Diversity, Pattern, and Environmental Drivers of Climbing Plants in China
Abstract
1. Introduction
2. Results
2.1. Floristic Composition of Climbing Plants in China
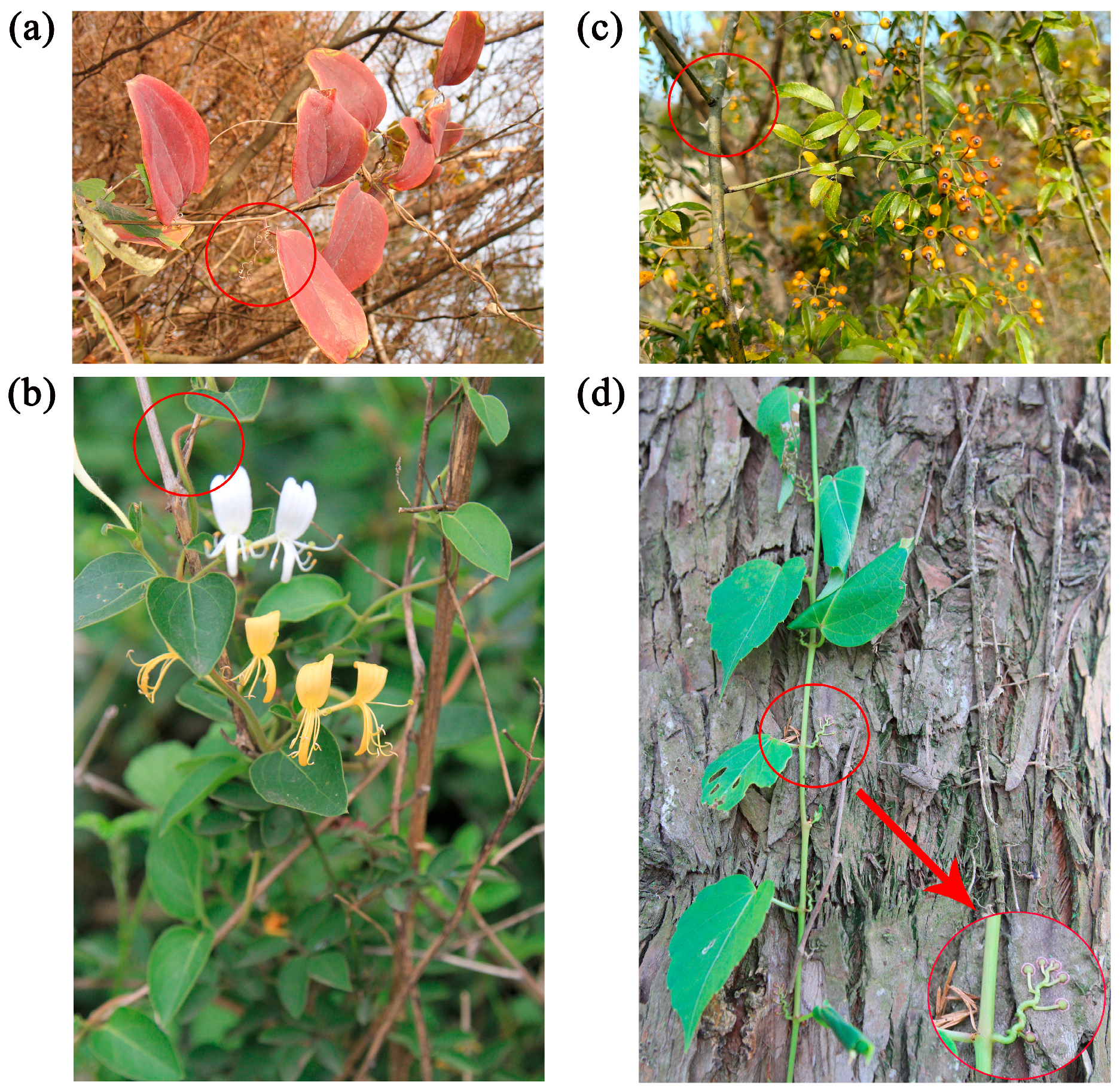
2.2. Life Forms and Climbing Methods of Climbing Plants in China
2.3. Spatial Distribution Patterns of Climbing Plant Diversity at the Family and Genus Levels in China
2.4. Spatial Distribution Patterns of Climbing Plant Species Richness at the Provincial Scale in China
2.5. Correlations Among the Entire, Endemic, Threatened, and Invasive Climbing Plant Species Density in China
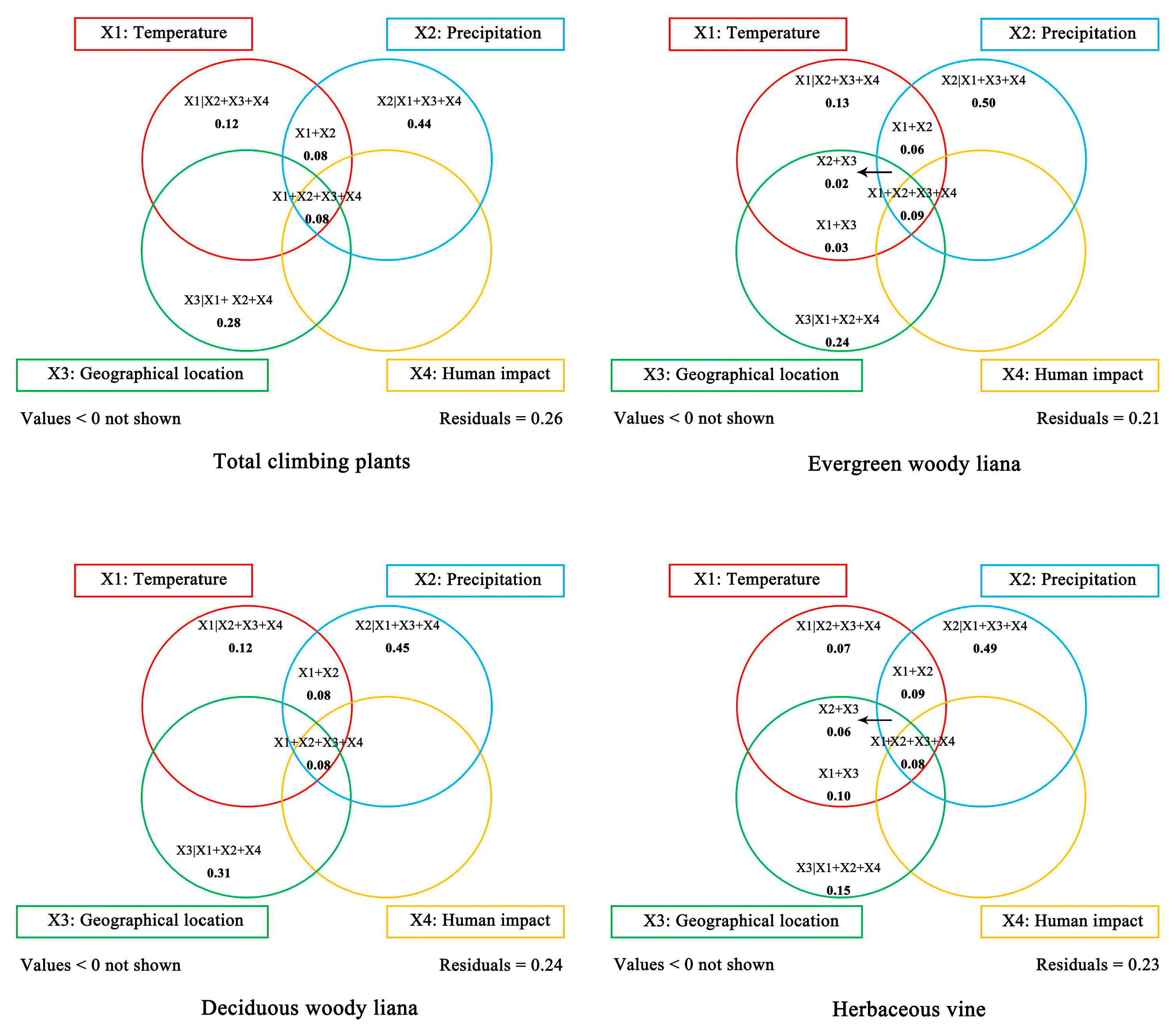
2.6. Determinative Environmental Factors Influencing the Density of Climbing Plants in China
3. Discussion
3.1. Diversity Characteristics of Climbing Plants in China
3.2. Spatial Distribution Pattern of Climbers in China
3.3. Key Environmental Factors Influencing the Distribution Pattern of Climbers in China
3.4. Conservation Implications for Climbing Plants in China
4. Materials and Methods
4.1. Study Area
4.2. Data Collection and Organization of Climbing Plant Species
4.3. Environmental Variable Data
4.4. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gentry, A.H. The distribution and evolution of climbing plants. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 3–52. [Google Scholar]
- Isnard, S.; Silk, W.K. Moving with Climbing Plants from Charles Darwin’s Time into the 21st Century. Am. J. Bot. 2009, 96, 1205–1221. [Google Scholar] [CrossRef]
- Chen, P.H.; Chung, A.C.; Lin, H.C.; Yang, S.Z. Climbing Strategies of Taiwan Climbers. Bot. Stud. 2023, 64, 26. [Google Scholar] [CrossRef]
- Darwin, C. The Movements and Habits of Climbing Plants; John Murray: London, UK, 1875. [Google Scholar]
- Gianoli, E. Evolutionary implications of the climbing habit in plants. In Ecology of Lianas; Schnitzer, S.A., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; Wiley Blackwell: Oxford, UK, 2015; pp. 239–250. [Google Scholar]
- Burris, J.N.; Lenaghan, S.C.; Stewart, C.N., Jr. Climbing Plants: Attachment Adaptations and Bioinspired Innovations. Plant Cell Rep. 2018, 37, 565–574. [Google Scholar] [CrossRef]
- Ceballos, S.J.; Aráoz, E.; Rojas, T.N. Exploring Co-Occurrence Patterns to Understand Epiphyte–Liana Interactions. Plants 2025, 14, 140. [Google Scholar] [CrossRef]
- Buckton, G.; Cheesman, A.W.; Munksgaard, N.C.; Wurster, C.M.; Liddell, M.J.; Cernusak, L.A. Functional Traits of Lianas in an Australian Lowland Rainforest align with Post-Disturbance Rather Than Dry Season Advantage. Austral Ecol. 2019, 44, 983–994. [Google Scholar] [CrossRef]
- Ngute, A.S.; Schoeman, D.S.; Pfeifer, M.; van der Heijden, G.M.; Phillips, O.L.; van Breugel, M.; Campbell, M.J.; Chandler, C.J.; Enquist, B.J.; Gallagher, R.V.; et al. Global Dominance of Lianas over Trees is Driven by Forest Disturbance, Climate and Topography. Glob. Change Biol. 2024, 30, e17140. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Vargas, B.; Grombone-Guaratini, M.T.; Morellato, L.P. Lianas Research in the Neotropics: Overview, Interaction with Trees, and Future Perspectives. Trees 2021, 35, 333–345. [Google Scholar] [CrossRef]
- Liu, M.C.; Zhang, J.N.; Zuo, Y.J.; Yang, Z.R.; Jin, X.B.; Pan, B.R.; Chang, Q.; Suo, Z.L. A Novel Method for Molecular Identification of Genetic Diversity of Plant Resources in Cucurbitaceae Based on Taxon-Specific Variable Nucleotide Characters from Complete Chloroplast Genomes. Bot. Res. 2024, 13, 289–314. [Google Scholar] [CrossRef]
- Delgado, D.L.; Figueroa, J.; Restrepo, C. Using Multiple Traits to Assess the Potential of Introduced and Native Vines to Proliferate in a Tropical Region. Ecol. Evol. 2016, 6, 8832–8845. [Google Scholar] [CrossRef] [PubMed]
- Waite, C.E.; van der Heijden, G.M.; Field, R.; Burslem, D.F.; Dalling, J.W.; Nilus, R.; Rodríguez-Ronderoset, M.E.; Marshall, A.R.; Boyd, D.S. Landscape-scale Drivers of Liana Load across a Southeast Asian Forest Canopy Differ to the Neotropics. J. Ecol. 2023, 111, 77–89. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.G. Diversity and distribution of climbing plants in Eurasia and North Africa. In Biodiversity of lianas; Parthasarathy, N., Ed.; Springer: Cham, Switzerland, 2015; pp. 57–79. [Google Scholar]
- Gianoli, E. The Behavioural Ecology of Climbing Plants. AoB Plants 2015, 7, plv013. [Google Scholar] [CrossRef] [PubMed]
- Vivek, P.; Parthasarathy, N. Liana Community and Functional Trait Analysis in Tropical Dry Evergreen Forest of India. J. Plant Ecol. 2015, 8, 501–512. [Google Scholar] [CrossRef]
- Zulqarnain; Silva, I.A.; Sfair, J.C.; van Melis, J.; Weiser, V.L.; Martins, F.R. Does Phylogeny Have a Role in the Liana-Phorophyte Interaction in Tropical Forests? Perspect. Plant Ecol. Evol. Syst. 2016, 21, 14–22. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Bongers, F. The Ecology of Lianas and their Role in Forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef]
- Schnitzer, S.A. A Mechanistic Explanation for Global Patterns of Liana Abundance and Distribution. Am. Nat. 2005, 166, 262–276. [Google Scholar] [CrossRef]
- Malizia, A.; Campanello, P.I.; Villagra, M.; Ceballos, S. Geographical, taxonomical and ecological aspects of lianas in subtropical forests of Argentina. In Biodiversity of Lianas; Parthasarathy, N., Ed.; Springer: Cham, Switzerland, 2015; pp. 17–42. [Google Scholar]
- Alves, L.F.; Assis, M.A.; van Melis, J.; Barros, A.L.; Vieira, S.A.; Martins, F.R.; Martinelli, L.A.; Joly, C.A. Variation in Liana Abundance and Biomass along an Elevational Gradient in the Tropical Atlantic Forest (Brazil). Ecol. Res. 2012, 27, 323–332. [Google Scholar] [CrossRef]
- Lobos-Catalán, P.; Jiménez-Castillo, M. Temperature Shapes Liana Diversity Pattern along a Latitudinal Gradient in Southern Temperate Rainforest. Plant Ecol. 2019, 220, 1109–1117. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Li, X.K.; Guo, Y.L.; Ding, T.; Wang, B.; Xiang, W.S. Diversity of Climbing Seed Plants and Their Reproductive Habit in a Karst Seasonal Rain Forest in Nonggang, Guangxi, China. Chin. J. Plant Ecol. 2017, 41, 716. [Google Scholar]
- Mackintosh, E.J.; Waite, C.E.; Putz, F.E.; Pfeifer, M.; Chen, C.R.; Lan, Z.M.; Brennan, S.; Marshall, A.R. Effects of Climate, Soil, Topography and Disturbance on Liana Prevalence. Ecol. Evol. 2024, 14, e70374. [Google Scholar] [CrossRef]
- Rowe, N. Lianas. Curr. Biol. 2018, 28, R249–R252. [Google Scholar] [CrossRef]
- DeWalt, S.J.; Schnitzer, S.A.; Alves, L.F.; Bongers, F.; Burnham, R.J.; Cai, Z.Q.; Carson, W.P.; Chave, J.; Chuyong, G.B.; Costa, F.R.; et al. Biogeographical patterns of liana abundance and diversity. In Ecology of Lianas; Schnitzer, S.A., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; Wiley Blackwell: Oxford, UK, 2015; pp. 131–146. [Google Scholar]
- Hu, L. Distribution and Diversity of Climbing Plants in Temperate East Asia. Biodivers. Sci. 2011, 19, 567. [Google Scholar] [CrossRef]
- Durigon, J.; Miotto, S.T.; Gianoli, E. Distribution and Traits of Climbing Plants in Subtropical and Temperate South America. J. Veg. Sci. 2014, 25, 1484–1492. [Google Scholar] [CrossRef]
- Ibarra-Manríquez, G.; Carrillo-Reyes, P.; Rendón-Sandoval, F.J.; Cornejo-Tenorio, G. Diversity and distribution of lianas in Mexico. In Ecology of Lianas; Schnitzer, S.A., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; Wiley Blackwell: Oxford, UK, 2015; pp. 91–103. [Google Scholar]
- Pandi, V.; Babu, K.N.; Anbarashan, M.; Reddy, C.S.; Borgohain, J.; Shynyan, K.; Mathew, A.A.; Rakshith, H.; Joseph, J.; Kennedy, V.N.; et al. Taxonomic Estimates of Climbing Plants in India: How Many Species are out There? Écoscience 2022, 29, 325–343. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.G.; Li, Z. The Diversity of Climbing Plants in the Spermatophyte Flora of China. Biodivers. Sci. 2010, 18, 198–207. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.G.; Li, Z. Geographical and Environmental Gradients of Lianas and Vines in China. Glob. Ecol. Biogeogr. 2010, 19, 554–561. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Mangan, S.A.; Dalling, J.W.; Baldeck, C.A.; Hubbell, S.P.; Ledo, A.; Muller-Landau, H.; Tobin, M.F.; Aguilar, S.; Brassfield, D.; et al. Liana Abundance, Diversity, and Distribution on Barro Colorado Island, Panama. PLoS ONE 2012, 7, e52114. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Rahmad, Z.B.; Shahrul, A.M. Environmental Factors Influencing Liana Community Diversity, Structure and Habitat Associations in a Tropical Hill Forest, Malaysia. Plant Ecol. Divers. 2014, 7, 485–496. [Google Scholar] [CrossRef]
- Bruy, D.; Ibanez, T.; Munzinger, J.; Isnard, S. Abundance, Richness and Composition of Lianas in Forest Communities along an Elevation Gradient in New Caledonia. Plant Ecol. Divers. 2017, 10, 469–481. [Google Scholar] [CrossRef]
- Tang, Y.S.; Shi, W.; Zeng, W.H.; Zheng, W.Y.; Cao, K.F. Floristic Composition and Phylogenetic Diversity of Climbing Plants in Natural Forests across Guangxi. Acta Ecol. Sin. 2018, 38, 8750–8757. [Google Scholar] [CrossRef]
- Dai, K.Y.; Xiao, J.H.; Zeng, Y.J.; Li, Y.Q.; Rao, X.Q.; Cai, X.A. Diversity of Lianas Resources in the Shimentai National Nature Reserve, Guangdong. Chin. J. Trop. Agr. 2024, 44, 91–97. [Google Scholar]
- Li, Z.R.; Weng, S.F.; Jiang, P.; Liang, Q.T. Analysis of the Geographical Composition and Ornamental Application of Climbing Plants in Guangdong Province. J. Trop. Subtrop. Bot. 2024, 32, 807–812. [Google Scholar]
- Villagra, B.L.; Gomes, E.P.; Burnham, R.J.; Neto, S.R. Diversity and Abundance of Climbers from the Atlantic Forest, Southeastern Brazil. Biodivers. Conserv. 2013, 22, 2505–2517. [Google Scholar] [CrossRef]
- Schnitzler, A.; Amigo, J.; Hale, B.; Schnitzler, C. Patterns of Climber Distribution in Temperate Forests of the Americas. J. Plant Ecol. 2016, 9, 724–733. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Ma, K.P. Geographic Distribution Patterns and Status Assessment of Threatened Plants in China. Biodivers. Conserv. 2008, 17, 1783–1798. [Google Scholar] [CrossRef]
- Zhao, F.W.; Li, Y.S.; Chen, H. Reflections on Biodiversity Legislation in China’s New Era. Biodivers. Sci. 2024, 32, 24027. [Google Scholar] [CrossRef]
- Huang, J.H.; Ma, K.P.; Chen, B. The Diversity and Geographical Distribution of Seed Plants Endemic to China; Higher Education Press: Beijing, China, 2014; ISBN 9787040411850. [Google Scholar]
- Wang, G.H.; Bai, F.; Sang, W.G. Spatial Distribution of Invasive Alien Animal and Plant Species and its Influencing Factors in China. Plant Sci. J. 2017, 35, 513–524. [Google Scholar]
- Xie, D.; Liu, B.; Zhao, L.N.; Pandey, T.R.; Liu, H.Y.; Shan, Z.J.; Qin, H.N. Diversity of Higher Plants in China. J. Syst. Evol. 2021, 59, 1111–1123. [Google Scholar] [CrossRef]
- Zhu, X.X.; Wang, J.; Liao, S.; Ma, J.S. Synopsis of Aristolochia L. and Isotrema Raf. (Aristolochiaceae) in China. Biodivers. Sci. 2019, 27, 1143–1146. [Google Scholar] [CrossRef]
- Gu, S.R.; Zeng, Q.B.; Clark, R.; Jiang, K.W.; Pérez-Escobar, O.A.; Li, S.J.; Tan, W.N.; Xie, Z.; Mattapha, S.; Shi, M.M.; et al. Phylogeny and Re-Circumscription of Cheniella (Leguminosae: Cercidoideae) Based on Plastome Data and Morphology, with Description of Three New Species. Taxon 2024, 73, 475–502. [Google Scholar] [CrossRef]
- Wang, L.S.; Jia, Y.; Zhang, X.C.; Qin, H.N. Overview of Higher Plant Diversity in China. Biodivers. Sci. 2015, 23, 217. [Google Scholar] [CrossRef]
- Gallagher, R.V. Climbing plant diversity in Australia: Taxonomy, biogeography and functional traits. In Ecology of Lianas; Schnitzer, S.A., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; Wiley Blackwell: Oxford, UK, 2015; pp. 104–115. [Google Scholar]
- Huang, J.H.; Huang, J.H.; Lu, X.H.; Ma, K.P. Diversity Distribution Patterns of Chinese Endemic Seed Plant Species and Their Implications for Conservation Planning. Sci. Rep. 2016, 6, 33913. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.X.; Quan, Q.; Du, Y.B.; Xia, L.; Ge, D.Y.; Yang, Q.S. Dispersal, Niche, and Isolation Processes Jointly Explain Species Turnover Patterns of Nonvolant Small Mammals in a Large Mountainous Region of China. Ecol. Evol. 2016, 6, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Li, Q.; Sun, S.C. Diversity and Distribution of Parasitic Angiosperms in China. Ecol. Evol. 2018, 8, 4378–4386. [Google Scholar] [CrossRef]
- Parthasarathy, N.; Vivek, P.; Muthumperumal, C.; Muthuramkumar, S.; Ayyappan, N. Biodiversity of lianas and their functional traits in tropical forests of Peninsular India. In Biodiversity of Lianas; Parthasarathy, N., Ed.; Springer: Cham, Switzerland, 2015; pp. 123–148. [Google Scholar]
- Sperotto, P.; Roque, N.; Acevedo-Rodríguez, P.; Vasconcelos, T. Climbing Mechanisms and the Diversification of Neotropical Climbing Plants across Time and Space. New Phytol. 2023, 240, 1561–1573. [Google Scholar] [CrossRef]
- Huang, E.H.; Chen, Y.X.; Fang, M.; Zheng, Y.; Yu, S.X. Environmental Drivers of Plant Distributions at Global and Regional Scales. Glob. Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar] [CrossRef]
- Qian, H. Environmental Determinants of Woody Plant Diversity at a Regional Scale in China. PLoS ONE 2013, 8, e75832. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth Century Redistribution in Climatic Drivers of Global Tree Growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef]
- Tao, Y.; Fan, F.; Duan, X.; He, H.; He, Q. On the Features of Different Climate Zones Temperature Change in Yunnan. J. Yunnan Univ. Nat. Sci. Ed. 2013, 35, 652–660. [Google Scholar]
- Chen, L.; Dong, H.J.; Peng, H. Diversity and Distribution of Higher Plants in Yunnan, China. Biodivers. Sci. 2013, 21, 359. [Google Scholar] [CrossRef]
- Zhao, L.N.; Li, J.Y.; Liu, H.Y.; Qin, H.N. Distribution, Congruence and Hotspots of Higher Plants in China. Sci. Rep. 2016, 6, 19080. [Google Scholar] [CrossRef]
- Guo, Z.X.; Zhang, X.N.; Wang, Z.M.; Fang, W.H. Responses of Vegetation Phenology in Northeast China to Climate Change. Chin. J. Ecol. 2010, 29, 578. [Google Scholar]
- Dang, Y.C.; Qin, L.J.; Huang, L.R.; Wang, J.Q.; Li, B.; He, H.S. Water Footprint of Rain-fed Maize in Different Growth Stages and Associated Climatic Driving Forces in Northeast China. Agric. Water Manag. 2022, 263, 107463. [Google Scholar] [CrossRef]
- Dang, R.L.; Pan, X.L. Floristic Analysis of Seed Plant Families in West-North Desert of China. Acta Bot. Boreali-Occident. Sin. 2002, 22, 24–32. [Google Scholar]
- Huang, J.H.; Ma, K.P.; Huang, J.H. Species Diversity Distribution Patterns of Chinese Endemic Seed Plants Based on Geographical Regions. PLoS ONE 2017, 12, e0170276. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Lu, L.L.; Haq, S.M.; Waheed, M.; Sahito, H.A.; Fatima, S.; Aziz, R.; Bussmann, R.W.; Tariq, A.; Ullah, I.; et al. Spatiotemporal Distribution Patterns of Climbers along an Abiotic Gradient in Jhelum District, Punjab, Pakistan. Forests 2022, 13, 1244. [Google Scholar] [CrossRef]
- He, S.N.; Hao, C.Y. Analysis on Spatial-Temporal Variation Characteristics of Climate in Qinling-Huaihe Demarcation Zone since 1961. Ecol. Indic. 2024, 158, 111345. [Google Scholar] [CrossRef]
- Xu, M.H.; Ma, L.; Jia, Y.Y.; Liu, M. Integrating the Effects of Latitude and Altitude on the Spatial Differentiation of Plant Community Diversity in a Mountainous Ecosystem in China. PLoS ONE 2017, 12, e0174231. [Google Scholar] [CrossRef]
- Schulz, T.; Saastamoinen, M.; Vanhatalo, J. Model-based Variance Partitioning for Statistical Ecology. Ecol. Monogr. 2025, 95, e1646. [Google Scholar] [CrossRef]
- Parolari, A.J.; Paul, K.; Griffing, A.; Condit, R.; Perez, R.; Aguilar, S.; Schnitzer, S.A. Liana Abundance and Diversity Increase with Rainfall Seasonality along a Precipitation Gradient in Panama. Ecography 2020, 43, 25–33. [Google Scholar] [CrossRef]
- Chen, Y.J.; Cao, K.F.; Schnitzer, S.A.; Fan, Z.X.; Zhang, J.L.; Bongers, F. Water-use Advantage for Lianas over Trees in Tropical Seasonal Forests. New Phytol. 2015, 205, 128–136. [Google Scholar] [CrossRef]
- Ewers, F.; Fisher, J.; Fichtner, K. Water flux and xylem structure in vines. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 127–160. [Google Scholar]
- Parkhurst, D.F.; Loucks, O.L. Optimal Leaf Size in Relation to Environment. J. Ecol. 1972, 60, 505–537. [Google Scholar] [CrossRef]
- Lusk, C.H.; Grierson, E.R.; Laughlin, D.C. Large Leaves in Warm, Moist Environments Confer an Advantage in Seedling Light Interception Efficiency. New Phytol. 2019, 223, 1319–1327. [Google Scholar] [CrossRef]
- Chen, S.B.; Jiang, G.M.; Ouyang, Z.Y.; Xu, W.H.; Xiao, Y. Relative Importance of Water, Energy, and Heterogeneity in Determining Regional Pteridophyte and Seed Plant Richness in China. J. Syst. Evol. 2011, 49, 95–107. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; et al. Energy, Water, and Broad-Scale Geographic Patterns of Species Richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef]
- Lu, C.; Ma, L.; Liu, T.X.; Huang, X. Temporal and Spatial Variations of Annual Precipitation and Meteorological Drought in China During 1951–2018. Chin. J. Appl. Ecol. 2022, 33, 1572–1580. [Google Scholar]
- Zhu, S.D.; Cao, K.F. Hydraulic Properties and Photosynthetic Rates in Co-Occurring Lianas and Trees in a Seasonal Tropical Rainforest in Southwestern China. Plant Ecol. 2009, 204, 295–304. [Google Scholar] [CrossRef]
- Bai, S.Q. Prediction of Annual Precipitation Based on the Weighted Markov Chain in Xinjiang in recent 10 years. Clim. Change Res. Lett. 2022, 11, 989. [Google Scholar] [CrossRef]
- Schnitzer, S.A. The contribution of lianas to forest ecology, diversity, and dynamics. In Biodiversity of Lianas; Parthasarathy, N., Ed.; Springer: Cham, Switzerland, 2015; pp. 149–160. [Google Scholar]
- Geng, Y.J.; Li, Z.Y.; Tian, Y. Convention on Biological Diversity: The Current Status, Ongoing Challenges, and Future Prospects of Marine Biodiversity Conservation. Biodivers. Sci. 2023, 31, 22645. [Google Scholar] [CrossRef]
- Ren, H.; Corlett, R.T.; Ouyang, Z.Y.; Blackmore, S. How can China Protect 30% of its Land? Trends Ecol. Evol. 2025, 40, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.S. In-situ Conservation of Biodiversity in China: Advances and Prospects. Biodivers. Sci. 2021, 29, 133–149. [Google Scholar] [CrossRef]
- Ye, C.; Liu, H.Y.; Qin, H.N.; Shu, J.P.; Zhou, Z.H.; Jin, X.H. Geographical Distribution and Conservation Strategy of National Key Protected Wild Plants of China. iScience 2023, 26, 107364. [Google Scholar] [CrossRef]
- Ji, M.T.; Dong, R.; Zhang, J.H.; Shi, X.L.; Wang, Y.C.; Huang, Q.Q.; Qu, D.; Wang, Y.Y. Diversity and Environmental Determinants of Aquatic Plants across China. Hydrobiologia 2024, 851, 3453–3469. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, L.B. Global New Species of Vascular Plants Published in 2020. Biodivers. Sci. 2021, 29, 1003–1010. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, L.B. Global New Taxa of Vascular Plants Published in 2021. Biodivers. Sci. 2022, 30, 22116. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, L.B. Global New Taxa of Vascular Plants Published in 2022. Biodivers. Sci. 2023, 31, 23162. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, L.B. Global New Taxa of Vascular Plants Published in 2023. Biodivers. Sci. 2024, 32, 24322. [Google Scholar] [CrossRef]
- Zheng, B.H.; Chen, X.Y.; Ni, J. A Dataset on the Plant Growth Form and Life Form of Vascular Plants in China. Biodivers. Sci. 2024, 32, 23468. [Google Scholar] [CrossRef]
- Qin, F.; Han, B.C.; Bussmann, R.W.; Xue, T.T.; Liang, Y.F.; Zhang, W.D.; Liu, Q.; Chen, T.X.; Yu, S.X. Present Status, Future Trends, and Control Strategies of Invasive Alien Plants in China Affected by Human Activities and Climate Change. Ecography 2024, 2024, e06919. [Google Scholar] [CrossRef]
- Dias, A.S.; Oliveira, R.S.; Martins, F.R.; Bongers, F.; Anten, N.P.; Sterck, F.J. Climbing Mechanisms as a Central Trait to Understand the Ecology of Lianas across the Tropics. Glob. Ecol. Biogeogr. 2024, 33, e13846. [Google Scholar] [CrossRef]
- Jiang, R.Y.; Zhang, G.F. Distribution Patterns and Influencing Factors of Different Parasitic Angiosperm Types in China. Glob. Ecol. Conserv. 2021, 27, e01533. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Ji, L.Q. Avian-Mammalian Species Diversity in Nine Representative Sites in China. Biodivers. Sci. 1999, 7, 220. [Google Scholar] [CrossRef]
- Li, S.C.; Li, T.T.; Wang, Z.X.; Tian, K.; Chen, Y.; Peng, Q.Q.; Xiong, B.M. Mammalian Species Diversity in Wanchaoshan Nature Reserve, Hubei Province of Central China Based on G-F Index. Ecol. Sci. 2018, 37, 72–80. [Google Scholar]
- Xing, Y.C.; Zhang, C.G.; Fan, E.Y.; Zhao, Y.H. Freshwater Fishes of China: Species Richness, Endemism, Threatened Species and Conservation. Divers. Distrib. 2016, 22, 358–370. [Google Scholar] [CrossRef]
- Qian, H.; Deng, T.; Jin, Y.; Mao, L.F.; Zhao, D.; Ricklefs, R.E. Phylogenetic Dispersion and Diversity in Regional Assemblages of Seed Plants in China. Proc. Natl. Acad. Sci. USA 2019, 116, 23192–23201. [Google Scholar] [CrossRef] [PubMed]


| Taxa | Family | Genus | Species | |||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| Lycophytes and Ferns | 3 | 2.86% | 3 | 0.54% | 11 | 0.32% |
| Gymnosperms | 1 | 0.95% | 1 | 0.18% | 10 | 0.29% |
| Angiosperms | 101 | 96.19% | 547 | 99.28% | 3464 | 99.39% |
| Total | 105 | 100.00% | 551 | 100.00% | 3485 | 100.00% |
| Types | Family | Genus | Species | |||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| Large family (N > 100) | 9 | 8.57% | 242 | 43.92% | 1893 | 54.32% |
| Medium family (6 ≤ N < 100) | 56 | 53.33% | 254 | 46.10% | 1515 | 43.47% |
| Oligospecific family (2 ≤ N ≤ 5) | 19 | 18.10% | 34 | 6.17% | 56 | 1.61% |
| Monotypic family (N = 1) | 21 | 20.00% | 21 | 3.81% | 21 | 0.60% |
| Total | 105 | 100.00% | 551 | 100.00% | 3485 | 100.00% |
| Life Form | Climbing Method | Total (%) | |||
|---|---|---|---|---|---|
| Active | Passive | ||||
| Twining Climbers | Tendrillar Climbers | Adhesive Climbers | Sprawling Climbers | ||
| Evergreen woody liana | 712 | 148 | 129 | 373 | 1362 (39.08%) |
| Deciduous woody liana | 431 | 148 | 39 | 276 | 894 (25.65%) |
| Herbaceous vine | 686 | 269 | 46 | 228 | 1229 (35.27%) |
| Total | 1829 (52.48%) | 565 (16.21%) | 214 (6.14%) | 877 (25.17%) | 3485 (100.00%) |
| Species Density | Endemic | Threatened | Invasive | |
|---|---|---|---|---|
| Species density | 1 | |||
| Endemic | 0.950 ** | 1 | ||
| Threatened | 0.963 ** | 0.912 ** | 1 | |
| Invasive | 0.711 ** | 0.499 ** | 0.617 ** | 1 |
| Variables | Climbing Plants | Evergreen Woody Liana | Deciduous Woody Liana | Herbaceous Vine |
|---|---|---|---|---|
| Bio1 | 0.722 | 0.695 | 0.755 | 0.681 |
| Bio2 | −0.696 | −0.647 | −0.748 | −0.676 |
| Bio3 | 0.610 | 0.620 | 0.496 | 0.629 |
| Bio4 | −0.862 | −0.822 | −0.863 | −0.854 |
| Bio5 | 0.216 | 0.216 | 0.272 | 0.158 |
| Bio6 | 0.815 | 0.778 | 0.846 | 0.783 |
| Bio7 | −0.876 | −0.831 | −0.887 | −0.864 |
| Bio8 | 0.352 | 0.347 | 0.359 | 0.326 |
| Bio9 | 0.821 | 0.787 | 0.847 | 0.787 |
| Bio10 | 0.365 | 0.353 | 0.417 | 0.316 |
| Bio11 | 0.828 | 0.793 | 0.855 | 0.796 |
| Bio12 | 0.754 | 0.699 | 0.766 | 0.767 |
| Bio13 | 0.709 | 0.652 | 0.694 | 0.749 |
| Bio14 | 0.565 | 0.505 | 0.626 | 0.566 |
| Bio15 | −0.511 | −0.455 | −0.650 | −0.452 |
| Bio16 | 0.766 | 0.713 | 0.747 | 0.796 |
| Bio17 | 0.546 | 0.486 | 0.617 | 0.540 |
| Bio18 | 0.760 | 0.701 | 0.728 | 0.812 |
| Bio19 | 0.535 | 0.478 | 0.600 | 0.530 |
| Latitude | −0.864 | −0.835 | −0.886 | −0.822 |
| Longitude | −0.049 | −0.082 | −0.032 | −0.007 |
| Elevation | −0.198 | −0.174 | −0.252 | −0.178 |
| Human impact | 0.227 | 0.162 | 0.300 | 0.255 |
| Category | Variable | Description | Unit |
|---|---|---|---|
| Temperature | Bio1 | Annual mean temperature | °C |
| Bio2 | Mean diurnal range (mean of monthly (max temp–min temp)) | °C | |
| Bio3 | Isothermality ((Bio2/Bio7) × 100) | % | |
| Bio4 | Temperature seasonality (standard deviation × 100) | - | |
| Bio5 | Max temperature of warmest month | °C | |
| Bio6 | Min temperature of coldest month | °C | |
| Bio7 | Temperature annual range (Bio5–Bio6) | °C | |
| Bio8 | Mean temperature of wettest quarter | °C | |
| Bio9 | Mean temperature of driest quarter | °C | |
| Bio10 | Mean temperature of warmest quarter | °C | |
| Bio11 | Mean temperature of coldest quarter | °C | |
| Precipitation | Bio12 | Annual precipitation | mm |
| Bio13 | Precipitation of wettest month | mm | |
| Bio14 | Precipitation of driest month | mm | |
| Bio15 | Precipitation seasonality (coefficient of variation) | - | |
| Bio16 | Precipitation of wettest quarter | mm | |
| Bio17 | Precipitation of driest quarter | mm | |
| Bio18 | Precipitation of warmest quarter | mm | |
| Bio19 | Precipitation of coldest quarter | mm | |
| Geographical location | Latitude | midpoint values of latitude | ° |
| Longitude | midpoint values of longitude | ° | |
| Elevation | Average elevation | m | |
| Human impact | HI | Human footprint | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, G. Diversity, Pattern, and Environmental Drivers of Climbing Plants in China. Plants 2025, 14, 3281. https://doi.org/10.3390/plants14213281
Wang H, Zhang G. Diversity, Pattern, and Environmental Drivers of Climbing Plants in China. Plants. 2025; 14(21):3281. https://doi.org/10.3390/plants14213281
Chicago/Turabian StyleWang, Haoran, and Guangfu Zhang. 2025. "Diversity, Pattern, and Environmental Drivers of Climbing Plants in China" Plants 14, no. 21: 3281. https://doi.org/10.3390/plants14213281
APA StyleWang, H., & Zhang, G. (2025). Diversity, Pattern, and Environmental Drivers of Climbing Plants in China. Plants, 14(21), 3281. https://doi.org/10.3390/plants14213281






