Pleurotus eryngii Stipe Base-Derived Carbon Dots Enhanced the Growth and Salt Tolerance of Tomato
Abstract
1. Introduction
2. Results
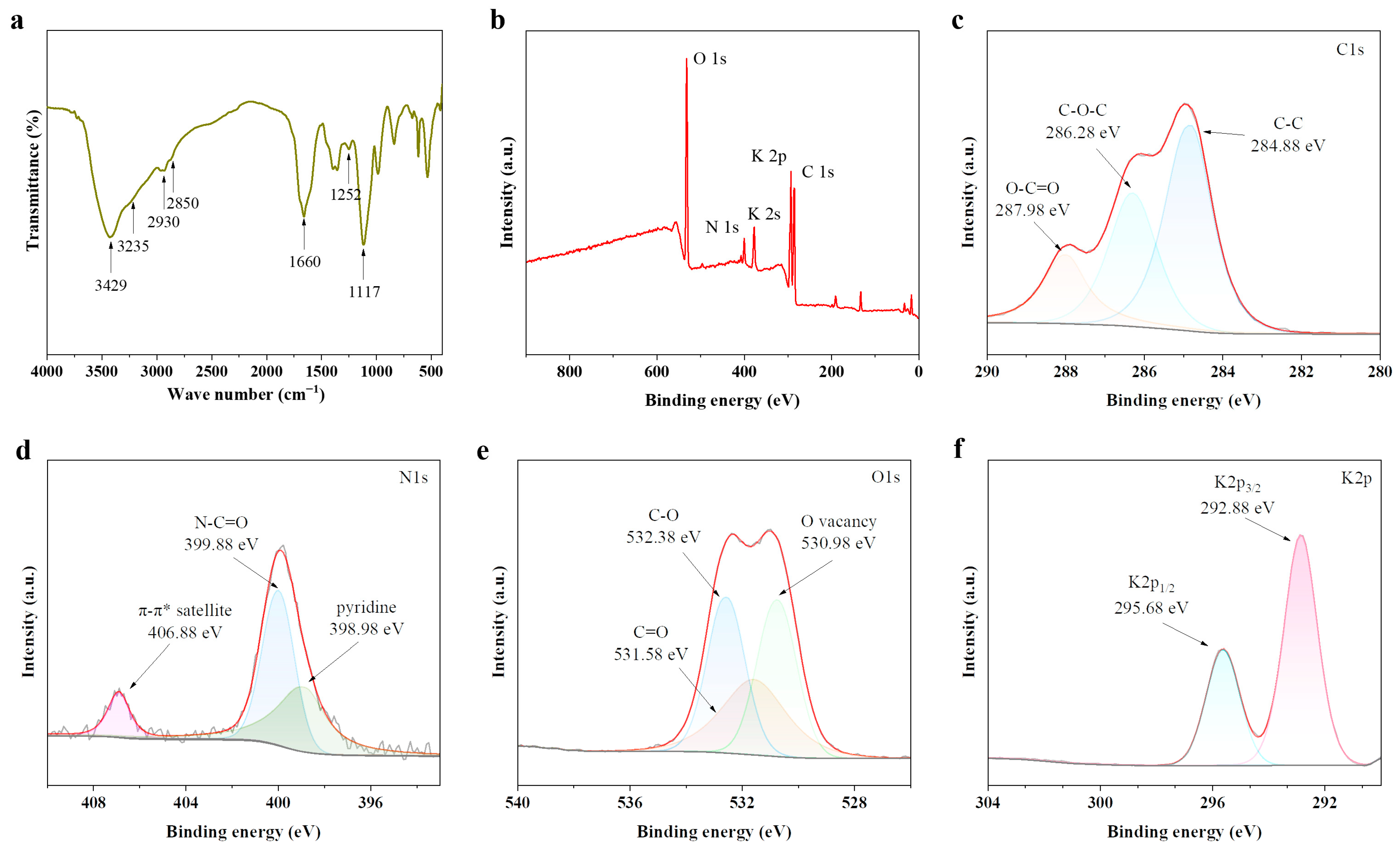
2.1. Structural and Morphologic Characterization of PbCDs
2.2. PbCD Treatment Promoted Tomato Growth and Enhanced Tomato Salt Tolerance
2.3. PbCD Treatment Affected Tomato Cell Expansion and Division by Altering Cell Cycle Progression Under Control and Salt Conditions
2.4. PbCDs Enhanced Photosynthetic Capacity, Thereby Promoting Tomato Growth and Increasing Salt Tolerance
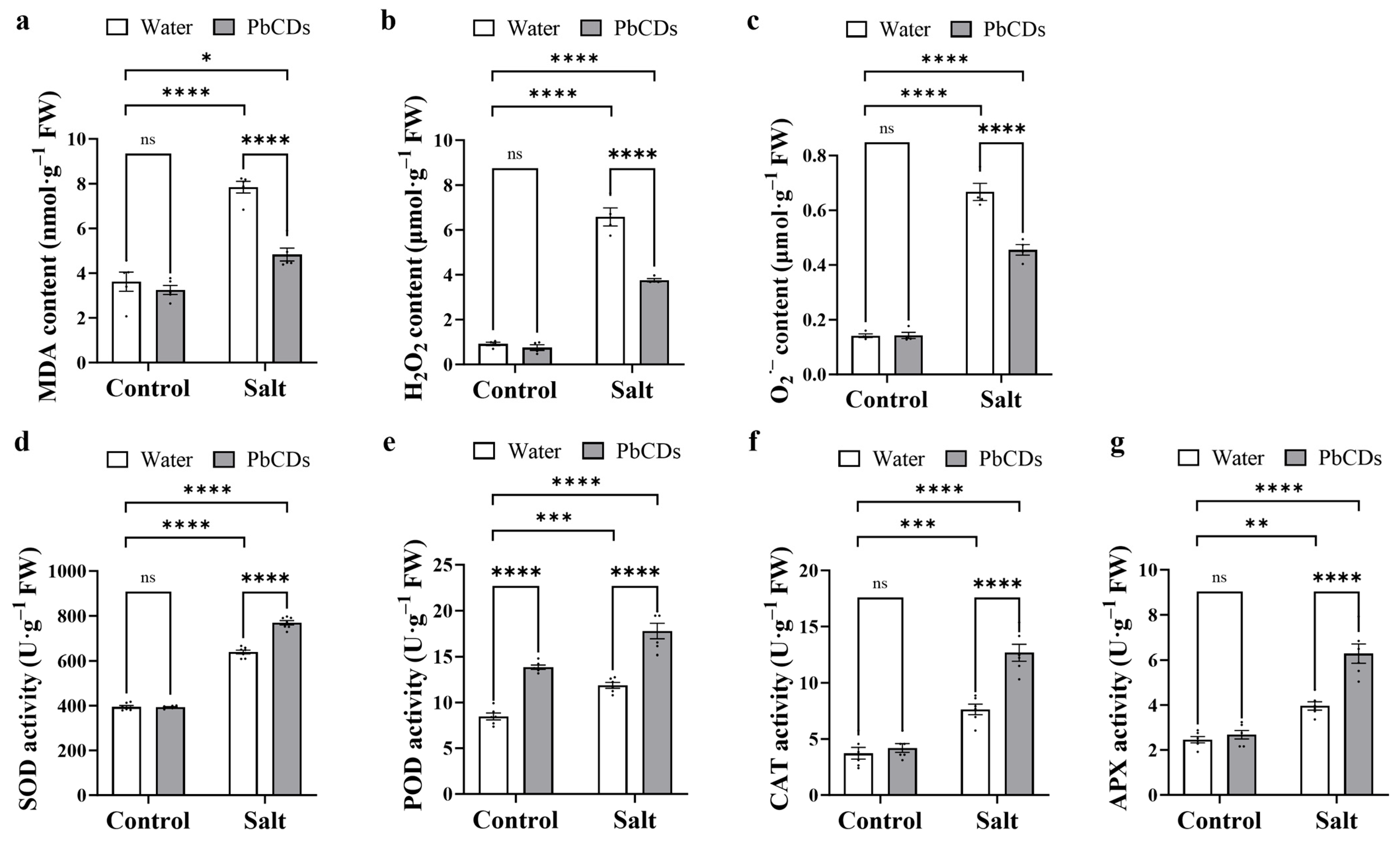
2.5. PbCD Treatment Alleviated Salt Stress-Induced Oxidative Stress and Activated the Antioxidant Enzyme Activity in Tomato
2.6. Correlation Analysis of Physiological Parameters of Tomato Treated with PbCDs Under Salt Stress
3. Discussion
4. Materials and Methods
4.1. The Synthesis and Characterization of PbCDs
4.2. Plant Materials Condition
4.3. Salt Treatment
4.4. Degree of Growth Inhibition Analysis
4.5. Cell Area, Cell Number, and DNA Ploidy Analysis
4.6. Photosynthetic-Related Parameter Analysis
4.7. Assessment of Resistance-Related Indicators
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Chen, L.C.; Shi, Q.H.; Ren, Z.H. SlMYB102, an R2R3-type MYB Gene, Confers Salt Tolerance in Transgenic Tomato. Plant Sci. 2020, 291, 110356. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.K.; Zhao, C.Z.; Zhao, Y.; Persson, S. The Cell Biology of Primary Cell Walls during Salt Stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Singh, A.; Rajput, V.D.; Sharma, R.; Ghazaryan, K.; Minkina, T. Salinity Stress and Nanoparticles: Insights into Antioxidative Enzymatic Resistance, Signaling, and Defense Mechanisms. Environ. Res. 2023, 235, 116585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schimel, J.P.; Nisbet, R.M.; Gardea-Torresdey, J.L.; Holden, P.A. Soybeans Grown with Carbonaceous Nanomaterials Maintain Nitrogen Stoichiometry by Assimilating Soil Nitrogen to Offset Impaired Dinitrogen Fixation. ACS Nano 2020, 14, 585–594. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.T.; Jia, H.Y.; Liu, C.Y.; Wang, D.B.; Sun, X.C. Foliar Exposure of FeO Nanoparticles on: Evidence for Nanoparticles Uptake, Plant Growth Promoter and Defense Response Elicitor against Plant Virus. J. Hazard. Mater. 2020, 393, 122415. [Google Scholar] [CrossRef]
- Li, Y.J.; Tang, Z.H.; Pan, Z.Y.; Wang, R.G.; Wang, X.; Zhao, P.; Liu, M.; Zhu, Y.X.; Liu, C.; Wang, W.C.; et al. Calcium-Mobilizing Properties of Derived Carbon Dots Confer Enhanced Environmental Adaptability in Plants. ACS Nano 2022, 16, 4357–4370. [Google Scholar] [CrossRef]
- Li, G.; Xu, J.; Xu, K. Physiological Functions of Carbon Dots and Their Applications in Agriculture: A Review. Nanomaterials 2023, 13, 2684. [Google Scholar] [CrossRef]
- Li, Y.D.; Xu, X.K.; Wu, Y.; Zhuang, J.L.; Zhang, X.J.; Zhang, H.R.; Lei, B.F.; Hu, C.F.; Liu, Y.L. A Review on the Effects of Carbon Dots in Plant Systems. Mater. Chem. Front. 2020, 4, 437–448. [Google Scholar] [CrossRef]
- Swift, T.A.; Fagan, D.; Benito-Alifonso, D.; Hill, S.A.; Yallop, M.L.; Oliver, T.A.A.; Lawson, T.; Galan, M.C.; Whitney, H.M. Photosynthesis and Crop Productivity are Enhanced by Glucose-functionalised Carbon Dots. New Phytol. 2021, 229, 783–790. [Google Scholar] [CrossRef]
- Kou, E.F.; Yao, Y.Y.; Yang, X.; Song, S.W.; Li, W.; Kang, Y.Y.; Qu, S.N.; Dong, R.Y.; Pan, X.Q.; Li, D.N.; et al. Regulation Mechanisms of Carbon Dots in the Development of Lettuce and Tomato. ACS Sustain. Chem. Eng. 2021, 9, 944–953. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, X.F.; Nie, X.K.; Li, Y.Y.; Liang, T.B.; Ci, L.J. Alleviation Role of Functional Carbon Nanodots for Tomato Growth and Soil Environment under Drought Stress. J. Hazard. Mater. 2022, 423, 127260. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, X.F.; Li, Y.Y.; Sun, Q.; Dai, L.N.; Li, J.W.; Guo, Z.J.; Zhang, L.; Ci, L.J. Functional Carbon Nanodots Improve Soil Quality and Tomato Tolerance in Saline-Alkali Soils. Sci. Total Environ. 2022, 830, 154817. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Bystrzejewski, M.; De Adhikari, A.; Huczko, A.; Wang, N.N. Methods for the Conversion of Biomass Waste into Value-Added Carbon Nanomaterials: Recent Progress and Applications. Prog. Energy Combust. 2022, 92, 101023. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Biotechnological, Nutritional and Therapeutic Uses of Pleurotus spp. (Oyster mushroom) Related with its Chemical Composition: A review on the Past Decade Findings. Trends Food Sci. Tech. 2016, 50, 103–117. [Google Scholar]
- Duan, Q.X.; He, Y.S.; Long, X.; Wang, J.Y.; Ni, C.J.; Wu, S.G. Synthesis of Green Photoluminescence Carbon Dots Using Edible Seafood Mushroom and Their Anti-Counterfeiting Applications. Adv. Sustain. Syst. 2024, 8, 536. [Google Scholar]
- Wang, W.J.; Xia, J.M.; Feng, J.; He, M.Q.; Chen, M.L.; Wang, J.H. Green Preparation of Carbon Dots for Intracellular pH Sensing and Multicolor Live Cell Imaging. J. Mater. Chem. B 2016, 4, 7130–7137. [Google Scholar] [CrossRef]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-Derived Carbon Dots for Toxic Metal Ion Detection and as Antibacterial and Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M. Fungus-Derived Photoluminescent Carbon Nanodots for Ultrasensitive Detection of Hg Ions and Photoinduced Bactericidal Activity. Sensor Actuat B-Chem. 2018, 258, 172–183. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Caglar, O.; Sargin, I.; Arslan, G. Synergistic Role of Carbon Quantum Dots in the Activity and Stability of Candida rugosa Lipase Encapsulated within Metal–Organic Frameworks (ZIF-8). Mater. Today Commun. 2022, 30, 103066. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.X.; Yang, Q.C. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Yang, K.C.; Liu, M.X.; Wang, Y.Y.; Wang, S.S.; Miao, H.; Yang, L.Q.; Yang, X.M. Carbon Dots Derived from Fungus for Sensing Hyaluronic Acid and Hyaluronidase. Sensor Actuat B-Chem. 2017, 251, 503–508. [Google Scholar] [CrossRef]
- Sargin, I.; Yanalak, G.; Arslan, G.; Patir, I.H. Green Synthesized Carbon Quantum Dots as TiO Sensitizers for Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2019, 44, 21781–21789. [Google Scholar] [CrossRef]
- Gurusamy, L.; Karuppasamy, L.; Anandan, S.; Barton, S.C.; Chuang, Y.H.; Liu, C.H.; Wu, J.J. Review of Oxygen-Vacancies Nanomaterials for Non-Enzymatic Electrochemical Sensors Application. Coord. Chem. Rev. 2023, 484, 215102. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Budka, A.; Niedzielski, P.; Mleczek, P.; Kuczynska-Kippen, N.; Budzynska, S.; Karolewski, Z.; Kalac, P.; Jedryczka, M. Can the Concentration of Elements in Wild-Growing Mushrooms be Deduced from the Taxonomic Rank? Environ. Res. 2024, 252, 119079. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth Regulation, Signaling, and Environmental Stress Tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Liang, X.Y.; Li, J.F.; Yang, Y.Q.; Jiang, C.F.; Guo, Y. Designing Salt Stress-Resilient Crops: Current Progress and Future Challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; Testerink, C. Tuning Plant Signaling and Growth to Survive Salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.C.; Stojilkovic, B.; Zhang, X.Y.; Sablowski, R. Plant cell size: Links to Cell Cycle, Differentiation and Ploidy. Curr. Opin. Plant Biol. 2024, 78, 102527. [Google Scholar] [CrossRef]
- Shabala, S.; Wu, H.H.; Bose, J. Salt Stress Sensing and Early Signalling Events in Plant Roots: Current Knowledge and Hypothesis. Plant Sci. 2015, 241, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Pan, X.Q.; Xu, X.K.; Wu, Y.; Zhuang, J.L.; Zhang, X.J.; Zhang, H.R.; Lei, B.F.; Hu, C.F.; Liu, Y.L. Carbon Dots as Light Converter for Plant Photosynthesis: Augmenting Light Coverage and Quantum Yield Effect. J. Hazard. Mater. 2021, 410, 124534. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Z.L.; Gong, B.; Shi, Q.H. S-Adenosylmethionine Synthetase 1 Confers Drought and Salt Tolerance in Transgenic Tomato. Environ. Exp. Bot. 2020, 179, 104226. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.X.; Chen, L.C.; Ren, Z.H. Comprehensive Analysis of Multiprotein Bridging Factor 1 Family Genes and SlMBF1c Negatively Regulate the Resistance to Botrytis cinerea in Tomato. BMC Plant Biol. 2019, 19, 437. [Google Scholar] [CrossRef]
- Czerednik, A.; Busscher, M.; Angenent, G.C.; de Maagd, R.A. The Cell Size Distribution of Tomato Fruit can be Changed by Overexpression of CDKA1. Plant Biotechnol. J. 2015, 13, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, Y.; Wang, H.; Dang, Y.; Wang, W.; Gao, Y.; Han, Y.; Kang, R.; Shi, Q.; Du, H. Nitrogen-Doped Car-bon Dots Alleviate Pesticide Toxicity in Tomato by Regulating Antioxidant Systems. Int. J. Mol. Sci. 2025, 26, 9916. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gao, Y.; Wang, W.; Wang, H.; Xin, Y.; Kang, R.; Nie, W.; Du, H.; Shi, Q. Pleurotus eryngii Stipe Base-Derived Carbon Dots Enhanced the Growth and Salt Tolerance of Tomato. Plants 2025, 14, 3227. https://doi.org/10.3390/plants14203227
Zhang X, Gao Y, Wang W, Wang H, Xin Y, Kang R, Nie W, Du H, Shi Q. Pleurotus eryngii Stipe Base-Derived Carbon Dots Enhanced the Growth and Salt Tolerance of Tomato. Plants. 2025; 14(20):3227. https://doi.org/10.3390/plants14203227
Chicago/Turabian StyleZhang, Xu, Yi Gao, Wenhui Wang, Hao Wang, Yu Xin, Rongrui Kang, Wenfeng Nie, Han Du, and Qinghua Shi. 2025. "Pleurotus eryngii Stipe Base-Derived Carbon Dots Enhanced the Growth and Salt Tolerance of Tomato" Plants 14, no. 20: 3227. https://doi.org/10.3390/plants14203227
APA StyleZhang, X., Gao, Y., Wang, W., Wang, H., Xin, Y., Kang, R., Nie, W., Du, H., & Shi, Q. (2025). Pleurotus eryngii Stipe Base-Derived Carbon Dots Enhanced the Growth and Salt Tolerance of Tomato. Plants, 14(20), 3227. https://doi.org/10.3390/plants14203227







