WRKY61 Negatively Regulates Aluminum Resistance by Inhibiting the Expression of ALMT1 in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
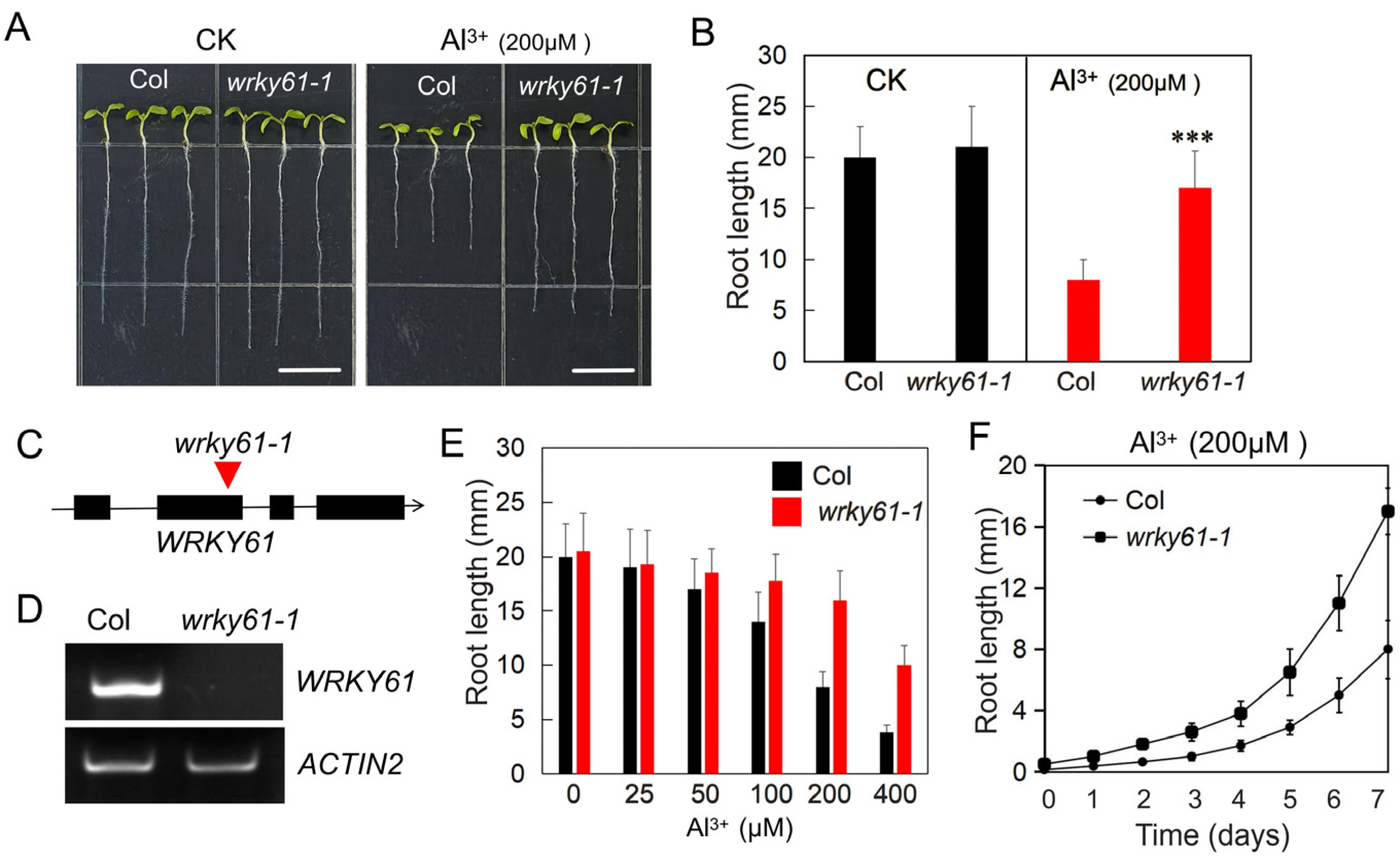
2.1. Wrky61-1 Mutant Shows Increased Resistance to Aluminum Toxicity
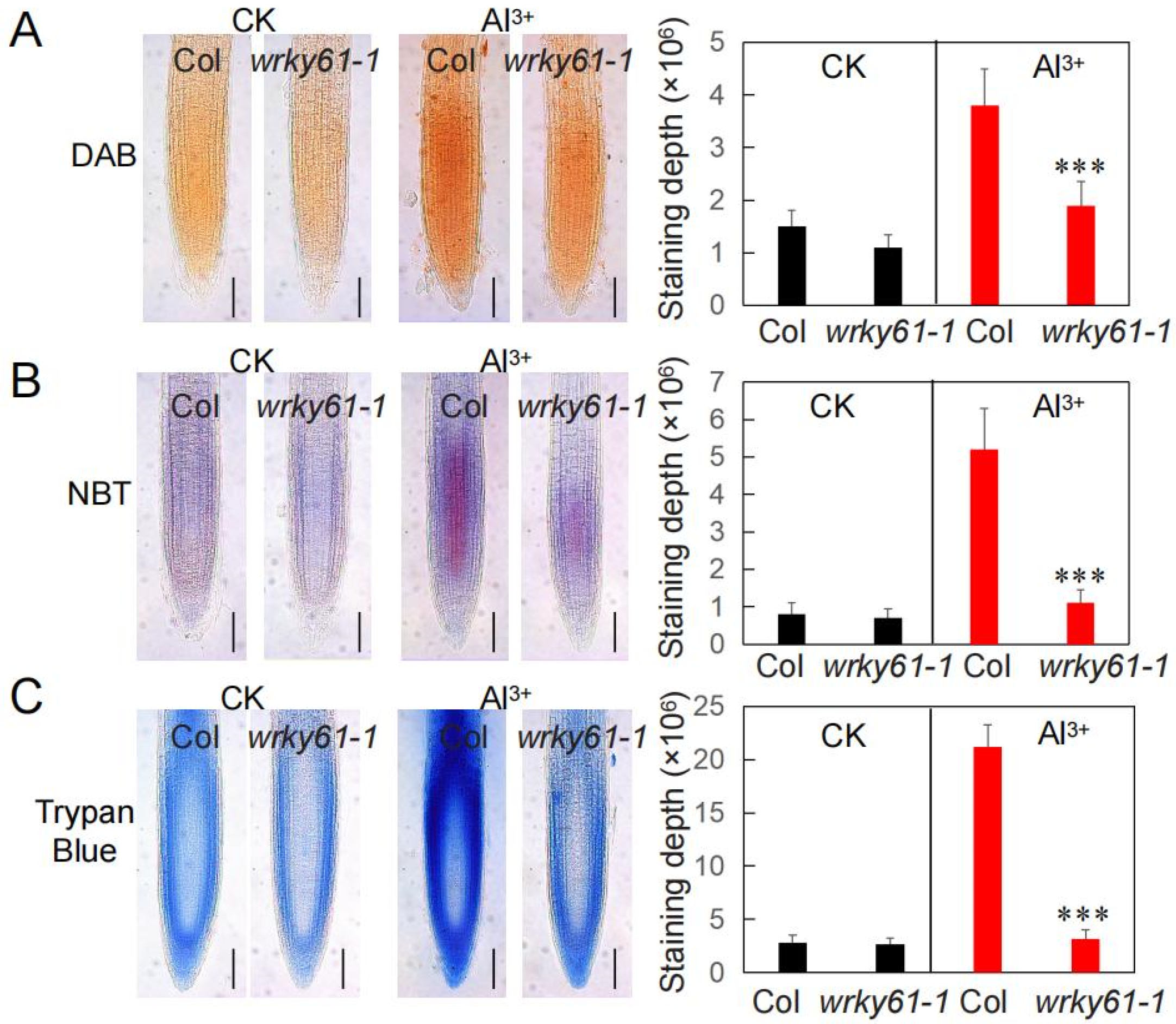
2.2. Reduced ROS Accumulation Under Aluminum Toxicity in Wrky61-1 Mutant
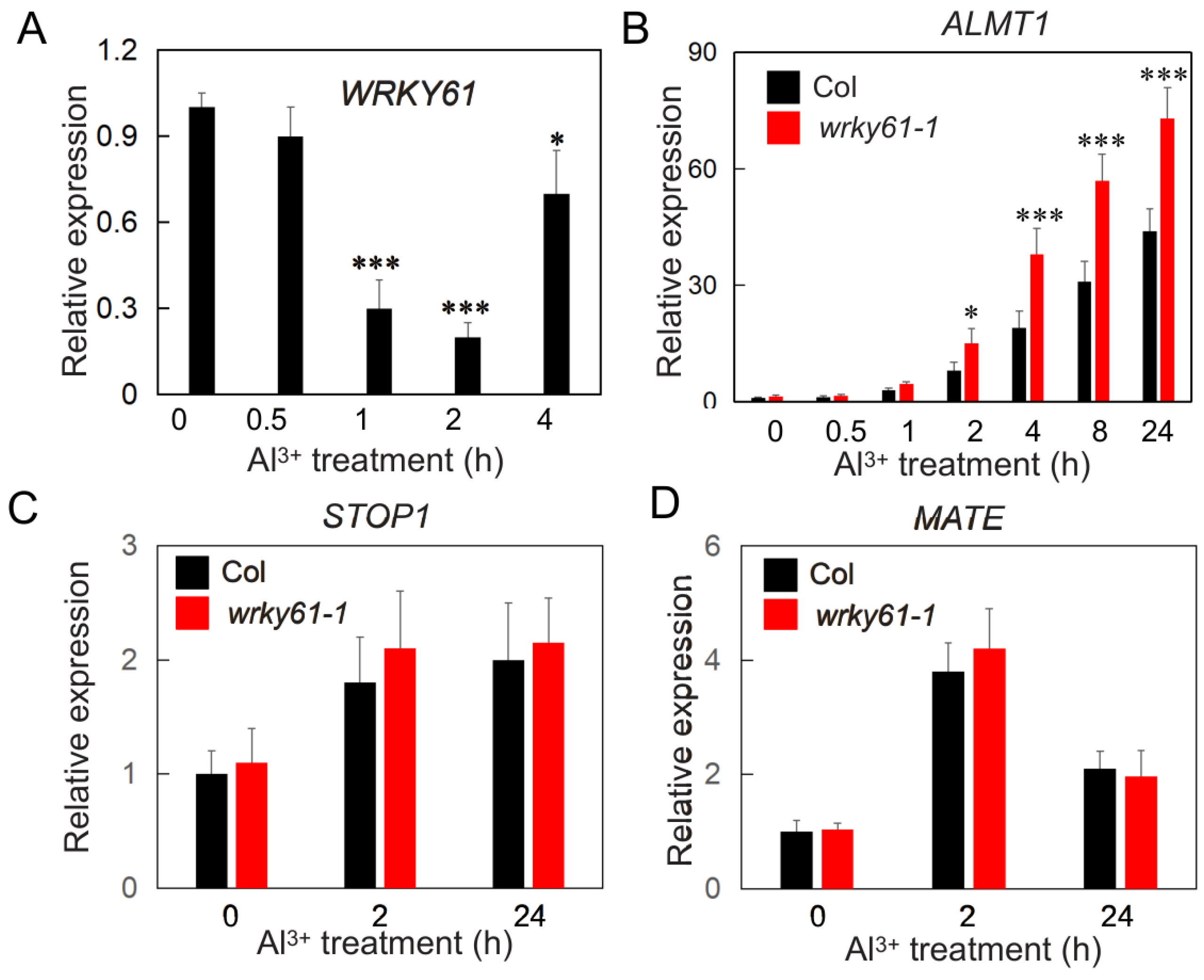
2.3. WRKY61 Negatively Regulates the Expression of ALMT1
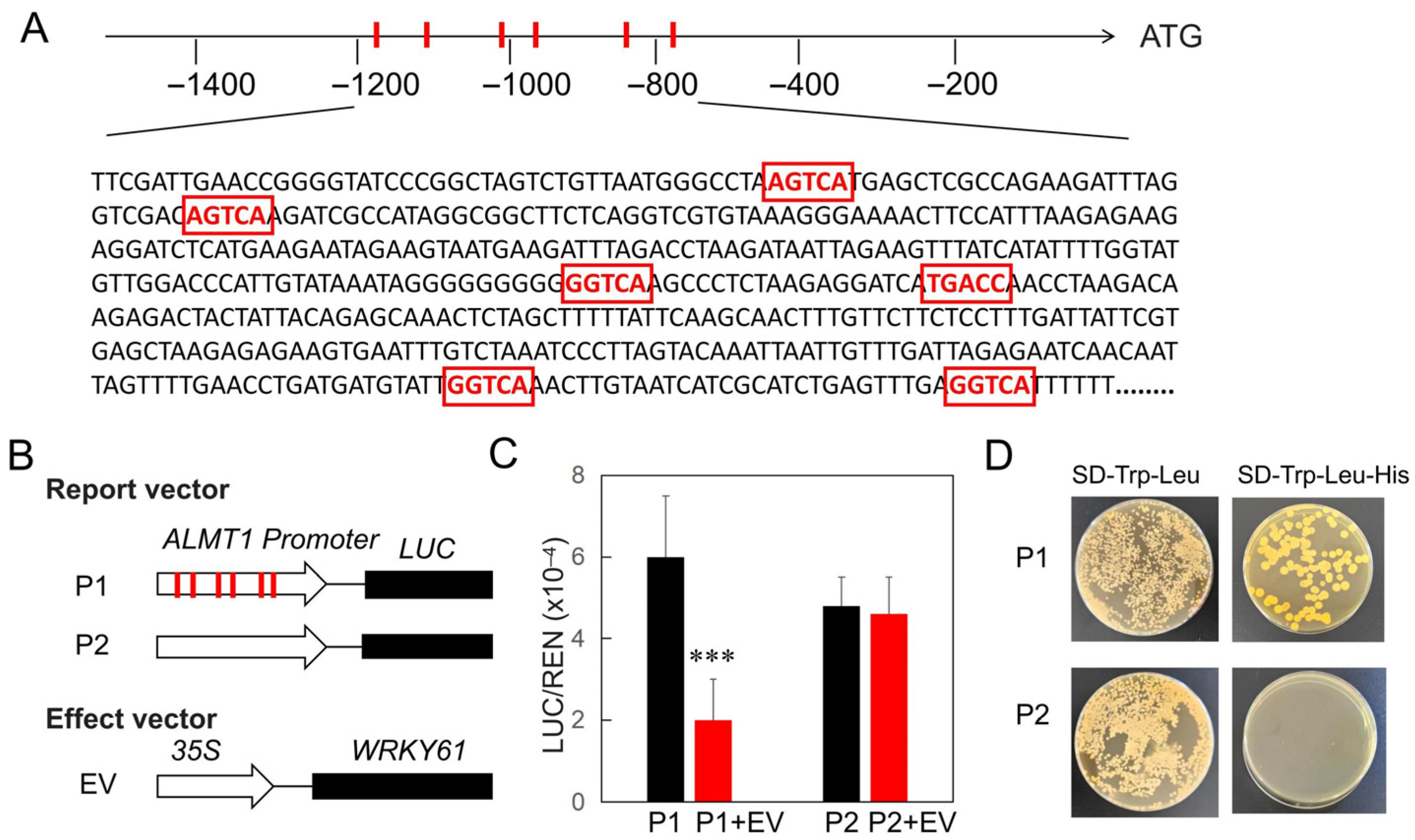
2.4. WRKY61 Binds to the W-Box in the ALMT1 Promoter
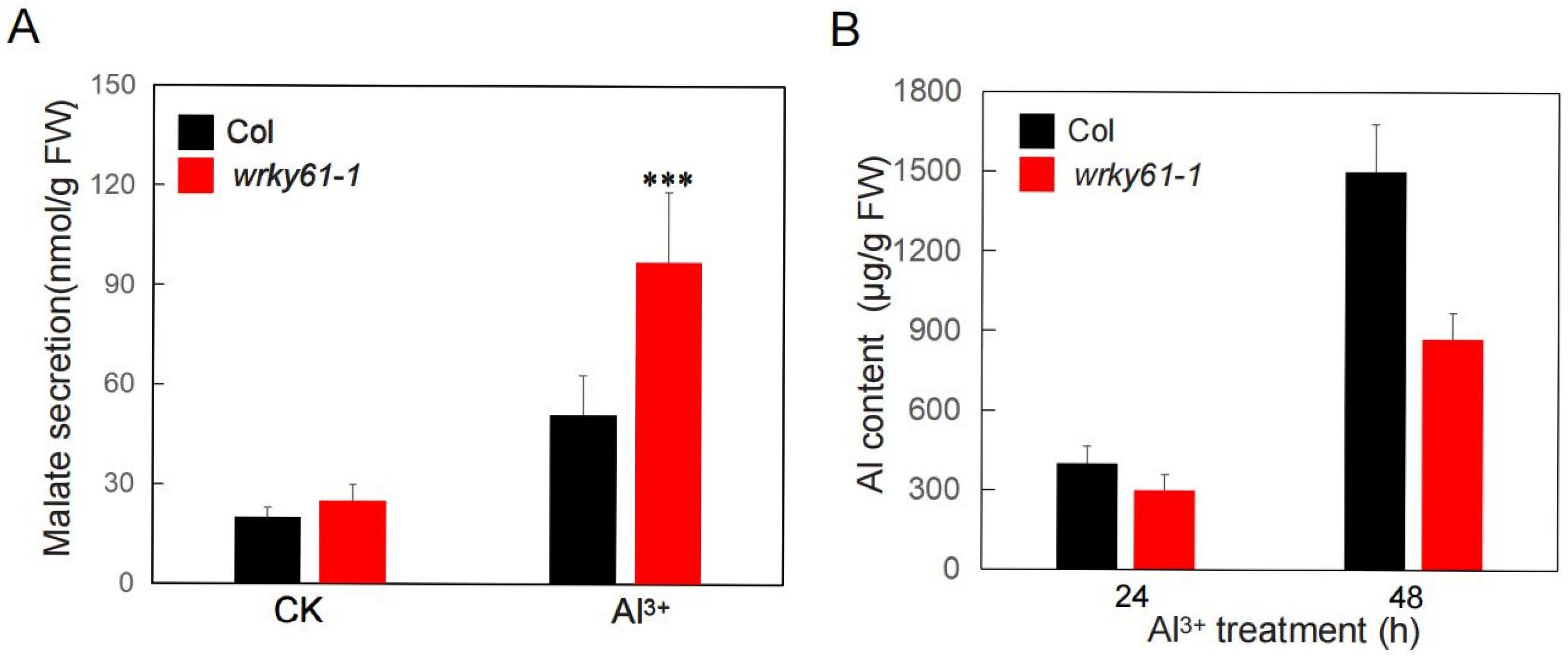
2.5. WRKY61 Suppresses Malate Secretion
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Histochemical Staining and Cytological Observation
4.3. Gene Expression Analysis
4.4. Quantification of Malate Secretion by HPLC-MS
4.5. Dual-Luciferase Assay
4.6. Yeast One-Hybrid Assay
4.7. Determination of Al Content in Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WRKY61 | WRKY DNA-BINDING PROTEIN 61 |
| ALMT1 | ALUMINUM-ACTIVATED MALATE TRANSPORTER 1 |
| STOP1 | SENSITIVE TO PROTON RHIZOTOXICITY 1 |
| MATE | MULTI-DRUG AND TOXIC COMPOUND EXTRUSION |
| RAE1 | REGULATION OF ATALMT1 EXPRESSION 1 |
| CAMTA2 | CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR 2 |
| CML24 | CALMODULIN-LIKE 24 |
References
- von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571. [Google Scholar] [CrossRef]
- Ryan, P.R. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Chen, Z.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Li, D.; Jia, X.; Zhou, D.; Li, J.; Lyi, S.M.; Hou, S.; Huang, Y.; Kochian, L.V.; et al. NIP1;2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 5047–5052. [Google Scholar] [CrossRef]
- Fan, N.; Li, X.; Xie, W.; Wei, X.; Fang, Q.; Xu, J.; Huang, C.F. Modulation of external and internal aluminum resistance by ALS3-dependent STAR1-mediated promotion of STOP1 degradation. New Phytol. 2024, 244, 17. [Google Scholar] [CrossRef]
- Hoekenga, O.A. Identification and characterization of aluminum tolerance loci in Arabidopsis (Landsberg erecta x Columbia) by quantitative trait locus mapping. A physiologically simple but genetically complex trait. Plant Physiol. 2003, 132, 936–948. [Google Scholar] [CrossRef]
- Liu, J.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Kochian, L.V. Aluminum Inhibition of the Inositol 1,4,5-Trisphosphate Signal Transduction Pathway in Wheat Roots: A Role in Aluminum Toxicity? Plant Cell 1995, 7, 1913–1922. [Google Scholar] [CrossRef]
- Liu, J.; Tian, H.; Zhang, M.; Sun, Y.; Wang, J.; Yu, Q.; Ding, Z. STOP1 attenuates the auxin response to maintain root stem cell niche identity. Cell Rep. 2024, 43, 113617. [Google Scholar] [CrossRef]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, J.; Zhang, Y.; Fan, N.; van den Burg, H.A.; Huang, C.F. Regulation of Aluminum Resistance in Arabidopsis Involves the SUMOylation of the Zinc Finger Transcription Factor STOP1. Plant Cell 2020, 32, 3921–3938. [Google Scholar] [CrossRef]
- Zhou, F.; Singh, S.; Zhang, J.; Fang, Q.; Li, C.; Wang, J.; Zhao, C.; Wang, P.; Huang, C.F. The MEKK1-MKK1/2-MPK4 cascade phosphorylates and stabilizes STOP1 to confer aluminum resistance in Arabidopsis. Mol. Plant 2023, 16, 337–353. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, Y.; Xie, W.; Ren, W.; Zhang, Y.; Zhang, H.; Dai, S.; Huang, C.F. H2O2 negatively regulates aluminum resistance via oxidation and degradation of the transcription factor STOP1. Plant Cell 2024, 36, 688–708. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Cui, R.; Guo, H.; Xia, Q.; Zhang, J.; Liu, W.; Yang, Z.B. Ca(2+)-dependent cytoplasmic and nuclear phosphorylation of STOP1 by CPK21 and CPK23 confers ALMT1-dependent aluminum resistance. Nat. Commun. 2025, 16, 5225. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, H.; Schmitz-Thom, I.; Wang, J.; Zhou, F.; Li, C.; Zhang, Y.; Cheng, Y.; Miki, D.; Kudla, J.; et al. CPK28-mediated Ca(2+) signaling regulates STOP1 localization and accumulation to facilitate plant aluminum resistance. Nat. Commun. 2025, 16, 5224. [Google Scholar] [CrossRef]
- Ding, Z.J.; Yan, J.Y.; Xu, X.Y.; Li, G.X.; Zheng, S.J. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013, 76, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, P.; Bai, Z.; Herde, M.; Ma, Y.; Li, N.; Liu, S.; Huang, C.F.; Cui, R.; Ma, H.; et al. Calmodulin-like protein CML24 interacts with CAMTA2 and WRKY46 to regulate ALMT1-dependent Al resistance in Arabidopsis thaliana. New Phytol. 2022, 233, 2471–2487. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef]
- Yang, L.; Fang, S.; Liu, L.; Zhao, L.; Chen, W.; Li, X.; Xu, Z.; Chen, S.; Wang, H.; Yu, D. WRKY transcription factors: Hubs for regulating plant growth and stress responses. J. Integr. Plant Biol. 2025, 67, 488–509. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Viana, V.E.; Busanello, C.; da Maia, L.C.; Pegoraro, C.; Costa de Oliveira, A. Activation of rice WRKY transcription factors: An army of stress fighting soldiers? Curr. Opin. Plant Biol. 2018, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Xu, Z.; Chen, M.; Yu, D. Functions of WRKYs in plant growth and development. Trends Plant Sci. 2023, 28, 630–645. [Google Scholar] [CrossRef]
- Gao, R.; Liu, P.; Yong, Y.; Wong, S.M. Genome-wide transcriptomic analysis reveals correlation between higher WRKY61 expression and reduced symptom severity in Turnip crinkle virus infected Arabidopsis thaliana. Sci. Rep. 2016, 6, 24604. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef]
- Hoekenga, O.A.; Maron, L.G.; Piñeros, M.A.; Cançado, G.M.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef]
- Ligaba, A.; Katsuhara, M.; Ryan, P.R.; Shibasaka, M.; Matsumoto, H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006, 142, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, R.; Qi, M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000, 22, 543–551. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, A.; Li, J.; Liu, S.; Du, Z.; Zeng, J.; Xiao, Y.; Feng, G. WRKY61 Negatively Regulates Aluminum Resistance by Inhibiting the Expression of ALMT1 in Arabidopsis thaliana. Plants 2025, 14, 3286. https://doi.org/10.3390/plants14213286
Ma A, Li J, Liu S, Du Z, Zeng J, Xiao Y, Feng G. WRKY61 Negatively Regulates Aluminum Resistance by Inhibiting the Expression of ALMT1 in Arabidopsis thaliana. Plants. 2025; 14(21):3286. https://doi.org/10.3390/plants14213286
Chicago/Turabian StyleMa, Aolin, Jie Li, Siqi Liu, Zhixuan Du, Jianjun Zeng, Yonghong Xiao, and Guanping Feng. 2025. "WRKY61 Negatively Regulates Aluminum Resistance by Inhibiting the Expression of ALMT1 in Arabidopsis thaliana" Plants 14, no. 21: 3286. https://doi.org/10.3390/plants14213286
APA StyleMa, A., Li, J., Liu, S., Du, Z., Zeng, J., Xiao, Y., & Feng, G. (2025). WRKY61 Negatively Regulates Aluminum Resistance by Inhibiting the Expression of ALMT1 in Arabidopsis thaliana. Plants, 14(21), 3286. https://doi.org/10.3390/plants14213286




