Effects and Mechanisms of Attapulgite Clay-g-(AA-co-AAm) Hydrogel (ACH) in Alleviating Saline Stress in Spinach
Abstract
1. Introduction
2. Results
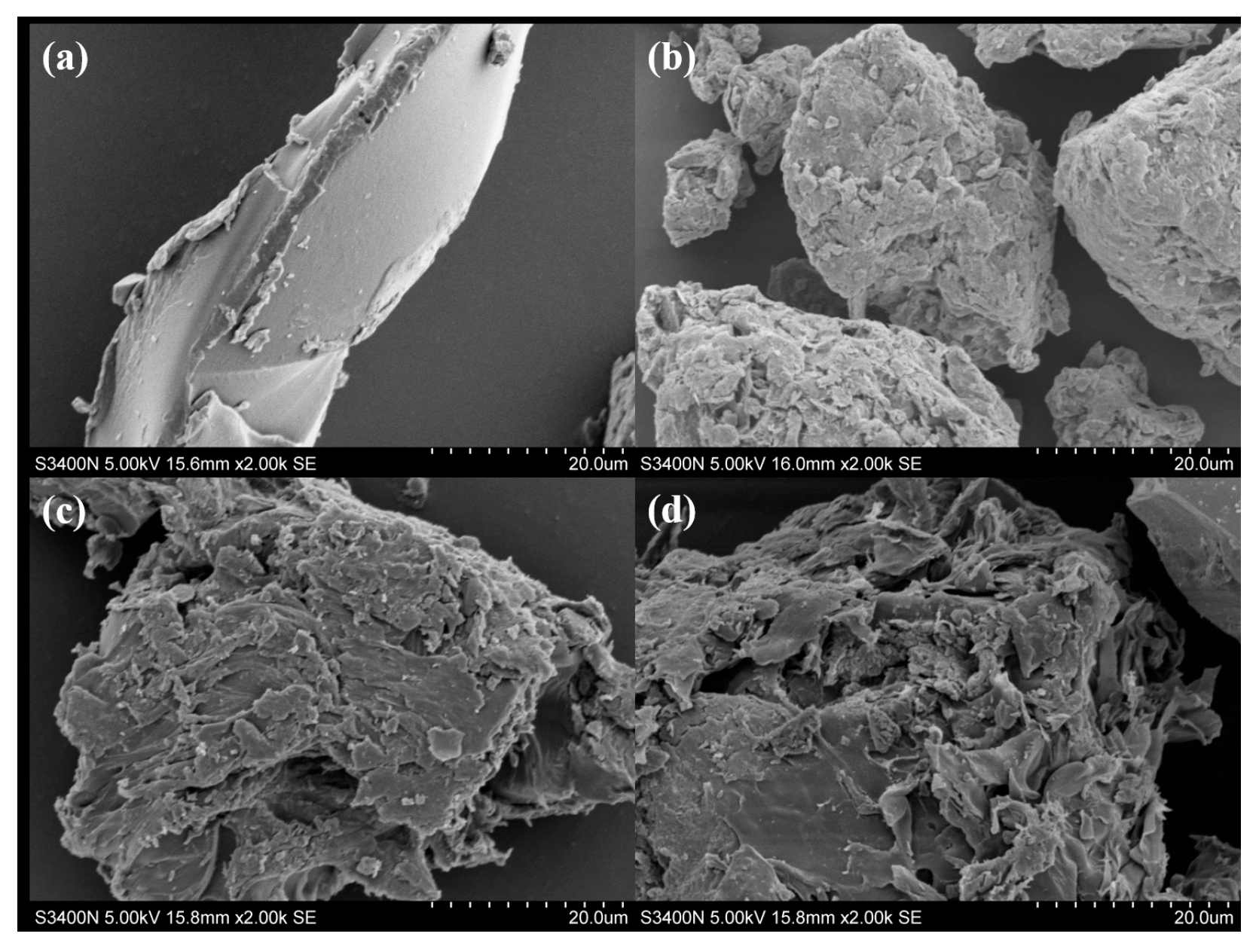
2.1. Scanning Electron Microscopy (SEM)
2.2. ACH Surface Functional Groups
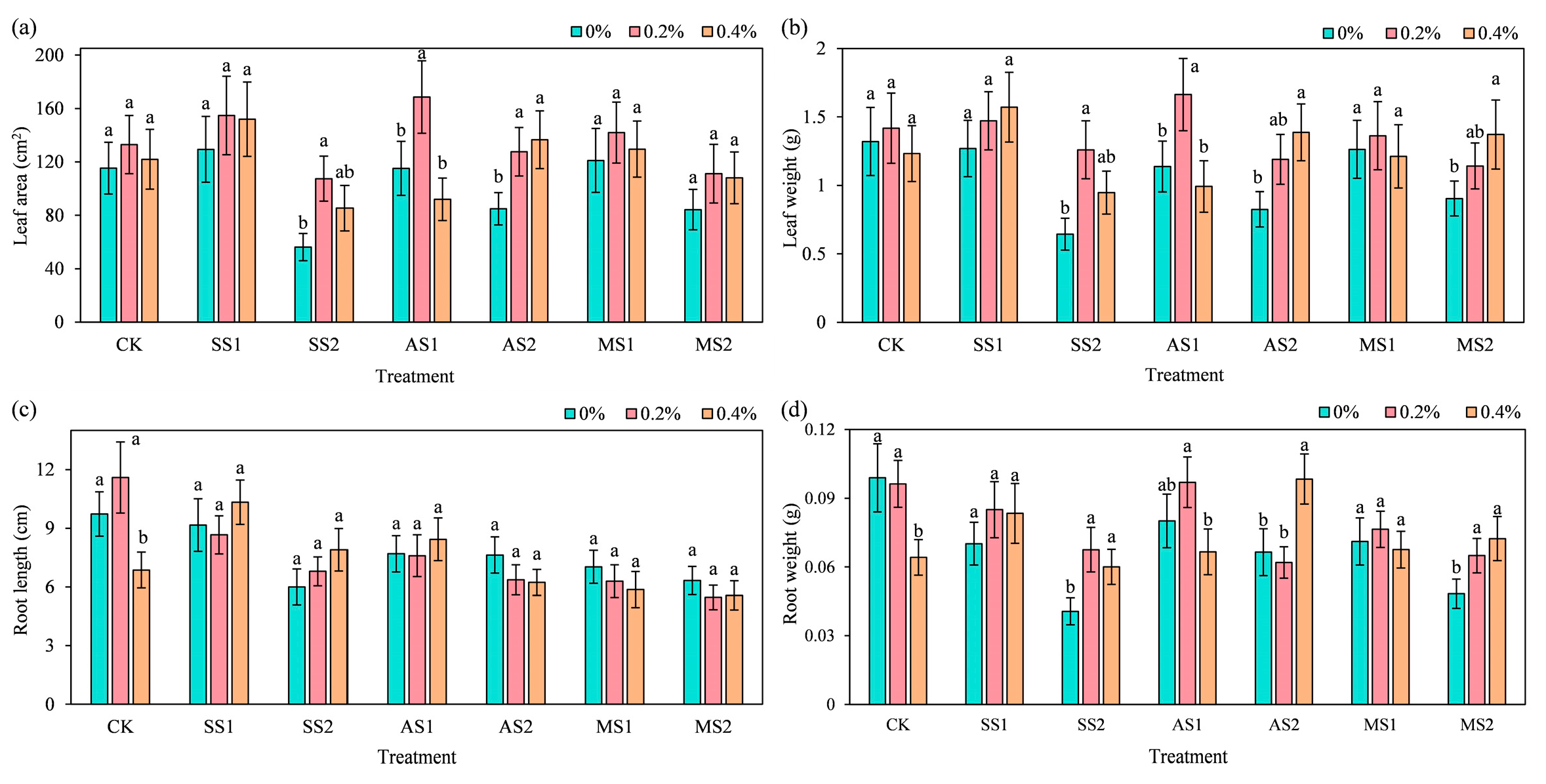
2.3. Effect of ACH Addition on Growth Indices of Spinach
2.3.1. Leaf Parameters
2.3.2. Root Parameters
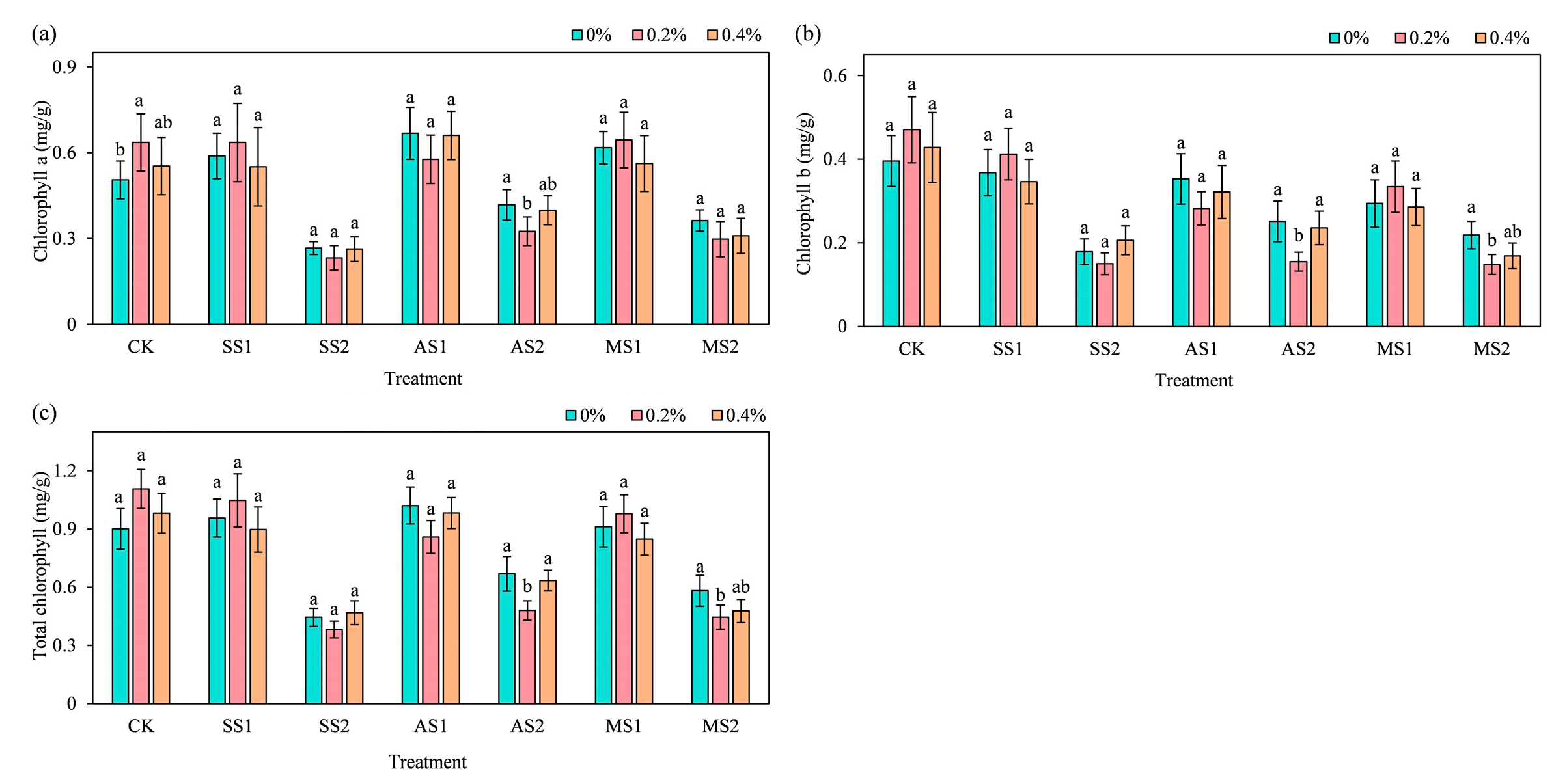
2.4. Effect of ACH Addition on Chlorophyll Content of Spinach
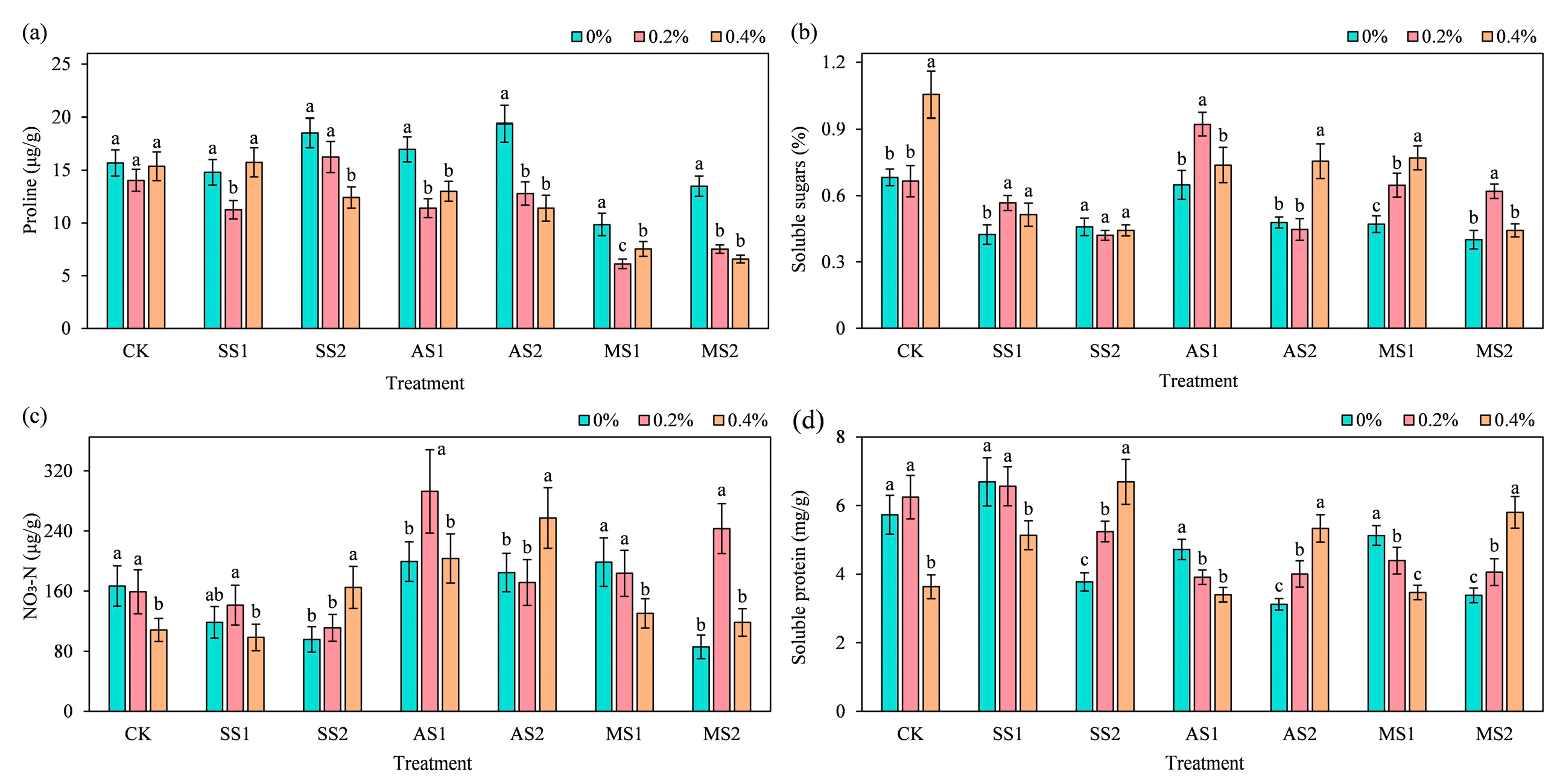
2.5. Effect of ACH Addition on the Osmoregulatory Substances in Spinach
2.5.1. Proline
2.5.2. Soluble Sugars
2.5.3. Nitrate Nitrogen (NO3−-N)
2.5.4. Soluble Protein
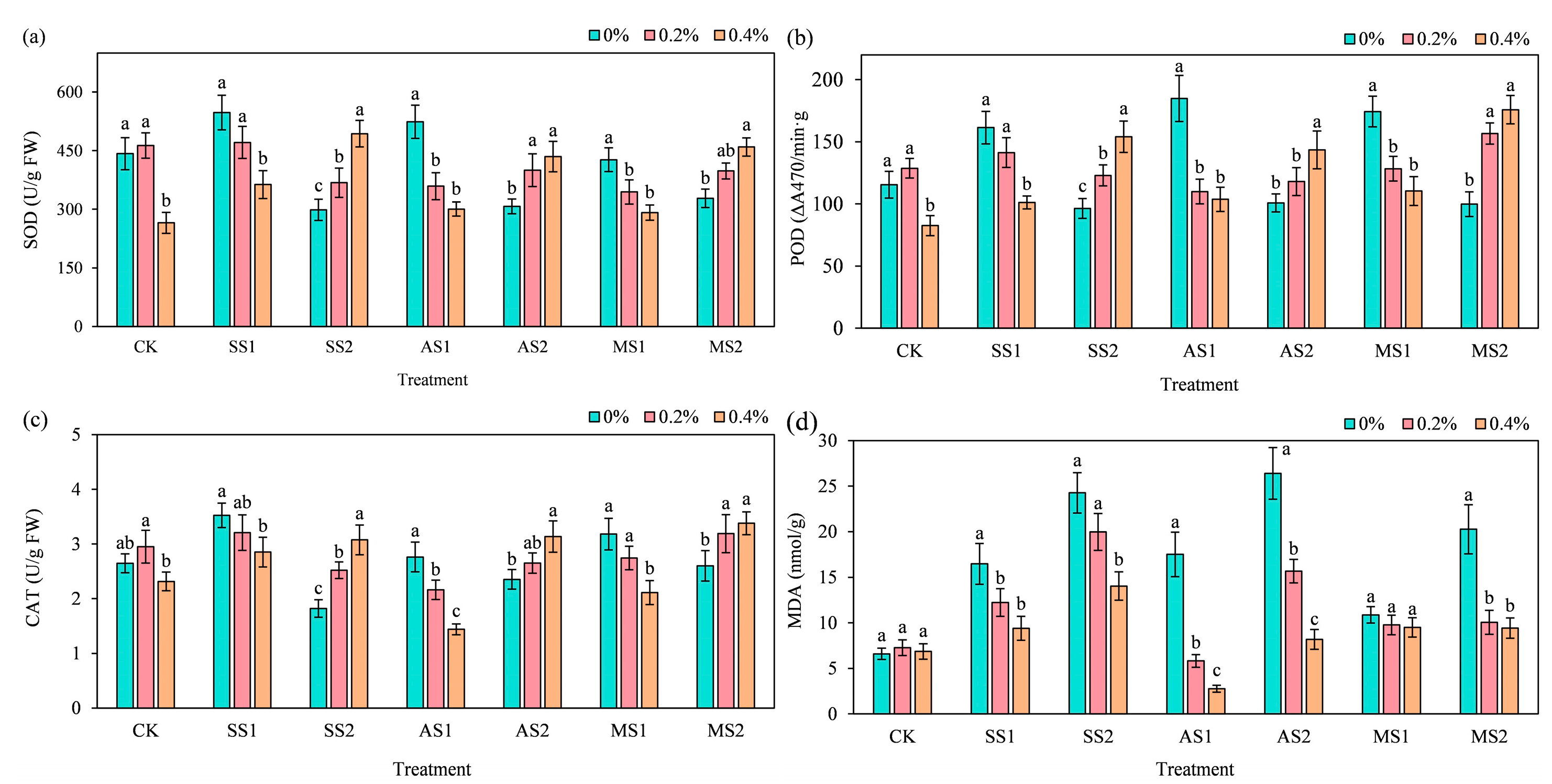
2.6. Effect of ACH Addition on Antioxidant Enzymes and Membrane Damage Indices in Spinach
2.6.1. Superoxide Dismutase
2.6.2. Peroxidase
2.6.3. Catalase
2.6.4. Malondialdehyde
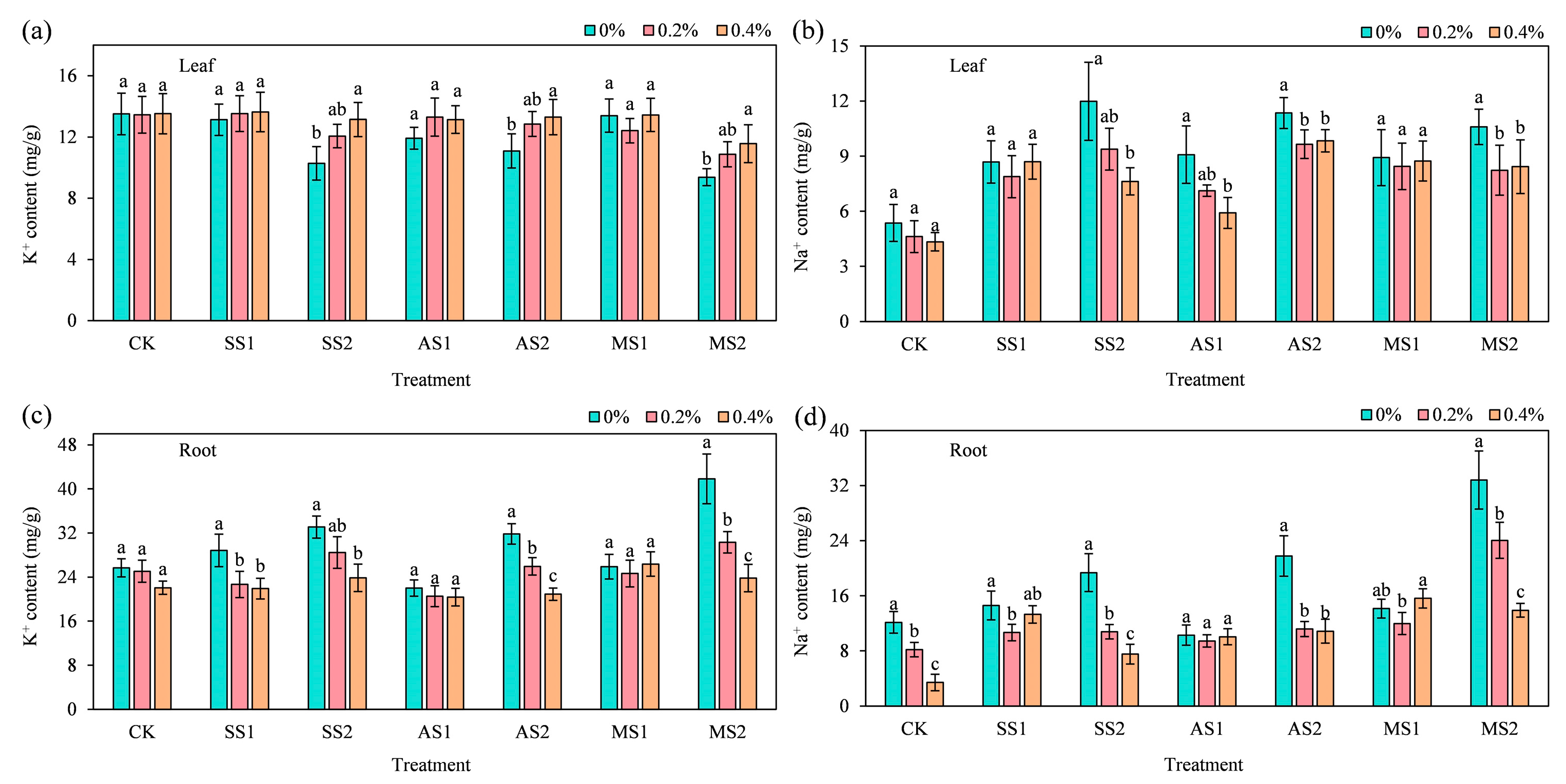
2.7. Effect of ACH on the Distribution of K+ and Na+ in Spinach
2.7.1. K+ and Na+ Content in Spinach Leaves
2.7.2. K+ and Na+ Content in Spinach Roots
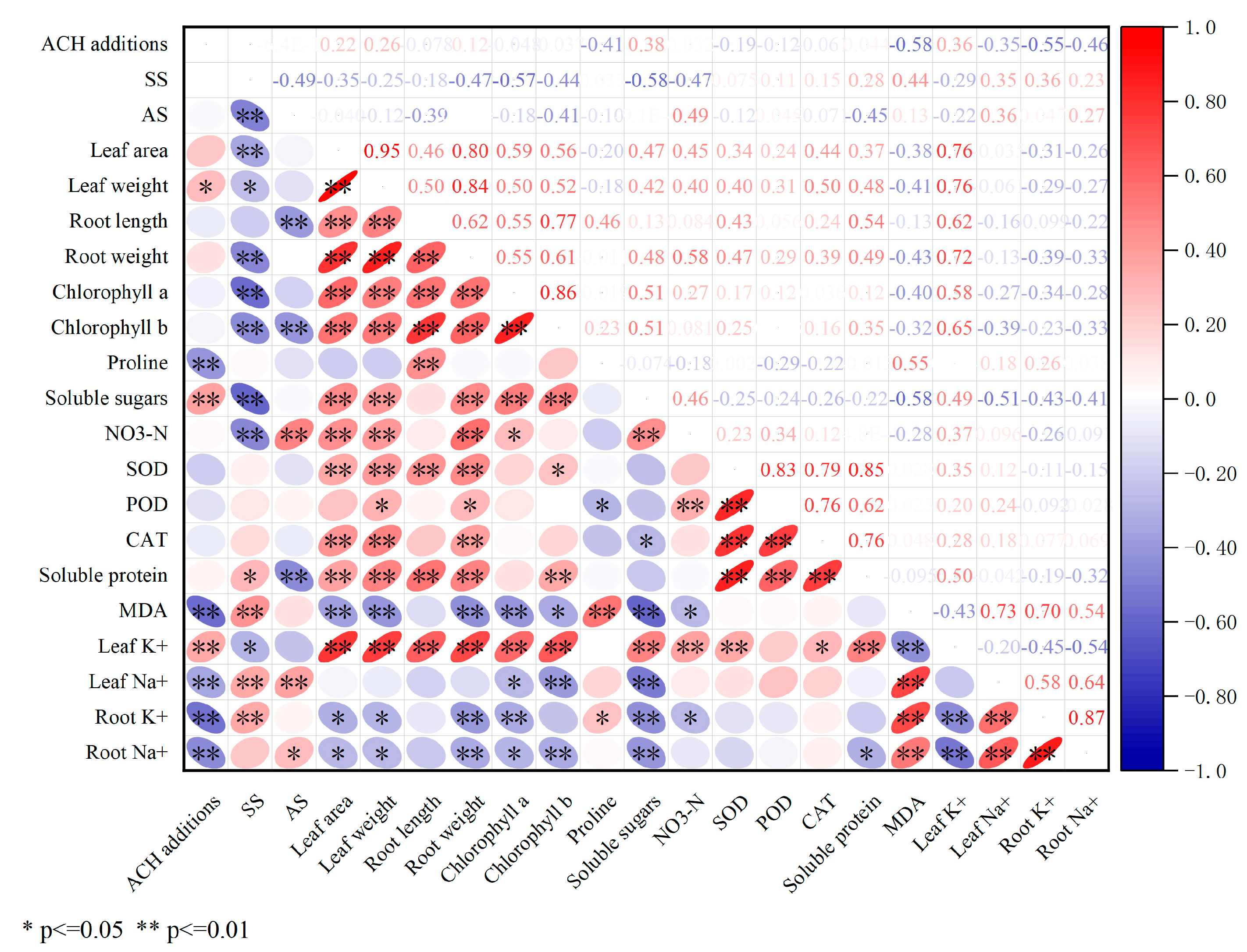
2.8. Correlation Analysis
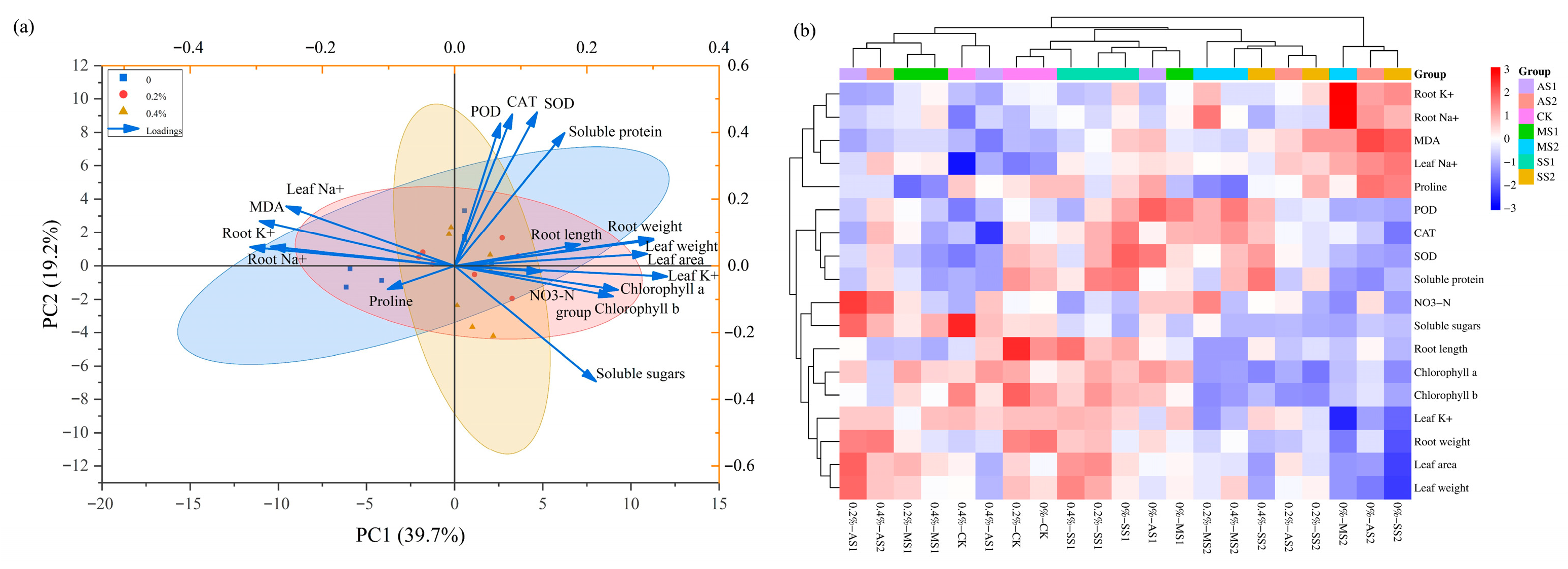
2.9. Principal Component Analysis (PCA) and Cluster Analysis
2.10. Cluster Analysis
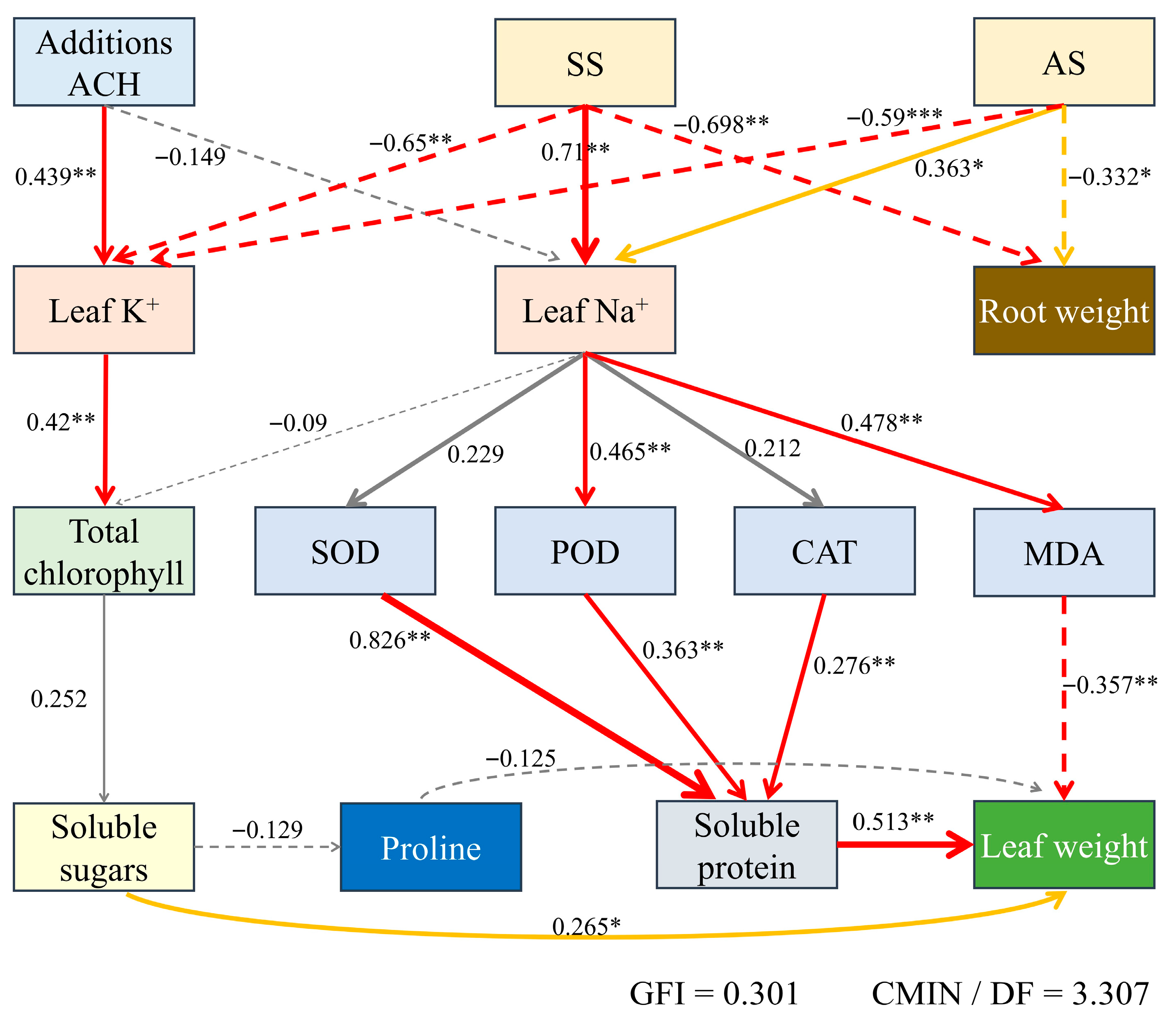
2.11. Path Analysis
3. Discussion
3.1. Surface Characteristics and Properties of ACH
3.2. Mechanisms of ACH Effect on Physiological Growth Indices of Spinach Tolerant to Salinity Stress
3.3. The Regulatory Mechanism of ACH on Na+ and K+ in Spinach
4. Materials and Methods
4.1. Experimental Material
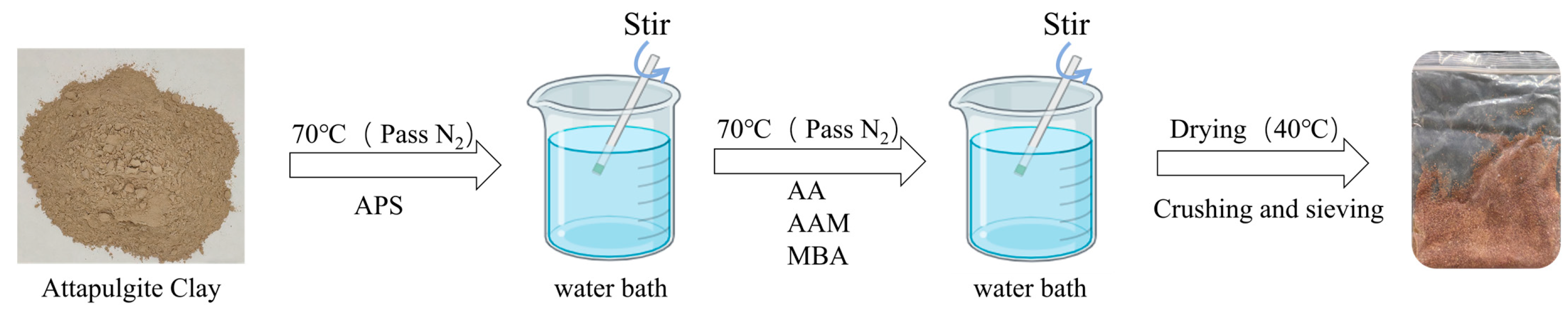
4.2. Preparation of Attapulgite Clay-P (AA-co-AAm) Hydrogels (ACHs)
4.3. ACH Characterization Tests
4.4. Testing of Salinity Tolerance of Spinach by ACH Addition
4.5. Measurement of Growth Indices
4.5.1. Biomass Determination
4.5.2. Leaf Area Measurement (Paper Weight Method)
4.6. Measurement of Physiological Indices
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACH | Attapulgite Clay-g-(AA-co-AAm) Hydrogel |
| MDA | Malondialdehyde |
| SOD | Superoxide Dismutase |
| POD | Peroxidase |
| CAT | Catalase |
References
- Cuevas, J.; Daliakopoulos, I.N.; del Moral, F.; Hueso, J.J.; Tsanis, I.K. A review of soil-improving cropping systems for soil salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef]
- Huang, J.; Shang, Y.; Chen, Y.; Xu, L.; Yang, Y.; Zhao, X. Analysis of Research Trends and Comprehensive Utilization Solutions for Saline–Alkali Land. Sustainability 2025, 17, 5202. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid. Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Zhang, Z.; Abuduwaili, J.; Yimit, H. The occurrence, sources and spatial characteristics of soil salt and assessment of soil salinization risk in Yanqi Basin, Northwest China. PLoS ONE 2014, 9, e106079. [Google Scholar]
- Nassaj-Bokharaei, S.; Motesharezedeh, B.; Etesami, H.; Motamedi, E. Effect of hydrogel composite reinforced with natural char nanoparticles on improvement of soil biological properties and the growth of water deficit-stressed tomato plant. Ecotoxicol. Environ. Saf. 2021, 223, 112576. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H.; Hou, C.; Guan, H.; Gu, S.; Yin, Y.; Jing, H.; Wang, Y.; Wang, M. Preparation of an attapulgite-modified composite hydrogel and application in an environmentally responsive green fertilizer. ACS Appl. Polym. Mater. 2023, 5, 10217–10225. [Google Scholar] [CrossRef]
- Cai, Z.; Zhan, F.; Wang, Y.; Wu, M.; Kong, L.; Wang, A.; Huang, Z. Study on Adsorption Characteristics and Water Retention Properties of Attapulgite–Sodium Polyacrylate and Polyacrylamide to Trace Metal Cadmium Ion. Polymers 2024, 16, 1756. [Google Scholar] [CrossRef]
- Li, H.; Gao, D. Characterisation of poly (acrylamide)/montmorillonite nanocomposite synthesised by polymerisation induced by UV radiation. Pigment. Resin Technol. 2011, 40, 79–83. [Google Scholar]
- Chen, S.; Zommorodi, M.; Fritz, E.; Wang, S.; Hüttermann, A. Hydrogel modified uptake of salt ions and calcium in Populus euphratica under saline conditions. Trees 2004, 18, 175–183. [Google Scholar]
- Kant, C.; Aydin, A.; Turan, M. Ameliorative effect of hydro gel substrate on growth, inorganic ions, proline, and nitrate contents of bean under salinity stress. J. Plant Nutr. 2008, 31, 1420–1439. [Google Scholar] [CrossRef]
- Hu, B.; Ouyang, Y.; Zhao, T.; Wang, Z.; Yan, Q.; Qian, Q.; Wang, W.; Wang, S. Antioxidant hydrogels: Antioxidant mechanisms, design strategies, and applications in the treatment of oxidative stress-related diseases. Adv. Healthc. Mater. 2024, 13, 2303817. [Google Scholar] [CrossRef]
- Pathak, V.; Ambrose, R.K. Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage. J. Appl. Polym. Sci. 2020, 137, 48523. [Google Scholar] [CrossRef]
- Zha, X.; Hou, X.; Li, Q.; Nan, H.; Ge, F.; Liu, Y.; Li, F.; Zhang, D.; Tian, J. Loading glyphosate in attapulgite and sodium alginate hydrogels to construct pH-responsive controlled release microsphere for enhanced soil sustained release. ACS Agric. Sci. 2022, 2, 1090–1100. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Xie, W.; Cheng, T.; Chen, C.; Sun, C.; Qi, L.; Zhang, Z. Optimizing and modeling Cu (II) removal from simulated wastewater using attapulgite modified with Keggin ions with the aid of RSM, BP-ANN, and GA-BP. Desalination Water Treat. 2020, 207, 270–286. [Google Scholar] [CrossRef]
- Archibong, F.N.; Orakwe, L.C.; Ogah, O.A.; Mbam, S.O.; Ajah, S.A.; Okechukwu, M.E.; Igberi, C.O.; Okafor, K.J.; Chima, M.O.; Ikelle, I.I. Emerging progress in montmorillonite rubber/polymer nanocomposites: A review. J. Mater. Sci. 2023, 58, 2396–2429. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K.; Kato, C. Preparation Of Montmorillonitepolyacrylamide Intercalation Compounds and the Water Absorbing Property. Clay Sci. 1989, 7, 243–251. [Google Scholar]
- Ruiz-Fresneda, M.A.; González-Morales, E.; Gila-Vilchez, C.; Leon-Cecilla, A.; Merroun, M.L.; Medina-Castillo, A.L.; Lopez-Lopez, M.T. Clay–polymer hybrid hydrogels in the vanguard of technological innovations for bioremediation, metal biorecovery, and diverse applications. Mater. Horiz. 2024, 11, 5533–5549. [Google Scholar] [CrossRef]
- Wang, J.; Ren, S.; Guo, M. Preparation and humidity controlling behaviors of sepiolite/polyacrylic acid (sodium) composite. Procedia Eng. 2012, 27, 423–430. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Khan, T.K.; Abd El-Aziz, G.H.; Shoala, T.; El-Garhy, H.A.; Fahmy, A.H. Implementation of biopolymeric nanomaterials to reduce the negative impacts of salinity on tomato quantity and quality. Molecules 2023, 28, 1594. [Google Scholar] [CrossRef]
- Errahali, S.; Chtouki, M.; Qetrani, S.; Oukarroum, A.; Latifi, M.; Belachemi, L.; Benyoucef, H.; Kaddami, H. Effects of Superabsorbent Hydrogel on Soil Porosity, Bulk Density, and Water Productivity of Tomato Grown Under Drought Stress in Clay Loam and Sandy Loam Soils. J. Soil Sci. Plant Nutr. 2025, 25, 6474–6493. [Google Scholar] [CrossRef]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective strategies for reclamation of saline-alkali soil and response mechanisms of the soil-plant system. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Wang, Y.; Li, J.; Xue, H.J. Effects of saline-alkali stress on the functional traits and physiological characteristics of Leymus chinensis leaves. Grassl. Sci. 2022, 68, 336–342. [Google Scholar] [CrossRef]
- Rychter, P.; Rogacz, D.; Lewicka, K.; Kollár, J.; Kawalec, M.; Mosnáček, J. Ecotoxicological Properties of Tulipalin A-Based Superabsorbents versus Conventional Superabsorbent Hydrogels. Adv. Polym. Technol. 2019, 2019, 2947152. [Google Scholar] [CrossRef]
- Li, H.; Tan, Z. Preparation of high water-retaining biochar and its mechanism of alleviating drought stress in the soil and plant system. Biochar 2021, 3, 579–590. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, J.; Song, W.; Zhao, D.; Kang, M.; Shen, H.; Li, Z. Insights into the surface oxygen functional group-driven fast and stable sodium adsorption on carbon. ACS Appl. Mater. Interfaces 2020, 12, 6991–7000. [Google Scholar] [CrossRef]
- Womack, N.; Cabrera, M.; Franklin, D.; Piccoli, I.; Camarotto, C.; Maggini, M.; Guerrini, G.; Morari, F. Hydrogel Effects on Soil Nitrogen Transformations in pH-Contrasting Soils. Commun. Soil Sci. Plant Anal. 2024, 55, 2883–2893. [Google Scholar] [CrossRef]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.A.R.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Sayed, H.E.; Kirkwood, R.; Graham, N. The effects of a hydrogel polymer on the growth of certain horticultural crops under saline conditions. J. Exp. Bot. 1991, 42, 891–899. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Ren, H.; Wang, Z.; OuYang, Y.; Wang, S.; Hussain, J.; Zeb, I.; Kong, Y.; Liu, S.; et al. Identification of potassium transport proteins in algae and determination of their role under salt and saline-alkaline stress. Algal Res. 2023, 69, 102923. [Google Scholar] [CrossRef]
- Rains, D.; Epstein, E. Sodium absorption by barley roots: Role of the dual mechanisms of alkali cation transport. Plant Physiol. 1967, 42, 314–318. [Google Scholar] [CrossRef]
- Wang, X.; An, M.; Wang, K.; Fan, H.; Shi, J.; Chen, K. Effects of organic polymer compound material on K+ and Na+ distribution and physiological characteristics of cotton under saline and alkaline stresses. Front. Plant Sci. 2021, 12, 636536. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Tang, Y.; Cui, X.; Hou, D.; Jia, H.; Wang, S.; Guo, L.; Wang, J.; Lin, A. Mitigating soil salinity stress with titanium gypsum and biochar composite materials: Improvement effects and mechanism. Chemosphere 2023, 321, 138127. [Google Scholar] [CrossRef]
- Malakar, P.; Chattopadhyay, D. Adaptation of plants to salt stress: The role of the ion transporters. J. Plant Biochem. Biotechnol. 2021, 30, 668–683. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and transport of sodium and potassium in plants. In The Alkali Metal ions: Their Role for Life; Springer: Berlin/Heidelberg, Germany, 2016; pp. 291–324. [Google Scholar]
- Song, C.-P.; Guo, Y.; Qiu, Q.; Lambert, G.; Galbraith, D.W.; Jagendorf, A.; Zhu, J.-K. A probable Na+ (K+)/H+ exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 10211–10216. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in agriculture: Prospects and challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Huang, J.; Gotoh, T.; Nakai, S.; Ueda, A. A Novel Composite Hydrogel Material for Sodium Removal and Potassium Provision. Polymers 2023, 15, 3568. [Google Scholar] [CrossRef]


| Treatment | ACH Addition | NaCl Addition | NaHCO3 Addition | Treatment | ACH Addition | NaCl Addition | NaHCO3 Addition |
|---|---|---|---|---|---|---|---|
| 0%-CK | 0% | 0% | - | - | - | - | |
| 0.2%-CK | 0.2% | 0% | - | - | - | ||
| 0.4%-CK | 0.4% | - | - | - | |||
| 0%-SS1 | 0% | 0.2% | 0%-SS2 | 0% | 0% | ||
| 0.2%-SS1 | 0.2% | 0% | 0.2%-SS2 | 0.2% | 0.4% | ||
| 0.4%-SS1 | 0.4% | 0.4%-SS2 | 0.4% | ||||
| 0%-AS1 | 0% | 0%-AS2 | 0% | 0.4% | |||
| 0.2%-AS1 | 0.2% | 0% | 0.2% | 0.2%-AS2 | 0.2% | 0% | |
| 0.4%-AS1 | 0.4% | 0.4%-AS2 | 0.4% | ||||
| 0%-MS1 | 0% | 0%-MS2 | 0% | ||||
| 0.2%-MS1 | 0.2% | 0.1% | 0.1% | 0.2%-MS2 | 0.2% | 0.2% | 0.2% |
| 0.4%-MS1 | 0.4% | 0.4%-MS2 | 0.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Teng, B.; Zhang, H.; Zhou, Z.; Xin, Y.; Cai, L.; Wu, J. Effects and Mechanisms of Attapulgite Clay-g-(AA-co-AAm) Hydrogel (ACH) in Alleviating Saline Stress in Spinach. Plants 2025, 14, 3330. https://doi.org/10.3390/plants14213330
Wang Y, Teng B, Zhang H, Zhou Z, Xin Y, Cai L, Wu J. Effects and Mechanisms of Attapulgite Clay-g-(AA-co-AAm) Hydrogel (ACH) in Alleviating Saline Stress in Spinach. Plants. 2025; 14(21):3330. https://doi.org/10.3390/plants14213330
Chicago/Turabian StyleWang, Yinhua, Bingqin Teng, Haodong Zhang, Zhengqian Zhou, Yangbin Xin, Liqun Cai, and Jun Wu. 2025. "Effects and Mechanisms of Attapulgite Clay-g-(AA-co-AAm) Hydrogel (ACH) in Alleviating Saline Stress in Spinach" Plants 14, no. 21: 3330. https://doi.org/10.3390/plants14213330
APA StyleWang, Y., Teng, B., Zhang, H., Zhou, Z., Xin, Y., Cai, L., & Wu, J. (2025). Effects and Mechanisms of Attapulgite Clay-g-(AA-co-AAm) Hydrogel (ACH) in Alleviating Saline Stress in Spinach. Plants, 14(21), 3330. https://doi.org/10.3390/plants14213330






