Physiological Mechanisms Underlying Maize Yield Enhancement by Straw Return in the Thin-Layer Mollisol Region of the Songnen Plain
Abstract
1. Introduction
2. Results
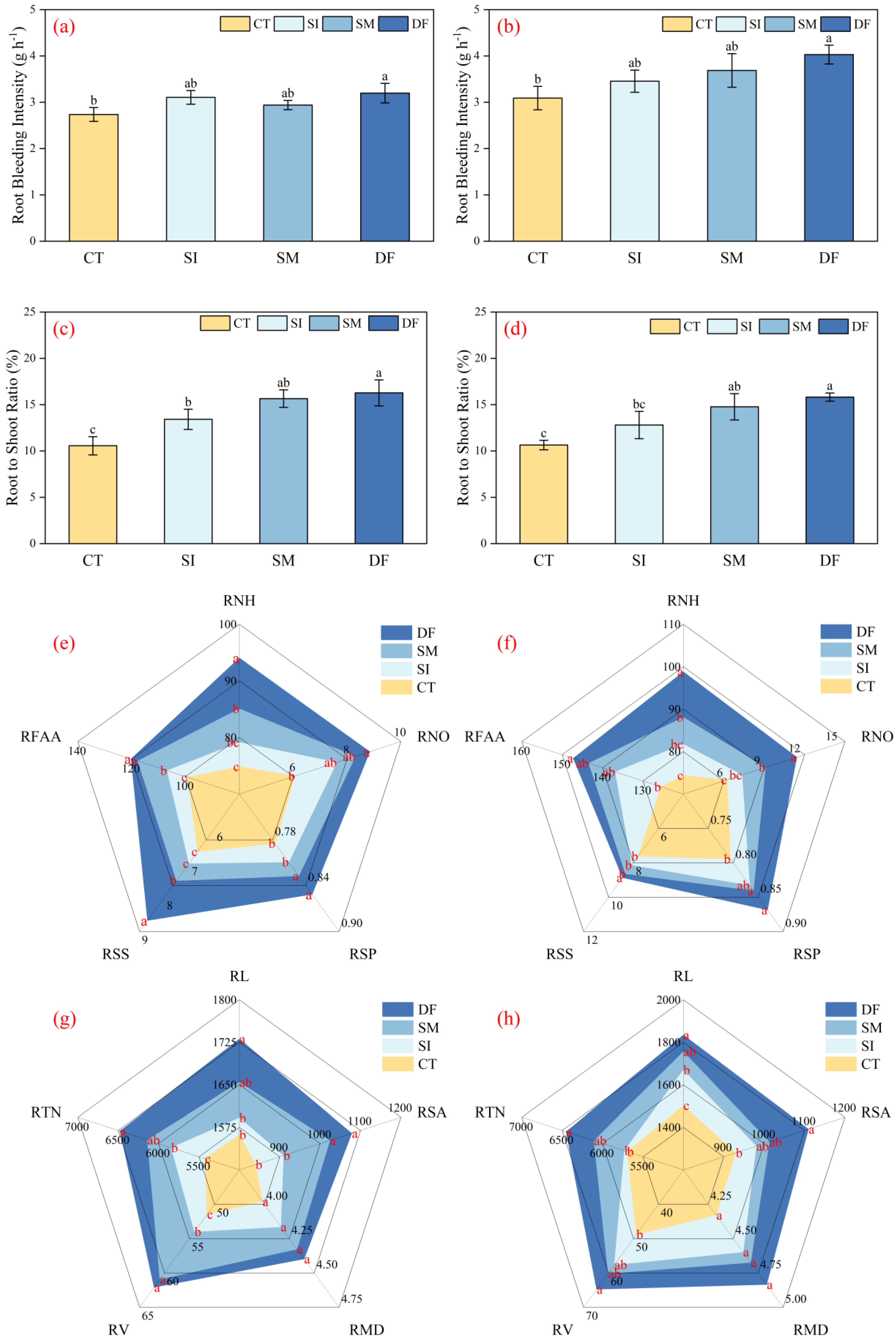
2.1. Effects of Different Straw Return Methods on Root Morphology and Physiological Traits
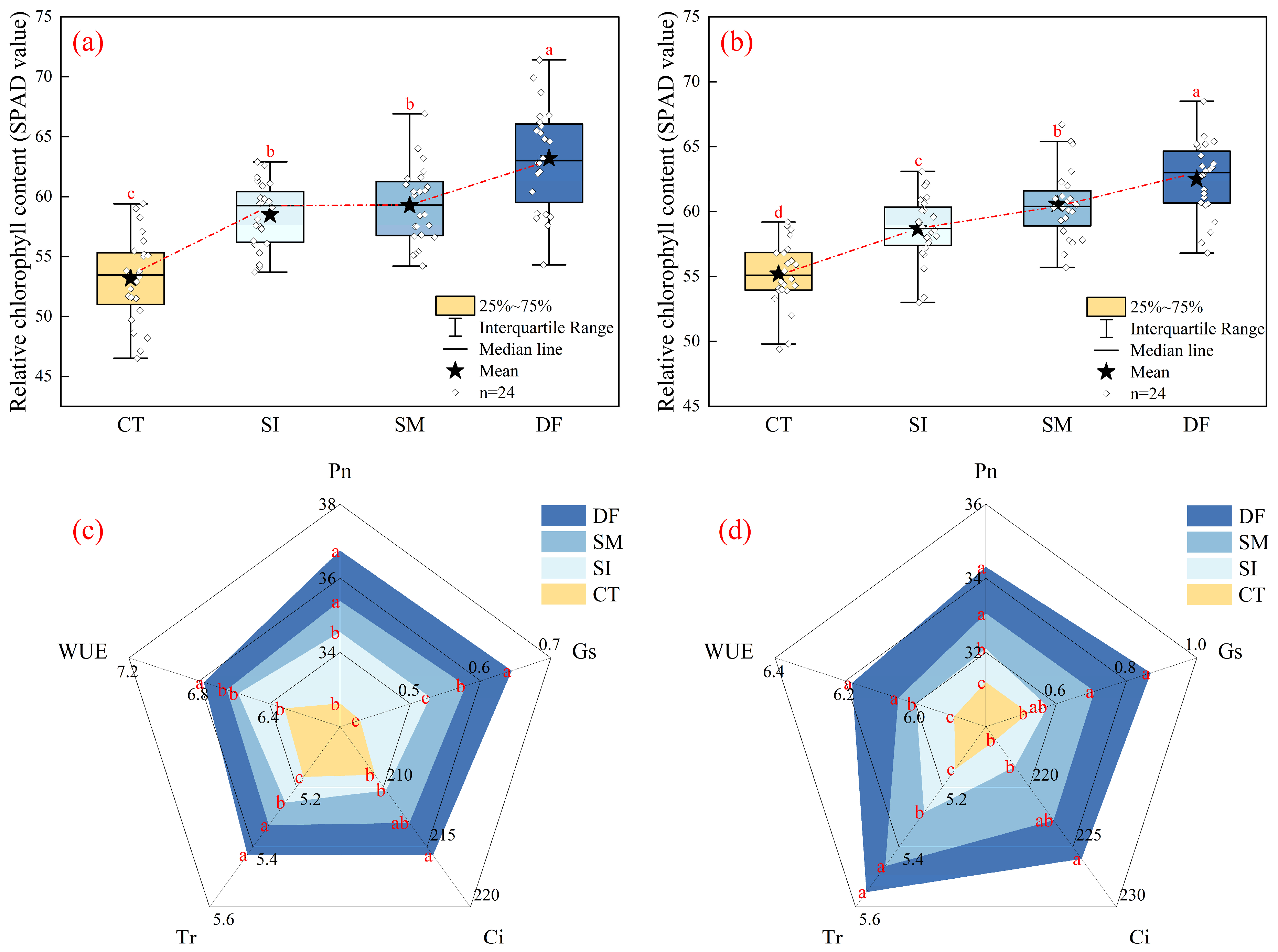
2.2. Leaf Photosynthetic Characteristics and Carbon–Nitrogen Metabolic Enzyme Responses to Straw Return Methods
2.3. Plant Nitrogen Accumulation and Translocation in Response to Straw Return
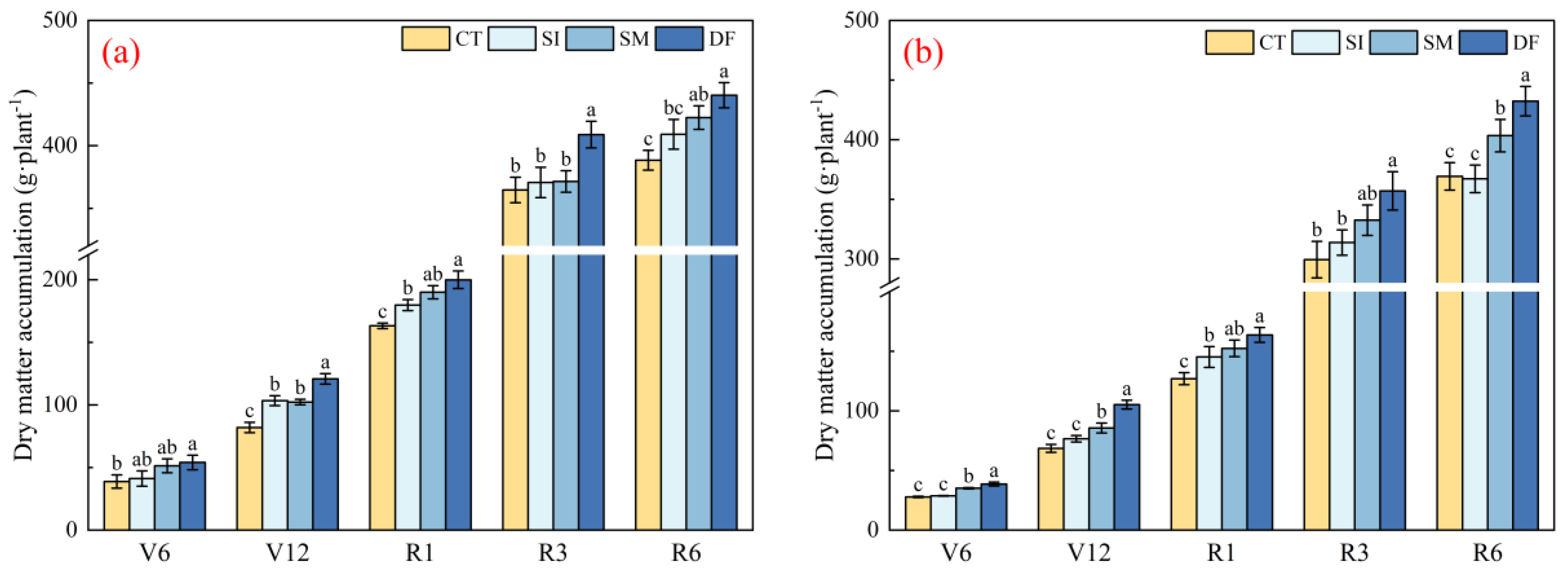
2.4. Dry Matter Accumulation and Yield Response to Straw Return
2.5. Drivers and Regulatory Pathways of Yield Formation
3. Discussion
3.1. Effects of Straw Return on the Root System: Synergistic Optimization of Morphology and Physiology
3.2. From Roots to Canopy: Systemic Responses in Photosynthesis and Carbon–Nitrogen Metabolism
3.3. Yield Formation Mechanisms: Statistical and Model Evidence Based on Root–Shoot Interactions
4. Materials and Methods
4.1. Experimental Design
4.2. Root Bleeding Sap Collection and Morphological Measurements
4.3. Leaf Photosynthetic Measurements
4.4. Leaf carbon and nitrogen metabolism enzymes
4.5. Nitrogen Accumulation and Translocation
4.6. Dry Matter Accumulation
4.7. Yield and Yield Components
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Yan, Z.; Zhiyuan, T.; Rui, M.; Lili, Q.; Yihang, W. Impact of soil erosion-driven degradation on crop yield in black soils of Northeast China’s Songnen Plain. Pedosphere 2025, in press. [Google Scholar] [CrossRef]
- Wang, H.; Shen, M.; Hui, D.; Chen, J.; Sun, G.; Wang, X.; Lu, C.; Sheng, J.; Chen, L.; Luo, Y. Straw incorporation influences soil organic carbon sequestration, greenhouse gas emission, and crop yields in a Chinese rice (Oryza sativa L.)–wheat (Triticum aestivum L.) cropping system. Soil Tillage Res. 2019, 195, 104377. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, L.; Xie, J.; Li, L.; Coulter, J.A.; Zhang, R.; Luo, Z.; Cai, L.; Carberry, P.; Whitbread, A. Conservation tillage increases yield and precipitation use efficiency of wheat on the semi-arid Loess Plateau of China. Agric. Water Manag. 2020, 231, 106024. [Google Scholar] [CrossRef]
- Wei, X.; He, K.; Ma, B.-L.; Guo, S.; Feng, C.; Liu, C.; Ma, Y.; Li, P. Straw return significantly enhances wheat yield in higher precipitation environment by promoting larger root diameters, wider xylem channels, and thinner root cortex. Plant Soil 2025, 514, 2217–2233. [Google Scholar] [CrossRef]
- Wang, X.-X.; Li, J.; Wang, D.; An, T.; Qin, W.; Zou, H.; Rengel, Z. Straw incorporation effects on net photosynthetic carbon assimilation and maize growth. Front. Agron. 2022, 4, 805320. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Chen, X.; Zhang, Z.; Zhou, Z.; Zhou, Y.; Qi, Z. Heterosis Analysis in Endogenous Substances in Root Bleeding Sap of Sorghum. Phyton 2024, 93, 1963–1980. [Google Scholar] [CrossRef]
- Wang, J.; Fan, J.; Wang, H.; Wang, X.; Xing, Y.; Gao, Y.; Hao, M. Dual-mulching under no-tillage promotes maize root growth and improves yield by optimizing soil hydrothermal conditions in semi-arid regions. Agric. Water Manag. 2025, 312, 109428. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, A.; Wang, P.; Shang, Y.; Wang, F.; Lyu, H.; Pang, X.; Li, Y.; Liu, Y.; Yin, B. No-tillage with total green manure mulching can improve soil moisture and temperature environment, promote maize root structure and photosynthetic capacity to increase maize yield. J. Integr. Agric. 2025, 24, 4211–4224. [Google Scholar] [CrossRef]
- Chen, X.; Ren, H.; Zhang, J.; Zhao, B.; Ren, B.; Wan, Y.; Liu, P. Deep phosphorus fertilizer placement increases maize productivity by improving root-shoot coordination and photosynthetic performance. Soil Tillage Res. 2024, 235, 105915. [Google Scholar] [CrossRef]
- Diacono, M.; Baldivieso-Freitas, P.; Sans Serra, F.X. Nitrogen utilization in a cereal-legume rotation managed with sustainable agricultural practices. Agron.J. 2019, 9, 113. [Google Scholar] [CrossRef]
- Kong, F.; Jiu, A.; Kan, Z.; Zhou, J.; Yang, H.; Li, F.-M. Deep tillage combined with straw biochar return increases rice yield by improving nitrogen availability and root distribution in the subsoil. Field Crops Res. 2024, 315, 109481. [Google Scholar] [CrossRef]
- Dong, S.; Bismark, A.-B.; Li, S.; Gao, Q.; Zhou, X.; Li, C. Ammonium Polyphosphate Promotes Maize Growth and Phosphorus Uptake by Altering Root Properties. Plants 2024, 13, 3407. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Gao, S.; Wang, X.; He, A.; He, P. Effects of water–nitrogen coupling on root distribution and yield of summer maize at different growth stages. Plants 2025, 14, 1278. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Du, Y.; Niu, W. Conservation tillage improves the yield of summer maize by regulating soil water, photosynthesis and inferior kernel grain filling on the semiarid Loess Plateau, China. J. Sci. Food Agric. 2022, 102, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Buivydienė, A.; Deveikytė, I.; Veršulienė, A.; Feiza, V. Tillage Practices Effect on Root Distribution and Variation of Soil CO2 Emission under Different Cropping Strategies. Agron. J. 2024, 14, 1768. [Google Scholar] [CrossRef]
- Wu, G.; Ling, J.; Liu, Z.-X.; Xu, Y.-P.; Chen, X.-M.; Wen, Y.; Zhou, S.-L. Soil warming and straw return impacts on winter wheat phenology, photosynthesis, root growth, and grain yield in the North China Plain. Field Crops Res. 2022, 283, 108545. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Sun, S.; Ji, X.; Wang, X.; Wang, Z.; Shang, J.; Jiang, Y.; Gong, X.; Qi, H. Optimization of the morphological, structural, and physicochemical properties of maize starch using straw returning and nitrogen fertilization in Northeast China. Int. J. Biol. Macromol. 2024, 265, 130791. [Google Scholar] [CrossRef]
- Jin, W.; Liu, Z.; Cheng, Z.; Wang, Q.; Hu, W.; Chen, B.; Meng, Y.; Zhou, Z. The trade-off between root growth redundancy and premature senescence under different straw returning modes affects boll formation and seedcotton yield. Eur. J. Agron. 2024, 156, 127175. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Borjigin, Q.; Gao, J.L.; Yu, X.F.; Hu, S.P.; Li, R.-P. Effects of different straw return methods on soil properties and yield potential of maize. Sci. Rep. 2024, 14, 28682. [Google Scholar] [CrossRef]
- Lian, Y.; Zhang, X.; Du, F.; Zhang, X.; Ali, S. Mulch drip fertigation with diverse tillage practices regulating root bleeding sap, root growth, lodging resistance and improve maize productivity. Agric. Water Manag. 2024, 306, 109186. [Google Scholar] [CrossRef]
- Jia, Q.; Chen, K.; Chen, Y.; Ali, S.; Sohail, A.; Fahad, S. Mulch covered ridges affect grain yield of maize through regulating root growth and root-bleeding sap under simulated rainfall conditions. Soil Tillage Res. 2018, 175, 101–111. [Google Scholar] [CrossRef]
- Ma, L.; Kong, F.; Wang, Z.; Luo, Y.; Lv, X.; Zhou, Z.; Meng, Y. Growth and yield of cotton as affected by different straw returning modes with an equivalent carbon input. Field Crops Res. 2019, 243, 107616. [Google Scholar] [CrossRef]
- Zhang, W.; Long, A.; Ji, X.; Sun, Z.; Tian, P.; Jin, C.; Gong, X.; Jiang, Y.; Qi, H.; Yu, H. Tillage combined with straw return increases maize yield and water use by regulating root morphological distribution and nitrogen metabolism in Northeast China. Soil Tillage Res. 2026, 256, 106876. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Z.; Zhou, Z.; Lu, T.; Chen, S.; He, M.; Zeng, X.; Chen, K.; Yu, H.; Shangguan, Y. Root system architecture differences of maize cultivars affect yield and nitrogen accumulation in Southwest China. Agriculture 2022, 12, 209. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Yu, Y.; Qian, C.; Li, C.; Wei, S.; Li, C.; Gu, W. Nitrogen and chemical control management improve yield and quality in high-density planting of maize by promoting root-bleeding sap and nutrient absorption. Front. Plant Sci. 2022, 13, 754232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xie, J.; Hou, H.; Li, M.; Wang, Y.; Wang, X. Effects of zeolite on physiological characteristics and grain quality in rice under alternate wetting and drying irrigation. Water 2023, 15, 2406. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, J.; Rengel, Z. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 2017, 416, 377–389. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Li, Y.; Yang, L.; Shi, W.; Liu, Y.; Chang, S.; Hou, F.; Jia, Q. Enhance root-bleeding sap flow and root lodging resistance of maize under a combination of nitrogen strategies and farming practices. Agric. Water Manag. 2019, 224, 105742. [Google Scholar] [CrossRef]
- Che, W.; Piao, J.; Gao, Q.; Li, X.; Li, X.; Jin, F. Response of soil physicochemical properties, soil nutrients, enzyme activity and rice yield to rice straw returning in highly saline-alkali paddy soils. Soil Sci. Plant Nutr. 2023, 23, 4396–4411. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Ding, Y.; Pan, R.; Shen, W.; Yu, X.; Xiong, F. The relationship between characteristics of root morphology and grain filling in wheat under drought stress. PeerJ 2021, 9, e12015. [Google Scholar] [CrossRef]
- Xu, G.w.; Song, K.j.; Lu, D.K.; Wang, H.Z.; Chen, M.c. Influence of water management and nitrogen application on rice root and shoot traits. Agron. J. 2019, 111, 2232–2244. [Google Scholar] [CrossRef]
- Wang, J.; Hussain, S.; Sun, X.; Zhang, P.; Javed, T.; Dessoky, E.S.; Ren, X.; Chen, X. Effects of nitrogen application rate under straw incorporation on photosynthesis, productivity and nitrogen use efficiency in winter wheat. Front. Plant Sci. 2022, 13, 862088. [Google Scholar] [CrossRef]
- Cui, H.; Luo, Y.; Chen, J.; Jin, M.; Li, Y.; Wang, Z. Straw return strategies to improve soil properties and crop productivity in a winter wheat-summer maize cropping system. Eur. J. Agron. 2022, 133, 126436. [Google Scholar] [CrossRef]
- Guo, Y.; Fan, H.; Li, P.; Wei, J.; Qiu, H. Photosynthetic physiological basis of no tillage with wheat straw returning to improve maize yield with plastic film mulching in arid irrigated areas. Plants 2023, 12, 1358. [Google Scholar] [CrossRef]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, D.; Ren, H.; Yang, Q.; Dong, S.; Zhang, J.; Zhao, B.; Liu, P. How delaying post-silking senescence in lower leaves of maize plants increases carbon and nitrogen accumulation and grain yield. Crop J. 2022, 10, 853–863. [Google Scholar] [CrossRef]
- Xiao, Y.; Luo, W.; Yang, K.; Fu, J.; Wang, P. Plow tillage with buried straw increases maize yield by regulating soil properties, root growth, photosynthetic capacity, and bacterial community assembly in semi-arid black soil farmlands. Eur. J. Agron. 2025, 164, 127532. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhang, S.; Liu, Z.; Liu, Z.; Shao, X.; Guo, L.; Geng, Y.; Wang, L.; Lv, Y. Mechanisms of topsoil depth drive differences in maize yield and photosynthetic carbon assimilation. Integr. Agric. 2025, in press. [Google Scholar] [CrossRef]
- Pan, S.; Liu, H.; Mo, Z.; Patterson, B.; Duan, M.; Tian, H.; Hu, S.; Tang, X. Effects of nitrogen and shading on root morphologies, nutrient accumulation, and photosynthetic parameters in different rice genotypes. Sci. Rep. 2016, 6, 32148. [Google Scholar] [CrossRef]
- Huang, M.; Xiao, H.; Zhang, J.; Li, S.; Peng, Y.; Guo, J.-H.; Jiang, P.; Wang, R.; Chen, Y.; Li, C. Effects of Long-Term Positioning Tillage Method and Straw Management on Crop Yield and Nutrient Accumulation and Utilization in Dryland Wheat–Maize Double-Cropping System. Agron. J. 2025, 15, 363. [Google Scholar] [CrossRef]
- Liao, C.; Tang, M.; Zhang, C.; Deng, M.; Li, Y.; Feng, S. Impacts of Various Straw Mulching Strategies on Soil Water, Nutrients, Thermal Regimes, and Yield in Wheat–Soybean Rotation Systems. Plants 2025, 14, 2233. [Google Scholar] [CrossRef]
- Fan, P.; Ming, B.; Evers, J.B.; Li, Y.; Li, S.; Xie, R.; Anten, N.P. Nitrogen availability determines the vertical patterns of accumulation, partitioning, and reallocation of dry matter and nitrogen in maize. Field Crops Res. 2023, 297, 108927. [Google Scholar] [CrossRef]
- Sartori, F.; Piccoli, I.; Polese, R.; Berti, A. Transition to conservation agriculture: How tillage intensity and covering affect soil physical parameters. Soil 2022, 8, 213–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gong, S.; Xu, D.; Mo, Y. Straw mulching enhanced the photosynthetic capacity of field maize by increasing the leaf N use efficiency. Agric. Water Manag. 2019, 218, 60–67. [Google Scholar] [CrossRef]
- USDA. Diagnosis and Improvement of Saline and Alkali Soils; USDA, USGPOW: Washington, DC, USA, 1954; Volume 18, p. 160. [Google Scholar] [CrossRef]
- Solorzano, L. Determination of Ammonia in Natural Waters by the Phenolhypochlorite Method. Master’s Thesis, Universidade Estadual da Paraíba, Campina Grande, Brazil, 1969. [Google Scholar] [CrossRef]
- Cawse, P. The determination of nitrate in soil solutions by ultraviolet spectrophotometry. Analyst 1967, 92, 311–315. [Google Scholar] [CrossRef]
- Kochert, G. Carbohydrate determination by the phenol-sulfuric acid method. In Handbook of Phycological Methods, Physiological and Biochemical Methods; Cambridge University Press: Cambridge, UK, 1978; p. 95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dai, H.L.; Wu, X.J. The nitrogen content determined in dry plant samples by Kjeldahl method. Jiangsu Agric. Res. 1995, 15, 70. [Google Scholar] [CrossRef]





| Year | Treatment | RuBP Carboxylase (U·g−1) | PEP Carboxylase (U·g−1) | NR Activity (µg·g−1·h−1) | GS Activity (A·mg−1·h−1) |
|---|---|---|---|---|---|
| 2023 | CT | 59.11 ± 2.39 c | 6.26 ± 0.16 d | 28.54 ± 0.64 c | 11.17 ± 0.36 c |
| SI | 61.22 ± 2.72 bc | 7.40 ± 0.24 b | 35.17 ± 0.88 b | 11.47 ± 0.30 bc | |
| SM | 64.44 ± 2.09 b | 6.96 ± 0.15 c | 33.21 ± 1.27 b | 11.94 ± 0.34 b | |
| DF | 69.51 ± 2.72 a | 8.35 ± 0.22 a | 38.39 ± 1.13 a | 12.19 ± 0.47 a | |
| 2024 | CT | 50.62 ± 3.23 b | 7.07 ± 0.23 c | 29.40 ± 0.40 c | 9.98 ± 0.12 c |
| SI | 56.78 ± 1.36 b | 7.82 ± 0.15 b | 33.77 ± 0.85 b | 10.54 ± 0.18 b | |
| SM | 68.37 ± 5.26 a | 7.77 ± 0.18 ab | 33.77 ± 0.85 b | 10.84 ± 0.31 b | |
| DF | 64.56 ± 3.57 a | 8.17 ± 0.21 a | 36.86 ± 0.76 a | 11.56 ± 0.27 a | |
| Year (Y) | * | ** | NS | ** | |
| Treatment (T) | ** | ** | ** | ** | |
| (Y) × (T) | NS | * | NS | NS | |
| Year | Treatment | Accumulation Before Silking (kg/hm2) | Accumulation After Silking (kg/hm2) | Volume of Transshipment (kg/hm2) | Transport Rate (%) |
|---|---|---|---|---|---|
| 2023 | CT | 127.99 ± 2.35 c | 70.34 ± 1.89 c | 57.65 ± 4.40 a | 44.98 ± 1.92 a |
| SI | 133.66 ± 1.93 b | 75.30 ± 2.37 bc | 58.35 ± 4.20 a | 43.62 ± 2.55 a | |
| SM | 132.34 ± 2.50 b | 76.87 ± 3.89 b | 55.46 ± 2.45 a | 41.93 ± 2.19 a | |
| DF | 145.18 ± 2.93 a | 85.61 ± 1.38 a | 59.57 ± 2.84 a | 41.00 ± 1.12 a | |
| 2024 | CT | 107.88 ± 3.38 b | 48.15 ± 3.38 c | 59.73 ± 1.27 a | 54.31 ± 2.24 a |
| SI | 111.51 ± 1.61 b | 56.06 ± 3.38 b | 55.45 ± 4.96 a | 49.67 ± 3.73 a | |
| SM | 112.78 ± 2.08 b | 55.78 ± 1.28 b | 57.00 ± 5.54 a | 50.49 ± 3.32 a | |
| DF | 126.30 ± 3.17 a | 64.89 ± 2.81 a | 61.40 ± 7.31 a | 48.47 ± 3.90 a | |
| Year (Y) | ** | ** | NS | ** | |
| Treatment (T) | ** | ** | NS | NS | |
| (Y) × (T) | NS | NS | NS | NS | |
| Year | Treatment | Number of Grains | Seed Yield (%) | 100-Grain Weight (g) | Yield (kg/hm2) |
|---|---|---|---|---|---|
| 2023 | CT | 578.40 ± 85.63 a | 88.14 ± 0.40 a | 35.38 ± 0.20 c | 11195.80 ± 144.96 b |
| SI | 590.26 ± 61.18 a | 88.22 ± 0.32 a | 37.53 ± 0.24 b | 11283.24 ± 207.27 b | |
| SM | 599.06 ± 77.99 a | 87.85 ± 0.65 a | 38.31 ± 0.43 ab | 11469.29 ± 136.16 ab | |
| DF | 608.00 ± 49.18 a | 88.23 ± 0.13 a | 38.79 ± 0.60 a | 11737.36 ± 180.59 a | |
| 2024 | CT | 515.73 ± 65.78 a | 87.44 ± 1.53 a | 33.07 ± 0.10 c | 11099.46 ± 229.48 c |
| SI | 528.00 ± 79.71 a | 86.61 ± 0.75 a | 34.67 ± 0.32 b | 11164.80 ± 300.94 bc | |
| SM | 536.53 ± 44.41 a | 87.69 ± 0.66 a | 34.54 ± 0.65 b | 11790.14 ± 297.92 ab | |
| DF | 556.80 ± 76.58 a | 88.50 ± 0.35 a | 37.80 ± 0.17 a | 12415.01 ± 302.27 a | |
| Year (Y) | NS | NS | ** | NS | |
| Treatment (T) | NS | NS | ** | ** | |
| (Y) × (T) | NS | NS | ** | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, C.; Ma, T.; Miao, M.; Chen, J.; Bao, Z.; Chen, B.; Lu, J.; Liu, F.; Wang, N.; Wang, H.; et al. Physiological Mechanisms Underlying Maize Yield Enhancement by Straw Return in the Thin-Layer Mollisol Region of the Songnen Plain. Plants 2025, 14, 3331. https://doi.org/10.3390/plants14213331
Guan C, Ma T, Miao M, Chen J, Bao Z, Chen B, Lu J, Liu F, Wang N, Wang H, et al. Physiological Mechanisms Underlying Maize Yield Enhancement by Straw Return in the Thin-Layer Mollisol Region of the Songnen Plain. Plants. 2025; 14(21):3331. https://doi.org/10.3390/plants14213331
Chicago/Turabian StyleGuan, Chenglong, Tai Ma, Ming Miao, Jiuhui Chen, Zhicheng Bao, Baoyu Chen, Jingkun Lu, Fangming Liu, Nan Wang, Hongjun Wang, and et al. 2025. "Physiological Mechanisms Underlying Maize Yield Enhancement by Straw Return in the Thin-Layer Mollisol Region of the Songnen Plain" Plants 14, no. 21: 3331. https://doi.org/10.3390/plants14213331
APA StyleGuan, C., Ma, T., Miao, M., Chen, J., Bao, Z., Chen, B., Lu, J., Liu, F., Wang, N., Wang, H., & Zhang, Z. (2025). Physiological Mechanisms Underlying Maize Yield Enhancement by Straw Return in the Thin-Layer Mollisol Region of the Songnen Plain. Plants, 14(21), 3331. https://doi.org/10.3390/plants14213331





