Evaluation of Phytotoxic and Cytotoxic Effects of Prenylated Phenol Derivatives on Tomato Plants (Solanum lycopersicum L.) and Botrytis cinerea B-05 Spores
Abstract
1. Introduction
2. Results and Discussion
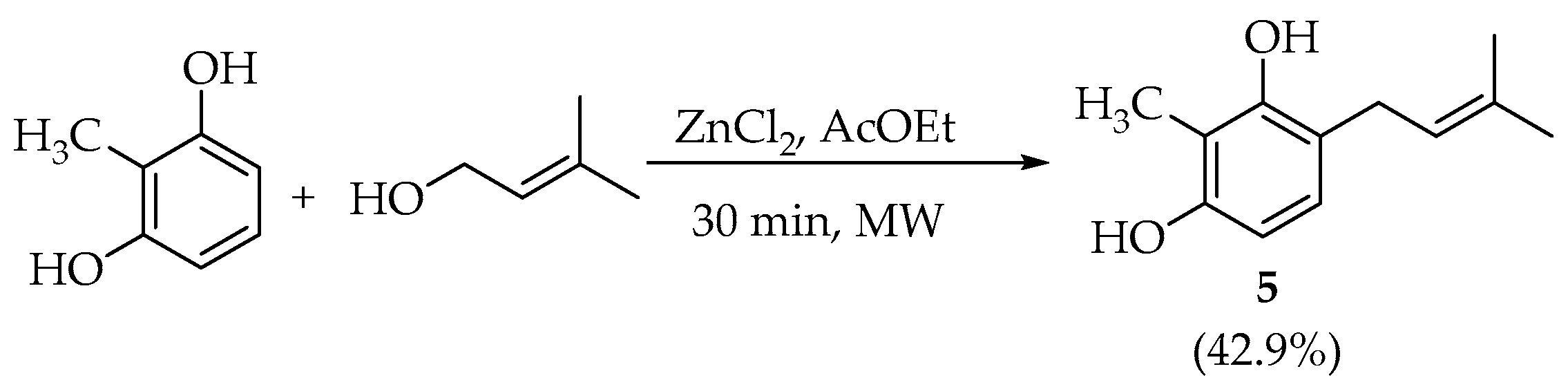
2.1. Synthesis
Structural Determination of Prenylated 2-Methylresorcinol (5)
2.2. Bioactivity Assays
2.2.1. Cytotoxicity Assay on B. cinerea Spores
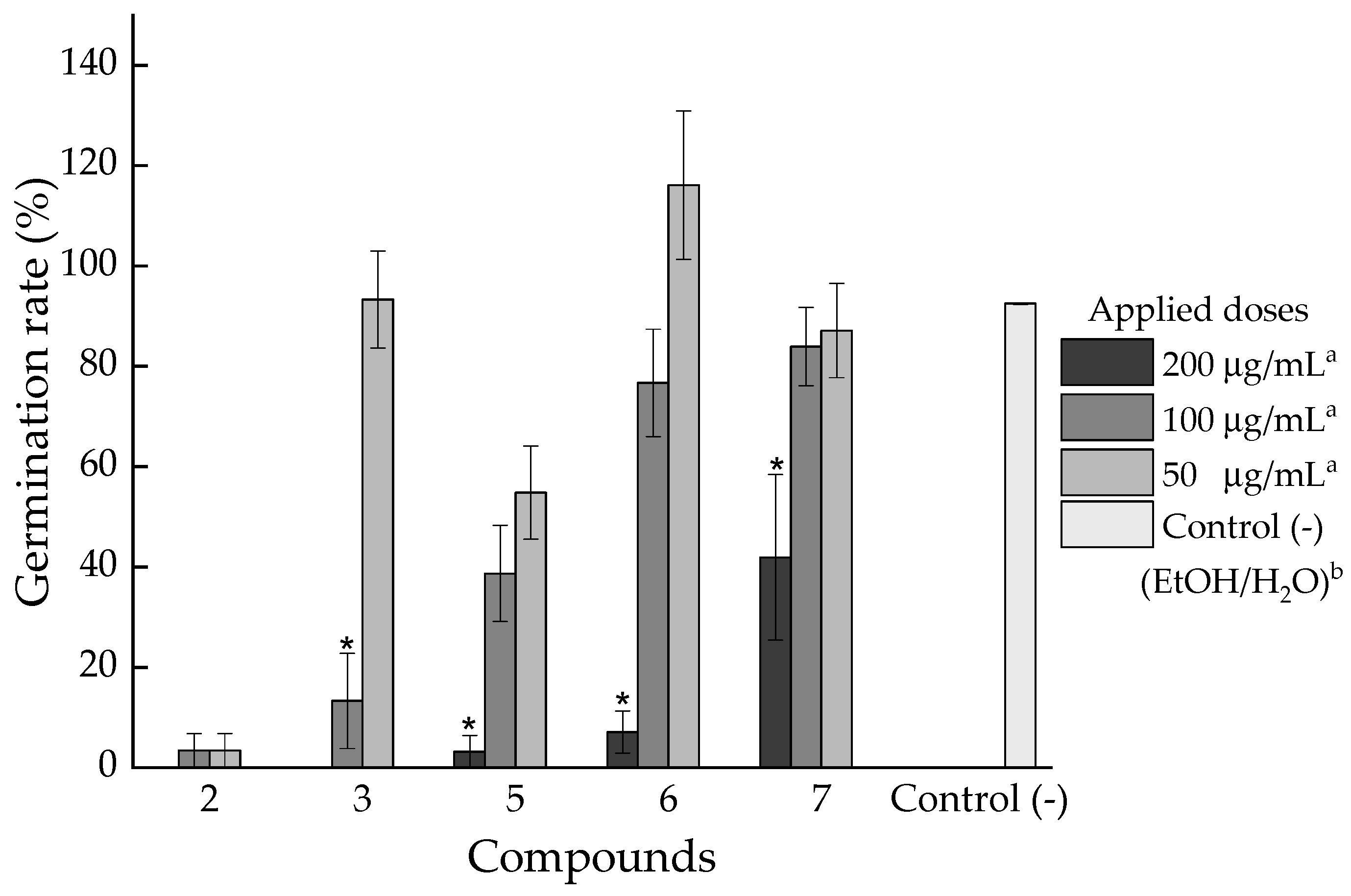
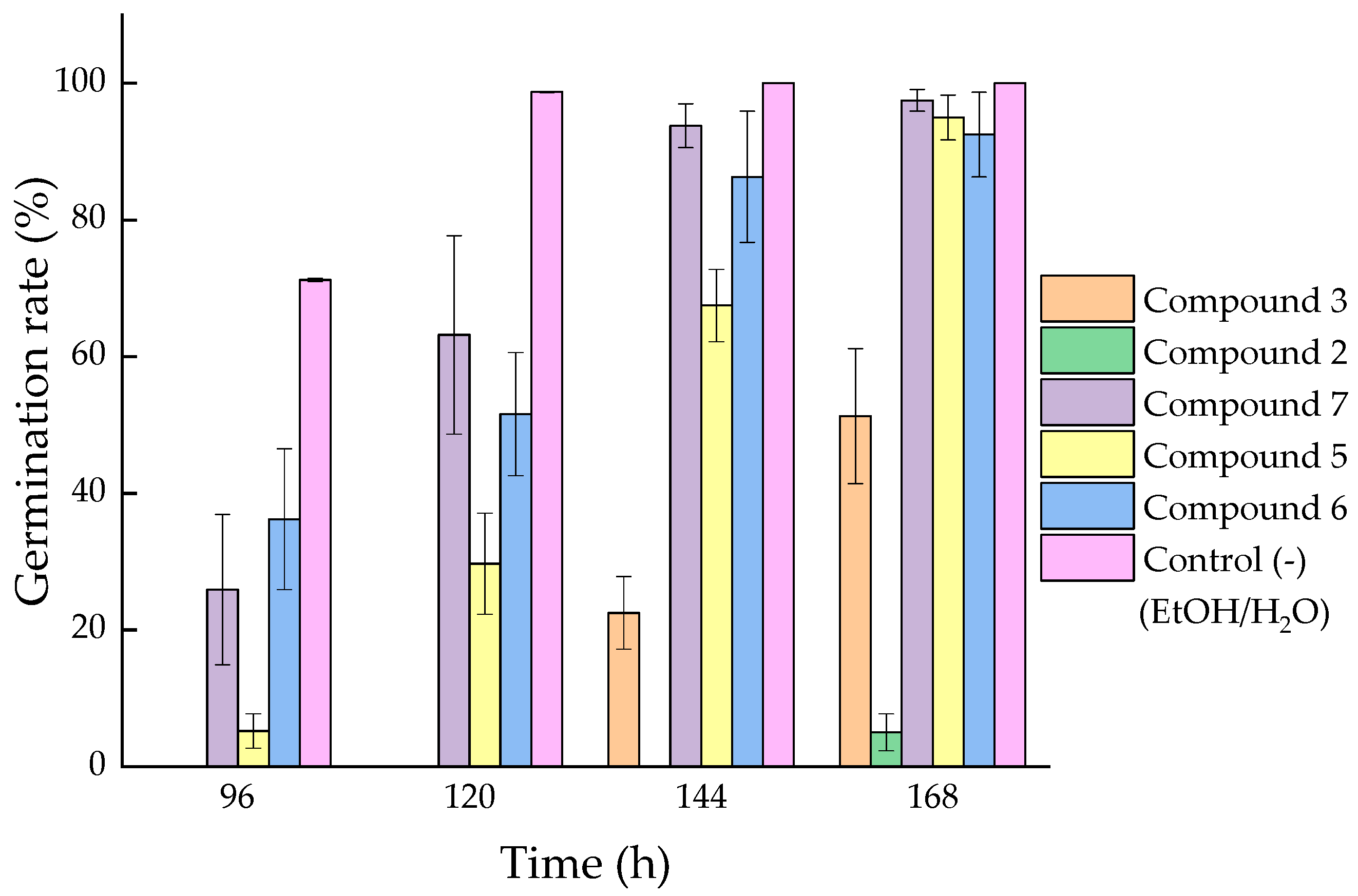
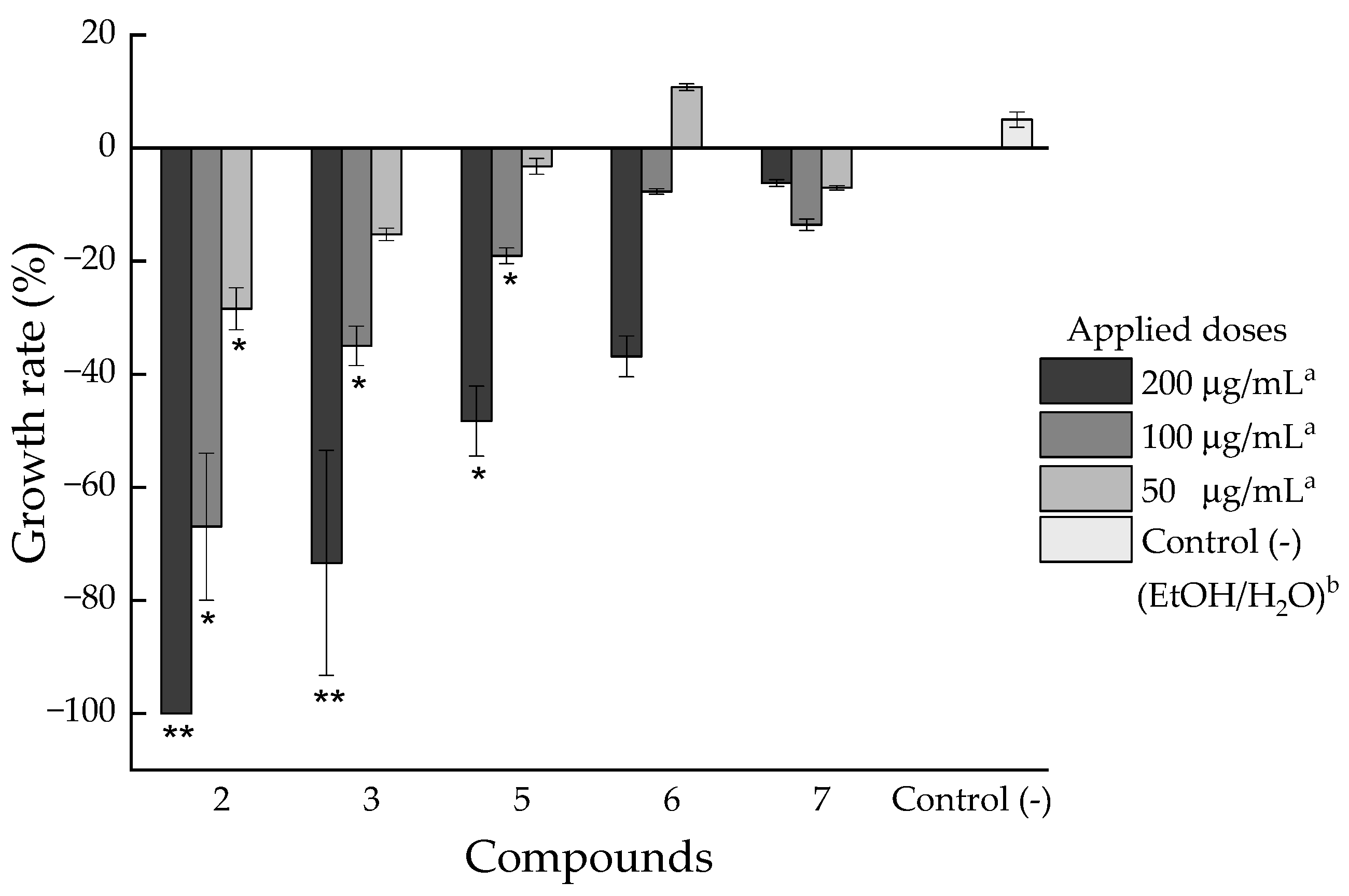
2.2.2. Phytotoxic Activity
3. Materials and Methods
3.1. Synthesis and Characterization
3.1.1. General Procedures
3.1.2. Microwave-Assisted Electrophilic Aromatic Substitution Reaction
- 2-(3-methylbut-2-en-1-yl)benzene-1,4-diol (1).
- 4-(3-methylbut-2-en-1-yl)benzene-1,3-diol (2).
- 4-(3-methylbut-2-en-1-yl)benzene-1,2-diol (3).
- 2-methyl-5-(3-methylbut-2-en-1-yl)benzene-1,4-diol (4).
- 2-methyl-4-(3-methylbut-2-en-1-yl)benzene-1,3-diol (5).
- 5-methyl-2-(3-methylbut-2-en-1-yl)benzene-1,3-diol (6).
- 4-methyl-5-(3-methylbut-2-en-1yl)benzene-1,2-diol (7).
3.1.3. Hydration Reaction
- 4-(3-hydroxy-3-methylbutyl)-5-methylbenzene-1,2-diol (8).
- 4-(3-hydroxy-3-methylbutyl)benzene-1,2-diol (9).
3.2. Bioactivity Assays
3.2.1. Cytotoxicity on B. cinerea Spores
3.2.2. Phytotoxic Activity on Tomato (Solanum lycopersicum L.) Seeds
3.2.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elad, Y.; Pertot, I.; Prado, A.M.C.; Stewart, A. Plant Hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar]
- Latorre, B.A.; Elfar, K.; Ferrada, E.E. Gray mold caused by Botrytis cinerea limits grape production in Chile. Cienc. E Investig. Agrar. 2015, 42, 305–330. [Google Scholar] [CrossRef]
- Elad, Y.; Gullino, M.L.; Shtienberg, D.; Aloi, C. Managing Botrytis cinerea on tomatoes in greenhouses in the Mediterranean. Crop Prot. 1995, 14, 105–109. [Google Scholar] [CrossRef]
- Romanazzi, G.; Droby, S. Control Strategies for Postharvest Grey Mould on Fruit Crops. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 217–228. [Google Scholar]
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Leroux, P. Chemical Control of Botrytis and its Resistance to Chemical Fungicides. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 195–222. [Google Scholar]
- Leroch, M.; Plesken, C.; Weber, R.W.S.; Kauff, F.; Scalliet, G.; Hahn, M. Gray Mold Populations in German Strawberry Fields Are Resistant to Multiple Fungicides and Dominated by a Novel Clade Closely Related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef]
- Latorre, B.A.; Torres, R. Prevalence of isolates of Botrytis cinerea resistant to multiple fungicides in Chilean vineyards. Crop Prot. 2012, 40, 49–52. [Google Scholar] [CrossRef]
- Hadacek, F.; Greger, H. Testing of antifungal natural products: Methodologies, comparability of results and assay choice. Phytochem. Anal. 2000, 11, 137–147. [Google Scholar] [CrossRef]
- Nxumalo, K.A.; Aremu, A.O.; Fawole, O.A. Potentials of Medicinal Plant Extracts as an Alternative to Synthetic Chemicals in Postharvest Protection and Preservation of Horticultural Crops: A Review. Sustainability 2021, 13, 5897. [Google Scholar] [CrossRef]
- Olea, A.F.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal Activity of Eugenol Derivatives against Botrytis Cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef]
- Olea, A.F.; Espinoza, L.; Sedan, C.; Thomas, M.; Martinez, R.; Mellado, M.; Carrasco, H.; Diaz, K. Synthesis and In Vitro Growth Inhibition of 2-Allylphenol Derivatives Against Phythopthora cinnamomi Rands. Molecules 2019, 24, 4196. [Google Scholar] [CrossRef]
- Rosero-Hernández, E.D.; Moraga, J.; Collado, I.G.; Echeverri, F. Natural Compounds That Modulate the Development of the Fungus Botrytis cinerea and Protect Solanum lycopersicum. Plants 2019, 8, 111. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest. Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Fujisawa, S.; Atsumi, T.; Kadoma, Y.; Sakagami, H. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology 2002, 177, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.; Aravena, J.; Vergara, A.; Taborga, L.; Baeza, E.; Catalan, K.; Gonzalez, C.; Carvajal, M.; Carrasco, H.; Espinoza, L. Synthesis and DPPH Radical Scavenging Activity of Prenylated Phenol Derivatives. Molecules 2012, 17, 556–570. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Wang, C.; Han, Z.; Shu, Y.; Li, X.; Zhou, L.; Qiu, M. Unusual prenylated phenols with antioxidant activities from Ganoderma cochlear. Food Chem. 2015, 171, 251–257. [Google Scholar] [CrossRef]
- Lin, K.-W.; Wang, B.-W.; Wu, C.-M.; Yen, M.-H.; Wei, B.-L.; Hung, C.-F.; Lin, C.-N. Antioxidant prenylated phenols of Artocarpus plants attenuate ultraviolet radiation-induced damage on human keratinocytes and fibroblasts. Phytochem. Lett. 2015, 14, 190–197. [Google Scholar] [CrossRef]
- Brezani, V.; Smejkal, K.; Hosek, J.; Tomasova, V. Anti-inflammatory Natural Prenylated Phenolic Compounds—Potential Lead Substances. Curr. Med. Chem. 2018, 25, 1094–1159. [Google Scholar] [CrossRef]
- Alavi, S.J.; Seyedi, S.M.; Saberi, S.; Safdari, H.; Eshghi, H.; Sadeghian, H. Allylphenols as a new class of human 15-lipoxygenase-1 inhibitors. Drug Dev. Res. 2021, 82, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, X.; Shao, X.; Wang, Z.; Du, Y.; Zhu, C.; Du, W.; Tang, D.; Ji, S. Prenylated phenolic compounds from licorice (Glycyrrhiza uralensis) and their anti-inflammatory activity against osteoarthritis. Food Funct. 2022, 13, 795–805. [Google Scholar] [CrossRef]
- Itoigawa, M.; Ito, C.; Tokuda, H.; Enjo, F.; Nishino, H.; Furukawa, H. Cancer chemopreventive activity of phenylpropanoids and phytoquinoids from Illicium plants. Cancer Lett. 2004, 214, 165–169. [Google Scholar] [CrossRef]
- Marín, M.; Máñez, S. Recent trends in the pharmacological activity of isoprenyl phenolics. Curr. Med. Chem. 2013, 20, 272–279. [Google Scholar] [CrossRef]
- Sugamoto, K.; Matsusita, Y.-i.; Matsui, K.; Kurogi, C.; Matsui, T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 2011, 67, 5346–5359. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives Against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; do Nascimento, P.G.G.; de Medeiros Costa, A.K.; de Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef]
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.A.; Monte, A. Antibacterial compounds from mushrooms I: A lanostane-type triterpene and prenylphenol derivatives from Jahnoporus hirtus and Albatrellus flettii and their activities against Bacillus cereus and Enterococcus faecalis. Planta Med. 2010, 76, 182–185. [Google Scholar] [CrossRef]
- de Souza, T.B.; Orlandi, M.; Coelho, L.F.L.; Malaquias, L.C.C.; Dias, A.L.T.; de Carvalho, R.R.; Silva, N.C.; Carvalho, D.T. Synthesis and in vitro evaluation of antifungal and cytotoxic activities of eugenol glycosides. Med. Chem. Res. 2014, 23, 496–502. [Google Scholar] [CrossRef]
- Soto, M.; Espinoza, L.; Chavez, M.I.; Diaz, K.; Olea, A.F.; Taborga, L. Synthesis of New Hydrated Geranylphenols and in Vitro Antifungal Activity against Botrytis cinerea. Int. J. Mol. Sci. 2016, 17, 840. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Gao, S.; Li, J.; Hao, J.J.; Ji, P. Synthesis and antifungal activity of 2-allylphenol derivatives against fungal plant pathogens. Pestic. Biochem. Physiol. 2017, 135, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Taborga, L.; Sortino, M.; Carrasco, H.; Butassi, E.; Zacchino, S.; Espinoza, L. Antifungal toxicity of linear geranylphenol. Influence of oxygenate substituents. Food Chem. Toxicol. 2017, 29, 827–835. [Google Scholar] [CrossRef]
- Gong, S.J.; Hao, J.J.; Xia, Y.Y.; Liu, X.L.; Li, J.Q. Inhibitory effect of bionic fungicide 2-allylphenol on Botrytis cinerea (Pers. ex Fr.) in vitro. Pest Manag. Sci. 2009, 65, 1337–1343. [Google Scholar] [CrossRef]
- De Rosa, S.; De Giulio, A.; Iodice, C. Biological Effects of Prenylated Hydroquinones—Structure-Activity Relationship Studies in Antimicrobial, Brine Shrimp, and Fish Lethality Assays. J. Nat. Prod. 1994, 57, 1711–1716. [Google Scholar] [CrossRef]
- Soto, M.; Estevez-Braun, A.; Amesty, Á.; Kluepfel, J.; Restrepo, S.; Diaz, K.; Espinoza, L.; Olea, A.F.; Taborga, L. Synthesis and Fungicidal Activity of Hydrated Geranylated Phenols against Botrytis cinerea. Molecules 2021, 26, 6815. [Google Scholar] [CrossRef]
- Garn, H.; Krause, H.; Enzmann, V.; Dröβler, K. An improved MTT assay using the electron-coupling agent menadione. J. Immunol. Methods 1994, 168, 253–256. [Google Scholar] [CrossRef]
- Lander, J.J.; Svirbely, W.J. The Dipole Moments of Catechol, Resorcinol and Hydroquinone1. J. Am. Chem. Soc. 1945, 67, 322–324. [Google Scholar] [CrossRef]
- Olea, A.F.; Rubio, J.; Sedan, C.; Carvajal, D.; Nuñez, M.; Espinoza, L.; Llovera, L.; Nuñez, G.; Taborga, L.; Carrasco, H. Antifungal Activity of 2-Allylphenol Derivatives on the Botrytis cinerea Strain: Assessment of Possible Action Mechanism. Int. J. Mol. Sci. 2023, 24, 6530. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Induction of oxidative stress as a possible mechanism of the antifungal action of three phenylpropanoids. FEMS Yeast Res. 2011, 11, 114–122. [Google Scholar] [CrossRef]
- Shahina, Z.; Ndlovu, E.; Persaud, O.; Sultana, T.; Dahms, T.E.S. Candida albicans Reactive Oxygen Species (ROS)-Dependent Lethality and ROS-Independent Hyphal and Biofilm Inhibition by Eugenol and Citral. Microbiol. Spectr. 2022, 10, e0318322. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Zhan, P.; Shao, X. ROS Stress and Cell Membrane Disruption are the Main Antifungal Mechanisms of 2-Phenylethanol against Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 14468–14479. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Boon, S.A.; Ijaz, M.K.; McKinney, J.; Gerba, C.P. Antifungal activity and mechanism of action of natural product derivates as potential environmental disinfectants. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad036. [Google Scholar] [CrossRef]
- Ruiz-Vásquez, L.; Ruiz Mesia, L.; Caballero Ceferino, H.D.; Ruiz Mesia, W.; Andrés, M.F.; Díaz, C.E.; Gonzalez-Coloma, A. Antifungal and Herbicidal Potential of Piper Essential Oils from the Peruvian Amazonia. Plants 2022, 11, 1793. [Google Scholar] [CrossRef]
- Sainz, P.; Andrés, M.F.; Martínez-Díaz, R.A.; Bailén, M.; Navarro-Rocha, J.; Díaz, C.E.; González-Coloma, A. Chemical Composition and Biological Activities of Artemisia pedemontana subsp. assoana Essential Oils and Hydrolate. Biomolecules 2019, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Lin, L.; Gao, Y.; Niu, X. Mulberrin inhibits Botrytis cinerea for strawberry storage by interfering with the bioactivity of 14α-demethylase (CYP51). Food Funct. 2022, 13, 4032–4046. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]


| Compounds | IC50 (μg/mL) a |
|---|---|
| 1 | >800 b |
| 2 | 461 |
| 3 | 325 |
| 4 | >800 b |
| 5 | 455 |
| 6 | 379 |
| 7 | <50 |
| 8 | >800 b |
| 9 | >800 b |
| Negative Control (1% DMSO) | >800 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez, G.; Llovera, L.; Arrieche, D.; Berrios, R.; Soto, M.; Osorio-Olivares, M.; Olea, A.F.; Sarmiento, E.; González, A.; Carrasco, H.; et al. Evaluation of Phytotoxic and Cytotoxic Effects of Prenylated Phenol Derivatives on Tomato Plants (Solanum lycopersicum L.) and Botrytis cinerea B-05 Spores. Plants 2025, 14, 3277. https://doi.org/10.3390/plants14213277
Núñez G, Llovera L, Arrieche D, Berrios R, Soto M, Osorio-Olivares M, Olea AF, Sarmiento E, González A, Carrasco H, et al. Evaluation of Phytotoxic and Cytotoxic Effects of Prenylated Phenol Derivatives on Tomato Plants (Solanum lycopersicum L.) and Botrytis cinerea B-05 Spores. Plants. 2025; 14(21):3277. https://doi.org/10.3390/plants14213277
Chicago/Turabian StyleNúñez, Gerard, Ligia Llovera, Dioni Arrieche, Romanet Berrios, Mauricio Soto, Mauricio Osorio-Olivares, Andrés F. Olea, Efraín Sarmiento, Azucena González, Héctor Carrasco, and et al. 2025. "Evaluation of Phytotoxic and Cytotoxic Effects of Prenylated Phenol Derivatives on Tomato Plants (Solanum lycopersicum L.) and Botrytis cinerea B-05 Spores" Plants 14, no. 21: 3277. https://doi.org/10.3390/plants14213277
APA StyleNúñez, G., Llovera, L., Arrieche, D., Berrios, R., Soto, M., Osorio-Olivares, M., Olea, A. F., Sarmiento, E., González, A., Carrasco, H., & Taborga, L. (2025). Evaluation of Phytotoxic and Cytotoxic Effects of Prenylated Phenol Derivatives on Tomato Plants (Solanum lycopersicum L.) and Botrytis cinerea B-05 Spores. Plants, 14(21), 3277. https://doi.org/10.3390/plants14213277






