Spraying Foliar Fertilizer Affect the Physiological Function of Leaf and Improve the Quality of ‘Snick’ Apple
Abstract
1. Introduction
2. Results
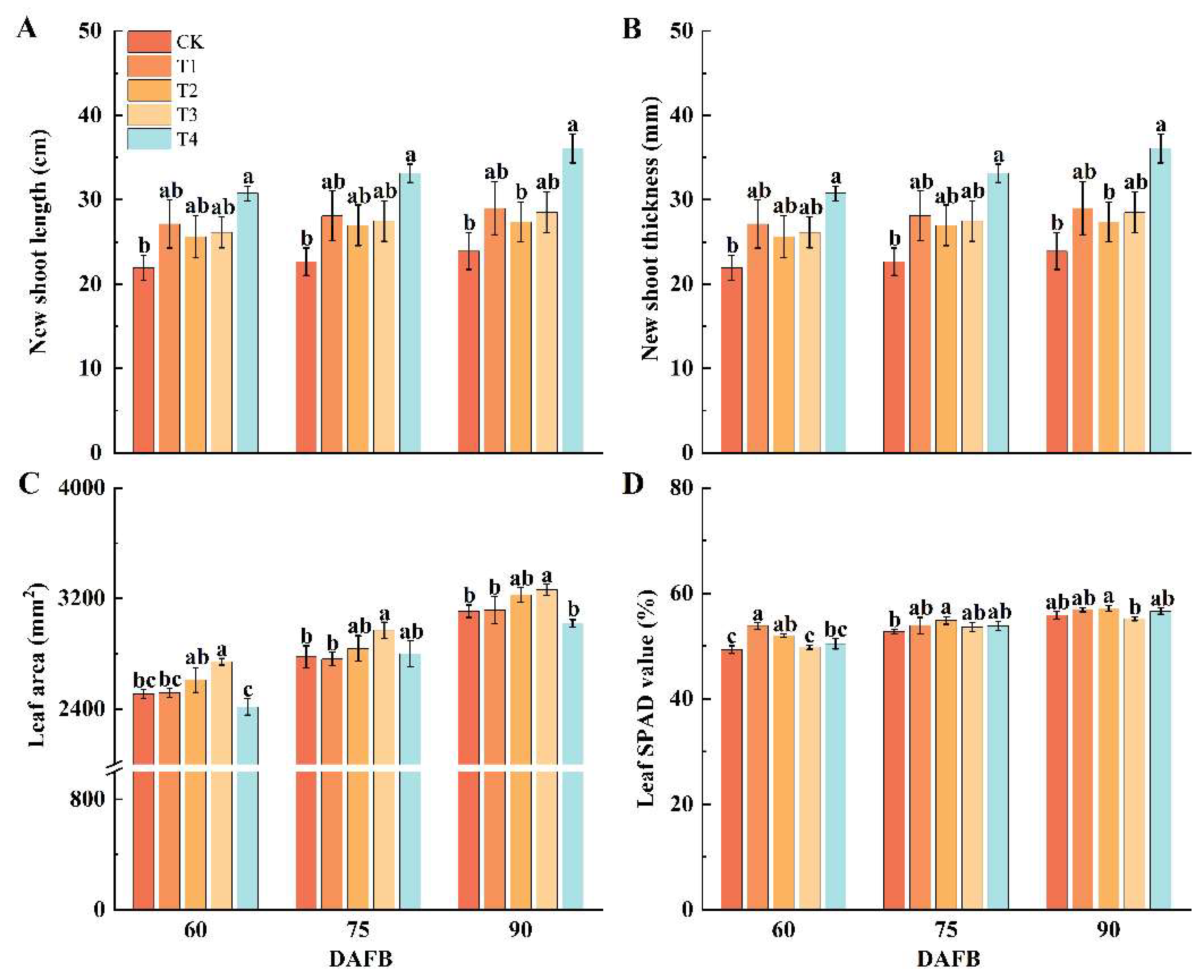
2.1. Effect of Different Foliar Fertilizers on Physiological Characteristics of Shoots and Leaves of ‘Snick’ Apple
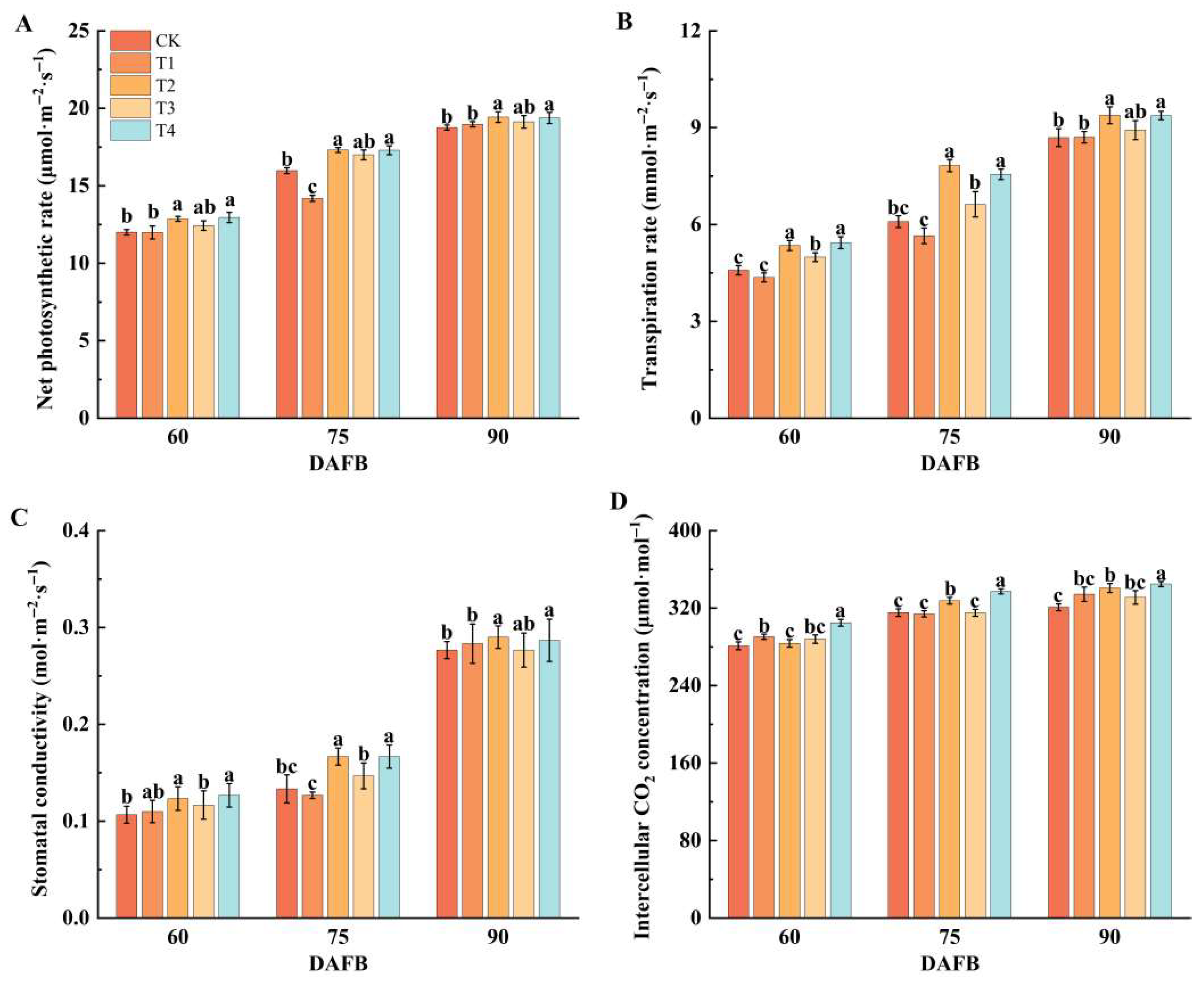
2.2. Effect of Different Foliar Fertilizers on Photosynthesis in ‘Snick’ Apple Leaves
2.3. Effect of Different Foliar Fertilizers on Fruit Appearance Quality at Harvest Time
2.4. Effect of Different Foliar Fertilizers on Fruit Internal Quality
2.4.1. Effect of Different Foliar Fertilisers on the Soluble Solids, Soluble Sugar, Titratable Acid and Sugar-Acid Ratios of Fruits
2.4.2. Effect of Different Foliar Fertilizers on Soluble Protein, Vitamin C Content, pH Value and Relative Water Content of Fruits
2.4.3. Effect of Different Foliar Fertilizers on the Concentrations of Total Phenolic Compounds, Tannins, Anthocyanins, and Flavonoids
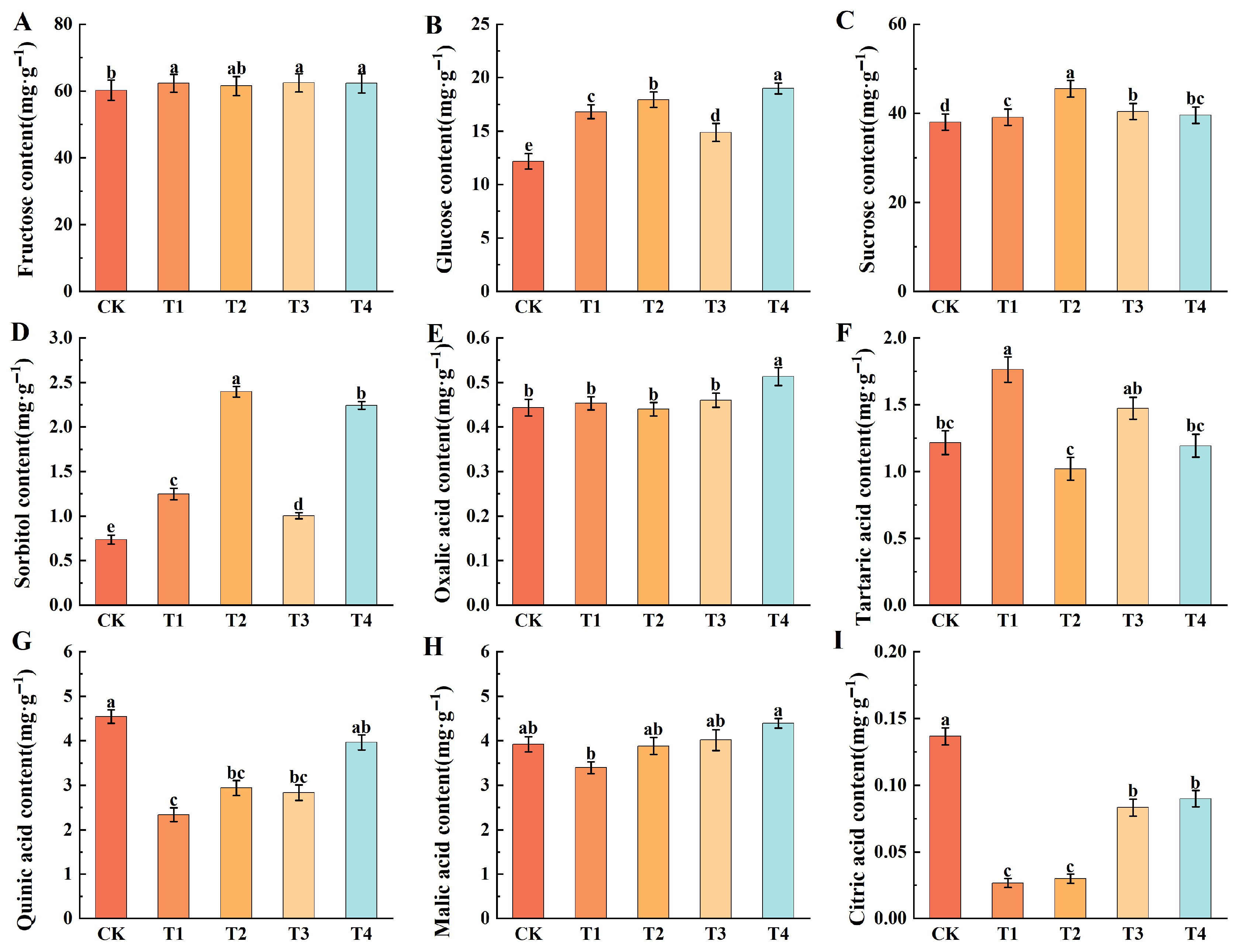
2.5. Effect of Different Foliar Fertilizers on the Sugar and Acid Components of Fruits
2.6. Effect of Different Foliar Fertilizers on Aroma Composition of Apple Fruits
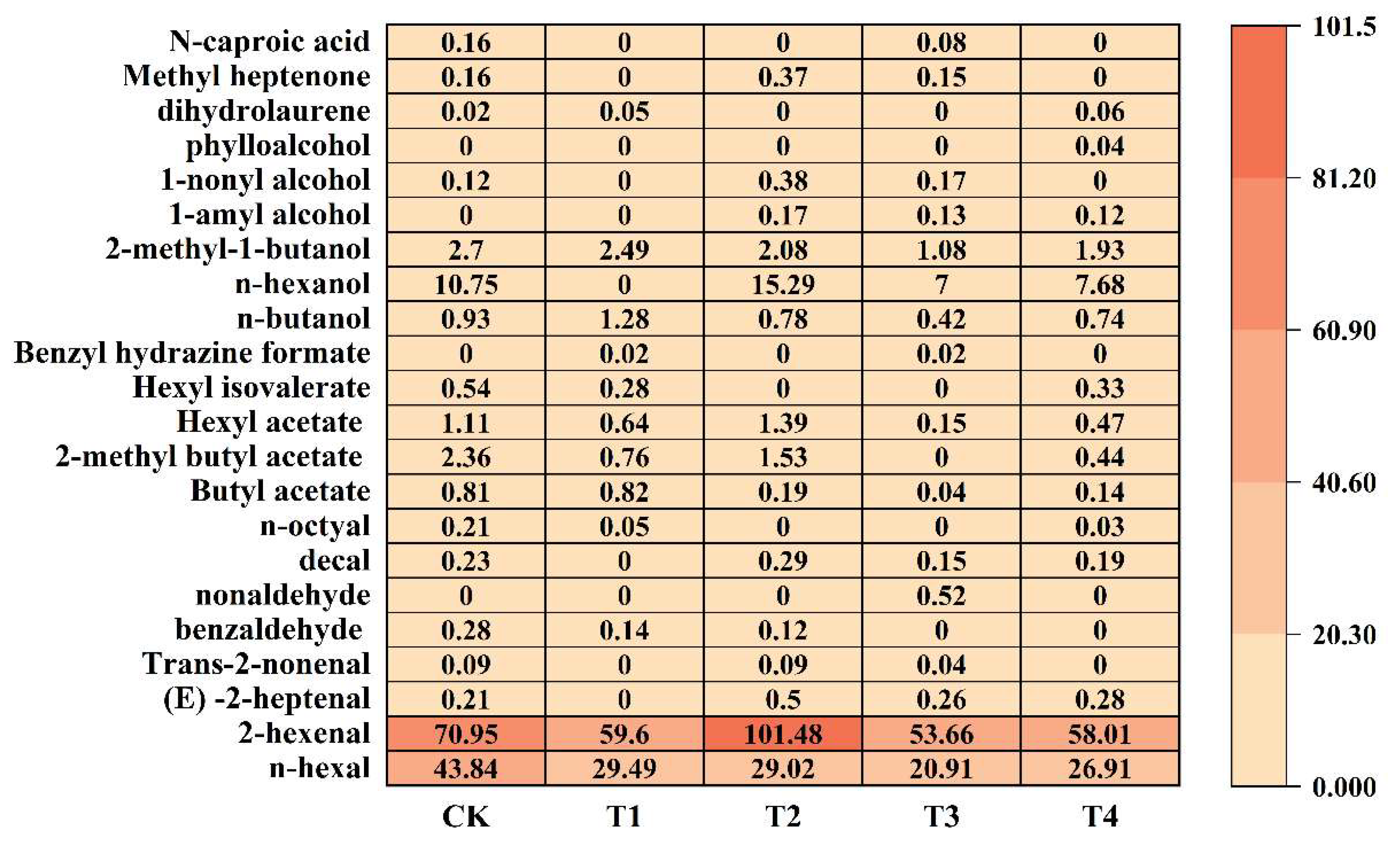
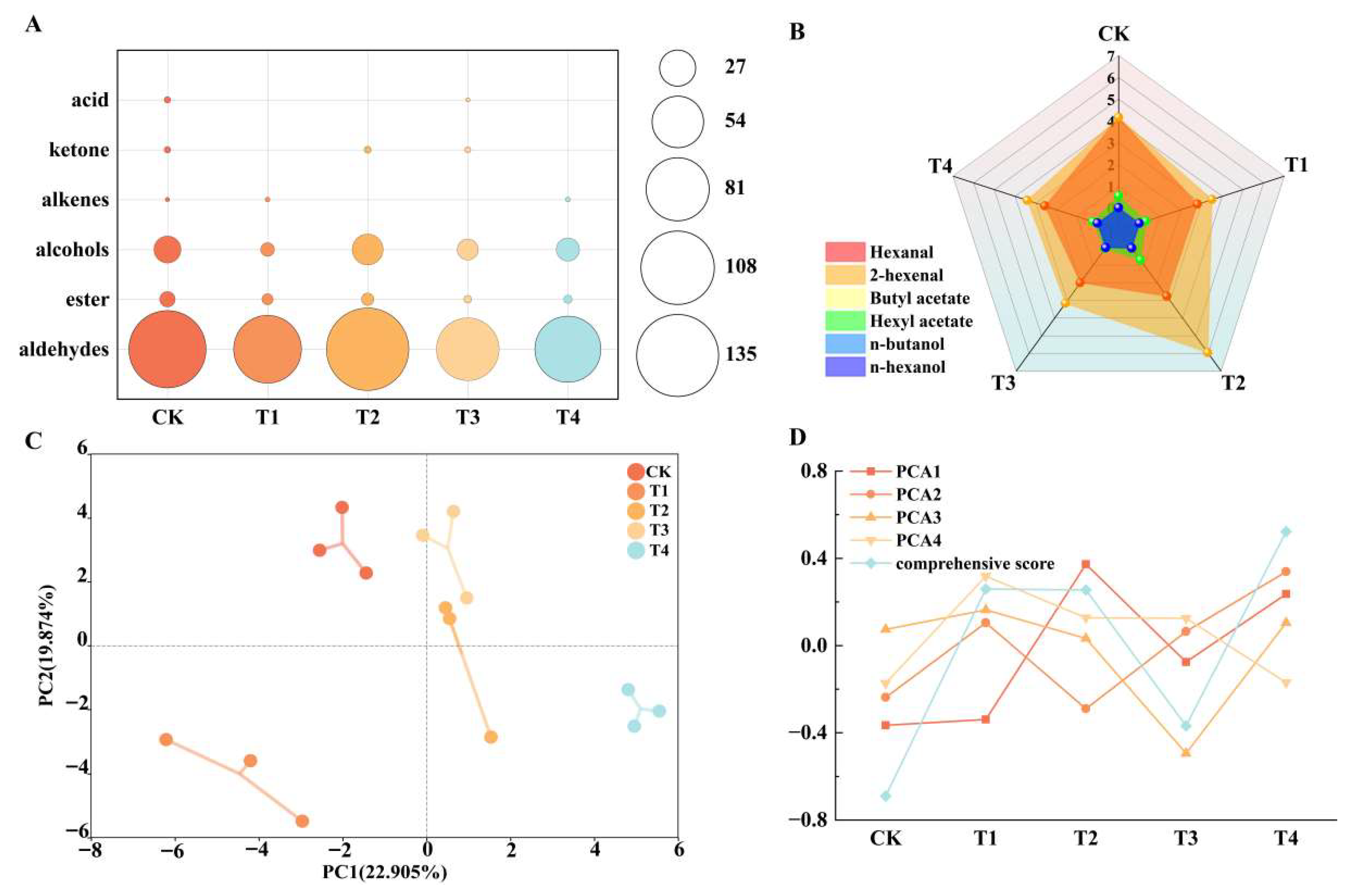
2.6.1. Effects of Different Foliar Fertilizers on the Contents of Aldehydes, Esters, Alcohols and Other Substances in Aroma Components
2.6.2. Changes of Total Aroma Substance Content and Characteristic Aroma Under Different Nutrient Foliar Fertilizer Treatments
2.7. Principal Component Analysis and Correlation Analysis
2.7.1. Principal Component Analysis
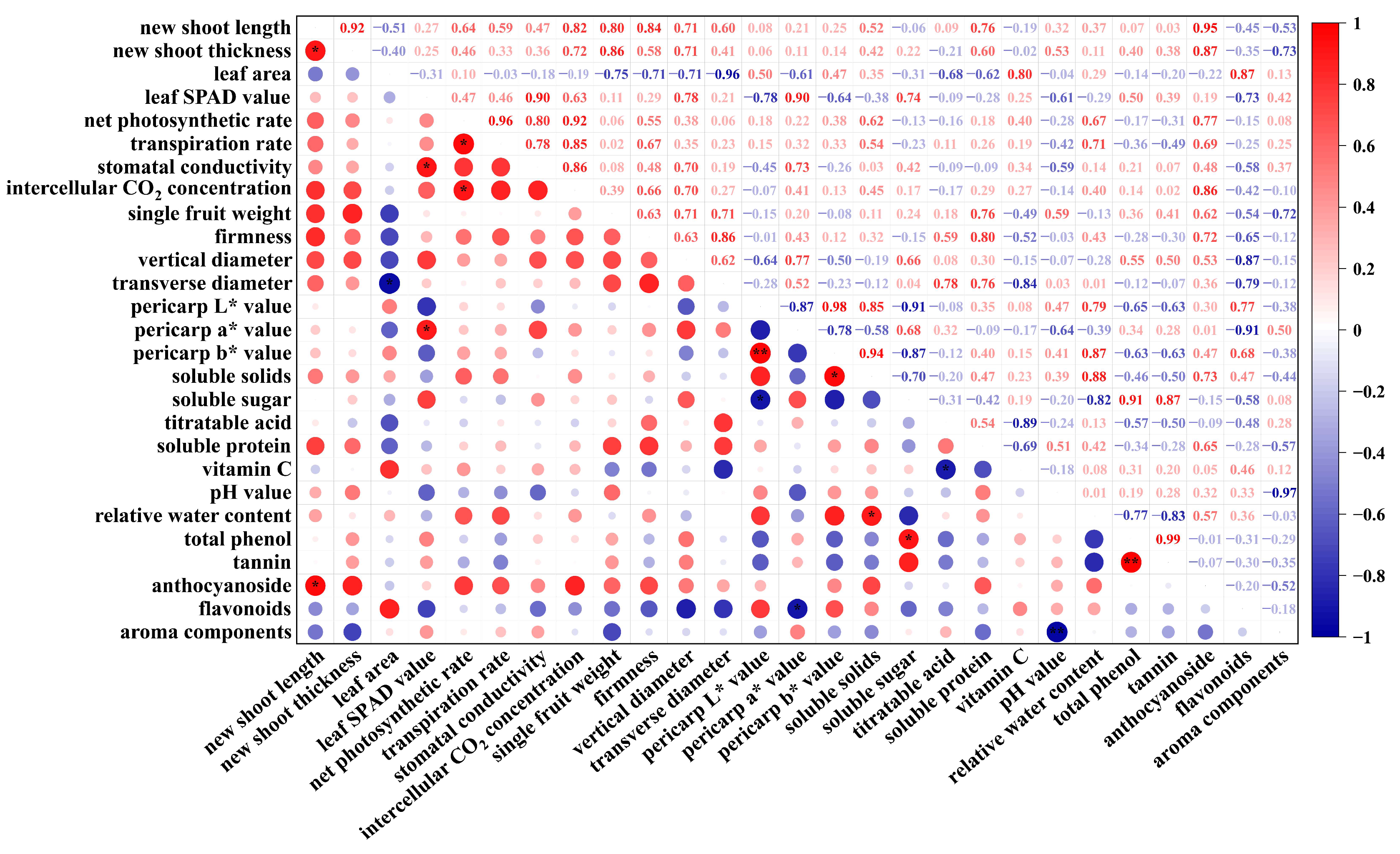
2.7.2. Analysis of Relationship
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Experimental Materials
4.2. Experimental Treatment and Design
4.3. Growth Index of New Shoots
4.4. Indices of Leaf Growth and Photosynthetic Characteristics
4.5. Determination of Fruit Appearance Quality
4.6. Determination of Soluble Solids, Soluble Sugar, Titratable Acidity, and Sugar-Acid Ratio
4.7. Determination of Soluble Protein, Vitamin C, pH and Relative Water Content
4.8. Determination of Total Phenols, Tannins, Anthocyanins and Flavonoids
4.9. High-Performance Liquid Chromatography (HPLC) Analysis of Sugars and Organic Acids
4.10. Determination of Aroma Substances and Their Thresholds
4.11. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, C.; Mu, Y.; Yuan, J.; Zhang, H.; Song, J.; Kang, S. Apple cultivar-influenced differences in cider: A comprehensive analysis of physicochemical and aroma compounds. Microchem. J. 2025, 208, 112382. [Google Scholar] [CrossRef]
- Wen, S.; Cui, N.; Wang, Y.; Gong, D.; Xing, L.; Wu, Z.; Zhang, Y.; Wang, Z. Deficit irrigation enhances yield and water productivity of apples by inhibiting excessive vegetative growth and improving photosynthetic performance. Agric. Water Manag. 2025, 307, 109220. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Wu, W.; Liao, Y. Mulching practices altered soil bacterial community structure and improved orchard productivity and apple quality after five growing seasons. Sci. Hortic. 2014, 172, 248–257. [Google Scholar] [CrossRef]
- Gao, X.; Mei, X.; Zhang, J.; Cai, J.; Gu, F.; Hao, W.; Gong, D. Comparison of three modified models in evapotranspiration and its components over a rainfed spring maize cropland on the Loess Plateau, China. Agric. For. Meteorol. 2023, 330, 109322. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Wang, W.; Zhang, L.; Wang, C.; Zhang, Q.; Wang, J.; Du, M.; Sheng, L.; Hu, D. MdCIbHLH1 modulates sugar metabolism and accumulation in apple fruits by coordinating carbohydrate synthesis and allocation. Hortic. Plant J. 2025, 11, 578–592. [Google Scholar] [CrossRef]
- Sredojevic, A.; Radivojevic, D.; Levic, S.M.; Aksic, M.F.; Milivojevic, J.; Djekic, I. Effects of the fruit harvest date and shelf-life nexus of apples on different quality perspectives. Appl. Sci. 2024, 14, 11737. [Google Scholar] [CrossRef]
- Hassan, M.R.; Arakawa, O.; Nissato, K.; Ito, D. Changes in the harvesting window and quality of apple fruit cultivated under long-term high temperature and CO2. Sci. Hortic. 2024, 338, 113611. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Q.; Feng, Y.; Yang, X. Foliar application protected vegetable against poisonous element cadmium and mitigated human health risks. Sci. Total Environ. 2024, 926, 171915. [Google Scholar] [CrossRef]
- Zaben Habib, J.; Nazhan Ali, O.; Ahmed Alsajri, F.; Hatta, M. Effect of shade covers and foliar fertilizer on the growth of pepper seeds. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 042011. [Google Scholar]
- Ishfaq, M.; Kiran, A.; Rehman, H.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- Khomphet, T.; Promwee, A.; Islam, S.S. Effects of foliar fertilizer application on the growth and fruit quality of commercial melon varieties grown in a soilless culture system. PeerJ 2023, 11, e14900. [Google Scholar] [CrossRef]
- Dong, H.; Li, F.; Xuan, X.; Ahiakpa, J.K.; Tao, J.; Zhang, X.; Ge, P.; Wang, Y.; Gai, W.; Zhang, Y. The genetic basis and improvement of photosynthesis in tomato. Hortic. Plant J. 2025, 11, 69–84. [Google Scholar] [CrossRef]
- Hong, T.; Cai, Z.; Li, R.; Liu, J.; Li, J.; Wang, Z.; Zhang, Z. Effects of water and nitrogen coupling on watermelon growth, photosynthesis and yield under CO2 enrichment. Agric. Water Manag. 2022, 259, 107229. [Google Scholar] [CrossRef]
- Therby-Vale, R.; Lacombe, B.; Rhee, S.Y.; Nussaume, L.; Rouached, H. Mineral nutrient signaling controls photosynthesis: Focus on iron deficiency-induced chlorosis. Trends Plant Sci. 2022, 27, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Y.; Liu, J.; Yan, J.; Zhu, K.; Sun, X.; Bu, X.; Wang, X.; Ahammed, G.J.; Liu, Y.; et al. Tetratricopeptide repeat protein SlREC2 positively regulates cold tolerance in tomato. Plant Physiol. 2023, 192, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Pan, C.; Li, X.; Huang, Z.; Shu, J.; Wang, X.; Lu, X.; Pan, F.; Hu, J.; Zhang, H.; et al. OBV (obscure vein), a C2H2 zinc finger transcription factor, positively regulates chloroplast development and bundle sheath extension formation in tomato (Solanum lycopersicum) leaf veins. Hortic. Res. 2021, 8, 230. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Khalid, M.H.; Zhou, X.B.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- El-Tanahy, A.M.M.; Mahmoud, S.H.; Elwahed, M.S.A.; Salama, D.M. Enhancing celery’s growth, production, quality, and nutritional status using tryptophan and glycine amino acids. Sci. Rep. 2024, 14, 26571. [Google Scholar] [CrossRef]
- Wang, T.; Tan, L.; Chen, Z.; Yang, Y.; Yuan, Y.; Zheng, Z.; Deng, L.; Zhang, M.; Sun, G.; He, S.; et al. Mitigating citrus fruit cracking: The efficacy of chelated calcium or silicon foliar fertilizers in ‘Okitsu no. 58’ citrus fruit. Front. Plant Sci. 2024, 15, 1402945. [Google Scholar] [CrossRef]
- Gholamnejad, S.; Haghighi, M.; Etemadi, N.; Pessarakli, M. Effects of boron on nutrient partitioning, Ca movement, and fruit quality of tomatoes. J. Plant Nutr. 2023, 46, 697–713. [Google Scholar] [CrossRef]
- Xu, W.; Wang, P.; Yuan, L.; Chen, X.; Hu, X. Effects of application methods of boron on tomato growth, fruit quality and flavor. Horticulturae 2021, 7, 223. [Google Scholar] [CrossRef]
- Singh, G.; Gschwend, A.R.; Dami, I.E. Effect of foliar application of potassium fertilizer on yield, fruit quality, and cold hardiness of Vitis spp. ‘Chambourcin’. Int. J. Fruit Sci. 2024, 24, 102–114. [Google Scholar] [CrossRef]
- Makale, A.R.; Mourice, S.K.; Kapinga, F.A. Assessment of farmers’ knowledge on the foliar fertilizer use for improved cashew (Anacardium occidentale L.) productivity in south-eastern Tanzania. J. Agric. Food Res. 2024, 16, 101092. [Google Scholar] [CrossRef]
- Pimentel, C.; Pina, C.M.; Müller, N.; Lara, L.A.; Melo Rodriguez, G.; Orlando, F.; Schoelkopf, J.; Fernández, V. Mineral particles in foliar fertilizer formulations can improve the rate of foliar uptake. Plants 2023, 13, 71. [Google Scholar] [CrossRef]
- Seo, H.J.; Sawant, S.S.; Lee, B.; Kim, K.; Song, J.; Choi, E.D. Mechanisms driving fruit cracking in ‘Sinhwa’ pears (Pyrus pyrifolia Nakai) and effect of foliar fertilizer application on fruit quality. Sci. Hortic. 2024, 332, 113232. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Xie, Z.; Zeng, G.; Wu, T.; Liu, J.; Lin, Z. Optimizing paclobutrazol application for regulating dwarfing in ougan (Citrus reticulata cv. Suavissima): Comprehensive insights from growth, photosynthesis, and physiological responses. Plants 2025, 14, 763. [Google Scholar] [CrossRef]
- Zheng, H.J.; Wang, X.; Ma, W.F.; Gou, H.M.; Liang, G.P.; Mao, J. Temporal variations in photosynthesis and leaf element contents of ‘Marselan’ grapevines in response to foliar fertilizer application. Plants 2025, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Gli, J.Q.H.; Khalaf, J.M. Effect of foliar application of humic acid and nano-NPK on some growth characteristic of apple trees Malus Domestica Borkh. IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 042027. [Google Scholar] [CrossRef]
- Hassan, A.; Margay, A.R.; Din, S. Effect of foliar sprays of phenylalanine, nano-potash and potassium sulphate on fruit quality attributes of apple (Malus × domestica Borkh.) cv. Ambri. Int. J. Plant Soil Sci. 2024, 36, 317–325. [Google Scholar] [CrossRef]
- Kufesh, S.A.; Abdul, A.S.; Al-Issawi, A.S. The impact of stem site and foliar application of zinc and boron in growth, flowers and yield of apples (Brahimi Class). IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 042038. [Google Scholar] [CrossRef]
- Seif El-Yazal, M.A.; Morsi, M.E. Metabolic changes in chemical constituents, flowering behavior, yield and fruit quality of ‘Anna’ apple (Malus sylvestris Mill) trees with foliar application some compounds. IJAGST 2022, 2, 30–39. [Google Scholar] [CrossRef]
- ValizadehKaji, B.; Mohammaei, M. Foliar application of biostimulants and potassium silicate enhances yield and fruit quality of mandarin Cv. ‘Page’. Appl. Fruit Sci. 2025, 67, 8. [Google Scholar] [CrossRef]
- Khan, A.S.; Munir, M.; Shaheen, T.; Tassawar, T.; Rafiq, M.A.; Ali, S.; Anwar, R.; Rehman, R.N.U.; Hasan, M.U.; Malik, A.U. Supplemental foliar applied mixture of amino acids and seaweed extract improved vegetative growth, yield and quality of citrus fruit. Sci. Hortic. 2022, 296, 110903. [Google Scholar] [CrossRef]
- Sajid, M.; Basit, A.; Shah, S.T.; Khan, A.; Ullah, I.; Bilal, M.; Khan, M.S.; Khan, W. Enhancing the quality and fruit yield of sweet cherry (Prunus avium) cultivars by foliar application of boron. Appl. Fruit Sci. 2024, 66, 485–494. [Google Scholar] [CrossRef]
- Guo, X.; Zhan, N.; Wang, Z.; Ding, F.; Yang, Z.; Cui, X. Effects of the combination of Bacillus aryabhattai and calcium peroxide on soil silicon and potassium contents, the yield and quality of facility tomato. Arch. Agron. Soil Sci. 2024, 70, 1–12. [Google Scholar] [CrossRef]
- Sediqui, N.; Amin, M.W.; Dawlatzai, N.; Gulab, G.; Poyesh, D.S.; Terada, N.; Sanada, A.; Kamata, A.; Koshio, K. Elucidation of shoot and root growth, physiological responses, and quality traits of tomato (Solanum lycopersicon L.) exposed to elevated calcium carbonate concentrations. Horticulturae 2024, 10, 573. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Wang, X.; Yan, L.; Hu, X.; Lian, M. Effect of foliar calcium fertilization on fruit quality, cell wall enzyme activity and expression of key genes in Chinese cherry. Int. J. Fruit Sci. 2023, 23, 200–216. [Google Scholar] [CrossRef]
- Walli, S.; Hafiz, I.A.; Khan, R.I.; Bashir, M.A.; Uddin, S.; Khan, S.U.; Ajmal, U.; Hussain, S.; Khalid, M.F. Zinc and boron application at different phenological stages alleviates tree growth, fruit yield and quality of sweet orange cv. ‘Blood Red’. Gesunde Pflanzen. 2022, 74, 385–396. [Google Scholar] [CrossRef]
- Thu, A.M.; Alam, S.M.; Khan, M.A.; Han, H.; Liu, D.H.; Tahir, R.; Ateeq, M.; Liu, Y.Z. Foliar spraying of potassium sulfate during fruit development comprehensively improves the quality of citrus fruits. Sci. Hortic. 2024, 338, 113696. [Google Scholar] [CrossRef]
- Malekzadeh, M.R.; Esmaeilizadeh, M.; Roosta, H.R. Optimizing strawberry growth and fruit quality through fertigation frequency and foliar application of potassium sulfate. J. Soil Sci. Plant Nutr. 2024, 24, 3042–3055. [Google Scholar] [CrossRef]
- Ali, Q.; Kurubas, M.S.; Ustun, H.; Balkhi, M.; Erkan, M. Evaluation of foliar organic fertilizer, biofertilizer and biological fungicide on the antioxidant compounds and postharvest quality attributes of strawberry fruit. Erwerbs-Obstbau 2022, 64, 365–376. [Google Scholar] [CrossRef]
- Yu, J.Q.; Ji, F.Y.; Yang, X.K.; Cheng, Y.; Gao, H.S.; Sheng, L.X. A genome-wide investigation of the mechanism underlying the effect of exogenous boron application on sugar content and overall quality of ‘Benihoppe’ strawberries. Plant Physiol. Biochem. 2024, 216, 109116. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chi, C.; Lv, S.; Tong, Y.; Yang, L. Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in ’Harward‘ kiwifruit (Actinidia deliciosa). Open Life Sci. 2025, 20, 20251114. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Du, J.J.; Ma, W.Q.; Chen, T.T.; Shui, X.; Zhou, K.B. Effect of calcium and magnesium foliar fertilizer on sugar contents and sugar metabolizing enzyme activities in ‘Feizixiao’ litchi pulp. J. Trop. Biol. 2024, 15, 217–223. [Google Scholar]
- Cook, B.S.; Brown, M.G.; Lin, Y.; Kwasniewski, M.T.; Ac-Pangan, M.F.; Stewart, A.C.; Peck, G.M. Foliar urea applications to apple trees increase yeast assimilable nitrogen, amino acids, and flavor volatiles in fruit and hard cider. J. Agric. Food Res. 2024, 16, 101136. [Google Scholar] [CrossRef]
- Henderson, B.C.R.; Sanderson, J.M.; Fowles, A. A review of the foliar application of individual amino acids as biostimulants in plants. Discov. Agric. 2025, 3, 69. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, X.; Gu, X.; Deng, M.; Li, X.; Zhou, A.; Suo, M.; Gao, W.; Lin, Y.; Wang, Y.; et al. Light quality and sucrose-regulated detached ripening of strawberry with possible involvement of abscisic acid and auxin signaling. Int. J. Mol. Sci. 2023, 24, 5681. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Zhang, X.; Hu, W.; Lin, L.; Deng, Q.; Xia, H.; Liang, D.; Lv, X. Effects of potassium-containing fertilizers on sugar and organic acid metabolism in grape fruits. Int. J. Mol. Sci. 2024, 25, 2828. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Li, W. Comparison of physiochemical characteristics of Lanzhou bulb of lily during storage periods. Postharvest Biol. Technol. 2025, 219, 113271. [Google Scholar] [CrossRef]
- Yang, T.; Samarakoon, U.; Altland, J.; Ling, P. Photosynthesis, biomass production, nutritional quality, and flavor-related phytochemical properties of hydroponic-grown arugula (Eruca sativa Mill.) ‘Standard’ under different electrical conductivities of nutrient solution. Agronomy 2021, 11, 1340. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Khomdram, S.D.; Singh, P.K. Polyphenolic compounds and free radical scavenging activity in eight Lamiaceae herbs of Manipur. Not. Sci. Biol. 2011, 3, 108–113. [Google Scholar] [CrossRef][Green Version]
- Nastasi, J.R.; Fitzgerald, M.A.; Kontogiorgos, V. Tuning the mechanical properties of pectin films with polyphenol-rich plant extracts. Int. J. Biol. Macromol. 2023, 253, 127536. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Y.; Wang, T.; Jiang, B.; Liao, M.; Lv, Y.; Li, J.; Zhong, Y. Dynamic analysis of the fruit sugar-acid profile in a fresh-sweet mutant and wild type in ‘Shatangju’ (Citrus reticulata cv.). Plants 2024, 13, 2722. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Q.; Tian, D.; Yuan, W.; Tang, X.; Deng, X.; Liu, Y.; Gao, C.; Fan, G.; Xiao, X.; et al. HS-SPME-GC-MS combined with relative odor activity value identify the key aroma components of flowery and fruity aroma in different types of GABA tea. Food Chem. X 2024, 24, 101965. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Foliar Fertilizer | Spray Concentration | Active Principle | Producer | Price (1000 g) |
|---|---|---|---|---|---|
| T1 | Ye Xiumei | 0.2 g·L−1 | amino acid content of 100 g·L−1, Cu + Fe + Mn + Zn + B ≥ 20 g·L−1 | Qingdao Qianhechun Biotechnology Co., Ltd., Qingdao, China | 50 yuan |
| T2 | Fluid calcium | 0.067 g·L−1 | Ca ≥ 180 g·L−1, sugar alcohol ≥ 50 g·L−1 | Shandong Ruipu Biotechnology Co., Ltd., Zouping, China | 30 yuan |
| T3 | Sugar alcohol boron | 0.05 g·L−1 | B ≥ 160 g·L−1 | Shandong Ruipu Biotechnology Co., Ltd., Zouping, China | 45 yuan |
| T4 | Mineral source potassium fulvic acid | 0.05 g·L−1 | soluble potassium fulvic acid ≥ 50%, and K2O ≥ 12% | Shandong Ruipu Biotechnology Co., Ltd., Zouping, China | 75 yuan |
| CK | water | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.-F.; Li, S.-M.; Ma, W.-F.; Lu, S.-X.; Bian, Z.-Y.; Liang, G.-P.; Mao, J. Spraying Foliar Fertilizer Affect the Physiological Function of Leaf and Improve the Quality of ‘Snick’ Apple. Plants 2025, 14, 2926. https://doi.org/10.3390/plants14182926
Xu H-F, Li S-M, Ma W-F, Lu S-X, Bian Z-Y, Liang G-P, Mao J. Spraying Foliar Fertilizer Affect the Physiological Function of Leaf and Improve the Quality of ‘Snick’ Apple. Plants. 2025; 14(18):2926. https://doi.org/10.3390/plants14182926
Chicago/Turabian StyleXu, Hong-Fu, Shi-Mei Li, Wei-Feng Ma, Shi-Xiong Lu, Zhi-Yuan Bian, Guo-Ping Liang, and Juan Mao. 2025. "Spraying Foliar Fertilizer Affect the Physiological Function of Leaf and Improve the Quality of ‘Snick’ Apple" Plants 14, no. 18: 2926. https://doi.org/10.3390/plants14182926
APA StyleXu, H.-F., Li, S.-M., Ma, W.-F., Lu, S.-X., Bian, Z.-Y., Liang, G.-P., & Mao, J. (2025). Spraying Foliar Fertilizer Affect the Physiological Function of Leaf and Improve the Quality of ‘Snick’ Apple. Plants, 14(18), 2926. https://doi.org/10.3390/plants14182926






