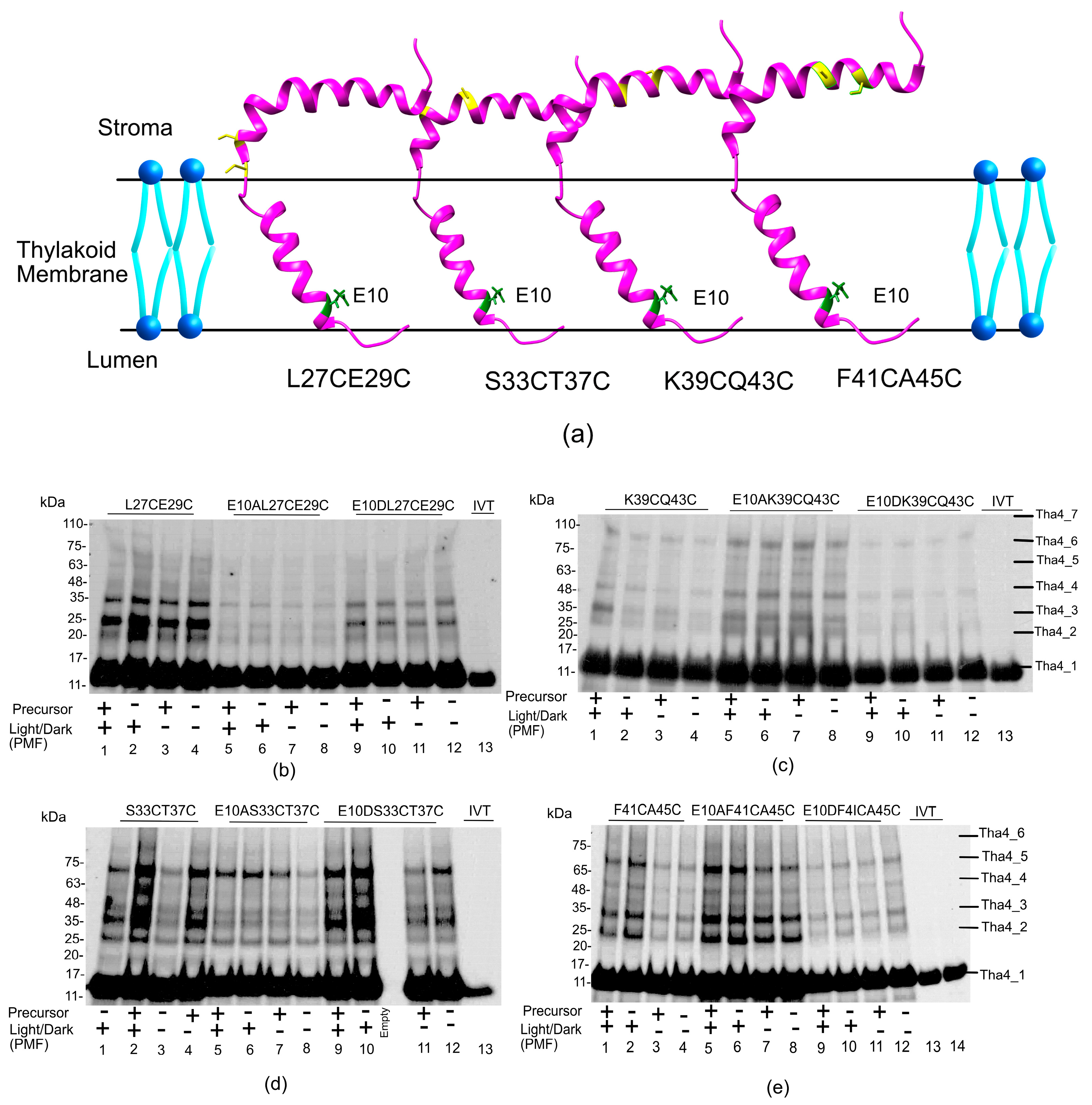
2.2.2. In the Hinge Region, Oligomer Formation Does Not Affect the Presence of Precursor or PMF
2.2.3. The Amphipathic Helix Region, the Residues in the Stroma, Exhibited Greater Oligomer Formation in Both Wild-Type and Aspartate-Substituted Cysteine Mutants
2.2.4. Oligomer Formation in the C Tail Mainly Responds to the Presence of a PMF, Regardless of the Presence of the Precursor
3. Discussion
3.1. Restoration of cpTAT Transport Defect by TMH Charge Variants of Tha4 E10 in IgG-Treated Thylakoids
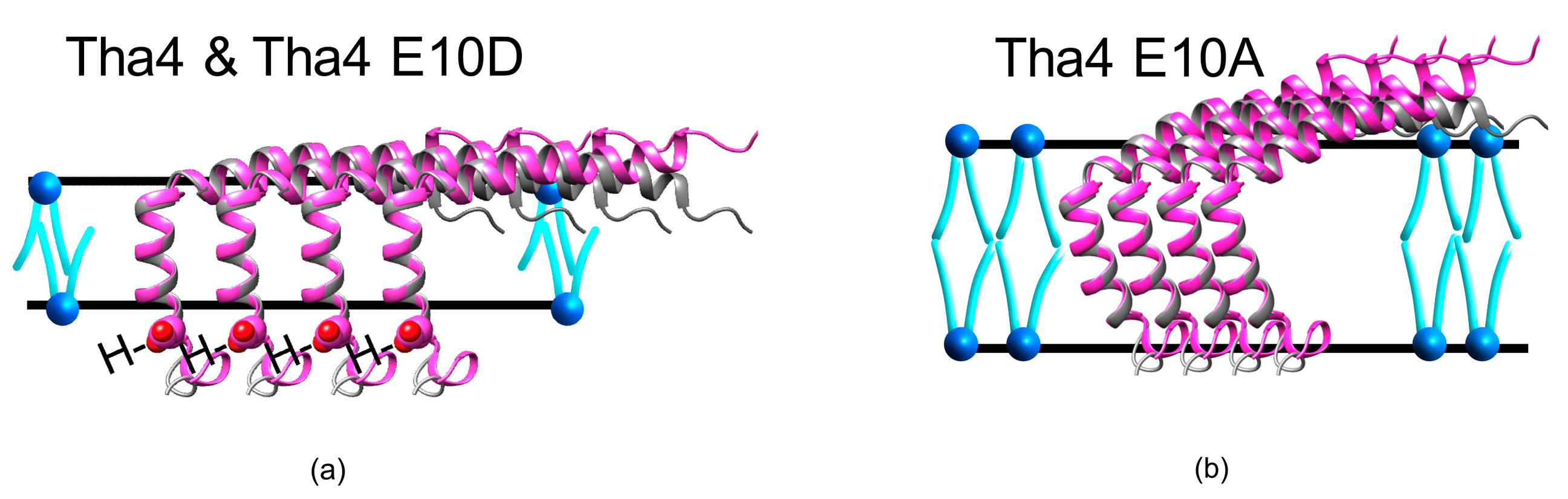
3.2. Crosslinking Formation of Tha4
3.3. Hydrophobic Mismatch and Tha4 TMH Hydrophobicity
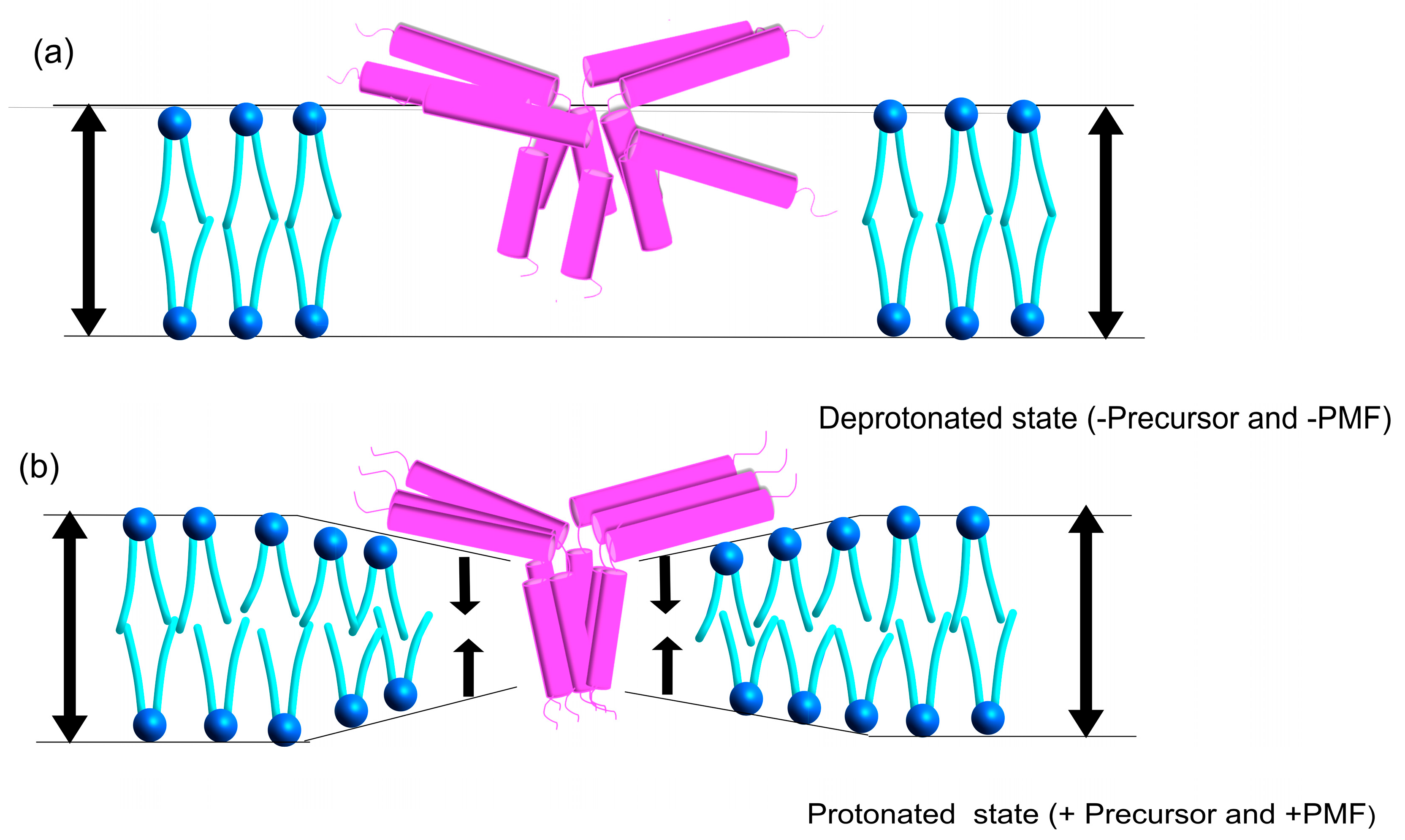
3.4. Proposed Model for Tha4 Oligomer Assembly
4. Materials and Methods
4.1. Chloroplast and Thylakoid Isolation
4.2. Generation and In Vitro Translation of Tha4 Double Cys Variants and Precursor DT23, (V-20F)tOE17
4.3. Substituting Endogenous Tha4 and Complementing cpTAT
4.4. Disulfide Crosslinking Assay
4.5. SDS-PAGE and Fluorography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cpTAT | Chloroplasts Twin Arginine Transport |
| PMF | Proton Motive Force |
| Cys | Cysteine |
| TMH | Transmembrane Helix |
| APH | Amphipathic helix |
| TM | Transmembrane |
| E10 | Glutamate at the 10th position |
| E10A | Glutamate substituted with alanine |
| E10D | Glutamate substituted with aspartate |
| E10XC | Wild type with glutamate substituted with single cysteine variants |
| E10A XC | Glutamate substituted with alanine with single cysteine variants |
| E10D XC | Glutamate substituted with aspartate with single cysteine variants |
| E10XCYC | Wild type with glutamate substituted with double cysteine variants |
| E10A XCYC | Glutamate substituted with alanine with double cysteine variants |
| E10D XCYC | Glutamate substituted with aspartate with double cysteine variants |
References
- Peltier, J.B. Proteomics of the chloroplast: Systematic identification and targeting analysis of lumenal and peripheral thylakoid proteins. Plant Cell 2000, 12, 319–341. [Google Scholar] [CrossRef][Green Version]
- Keegstra, K. Transport and Routing of Proteins into Chloroplasts. Cell 1989, 56, 247–253. [Google Scholar] [CrossRef]
- Flores-Pérez, Ú.; Jarvis, P. Molecular chaperone involvement in chloroplast protein import. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.; Andersson, M.X.; Hebert, C. Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat. Cell Biol. 2005, 7, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.; Dobberstein, B. Common principles of protein translocation across membranes. Science 1996, 271, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Von-Heijne, G. The signal peptide. J. Membr. Biol. 1990, 115, 195–201. [Google Scholar] [CrossRef]
- Richardson, L.G.L.; Schnell, D.J. Origins, function, and regulation of the TOC–TIC general protein import machinery of plastids. J. Exp. Bot. 2020, 71, 1226–1238. [Google Scholar] [CrossRef]
- Akimaru, J.; Matsuyama, S.-I.; Tokuda, H.; Mizushima, S. Reconstitution of a protein translocation system containing purified SecY, SecE, and SecA from Escherichia coli. J. Biol. Chem. 1991, 266, 2241–2247. [Google Scholar] [CrossRef]
- Robson, A.; Carr, B.; Sessions, R.B.; Collinson, I. Synthetic peptides identify a second periplasmic site for the plug of the SecYEG protein translocation complex. FEBS Lett. 2009, 583, 207–212. [Google Scholar] [CrossRef]
- Berks, B.C.; Sargent, F.; Palmer, T. The Tat protein export pathway. Mol. Microbiol. 2000, 35, 260–274. [Google Scholar] [CrossRef]
- Pugsley, A.P. The complete general secretory pathway in Gram-negative bacteria. Microbiol. Rev. 1993, 57, 50–108. [Google Scholar] [CrossRef]
- Fernandez, D.E. Two paths diverged in the stroma: Targeting to dual SEC translocase systems in chloroplasts. Photosynth. Res. 2018, 138, 261–268. [Google Scholar] [CrossRef]
- Karnauchov, I.; Cai, D.; Schmidt, I.; Herrmann, R.G.; Klösgen, R.B. The Thylakoid Translocation of Subunit 3 of Photosystem I, the psaF Gene Product, Depends on a Bipartite Transit Peptide and Proceeds along an Azide-sensitive Pathway. J. Biol. Chem. 1994, 269, 32871–32878. [Google Scholar] [CrossRef]
- Rodrigues, R.A.O.; Silva-Filho, M.C.; Cline, K. FtsH2 and FtsH5: Two homologous subunits use different integration mechanisms leading to the same thylakoid multimeric complex. Plant J. 2011, 65, 600–609. [Google Scholar] [CrossRef]
- Röhl, T.; Van Wijk, K.J. In vitro reconstitution of insertion and processing of cytochrome in a homologous chloroplast translation system. J. Biol. Chem. 2001, 276, 35465–35472. [Google Scholar] [CrossRef] [PubMed]
- New, C.P.; Ma, Q.; Dabney-Smith, C. Routing of thylakoid lumen proteins by the chloroplast twin arginine transport pathway. Photosynth. Res. 2018, 138, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Bolhuis, A. Tat-dependent protein targeting in prokaryotes and chloroplasts. Biochim. Biophys. Acta Bioenerg. 2004, 1694, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Cline, K.; Theg, S.M. The Sec and Tat protein translocation pathways in chloroplasts. Annu. Rev. Plant Biol. 2007, 58, 325–353. [Google Scholar]
- Sargent, F.; Bogsch, E.G.; Stanley, N.R.; Wexler, M.; Robinson, C.; Berks, B.C.; Palmer, T. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 1998, 17, 3640–3650. [Google Scholar] [CrossRef]
- Berks, B.C. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 1996, 22, 393–404. [Google Scholar] [CrossRef]
- Ifuku, K.; Ido, K.; Sato, F. Molecular functions of PsbP and PsbQ proteins in the photosystem II supercomplex. J. Photochem. Photobiol. B 2011, 103, 161–168. [Google Scholar] [CrossRef]
- Haldrup, A.; Naver, H.; Scheller, H.V. The interaction between plastocyanin and photosystem I is inefficient in transgenic Arabidopsis plants lacking the PSI-N subunit of photosystem I. Plant J. 1999, 17, 689–698. [Google Scholar] [CrossRef]
- Rollauer, S.E.; Tarry, M.J.; Graham, J.E.; Jaaskelainen, M.; Jager, F.; Johnson, S.; Krehenbrink, M.; Liu, S.M.; Lukey, M.J.; Marcoux, J.; et al. Structure of the TatC core of the twin-arginine protein transport system. Nature 2012, 492, 210–214. [Google Scholar] [CrossRef]
- Ramasamy, S.; Abrol, R.; Suloway, C.J.M.; Clemons, W.M. The glove-like structure of the conserved membrane protein TatC provides insight into signal sequence recognition in twin-arginine translocation. Structure 2013, 21, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Li, H.; Jin, C. Structural basis for TatA oligomerization: An NMR study of Escherichia coli TatA dimeric structure. PLoS ONE 2014, 9, e103157. [Google Scholar] [CrossRef] [PubMed]
- Settles, A.M.; Yonetani, A.; Baron, A.; Bush, D.R.; Cline, K.; Martienssen, R. Sec-independent protein translocation by the maize Hcf106 protein. Science 1997, 278, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Alcock, F.; Stansfeld, P.J.; Basit, H.; Habersetzer, J.; Bell, A.; Gahan, L.R.; Gor’kov, P.L.; Caffrey, M.; Diederichs, K.; Palmer, T.; et al. Assembling the Tat protein translocase. Elife 2016, 5, e20718. [Google Scholar] [CrossRef]
- Habersetzer, J.; Moore, K.; Cherry, J.; Buchanan, G.; Stansfeld, P.J.; Palmer, T. Substrate-triggered position switching of TatA and TatB during Tat transport in Escherichia coli. Open Biol. 2017, 7, 170091. [Google Scholar] [CrossRef]
- Cline, K.; Mori, H. Thylakoid ΔpH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J. Cell Biol. 2001, 154, 719–729. [Google Scholar] [CrossRef]
- Dabney-Smith, C.; Cline, K. Clustering of C-terminal stromal domains of Tha4 homo-oligomers during translocation by the Tat protein transport system. Mol. Biol. Cell 2009, 20, 3498–3507. [Google Scholar] [CrossRef][Green Version]
- Dabney-Smith, C.; Mori, H.; Cline, K. Oligomers of Tha4 organize at the thylakoid Tat translocase during protein transport. J. Biol. Chem. 2006, 281, 5476–5483. [Google Scholar] [CrossRef]
- Cline, K. Mechanistic aspects of folded protein transport by the twin arginine translocase (Tat). J. Biol. Chem. 2015, 290, 16530–16538. [Google Scholar] [CrossRef]
- Alcock, F.; Baker, M.A.B.; Greene, N.P.; Palmer, T.; Wallace, M.I.; Berks, B.C. Live cell imaging shows reversible assembly of the TatA component of the twin-arginine protein transport system. Proc. Natl. Acad. Sci. USA 2013, 110, 15376–15381. [Google Scholar] [CrossRef] [PubMed]
- Dabney-Smith, C.; Mori, H.; Cline, K. Requirement of a Tha4-conserved transmembrane glutamate in thylakoid Tat translocase assembly revealed by biochemical complementation. J. Biol. Chem. 2003, 278, 43027–43033. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Fite, K.; Dabney-Smith, C. Direct interaction between a precursor mature domain and transport component Tha4 during twin arginine transport of chloroplasts. Plant Physiol. 2013, 161, 990–1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aldridge, C.; Storm, A.; Cline, K.; Dabney-Smith, C. The chloroplast twin arginine transport (Tat) component, Tha4, undergoes conformational changes leading to Tat protein transport. J. Biol. Chem. 2012, 287, 34752–34763. [Google Scholar] [CrossRef]
- Aldridge, C.; Ma, X.; Gerard, F.; Cline, K. Substrate-gated docking of pore subunit Tha4 in the TatC cavity initiates Tat translocase assembly. J. Cell Biol. 2014, 205, 51–65. [Google Scholar] [CrossRef]
- Bocharov, E.V. Spatial structure and pH-dependent conformational diversity of dimeric transmembrane domain of the receptor tyrosine kinase EphA1. J. Biol. Chem. 2008, 283, 29385–29395. [Google Scholar] [CrossRef]
- Catterall, W.A.; Wisedchaisri, G.; Zheng, N. The chemical basis for electrical signaling. Nat. Chem. Biol. 2017, 13, 455–463. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Hu, Y.; Jin, C. Solution structure of the TatB component of the twin-arginine translocation system. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1881–1888. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.; Carrigan, M.; McCaffery, M.; Ma, X.; Cline, K. Targeting determinants and proposed evolutionary basis for the Sec and the ΔpH protein transport systems in chloroplast thylakoid membranes. J. Cell Biol. 1997, 136, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Celedon, J.M.; Cline, K. Stoichiometry for binding and transport by the twin arginine translocation system. J. Cell Biol. 2012, 197, 523–534. [Google Scholar] [CrossRef]
- Ma, X.; Cline, K. Precursors bind to specific sites on thylakoid membranes prior to transport on the delta pH protein translocation system. J. Biol. Chem. 2000, 275, 10016–10022. [Google Scholar] [CrossRef]
- Prahlad, J.; Hauser, D.N.; Milkovic, N.M.; Cookson, M.R.; Wilson, M.A. Use of cysteine-reactive cross-linkers to probe conformational flexibility of human DJ-1 demonstrates that Glu18 mutations are dimers. J. Neurochem. 2014, 130, 839–853. [Google Scholar] [CrossRef]
- Pettersson, P.; Ye, W.; Jakob, M.; Tannert, F.; Klösgen, R.B.; Mäler, L. Structure and dynamics of plant TatA in micelles and lipid bilayers studied by solution NMR. FEBS J. 2018, 285, 1886–1906. [Google Scholar] [CrossRef]
- Greene, N.P.; Porcelli, I.; Palmer, T.; Berks, B.C. Cysteine scanning mutagenesis and disulfide mapping studies of the TatA component of the bacterial twin arginine translocase. J. Biol. Chem. 2007, 282, 23937–23945. [Google Scholar] [CrossRef][Green Version]
- Pettersson, P.; Ye, W.; Jakob, M.; Tannert, F.; Klösgen, R.B.; Mäler, L. Soluble TatA forms oligomers that interact with membranes: Structure and insertion studies of a versatile protein transporter. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183529. [Google Scholar] [CrossRef]
- Stockwald, E.R.; Gohlke, H.; Ponnu, J.; Knapp, B.; Sinner, E.K.; Fath, S.; Warnecke, T.; Rief, M. Length matters: Functional flip of the short TatA transmembrane helix. Biophys. J. 2023, 122, 2125–2146. [Google Scholar] [CrossRef]
- Panahi, A.; Brooks, C.L. Membrane environment modulates the pKa values of transmembrane helices. J. Phys. Chem. B 2015, 119, 4601–4607. [Google Scholar] [CrossRef]
- Gayen, A.; Leninger, M.; Traaseth, N.J. Protonation of a glutamate residue modulates the dynamics of the drug transporter EmrE. Nat. Chem. Biol. 2016, 12, 141–145. [Google Scholar] [CrossRef]
- Fitch, C.A.; Karp, D.A.; Lee, K.K.; Stites, W.E.; Lattman, E.E.; García-Moreno, E.B. Experimental pKa values of buried residues: Analysis with continuum methods and role of water penetration. Biophys. J. 2002, 82, 3289–3304. [Google Scholar] [CrossRef]
- Trinh, M.D.L.; Masuda, S. Chloroplast pH homeostasis for the regulation of photosynthesis. Front. Plant Sci. 2022, 13, 919896. [Google Scholar] [CrossRef] [PubMed]
- Davletshina, L.N.; Semin, B.K. pH dependence of photosystem II photoinhibition: Relationship with structural transition of oxygen-evolving complex at the pH of thylakoid lumen. Photosynth. Res. 2020, 145, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hoh, D.; Armbruster, U. Redox regulation in chloroplast thylakoid lumen: The pmf changes everything again. Plant Cell Environ. 2023, 46, 2741–2761. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Cline, K. A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid ΔpH/Tat translocase. J. Cell Biol. 2002, 157, 205–210. [Google Scholar] [CrossRef]
- Rodriguez, F.; Buchanan, G.; Tarry, M.J.; Berks, B.C.; Palmer, T.; Lea, S.M. Structural model for the protein-translocating element of the twin-arginine transport system. Proc. Natl. Acad. Sci. USA 2013, 110, 454–459. [Google Scholar] [CrossRef]
- Han, S. Structural dynamics determine voltage and pH gating in human voltage-gated proton channel. Elife 2022, 11, e73093. [Google Scholar] [CrossRef]
- Chanda, B.; Bezanilla, F.A. Common Pathway for Charge Transport through Voltage-Sensing Domains. Neuron 2008, 57, 345–351. [Google Scholar] [CrossRef]
- Sigworth, F.J. Voltage gating of ion channels. Q. Rev. Biophys 1994, 27, 1–40. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Girvin, M.E. Structural changes linked to proton translocation by subunit c of the ATP synthase. Nature 1999, 402, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, K. Catalytic oxidation of sulfhydryl groups by o-phenanthroline copper complex. J. Biochem. 1968, 64, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Geula, S.; Naveeds, H.; Liangs, J.; Shoshan-Barmatz, V. Structure-based analysis of VDAC1 protein: Defining oligomer contact sites. J. Biol. Chem. 2012, 287, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.C.K.; Maillard, A.P.; Chan, K.K.Y.; Duong, F. Investigating the SecY plug movement at the SecYEG translocation channel. EMBO J. 2005, 24, 3380–3388. [Google Scholar] [CrossRef]
- Murphy, D.M.; Ivanenkov, V.V.; Kirley, T.L. Identification of cysteine residues responsible for oxidative cross-linking and chemical inhibition of human nucleoside-triphosphate diphosphohydrolase 3. J. Biol. Chem. 2002, 277, 6162–6169. [Google Scholar] [CrossRef]
- Hamsanathan, S.; Musser, S.M. The Tat protein transport system: Intriguing questions and conundrums. FEMS Microbiol. Lett. 2018, 365, 123. [Google Scholar] [CrossRef]
- Mitra, K.; Ubarretxena-Belandia, I.; Taguchi, T.; Warren, G.; Engelman, D.M. Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc. Natl. Acad. Sci. USA 2004, 101, 4083–4088. [Google Scholar] [CrossRef]
- Wilson, S. Hydrophobic mismatch in the thylakoid membrane regulates photosynthetic light harvesting. J. Am. Chem. Soc. 2024, 146, 14905–14914. [Google Scholar] [CrossRef]
- Hao, B.; Zhou, W.; Theg, S.M. Hydrophobic mismatch is a key factor in protein transport across lipid bilayer membranes via the Tat pathway. J. Biol. Chem. 2022, 298, 101991. [Google Scholar] [CrossRef]
- Huang, Q.; Palmer, T. Signal peptide hydrophobicity modulates interaction with the Twin-Arginine translocase. mBio 2017, 8, e00909-17. [Google Scholar] [CrossRef] [PubMed]
- Bruser, T.; Sanders, C. An alternative model of the twin arginine translocation system. Microbiol. Res. 2003, 158, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Alcock, F.; Berks, B.C. New insights into the Tat protein transport cycle from characterizing the assembled Tat translocon. Mol. Microbiol. 2023, 118, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Goral, T.K.; Duffy, C.D.P.; Brain, A.P.R.; Mullineaux, C.W.; Ruban, A.V. Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 2011, 23, 1468–1479. [Google Scholar] [CrossRef]
- Kirchhoff, H.; Mukherjee, U.; Galla, H.J. Dynamic control of protein diffusion within the granal thylakoid lumen. Proc. Natl. Acad. Sci. USA 2011, 108, 20248–20253. [Google Scholar] [CrossRef]
- Murakami, S.; Packer, L. Light-induced changes in the conformation and configuration of the thylakoid membrane of Ulva and Porphyra chloroplasts in vivo. Plant Physiol. 1970, 45, 289–295. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, E.; Li, H.; Xia, B.; Jin, C. Solution NMR structure of the TatA component of the twin-arginine protein transport system from gram-positive bacterium Bacillus subtilis. J. Am. Chem. Soc. 2010, 132, 15942–15944. [Google Scholar] [CrossRef]
- Torabifard, H.; Panahi, A.; Brooks III, C.L. M2 amphipathic helices facilitate pH-dependent conformational transition in influenza A virus. Proc. Natl. Acad. Sci. USA 2020, 117, 3583–3591. [Google Scholar] [CrossRef]
- Forsyth, W.R.; Antosiewicz, J.M.; Robertson, A.D. Empirical relationships between protein structure and carboxyl pKa values in proteins. Proteins 2002, 48, 388–403. [Google Scholar] [CrossRef]
- Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008, 9, 323–332. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, A.; Chen, J.; Ruta, V.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-ray structure of a voltage-dependent K+ channel. Nature 2003, 423, 33–41. [Google Scholar] [CrossRef]
- Cline, K. Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J. Biol. Chem. 1986, 261, 14804–14810. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weesinghe, V.S.; New, C.P.; Dabney-Smith, C. The Transmembrane Glutamate Serves as a pH Sensor for Tha4 Oligomerization During Twin Arginine Transport of Proteins. Plants 2025, 14, 3338. https://doi.org/10.3390/plants14213338
Weesinghe VS, New CP, Dabney-Smith C. The Transmembrane Glutamate Serves as a pH Sensor for Tha4 Oligomerization During Twin Arginine Transport of Proteins. Plants. 2025; 14(21):3338. https://doi.org/10.3390/plants14213338
Chicago/Turabian StyleWeesinghe, Vidusha S., Christopher Paul New, and Carole Dabney-Smith. 2025. "The Transmembrane Glutamate Serves as a pH Sensor for Tha4 Oligomerization During Twin Arginine Transport of Proteins" Plants 14, no. 21: 3338. https://doi.org/10.3390/plants14213338
APA StyleWeesinghe, V. S., New, C. P., & Dabney-Smith, C. (2025). The Transmembrane Glutamate Serves as a pH Sensor for Tha4 Oligomerization During Twin Arginine Transport of Proteins. Plants, 14(21), 3338. https://doi.org/10.3390/plants14213338





