Impact of Daily and Seasonal Variation on the Phytochemical Profile of Larrea cuneifolia in Northwestern Argentina
Abstract
1. Introduction
2. Results and Discussion
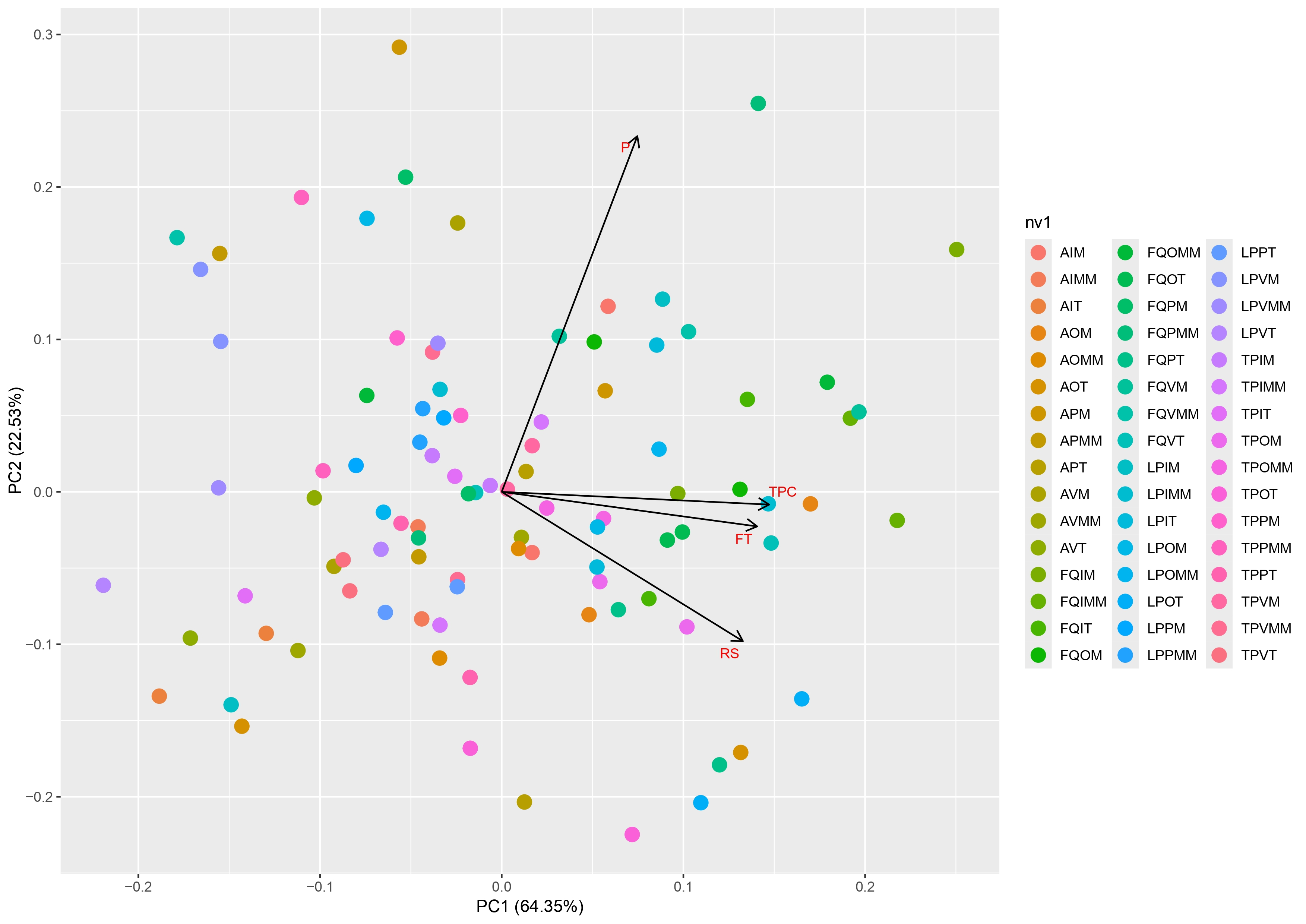
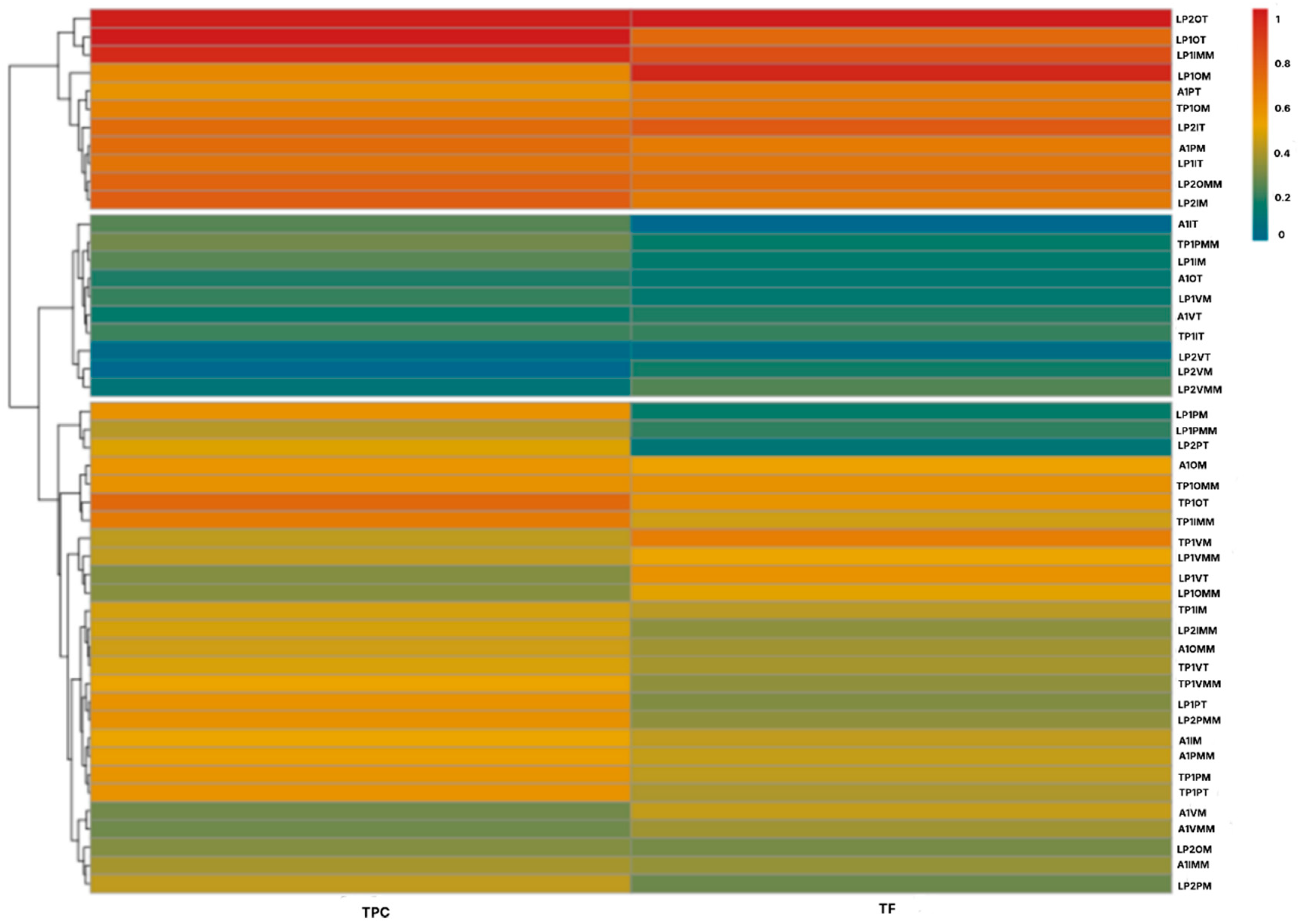
2.1. Phytochemical Characterization
2.2. Biological Activity
3. Materials and Methods
3.1. Chemicals, Reagents and Materials
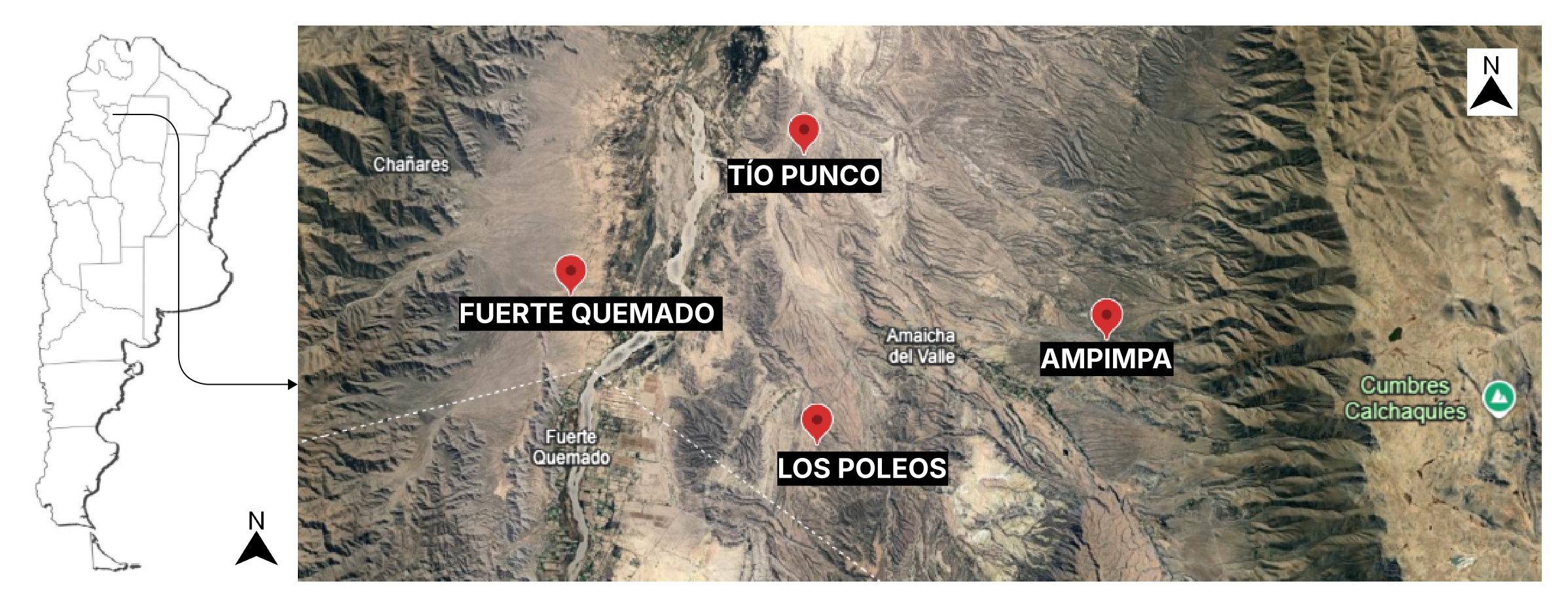
3.2. Plant Material
3.3. Extract Preparation
3.4. Phytochemical Analysis
3.4.1. Quantification of Total Phenolic Compounds
3.4.2. Quantification of Total Flavonoids
3.4.3. Quantification of Total Lignans
3.5. Chromatographic Profile, HPLC-DAD
3.6. Macronutrient Quantification
3.6.1. Quantification of Reducing Sugars
3.6.2. Quantification of Soluble Proteins
3.7. Determination of Antioxidant Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrera, M.C.; Kobak, C.P.; Uriburu, F.M.C.; Cuello, A.S.; Perea, M.C.; Torres, S.; Zampini, C.I.; Isla, M.I.; Rosa, M.D. Patterns of diversity of medicinal shrubs in the Monte desert in the Calchaquí Valleys (Tucumán, Argentina). Lilloa 2025, 62, 399–423. [Google Scholar] [CrossRef]
- Ceballos, S.J.; Perea, M.C. Plantas medicinales utilizadas por la comunidad indígena de Quilmes (Tucumán, Argentina). Bol. Latinoam. Caribe Plantas Med. Aromat. 2014, 13, 47–68. [Google Scholar]
- Carabajal, M.P.A.; Perea, M.C.; Isla, M.I.; Zampini, I.C. The use of jarilla native plants in a Diaguita-Calchaquí indigenous community from northwestern Argentina: An ethnobotanical, phytochemical and biological approach. J. Ethnopharm. 2020, 247, 112258. [Google Scholar] [CrossRef] [PubMed]
- Barboza, G.; Cantero, J.; Nuñez, C.; Pacciaroni, A.; Ariza Espinar, L. Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine flora. Kurtziana 2009, 34, 7–365. [Google Scholar]
- Ladio, A.H.; Gómez, D.D. Plantas medicinales en comunidades rurales de Argentina: Usos, conocimiento y manejo. Rev. Argent. Cienc. Nat. 2006, 13, 39–54. [Google Scholar]
- Conta, A.; Simirgiotis, M.J.; Martínez Chamás, J.; Isla, M.I.; Zampini, I.C. Extraction of Bioactive Compounds from Larrea cuneifolia Cav. Using Natural Deep Eutectic Solvents: A Contribution to the Plant Green Extract Validation of Its Pharmacological Potential. Plants 2025, 14, 1016. [Google Scholar] [CrossRef]
- Moreno, M.A.; Córdoba, S.; Zampini, I.C.; Mercado, M.I.; Ponessa, G.; Sayago, J.E.; Pino Ramos, L.L.; Schmeda-Hirschmanne, G.; Isla, M.I. Argentinean Larrea dry extracts with potential use in vaginal candidiasis. Nat. prod. commun. 2018, 13, 1934578X1801300215. [Google Scholar] [CrossRef]
- Moreno, M.A.; Zampini, I.C.; Isla, M.I. Antifungal, anti-inflammatory and antioxidant activity of bi-herbal mixtures with medicinal plants from Argentinean highlands. J. Ethnopharmacol. 2020, 253, 112642. [Google Scholar] [CrossRef]
- Quiroga, E.M.; Sampietro, A.R.; Vattuone, M.A. Screening antifungal activities of selected medicinal plants. J. Ethnopharmacol. 2001, 74, 89–96. [Google Scholar] [CrossRef]
- Svetaz, L.; Zuljan, F.; Derita, M.; Petenatti, E.; Tamayo, G.; Cáceres, A.; Cechinel Filho, V.; Giménez, A.; Pinzón, R.; Zacchino, S.A.; et al. Value of the ethnomedical information for the discovery of plants with antifungal properties. A survey among seven Latin American countries. J. Ethnopharmacol. 2010, 127, 137–158. [Google Scholar] [CrossRef]
- Batallán, G.; Torre, R.; Flores, F.; Konigheim, B.; Ludueña-Almeida, F.; Tonn, C.; Contagiani, M.; Almirón, W. Larvicidal activity of crude extracts from Larrea cuneifolia (Zygophyllaceae) and of its metabolite nordihydroguaiaretic acid against the vector Culex quinquefasciatus (Diptera: Culicidae). Rev. Soc. Bras. Med. Trop. 2013, 46, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Carabajal, M.P.A.; Isla, M.I.; Zampini, I.C. Evaluation of antioxidant and antimutagenic activity of herbal teas from native plants used in traditional medicine in Argentina. S. Afr. J. Botany. 2017, 110, 258–265. [Google Scholar] [CrossRef]
- Lorenzo, M.E.; Gómez, P.E.; Sabatino, E.; Segovia, A.F.; Figueroa, L.C.; Baroni, M.V. Phenolic Profile and Antioxidant Activity of Ethanolic Extract of Larrea cuneifolia Cav. Leaves. Proceedings 2020, 70, 37. [Google Scholar] [CrossRef]
- Torres, R.; Urbina, F.; Morales, C.; Modak, B.; Delle Monache, F. Antioxidant properties of lignans and ferulic acid from the resinous exudate of Larrea nitida. J. Chil. Chem. Soc. 2003, 48, 61–63. [Google Scholar] [CrossRef]
- Carabajal, M.P.A.; Piloto-Ferrer, J.; Nicollela, H.D.; Squarisi, I.S.; Guissone, A.P.P.; Esperandim, T.R.; Crispim Tavares, D.; Isla, M.I.; Zampini, I.C. Antigenotoxic, antiproliferative and antimetastatic properties of a combination of native medicinal plants from Argentina. J. Ethnopharm. 2021, 267, 13479. [Google Scholar] [CrossRef]
- Davicino, R.; Alonso, R.; Anesini, C. Activity of a combination of decaffeinated coffee and Larrea divaricata Cav. aqueous extract on hair growth. Pat. Bulletin. 2010, 44, P090101704. [Google Scholar]
- Iannicelli, J.; Guariniello, J.; Pitta Alvarez, S.I.; Escandón, A.S. Traditional uses, conservation estatus and biotechnological advances for a group of aromatic/medicinal native plants from America. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2018, 17, 453–491. [Google Scholar]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of seasonal variation on secondary metabolites of medicinal plants. Int. J. Pharm. Sci. Res. 2015, 6, 3654. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. JARMAP 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, M.S.A.; Ashraf, M.; Hussain, M.; Ashraf, M.Y. Seasonal variation in some medicinal and biochemical ingredients in Mentha longifolia (L.) Huds. Pak J Bot. 2011, 43, 69–77. [Google Scholar]
- Souto, C.P.; Zalazar, L.P.; Tadey, M.; Premoli, A.C. Modeling past, present and future: Species-specific responses to climate changes in three shrub congeners from South American drylands. J. Arid. Environ. 2024, 221, 105139. [Google Scholar] [CrossRef]
- Diaz, S.M.; Mangione, A.M. Paraheliotropismo en hojas de Jarilla Norte-Sur (Larrea cuneifolia Cav.). Métodos Ecol. Sistemática. 2015, 10, 67–75. [Google Scholar]
- Medeiros, J.S.; Begaye, A.; Hanson, D.T.; Logan, B.; Pockman, W.T. Photoprotective response to chilling differs among high and low latitude Larrea divaricata grown in a common garden J. Arid. Environ. 2015, 120, 51–54. [Google Scholar] [CrossRef]
- Available online: https://www.meteoblue.com/es/tiempo/historyclimate/climatemodelled/amaicha-del-valle_argentina_3865969 (accessed on 2 October 2025).
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Singh, R.; Iqbal, N.; Umar, S.; Ahmad, S. Lignan Enhancement: An Updated Review on the Significance of Lignan and Its Improved Production in Crop Plants. Phyton 2024, 93, 3237–3271. [Google Scholar] [CrossRef]
- Zitong, Z.; Manqun, L.; Binhong, Z.; Zhang, F.; Peijin, N.; Zhang, Z.; Sun, D.; Wang, Z.; Shi, G.; Ai, J. Dynamic Changes in Lignan Content and Antioxidant Capacity During the Development of Three Cultivars of Schisandra chinensis Seeds. Horticulturae 2025, 11, 1106. [Google Scholar] [CrossRef]
- Boiteux, J.; Espino, M.; Fernández, M.D.L.Á.; Pizzuolo, P.; Silva, M.F. Control sustentable poscosecha: Extractos de Larrea cuneifolia mediados por nades frente a Botrytis cinerea. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo. 2019, 51, 427–437. [Google Scholar]
- Craigo, J.; Callahan, M.; Huang, R.C.; DeLucia, A.L. Inhibition of human papillomavirus type 16 gene expression by nordihydroguaiaretic acid plant lignan derivatives. Antivir. Res. 2000, 47, 19–28. [Google Scholar] [CrossRef]
- Heller, J.D.; Kuo, J.; Wu, T.C.; Kast, W.M.; Huang, R.C. Tetra O-methyl nordihydroguaiaretic acid induces G2 arrest in mammalian cells and exhibits tumoricidal activity in vivo. Cancer Res. 2001, 61, 5499–5504. [Google Scholar]
- Lambert, J.D.; Dorr, R.; Timmermann, B.N. Nordihydroguaiaretic acid: A review of its numerous and varied biological activities. Pharm. Biol. 2004, 42, 149–158. [Google Scholar] [CrossRef]
- Lambert, J.D.; Meyers, R.O.; Timmermann, B.N.; Dorr, R.T. tetra-O-Methylnordihydroguaiaretic acid inhibits melanoma in vivo. Cancer Lett. 2001, 171, 47–56. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Scribner, K.; Gadbois, T.; Gowri, M.; Azhar, S.; Reaven, G.M. Masoprocol decreases serum triglyceride concentrations in rats with fructose-induced hypertriglyceridemia. Metab. Clin. Exp. 2000, 49, 1106–1110. [Google Scholar] [CrossRef]
- Carrizo, J.; Grau, A. Plantas Silvestres de los Valles Calchaquíes; Guía visual. Universidad Nacional de Tucumán: Tucumán, Argentina, 2014; 208p, ISBN 978-950-554-870-5. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Somogyi, M. A new reagent for the determination of sugars. J. Biol. Chem. 1945, 160, 61–68. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2015; Grupo InfoStat, FCA; Universidad Nacional de Córdoba: Córdoba, Argentina, 2012. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R.; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 20 July 2025).

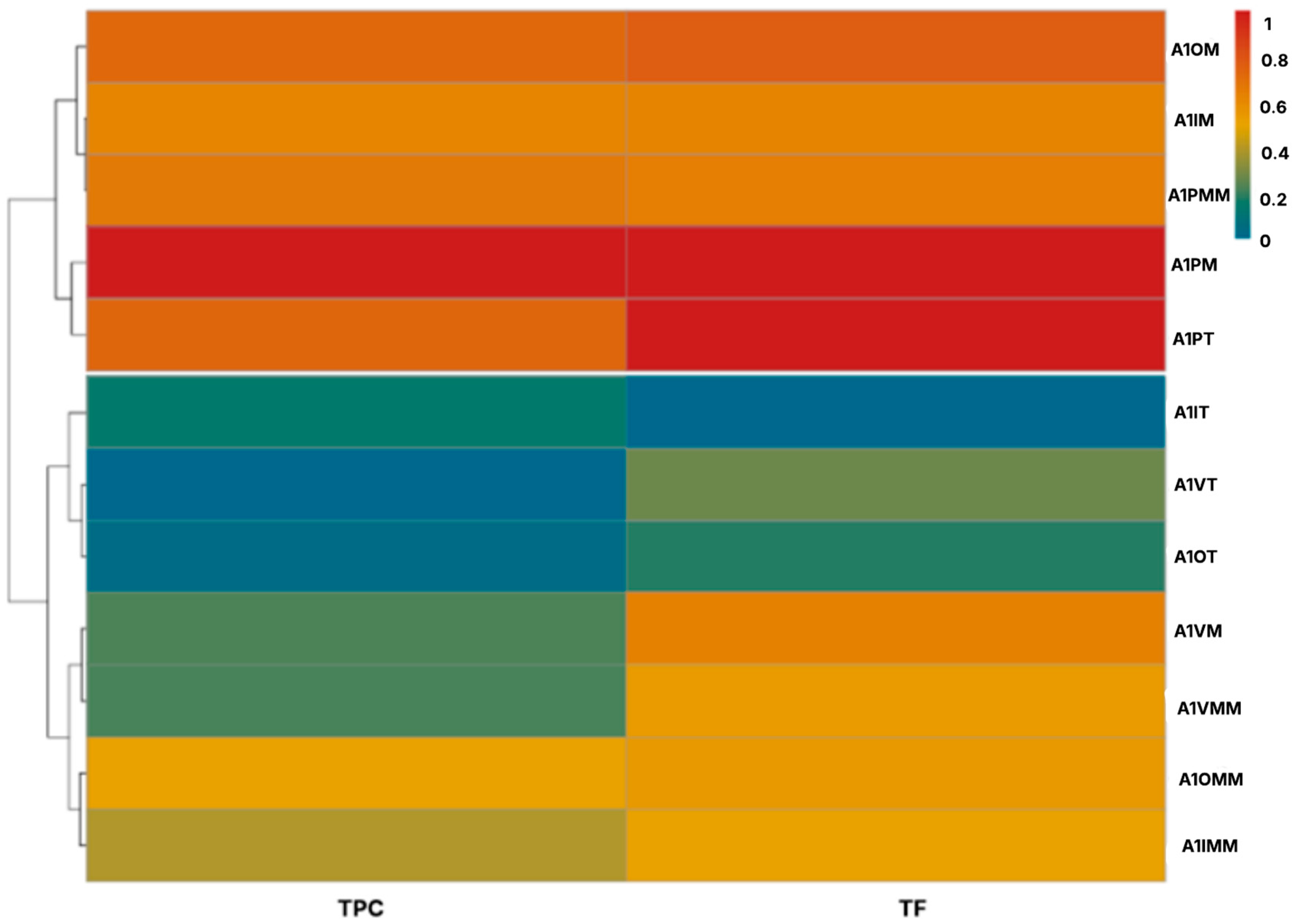
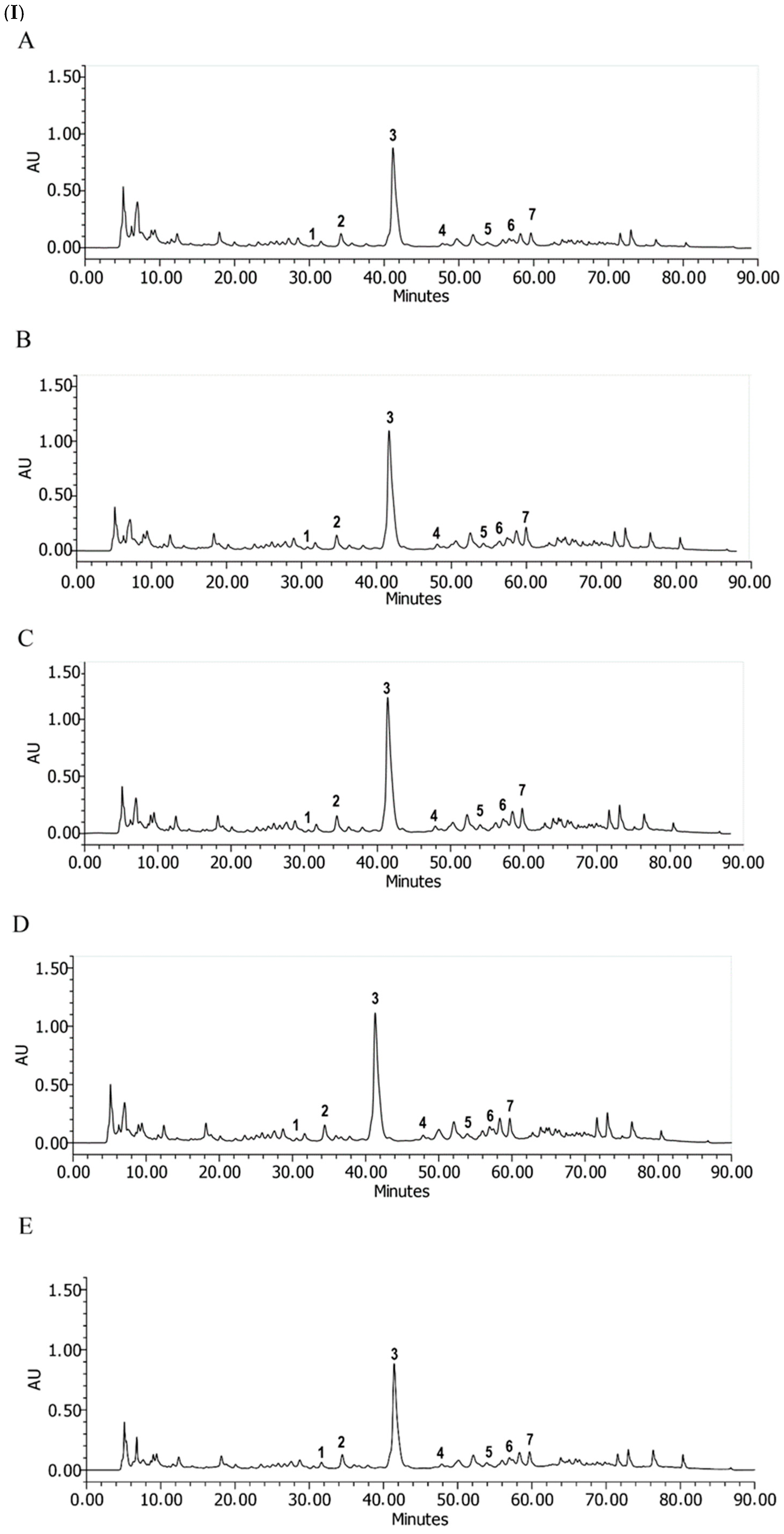
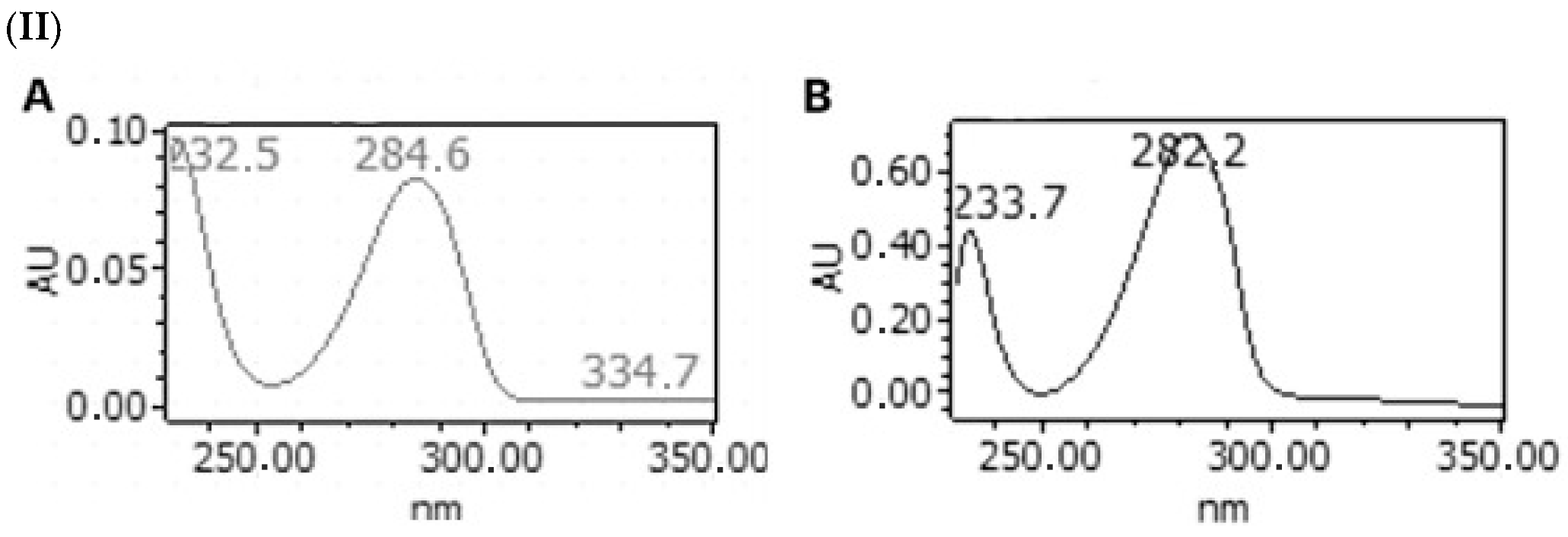
| Total Lignans (µg NDGAE/g DW) | ||||
|---|---|---|---|---|
| Summer | Autumn | Winter | Spring | |
| Morning | 518.5 ± 5.78 Ba | 496.60 ± 3.93 Ab | 511.24 ± 3.53 Bb | 517.84 ± 7.06 Ba |
| Midday | 558.7 ± 6.12 Cb | 533.24 ± 2.30 Bc | 519.73 ± 4.63 Ab | 564.36 ± 6.11 Cb |
| Afternoon | 517.42 ± 2.61 Ca | 282.59 ± 5.79 Aa | 384.89 ± 4.81 Ba | 581.84 ± 3.12 Db |
| Compounds | Autumn | Spring | Winter | ||||
|---|---|---|---|---|---|---|---|
| Morning | Morning | Midday | Afternoon | Morning | |||
| RT. (Minutes) | A | B | C | D | E | ||
| µg of Lignans in NDGA Equivalent/mg of Soluble Principle (Dry Weight of Extract) | |||||||
| 1 | 3,3′,4′-trihydroxy-4 methoxy 7,7′-epoxylignan | 31.5 | 10.2 ± 4.7 | 10.1 ± 4.8 | 9.9 ± 1.6 | 11.8 ± 7.9 | 8.5 ± 5.5 |
| 2 | 3,4,3′,4′-tetrahydroxy-6,7′ cyclolignan | 34.2 | 21.2 ± 3.6 | 73.0 ± 0.2 | 20.8 ± 0.0 | 24.6 ± 2.0 | 19.5 ± 5.2 |
| 3 | Nordihydroguaiaretic acid | 41.19 | 182.1 ± 2.9 | 273.4 ± 2.5 | 291.8 ± 2.8 | 253.6 ± 3.4 | 187.6 ± 3.1 |
| 4 | 3,3′-dihydroxy-4-methoxy 7,7′- epoxylignan | 47.7 | 4.2 ± 0.3 | 8.6 ± 0.3 | 9.3 ± 2.5 | 8.8 ± 4.8 | 6.5 ± 0.5 |
| 5 | 4-[4-(4-hydroxy-phenyl)-2,3 dimethyl-butyl]-benzene-1,2-diol | 53.7 | 10.1 ± 1.4 | 40.6 ± 4.2 | 14.6 ± 1.7 | 13.6 ± 1.3 | 8.3 ± 5.2 |
| 6 | Methyl nordihydroguaiaretic acid | 55.8 | 12.8 ± 3.6 | 14.8 ± 3.9 | 18.2 ± 5.1 | 17.5 ± 1.1 | 10.8 ± 0.5 |
| 7 | Methyl nordihydroguaiaretic acid isomer | 59.6 | 20.5 ± 0.5 | 28.4 ± 5.2 | 33.4 ± 1.3 | 30.9 ± 3.0 | 21.3 ± 3.7 |
| SC50 (µg GAE/mL) | ||||
|---|---|---|---|---|
| Summer | Autumn | Winter | Spring | |
| Morning | 2.323 ± 0.012 Ba | 3.838 ± 0.122 Db | 3.227 ± 0.011 Cb | 1.560 ± 0.021 Aa |
| Midday | 2.250 ± 0.009 Ba | 3.434 ± 0.009 Da | 3.071 ± 0.023 Ca | 1.647 ± 0.155 Aa |
| Afternoon | 2.299 ± 0.092 Aa | 4.977 ± 0.043 Cc | 3.644 ± 0.072 Bc | 2.116 ± 0.185 Ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera, M.C.; Rosa, M.D.; Zampini, I.C.; Isla, M.I. Impact of Daily and Seasonal Variation on the Phytochemical Profile of Larrea cuneifolia in Northwestern Argentina. Plants 2025, 14, 3332. https://doi.org/10.3390/plants14213332
Barrera MC, Rosa MD, Zampini IC, Isla MI. Impact of Daily and Seasonal Variation on the Phytochemical Profile of Larrea cuneifolia in Northwestern Argentina. Plants. 2025; 14(21):3332. https://doi.org/10.3390/plants14213332
Chicago/Turabian StyleBarrera, María Celeste, Mariana Daniela Rosa, Iris Catiana Zampini, and María Inés Isla. 2025. "Impact of Daily and Seasonal Variation on the Phytochemical Profile of Larrea cuneifolia in Northwestern Argentina" Plants 14, no. 21: 3332. https://doi.org/10.3390/plants14213332
APA StyleBarrera, M. C., Rosa, M. D., Zampini, I. C., & Isla, M. I. (2025). Impact of Daily and Seasonal Variation on the Phytochemical Profile of Larrea cuneifolia in Northwestern Argentina. Plants, 14(21), 3332. https://doi.org/10.3390/plants14213332







