1. Introduction
Grape (
Vitis spp.) is a perennial vine fruit tree that is widely planted throughout the world and plays an important role in the fields of fresh food, winemaking, and drying [
1]. Shine Muscat grape (
Vitis labrusca L. ×
Vitis vinifera L.) is favored in domestic and foreign markets due to its unique sweet taste, rich rose aroma, and good storage–transport resistance, creating substantial economic benefits for the grape industry [
2]. However, under the context of global climate change, low-temperature stress has become a key environmental constraint that limits the growth, development, yield, and quality improvement of Shine Muscat grape [
3]. Thus, exploring the regulatory mechanism of dopamine (Da) on its cold tolerance and proposing effective improvement strategies are of great practical significance.
Low-temperature stress causes multilevel harm to grapes: at the growth level, it inhibits germination, flowering, and fruit development, leading to reduced fruit set rate and deteriorated quality [
4]; at the physiological level, it destroys the synthesis of photosynthetic pigments and the function of photosystem II (PSII), thereby lowering photosynthetic efficiency [
5]; and at the cellular level, it increases cell membrane permeability, disrupts ion balance, and induces excessive accumulation of reactive oxygen species (ROS), ultimately resulting in membrane lipid peroxidation and metabolic imbalance [
6]. In recent years, using exogenous substances to regulate plant stress resistance has become a research focus. A widely existing bioactive substance, Da has been proven to alleviate low-temperature stress damage in multiple crops by regulating proline content, antioxidant enzyme activity, and polyamine metabolism [
7,
8,
9].
Despite the verified stress-resistant effect of Da in various crops [
10,
11], research on grapes—especially the Shine Muscat cultivar—remains insufficient. Dai et al. only reported the effect of Da on the photosynthetic performance and quality of Shine Muscat grape in the Hexi Corridor of Gansu Province without involving the regulatory mechanism of cold tolerance [
12]. Existing studies on Shine Muscat cold resistance mainly focus on rootstock screening [
13,
14], traditional cold prevention measures [
4], or other exogenous substances [
5,
11,
12], while the specific mechanism of Da regulating Shine Muscat response to low-temperature stress—including its synergistic role in photosynthetic system protection, ion homeostasis maintenance, antioxidant defense, and cold-responsive gene expression—remains unclear. Additionally, previous studies on the physiological response of Shine Muscat saplings grafted with different rootstocks under combined salt and low-temperature stress indicated that ion homeostasis is the core of stress resistance, but whether exogenous Da can directly regulate this process still needs exploration [
13]. Moreover, studies on the mechanism of exogenous substances improving grape cold tolerance point out that most regulatory substances have concentration-dependent threshold effects, yet the optimal concentration of Da for Shine Muscat and its multi-pathway synergistic mechanism under low-temperature stress are still unclear [
15].
To address the current gap in research on the regulatory mechanism of Da in the cold resistance of Shine Muscat grapes, this study used 1-year-old Shine Muscat grape seedlings as experimental material, set up five gradient Da concentration treatment groups, and explored the multi-pathway synergistic regulatory network of Da in regulating the cold resistance of Shine Muscat grapes. This study aimed to verify the concentration effect law of Da on the cold resistance of Shine Muscat grapes and clarify the optimal Da concentration threshold for cold-resistance regulation. We analyzed the multidimensional synergistic mechanisms of Da in protecting the photosynthetic system, maintaining cellular osmotic balance, preserving ion homeostasis, activating the antioxidant defense system, and regulating cold-responsive gene expression in grape plants under low-temperature stress. The results of this study will help fill the theoretical gap in Da research related to the cold resistance of Shine Muscat grapes, provide directly applicable and precise technical parameters for the application of Da in cold-resistant cultivation practice of grapes, and further offer important theoretical support and practical reference for stress resistance genetic improvement and cultivation technology optimization of cold-sensitive fruit crops, thereby contributing to promoting technological breakthroughs and industrial application upgrading in the field of fruit tree cold-resistance research.
2. Results
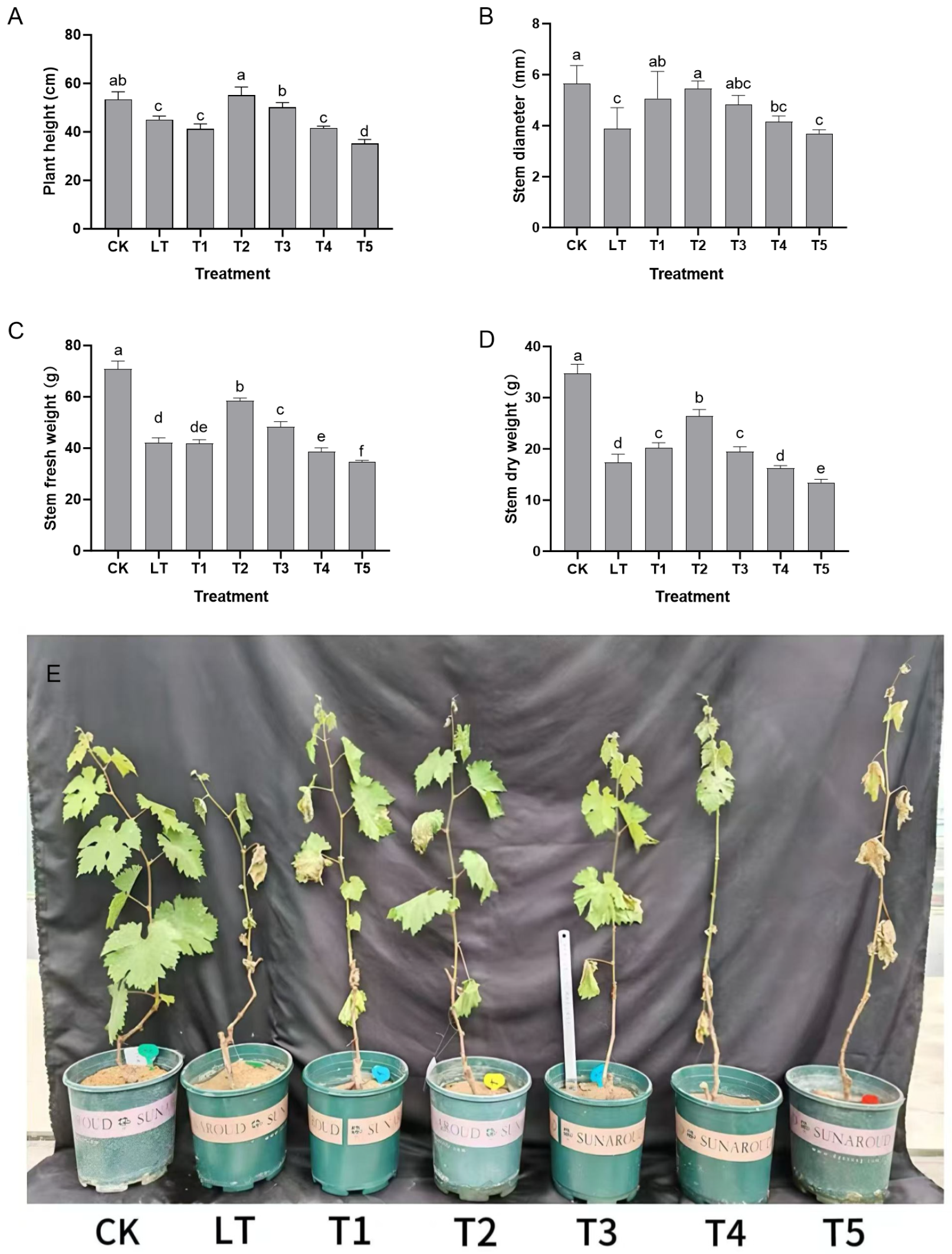
2.1. Effect of Exogenous Dopamine on the Growth of Shine Muscat Grapes Under Low-Temperature Stress
Under normal growth conditions (CK group), the plant height reached 53.4 cm, the stem diameter 5.65 mm, the fresh stem weight was 70.88 g, and the dry stem weight was 34.75 g. The overall growth was characterized by upright and straight new shoots, a robust and resilient stem base, fully spread and lustrous leaves, and a coordinated development of nutrient accumulation, cell elongation, and substance synthesis (
Figure 1).
Low-temperature stress (LT group) significantly disrupted this growth balance. The appearance of the seedlings showed a significant decline: the plant height dropped to 45.03 cm (a decrease of 15.7%), the stem diameter shrank to 3.89 mm (a reduction of 31.1%), and the fresh stem weight and the dry stem weight decreased to 42.13 g (a reduction of 40.6%) and 17.34 g (a reduction of 50.1%), respectively (
Figure 1A–D). The plant became shorter overall, the stem became thin and weak, prone to lodging, the leaf edges curled slightly, and the leaf color became pale and lacked luster (
Figure 1E). This directly demonstrated the overall inhibition of low temperature on the longitudinal elongation, lateral thickening, and substance accumulation of the plants.
After treatment with different concentrations of dopamine, the appearance of the seedlings showed a regular change pattern of “low promotion and high inhibition” with concentration: the 0.4 mmol L
−1 treatment (T2 group) had the best effect, with a plant height of 55.13 cm, a stem diameter of 5.46 mm, a fresh stem weight of 58.48 g, and a dry stem weight of 26.39 g. Compared with the LT group, these values increased by 22.4%, 40.6%, 38.8%, and 52.2%, respectively. At this time, the new shoots of the seedlings regained their uprightness, the stems became as thick as those of the CK group, the degree of leaf spread increased, and the leaf color deepened. The plant height and stem diameter even exceeded those of the CK group and all indicators were synergistically enhanced, highlighting the effective alleviation of low-temperature stress. When the concentration was increased to 0.6 mmol L
−1 (T3 group), the appearance of the seedlings began to decline, the stem was slightly thinner than that of the T2 group, and the degree of leaf spread decreased. For ≥0.8 mmol L
−1 treatment (T4 and T5 groups), the growth inhibition intensified: the plant height of the T5 group (1.0 mmol L
−1) was only 35.17 cm, the stem diameter was 3.69 mm, the fresh stem weight was 34.58 g, and the dry stem weight was 13.43 g (lower than the LT group). Overall, the plants became significantly shorter, the stems became thin, fragile, and prone to lodging, and the leaves showed mild wilting and a pale color, reflecting the comprehensive inhibition of growth by high concentrations of dopamine (
Figure 1).
In summary, the dynamic appearance of the seedlings in all six groups was highly synchronous with such growth indicators as plant height and stem diameter. This jointly confirmed the blocked growth state of Shine Muscat grapes under low-temperature stress and the concentration-dependent regulatory effect of exogenous dopamine. The 0.4 mmol L−1 treatment most effectively coordinate the longitudinal growth, lateral development, and substance accumulation of the plants and achieved the best alleviation of low-temperature stress by improving the plant type and growth condition.
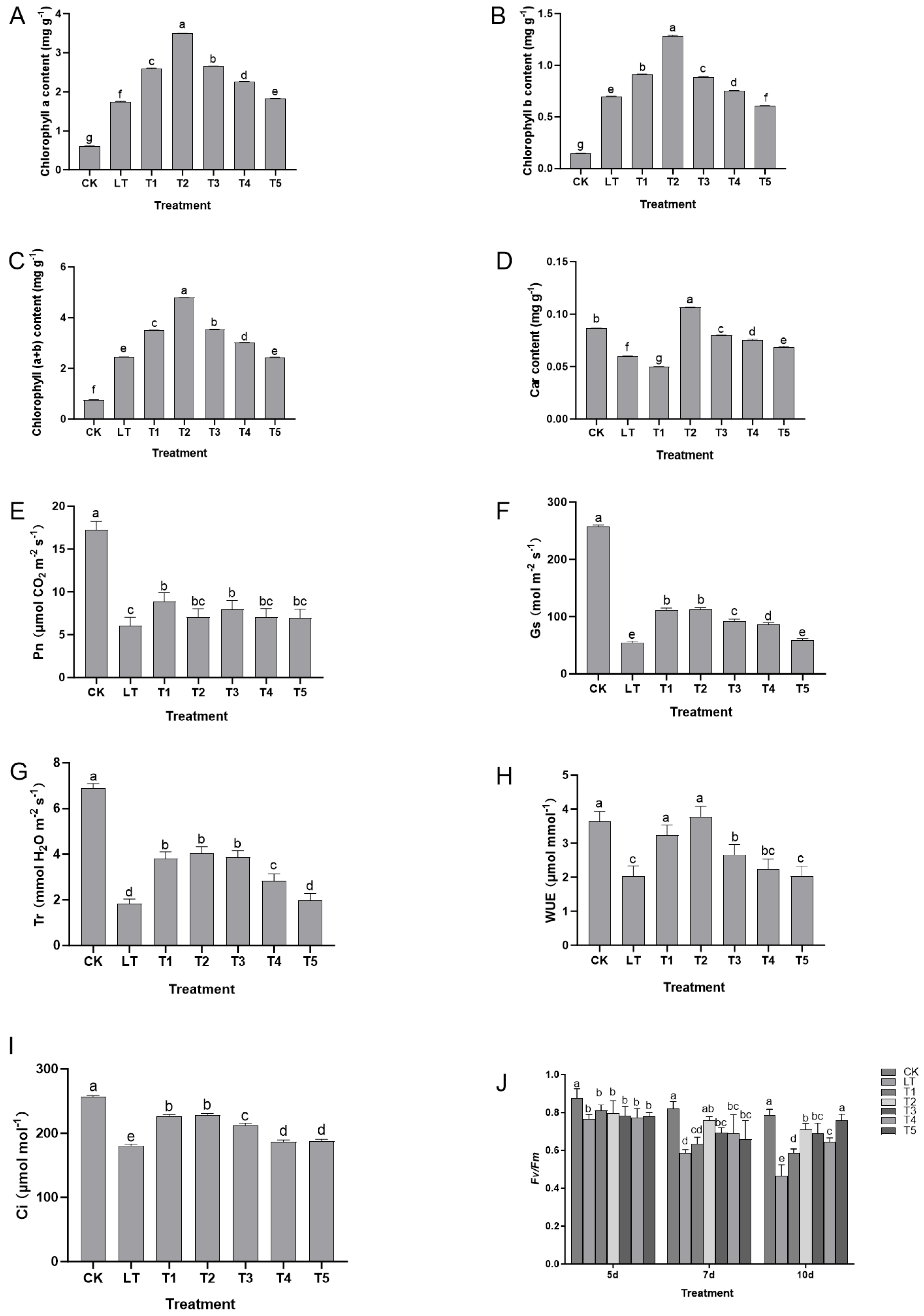
2.2. Effects of Exogenous Dopamine on the Protection and Efficiency Pathways of the Photosynthetic System of Shine Muscat Grapes Under Low-Temperature Stress
Low-temperature stress significantly disrupted the structure and function of the photosynthetic system of Shine Muscat grapes. However, exogenous dopamine treatment alleviated the damage caused by low temperature and maintained photosynthetic efficiency by regulating key photosynthetic indicators (
Figure 2). Under low-temperature stress, the content of chlorophyll (Chla, Chlb, and total Chl) in grape leaves significantly decreased (
Figure 2A–C), which might have been related to the enhanced activity of chlorophyll-degrading enzymes or the inhibition of synthesis under low temperature, directly weakening the material basis for light absorption and conversion. The content of carotenoids (Car) also decreased simultaneously (
Figure 2D), further affecting the light protection ability. The changes in photosynthetic gas exchange parameters corresponded to this: under low-temperature stress, the net photosynthesis rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and water use efficiency (WUE) significantly decreased, while the intercellular CO
2 concentration (Ci) showed an upward trend (
Figure 2E–I), indicating that the decrease in photosynthesis rate under low temperature was not solely caused by stomatal limitation (Gs decrease leading to insufficient CO
2 supply), but might have been related to non-stomatal limitations (such as PSII function impairment, inhibition of dark reaction enzyme activity). At the same time, the function of PSII was severely damaged, manifested as significant decreases in the maximum photochemical efficiency (Fv/Fm), actual photochemical efficiency (ΦPSII), and electron transfer rate (ETR) and an abnormal increase in non-photochemical quenching (NPQ) (
Figure 2J), This indicated the destruction of the PSII reaction center structure, a decline in light energy conversion efficiency, and an inability of excess light energy to be effectively consumed through the photochemical pathway, forcing it to be released as heat and exacerbating the potential damage to the photosynthetic system.
After exogenous dopamine treatment, the above indicators were significantly improved: the decrease in the content of chlorophyll (Chla, Chlb, and total Chl) and carotenoids (Car) was significantly inhibited (
Figure 2A–D), maintaining strong light capture and light protection ability. At the same time, the decline in Pn, Gs, Tr, and WUE was significantly alleviated and the increase in Ci was reduced (
Figure 2E–I), indicating that dopamine improved the photosynthetic system under low-temperature stress by optimizing stomatal behavior and alleviating non-stomatal limitations (such as stable PSII function), enhancing carbon assimilation efficiency. The decreases in Fv/Fm, ΦPSII, and ETR of PSII were reduced and the increase in NPQ was inhibited (
Figure 2J), suggesting that dopamine can stabilize the PSII reaction center structure, reduce light energy conversion obstacles and excessive light energy heat dissipation, and alleviate light damage to the photosynthetic system.
In summary, exogenous dopamine alleviated the damage of low temperature to the photosynthetic system of Shine Muscat grapes through a multi-pathway synergy of pigment synthesis maintenance—stomatal regulation optimization—photosystem protection: at an appropriate concentration (0.4 mmol L−1), it maintained the content of chlorophyll and carotenoids to ensure light absorption and light protection, stabilized the function of PSII to promote light energy conversion, and coordinated stomatal and non-stomatal processes to improve carbon assimilation efficiency, thereby achieving the protection of the photosynthetic system under low-temperature stress and increasing its photosynthetic efficiency.
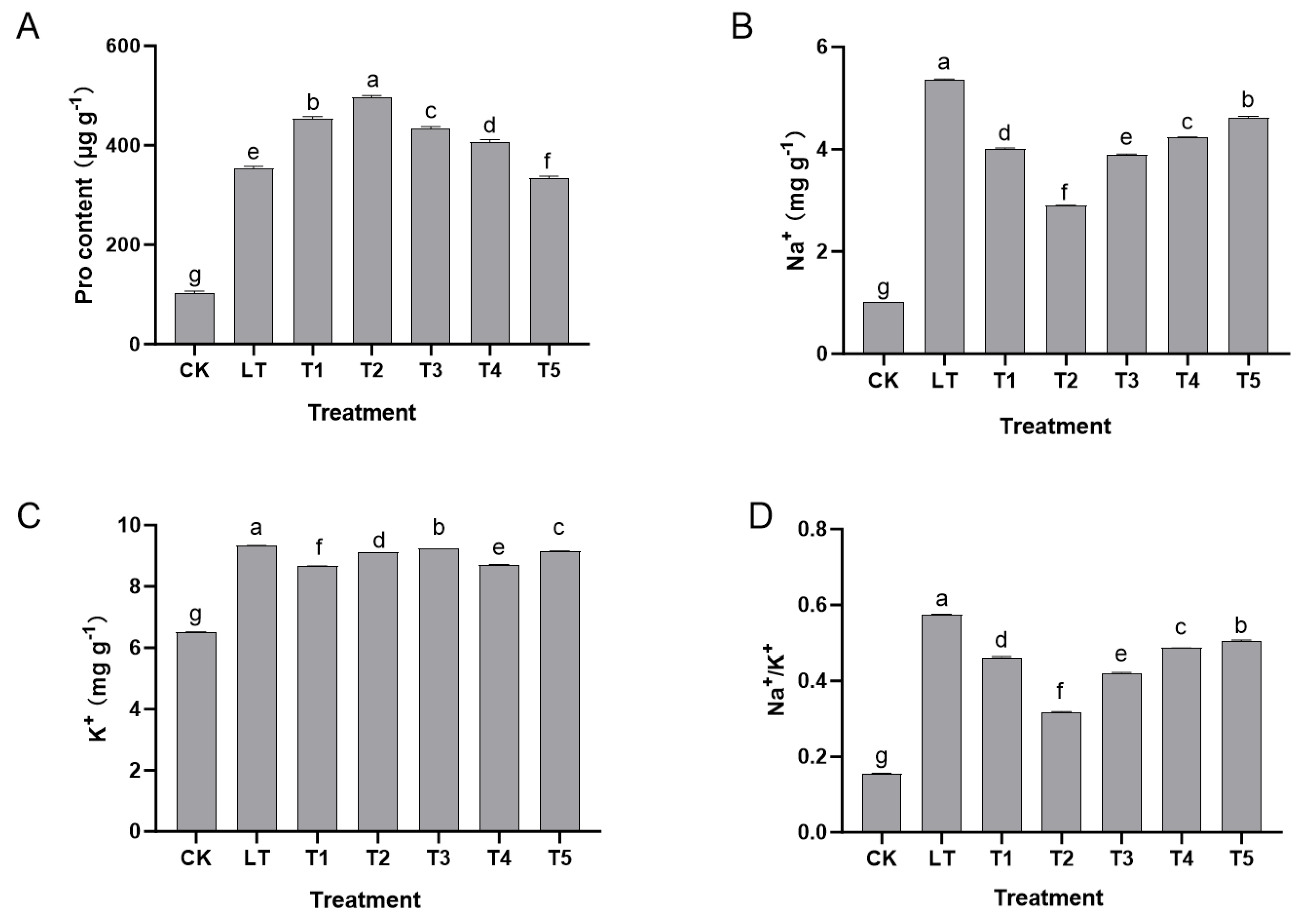
2.3. Effects of Exogenous Dopamine on Penetrative Regulation and Ion Homeostasis Maintenance Pathways of Shine Muscat Grapes Under Low-Temperature Stress
Low-temperature stress (LT group) significantly induced the accumulation of free proline in the leaves of Shine Muscat grapes (from 102.33 μg g−1 in the CK group to 353.23 μg g−1) while causing ion homeostasis imbalance, manifested as an increase in Na+ content from 1.01 mg g−1 to 5.37 mg g−1, and K+ from 6.50 mg g−1 to 9.33 mg g−1, but the Na+/K+ ratio increased sharply from 0.16 to 0.58, indicating that low temperature responded to dehydration stress by promoting the synthesis of permeation-regulating substances, but disrupted the selectivity of cell membranes and caused ion distribution disorder.
However, after exogenous dopamine treatment, these indicators significantly improved. Treatments of 0.2–0.6 mmol L
−1 (T1–T3) enhanced permeation regulation and improved ion balance, with the 0.4 mmol L
−1 (T2 group) treatment being the most effective: proline content reached 495.56 μg g
−1 (an increase of 40.3% compared to the LT group), Na
+ content decreased to 2.90 mg g
−1 (a reduction of 45.9%), K
+ remained at 9.12 mg g
−1, and the Na
+/K
+ ratio decreased to 0.32 (a reduction of 44.8%). At this concentration, dopamine enhanced cell osmotic pressure by synergistically promoting proline accumulation, inhibited Na
+ influx (or promote excretion), and stabilized K
+ levels, achieving dual optimization of permeation regulation ability and ion gradient homeostasis. High concentrations (0.8–1.0 mmol L
−1) of dopamine significantly reduced proline content (333.45–406.67 μg g
−1), and their improvement effect on ion balance also weakened, indicating that excessive dopamine can weaken the coordinated regulatory effect of permeation regulation substance (proline) synthesis accumulation and ion (Na
+, K
+) transmembrane transport balance (
Figure 3).
In summary, an appropriate concentration of exogenous dopamine (0.4 mmol L−1) optimized the Na+/K+ ratio by coordinating the synthesis accumulation of permeation-regulating substances (proline) and the transmembrane transport balance of ions (Na+, K+), thereby enhancing the adaptability of Shine Muscat grapes to low-temperature stress.
2.4. Effect of Exogenous Dopamine on the Antioxidant Defense Pathway of Shine Muscat Grapes Under Low-Temperature Stress
Low-temperature stress (LT group) significantly disrupted the oxidative balance of Shine Muscat grapes: compared with normal growth conditions (CK group), the activity of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) was significantly reduced, with SOD decreasing from 163.44 U g
−1 to 111.43 U g
−1, POD decreasing from 740.33 U g
−1 to 509.55 U g
−1, and CAT decreasing from 110.23 U g
−1 to 59.77 U g
−1, leading to enzymatic clearance of ROS. The ability had significantly weakened. At the same time, a large amount of oxidation products accumulated, with the content of superoxide anions increasing from 0.0112 μmol g
−1 to 0.1511 μmol g
−1, hydrogen peroxide (H
2O
2) increasing from 5.26 μmol g
−1 to 28.39 μmol g
−1, and membrane lipid peroxidation product malondialdehyde (MDA) increasing from 37.53 nmol g
−1 to 87.77 nmol g
−1. The relative conductivity (REC) also increased significantly, indicating that excessive accumulation of ROS under low-temperature stress had caused serious damage to cell membrane structure. The T2 group showed 31.7–49.5% higher SOD, POD, and CAT activity than the LT group, accompanied by 48.0–72.0% lower ROS and MDA content (
Figure 4A–F). Correlation analysis confirmed that SOD and CAT activity was highly positively correlated with fresh stem weight (r = 0.979, 0.956;
p < 0.01) and highly negatively correlated with MDA content (r = −0.941, −0.902;
p < 0.01;
Table 1), indicating that antioxidant enzyme activation is closely linked to growth recovery under low-temperature stress.
The regulation of oxidative imbalance by exogenous dopamine exhibited a significant concentration effect of “low promotion and high inhibition.” Treatment with 0.2–0.6 mmol L
−1 dopamine (T1–T3 groups) enhanced antioxidant capacity to varying degrees, with the most significant effect observed in the 0.4 mmol L
−1 (T2 group). At this concentration, the SOD activity reached 146.77 U g
−1 (an increase of 31.7% compared to the LT group), the POD reached 643.98 U g
−1 (an increase of 26.4%), and the CAT reached 89.36 U g
−1 (an increase of 49.5%). The synergistic enhancement of enzyme activity significantly improved ROS clearance efficiency, reducing O
2 content to 0.0423 μmol g
−1 (a decrease of 72.0% compared to the LT group), H
2O
2 to 8.33 μmol g
−1 (a decrease of 70.6%), and MDA to 45.67 nmol g
−1 (decreased by 48.0%), and REC also decreased synchronously, indicating effective protection of cell membrane integrity. However, high concentrations of dopamine (0.8–1.0 mmol L
−1, T4 and T5 groups) significantly weakened the antioxidant defense function, and the activity of SOD, POD, and CAT were significantly decreased compared to the T2 group (such as SOD in the T5 group being only 103.46 U g
−1). At the same time, the content of O
2, H
2O
2, MDA, and REC was significantly increased (such as MDA in the T5 group rising to 79.44 nmol g
−1), even approaching the level of the LT group, suggesting that excessive dopamine may inhibit the activation of the antioxidant system (
Figure 4).
In summary, an appropriate concentration of exogenous dopamine (0.4 mmol L−1) can synergistically enhance the activity of antioxidant enzymes such as SOD, POD, and CAT, effectively eliminate excessive ROS induced by low temperature, and reduce membrane lipid peroxidation damage, thereby strengthening the antioxidant defense system of Shine Muscat grapes and alleviating low-temperature stress damage. However, high concentrations of dopamine had negative effects due to the inhibition of antioxidant function. These results provide important evidence for analyzing the physiological mechanisms of dopamine enhancing grape low-temperature tolerance.
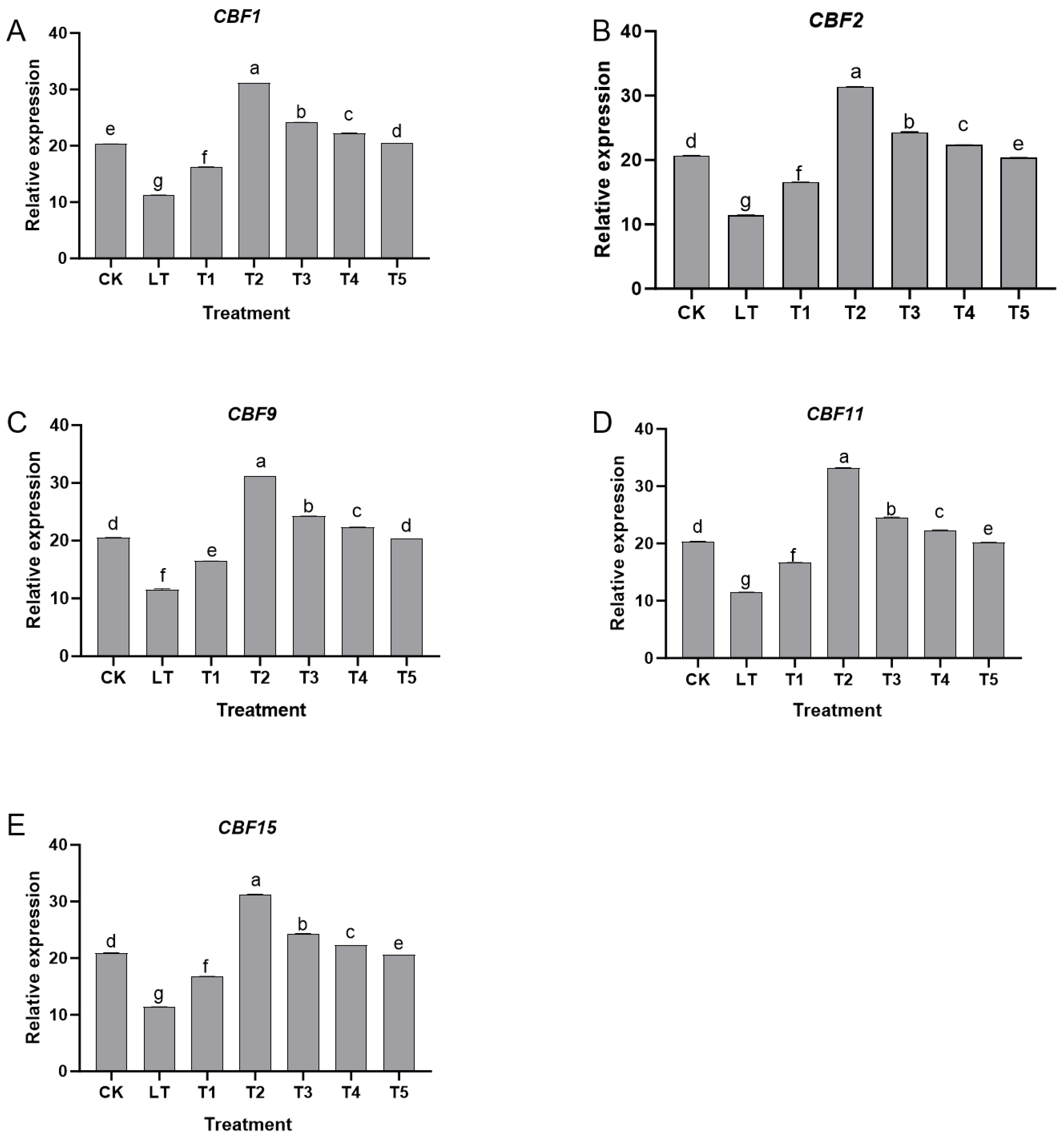
2.5. Effect of Exogenous Dopamine on the Regulation Pathway of Cold-Resistance Gene Expression in Shine Muscat Grapes Under Low-Temperature Stress
Under normal growth conditions (CK group), all genes in the
CBF family [
16] maintained a certain basal expression level. Low-temperature stress (LT group) significantly inhibited the expression of these genes. Compared with the CK group, the relative expression levels of
CBF1,
CBF2,
CBF9,
CBF11, and
CBF15 significantly decreased (
p < 0.05), indicating that the transcriptional activation ability of cold-resistant genes in Shine Muscat grapes was inhibited under low-temperature stress.
In contrast, the T5 group (poor physiological tolerance) showed
CBF expression close to the LT group (
Figure 5), indicating a potential link between
CBF gene activation and physiological cold tolerance enhancement.
CBF2 expression was positively correlated with ΦPSII (r = 0.823;
p < 0.01), suggesting
CBF genes may mediate Da’s regulation of photosynthesis and ion homeostasis. After treatment with exogenous dopamine, the expression levels of various genes showed regular changes with concentration: 0.2 mmol L
−1 (T1 group) and T2 group treatments significantly upregulated
CBF family gene expression, with the T2 group showing the most prominent effect. Compared with the LT group, the expression level of
CBF1 increased by about 2.8 times,
CBF2 increased by about 3.2 times,
CBF9 increased by about 2.5 times,
CBF11 increased by about 2.3 times, and
CBF15 increased by about 2.7 times in the T2 group, all of which were significantly higher than other treatment groups (
p < 0.05). This indicated that appropriate concentrations of dopamine effectively activated the transcription of
CBF-family cold-resistant genes and enhanced the plant’s cold-resistant molecular response. When the dopamine concentration was increased to 0.6 mmol L
−1 (T3 group), the expression levels of various genes were significantly decreased compared to the T2 group (
p < 0.05), but were still higher than the LT group. When the concentration was ≥0.8 mmol L
−1 (T4 and T5 groups), the gene expression level further decreased.
CBF-family gene expression level in the T5 group (1.0 mmol L
−1) was close to or even lower than that in the LT group, indicating that high-concentration dopamine had an inhibitory effect on
CBF gene expression.
In summary, exogenous dopamine participated in the low-temperature response of Shine Muscat grapes by regulating the expression of CBF-family cold-resistant genes. Treatment with 0.4 mmol L−1 most significantly upregulated the expression of key cold-resistant genes such as CBF1 and CBF2, thereby enhancing the plant’s cold-resistant molecular mechanism. However, high concentrations of dopamine weakened the cold-resistance regulation ability of plants by inhibiting the expression of these genes. This result was consistent with the changes in physiological pathways such as photosynthetic protection and antioxidant defense, which jointly confirmed the mechanism by which exogenous dopamine synergistically enhances the cold resistance of Shine Muscat grapes through multiple pathways.
2.6. Correlation Analysis of Growth Indicators and Photosynthesis-, Ion-, and Oxidation-Related Indicators in Grape Plants Treated with Exogenous Dopamine Under Low-Temperature Stress
The growth status of Shine Muscat grapes under low-temperature stress (plant height, stem diameter, fresh stem weight, dry stem weight) was significantly correlated with photosynthetic system function (especially PSII stability, stomatal regulation), ion homeostasis (low Na+/K+ ratio), antioxidant defense (high SOD, CAT activity), and degree of oxidative damage (low ROS, MDA content). The correlation between antioxidant enzyme activity, ion balance, and photosynthetic parameters (Gs, Ci, Tr) and growth indicators was the strongest (|r| ≥ 0.8, p < 0.01). This result confirmed the comprehensive mechanism by which exogenous dopamine enhanced cold resistance through synergistic regulation of photosynthetic protection, ion homeostasis, antioxidant defense, and other pathways, providing relevant evidence for the optimal regulatory effect of 0.4 mmol L−1 dopamine.
3. Discussion
This study found for the first time that exogenous dopamine has a significant “low promotion and high inhibition” effect on the cold resistance of Shine Muscat grapes: treatment with 0.4 mmol L
−1 can alleviate low-temperature stress to the greatest extent, while treatment with ≥0.8 mmol L
−1 inhibits growth and weakens stress resistance. The significant positive correlation between
CBF gene expression (upregulated 2.3–3.2 times in the T2 group) and physiological indicators (e.g., ΦPSII, r = 0.823;
p < 0.01) suggests that dopamine may indirectly regulate
CBF-mediated cold signaling to coordinate physiological defense pathways, though the direct molecular interaction between dopamine and
CBF transcription factors requires further verification. Although this pattern is consistent with the role of dopamine in various other crops [
7,
11], this study is the first to clarify that the optimal regulatory threshold for dopamine in the Shine Muscat grape is 0.4 mmol L
−1. Previously, Dai et al. [
12] only reported the effect of dopamine on the photosynthetic performance of grapes, without involving the effect of cold-resistance concentration. The hypothesis of “concentration-dependent threshold effect of exogenous substances” proposed by Wang et al. [
15] lacks grape-specific data, and this study provides key parameters for the precise application of dopamine in fruit crops, filling the research gap in this field in the grape genus. The potential mechanism may be that low concentrations of dopamine activate the antistress pathway as a signaling molecule, while high concentrations interfere with cellular redox balance or hormone signal interactions. This speculation provides direction for subsequent molecular mechanism research. This variation likely stems from differences in substance specificity: dopamine has higher biological activity in Shine Muscat and may trigger stress responses at lower concentrations.
Under low-temperature stress, the photosynthetic efficiency of Shine Muscat grapes decreased due to chlorophyll degradation, impaired PSII function, and non-stomatal limitation, which is consistent with the regulatory effect of methyl jasmonate on grapes [
5]. The increase in Ci and decrease in Gs under low temperature indicates that the decrease in photosynthesis rate is not solely caused by stomatal limitation, which is a common non-stomatal limitation phenomenon in grape low-temperature stress reported in previous studies [
5,
17]. However, this study mainly revealed the dopamine light energy conversion process at the leaf level. Compared with the study by Wang et al. [
17] in loquat, this study found that 0.4 mmol L
−1 dopamine promoted the increase of ΦPSII and ETR, which was significantly higher than the regulatory effect reported by Luo et al. [
18], indicating that dopamine has more advantages in stabilizing the structure of the photosystem and promoting light energy conversion. Low temperature induces PSII damage, and dopamine’s upregulation of
CBF genes (not observed in non-stress studies) may specifically protect photosystem structure. In addition, Wang et al. [
8] only revealed the correlation between exogenous substances and the cold resistance and photosynthetic parameters of grape branches, while this study focused on the dynamic changes in the leaf photosynthetic system, systematically analyzing the response mechanism of photosynthetic protection from dimensions such as chlorophyll synthesis, PSII functional stability, and light energy conversion efficiency, so has more advantages in terms of research specificity and depth. Dopamine synergistically regulates osmotic adjustment and ion homeostasis, two pathways previously thought to act independently under cold stress. First, it promotes proline accumulation to enhance cellular osmotic pressure, addressing dehydration stress. Second, it reduces Na
+ influx while stabilizing K
+ levels, maintaining ion gradient balance. This synergy ensures comprehensive cellular protection, which is more effective than single-pathway regulation reported in other grape stress studies.
In low-temperature stress, plants usually cope with stress by accumulating proline (osmotic regulation) and changing the Na
+/K
+ ratio (ion homeostasis) [
6], but the synergistic effect of the two is not yet clear in low-temperature stress research. This study found that 0.4 mmol L
−1 dopamine can increase proline content and decrease the Na
+/K
+ ratio, confirming for the first time the synergistic effect of osmoregulatory substances (proline) and ion balance under low-temperature stress. This is complementary to the ion regulation mechanism under salt stress reported by Afzal et al. [
19], providing a new perspective on the relationship between plant stress resistance and metabolism. Further research reveals that the core of dopamine maintaining ion homeostasis is to inhibit Na
+ influx rather than simply promote K
+ absorption. This mechanism is different from the main cause of ion imbalance in grape salt stress proposed by Lu et al. [
20], providing new ideas for understanding the regulation of ion transport for maintaining cellular ion gradient. In addition, Xing et al. [
13] found that ion homeostasis depends on rootstock improvement, while this study confirmed that dopamine can directly regulate ion balance without relying on rootstock, providing a practical basis for simplifying cold-resistant cultivation techniques. Combined with the complexity of the ion homeostasis regulation network revealed by Liu et al. [
21], the synergistic regulation results of this study further confirm that plant stress resistance is a process of multi-pathway integration. Dopamine can enhance the activity of SOD, POD, and CAT and reduce the accumulation of ROS, which is consistent with the antioxidant properties of dopamine in apples [
10] and cucumbers [
11]. The optimal dopamine concentration (0.4 mmol L
−1) simultaneously enhanced antioxidant enzyme activity and stabilized ion balance. This dual regulation is critical for alleviating low-temperature-induced oxidative damage and cellular osmotic disorder, addressing two core physiological bottlenecks of Shine Muscat under cold stress. Compared with the 20–30% increase in SOD activity by dopamine in cucumbers [
11], the 31.7% increase in SOD activity in the T2 group of this study indicates that dopamine has a stronger antioxidant regulatory effect in Shine Muscat grapes, which may be related to the variety-specific sensitivity of grapes to dopamine. This study confirmed for the first time through correlation analysis that the activity of SOD and CAT is significantly positively correlated with growth indicators such as plant height and stem diameter (r > 0.8,
p < 0.01), breaking the limitation of only observing changes in enzyme activity in the past and directly proving that antioxidant capacity is the core link for dopamine to enhance cold resistance. Compared with the melatonin reported by Tang et al. [
22], dopamine showed a higher increase in POD activity in the Shine Muscat grape, indicating its specific advantage in the grape enzymatic defense system. In addition, Kong [
23] found that dopamine can alleviate cadmium stress in
Pyrus betulifolia seedlings, and this study for the first time focused on the universal cross-stress response of antioxidant regulation to the low-temperature response of Shine Muscat, providing a new direction for understanding the broad-spectrum stress resistance mechanism of grapes [
24].
CBF transcription factors are the core of plant cold-resistance pathways [
16], but dopamine’s regulation of them in Shine Muscat is not yet clear. This study found that 0.4 mmol L
−1 dopamine can increase the expression level of
CBF2 gene by 3.2 times compared to the low-temperature group, and is the first to clarify the upregulation effect of dopamine on the
CBF family genes of Shine Muscat. This is consistent with the conservative function of
CBF studied by Cui et al. [
25], and further establishes its core position in the dopamine regulatory network. Based on the transcription factor network revealed by Lv et al. [
26], this study suggests that the
CBF family may collaborate with similar regulatory modules in grapes to enhance cold-resistance responses through multifactor interactions. This provides further research directions for understanding the molecular mechanisms of dopamine regulation.
It is necessary to acknowledge the limitations of this study. This study focused on 1-year-old seedlings under controlled artificial climate conditions. The regulatory effect of dopamine on mature grapevines in open-field environments (with fluctuating temperatures and other stresses) remains to be verified. We identified a correlation between CBF genes and physiological pathways, but the direct molecular targets of dopamine (e.g., whether it binds to CBF promoters) have not been clarified. Corresponding future research directions should include conducting field trials to validate the optimal dopamine concentration and application timing for mature Shine Muscat vines, considering regional climate differences, using molecular techniques (e.g., ChIP-seq, yeast two-hybrid) to explore the direct interaction between dopamine and CBF gene regulatory elements, clarifying the upstream signaling mechanism, investigating whether dopamine can be combined with other exogenous substances (e.g., melatonin) to achieve synergistic cold tolerance enhancement, and further improving application efficiency. These follow-up studies will help to further improve the theoretical system of dopamine regulating grape cold tolerance and provide more comprehensive technical support for cold-resistant grape cultivation, which is of great significance for promoting the stable production of cold-sensitive grape varieties in northern China.