Effects of Synergistic Regulation of Functional Fertilisers and Vermicompost on Soil Fertility and the Growth and Quality of Two Tomato Varieties
Abstract
1. Introduction
2. Results
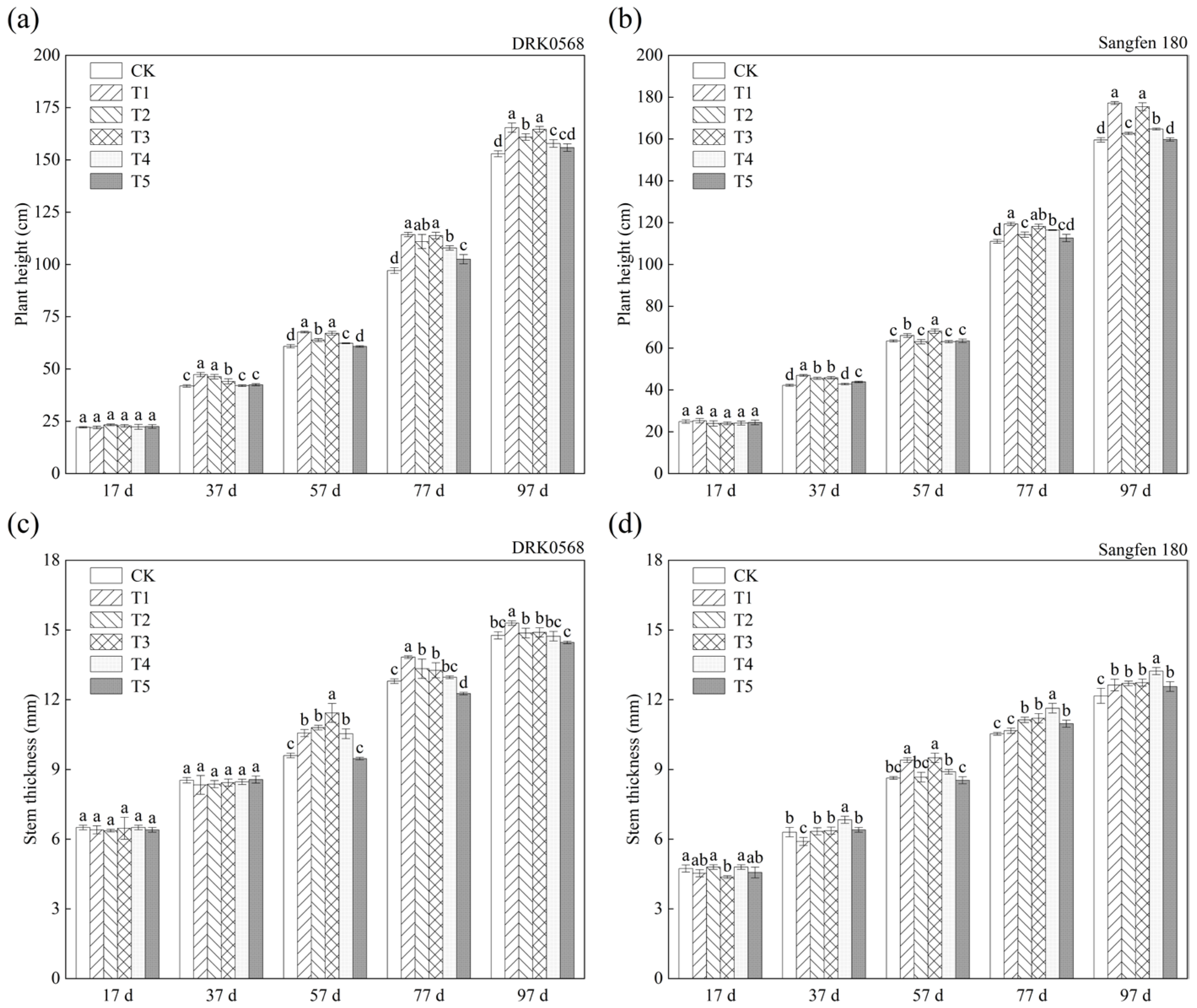
2.1. The Effects of Five Functional Fertiliser Treatments on Tomato Growth
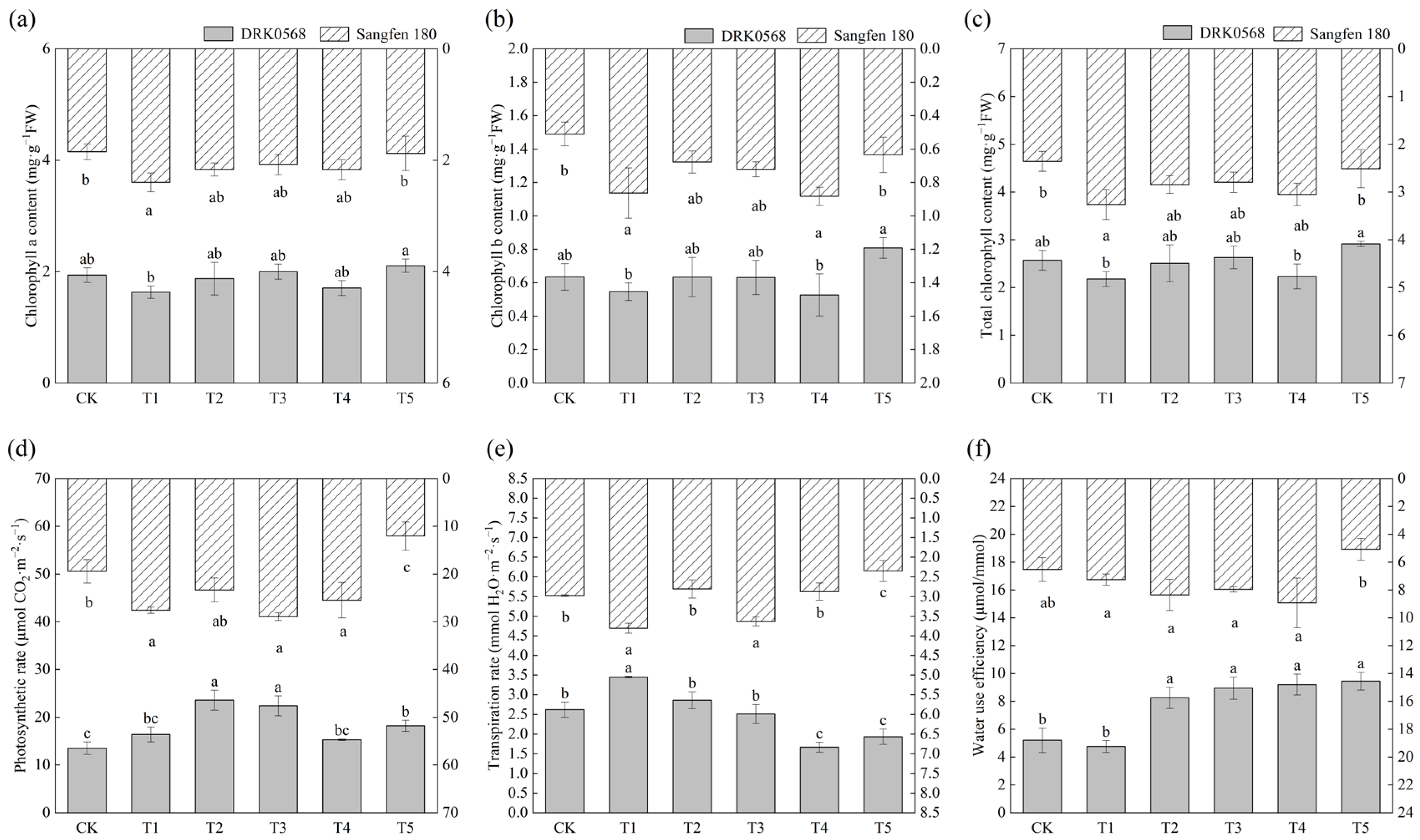
2.2. Effects of Five Functional Fertiliser Treatments on Photosynthetic Parameters of Two Tomato Cultivars
2.3. Effects of Five Functional Fertiliser Treatments on Tomato Quality Traits
2.4. Effects of Five Functional Fertiliser Treatments on Tomato Yield and Malformed Fruit Rate
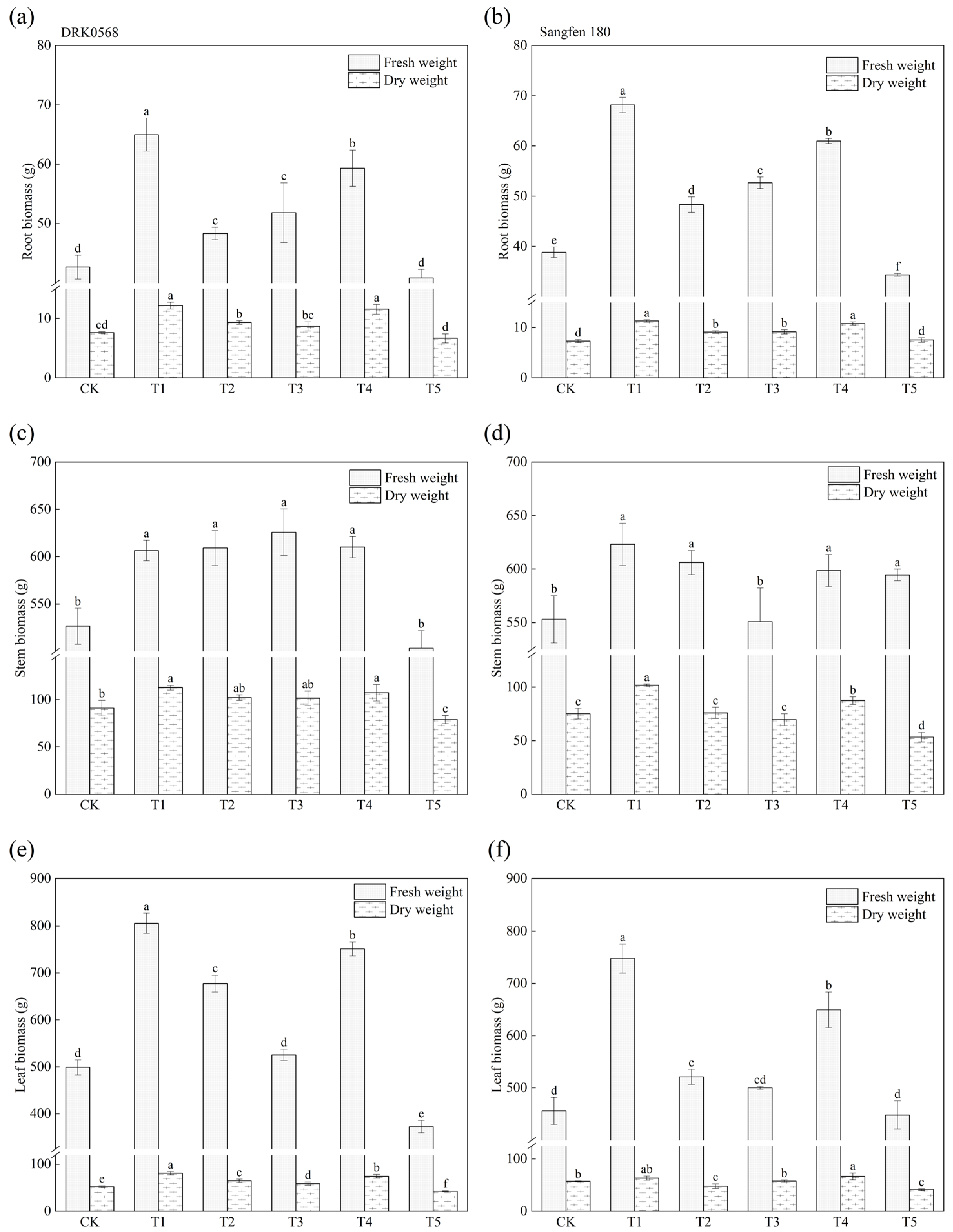
2.5. Effects of Five Functional Fertiliser Treatments on Tomato Biomass
2.6. Effects of Five Functional Fertiliser Treatments on Soil Fertility
2.6.1. Soil Physicochemical Properties
2.6.2. Soil Enzyme Activity
3. Discussion
3.1. Differential Regulation of Tomato Growth Physiology and Yield by Functional Fertilisers
3.2. Differences in Tomato Nutritional Indicators in Response to Functional Fertilisers
3.3. Soil Nutrient Transformation and Enzyme Activity Response Regulated by Fertiliser Type
4. Materials and Methods
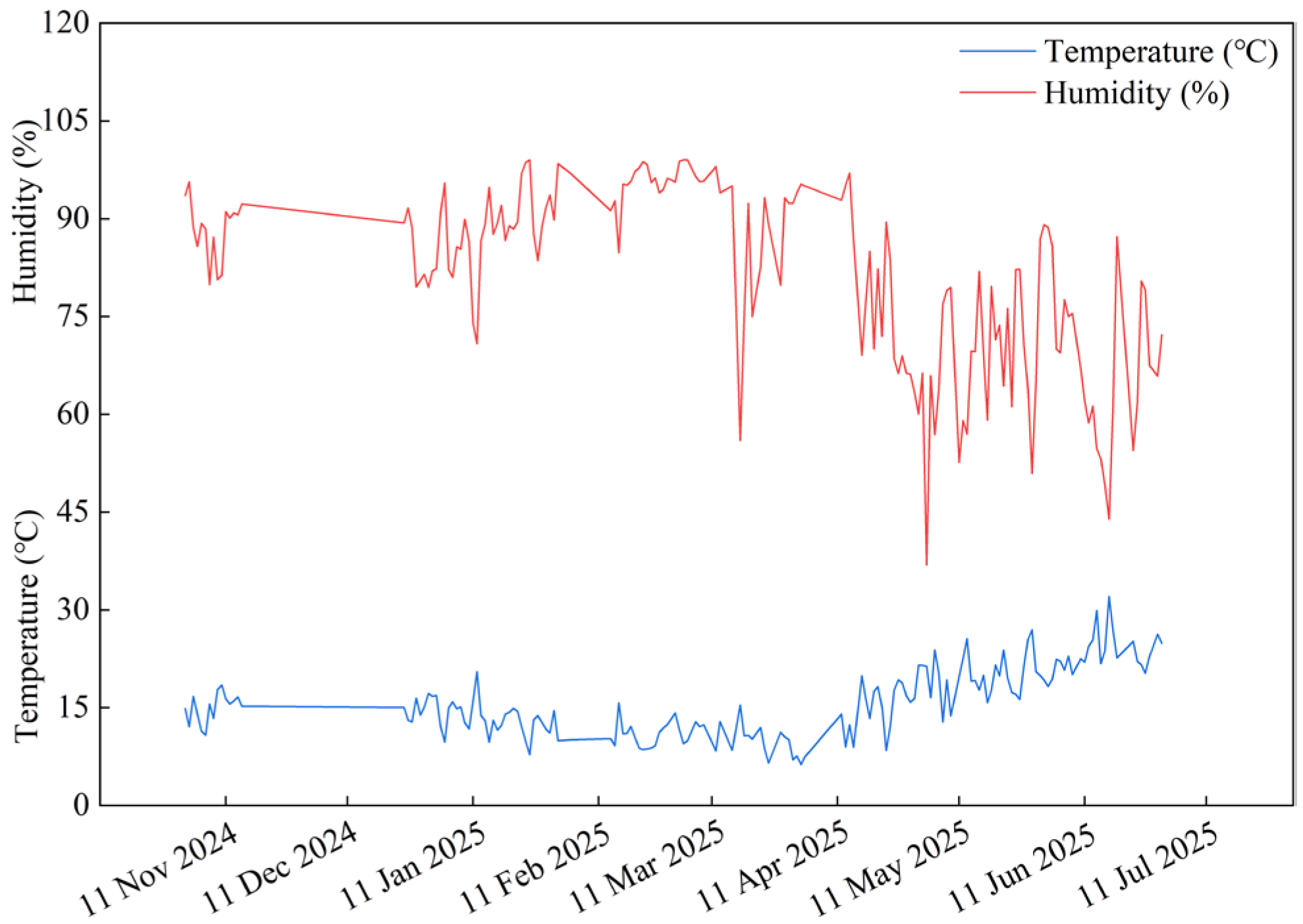
4.1. Experimental Conditions
4.2. Experimental Materials
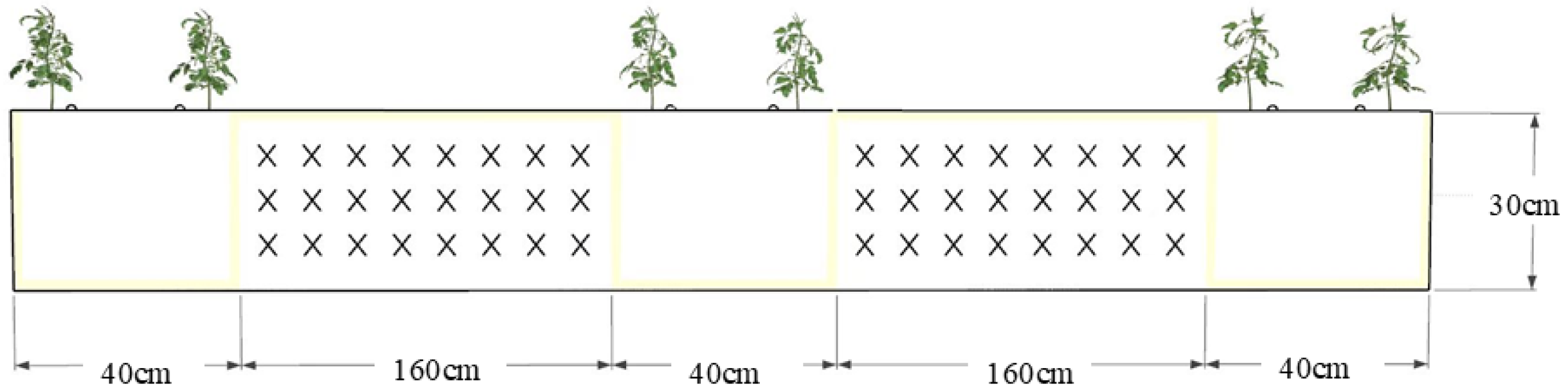
4.3. Experimental Design and Treatments
4.4. Data Collection
4.4.1. Plant Growth and Fruit Yield
4.4.2. Photosynthetic Parameters
4.4.3. Quality Indicators
4.4.4. Soil Fertility Indicators
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aldrich, H.T.; Salandanan, K.; Kendall, P.; Bunning, M.; Stonaker, F.; Külen, O.; Stushnoff, C. Cultivar choice provides options for local production of organic and conventionally produced tomatoes with higher quality and antioxidant content. J. Sci. Food Agric. 2010, 90, 2548–2555. [Google Scholar] [CrossRef]
- Chea, L.; Erika, C.; Naumann, M.; Smit, I.; Horneburg, B.; Pawelzik, E. Morphological, leaf nutrient, and fruit quality characteristics of diverse tomato cultivars under organic low-input management. Sustainability 2021, 13, 12326. [Google Scholar] [CrossRef]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An alarming decline in the nutritional quality of foods: The biggest challenge for future generations’ health. Foods 2024, 13, 877. [Google Scholar] [CrossRef]
- Nie, H.; Yang, X.; Zheng, S.; Hou, L. Gene-Based developments in improving quality of tomato: Focus on firmness, shelf life, and pre-and post-harvest stress adaptations. Horticulturae 2024, 10, 641. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Distefano, M.; Mauro, R.P.; Page, D.; Giuffrida, F.; Bertin, N.; Leonardi, C. Aroma volatiles in tomato fruits: The role of genetic, preharvest and postharvest factors. Agronomy 2022, 12, 376. [Google Scholar] [CrossRef]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Mu, L.; Zhou, H.; Yang, K.; Wang, J.; Sun, S.; Lu, Z.; Wang, L.; Zhang, N.; Bao, L. Effect of Biochar-Based Organic Fertilizer on the Growth of Maize in Cadmium-Contaminated Soil. Agriculture 2025, 15, 447. [Google Scholar] [CrossRef]
- Ge, J.; Du, Y.; Wang, Q.; Xu, X.; Li, J.; Tao, J.; Gao, F.; Yang, P.; Feng, B.; Gao, J. Effects of nitrogen fertilizer on the physicochemical, structural, functional, thermal, and rheological properties of mung bean (Vigna radiata) protein. Int. J. Biol. Macromol. 2024, 260, 129616. [Google Scholar] [CrossRef]
- Yan, X.; Wang, J.; Hu, X.; Yu, B.; Gao, B.; Li, Z.; Chen, J.; Zhang, M.; Liu, X. Contrasting effects of microbial fertiliser and organic fertiliser on soil bacterial community in coal mine dump of Inner Mongolia. Chem. Ecol. 2021, 37, 384–398. [Google Scholar] [CrossRef]
- Salomon, M.J.; Cavagnaro, T.R.; Burton, R.A. Potential of black soldier fly larvae frass (BSFL) as a novel fertilizer: Impacts on tomato growth, nutrient uptake, and mycorrhizal formation. Plant Soil 2025, 513, 417–434. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Valadez-Blanco, R.; Salas-Coronado, R.; Sustaita-Rivera, F.; Hernández-Carlos, B.; García-Ortega, S.; Santos-Sánchez, N.F. Effect of nitrogen fertilization and Bacillus licheniformis biofertilizer addition on the antioxidants compounds and antioxidant activity of greenhouse cultivated tomato fruits (Solanum lycopersicum L. var. Sheva). Sci. Hortic. 2016, 201, 338–345. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Mu, X.; Gao, H.; Zhang, Y.; Li, R.; Cao, K.; Ye, L. Effects of organic fertilizer application on tomato yield and quality: A meta-analysis. Appl. Sci. 2023, 13, 2184. [Google Scholar] [CrossRef]
- Dursun, A.; Yildirim, E.; Turan, M.; Ekinci, M.; Kul, R.; Parlakova Karagoz, F. Determination of the effects of bacterial fertilizer on yield and growth parameters of tomato. J. Agric. Sci. Technol. 2019, 21, 1227–1234. [Google Scholar]
- Mehlferber, E.C.; McCue, K.F.; Ferrel, J.E.; Koskella, B.; Khanna, R. Temporally selective modification of the tomato rhizosphere and root microbiome by volcanic ash fertilizer containing micronutrients. Appl. Environ. Microbiol. 2022, 88, e0004922. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Truong, V.K.; Zhang, D. Earthworm (Eisenia fetida) mucus inspired bionic fertilizer to stimulate maize (Zea mays L.) growth. Sustainability 2021, 13, 4299. [Google Scholar] [CrossRef]
- Bhadauria, T.; Ramakrishnan, P.S. Role of earthworms in nitrogen cycling during the cropping phase of shifting agriculture (Jhum) in north-east India. Biol. Fertil. Soils 1996, 22, 350–354. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Zhang, Y.; Chen, J.; Zhang, D.; Tong, J. Changes in fibrolytic enzyme activity during vermicomposting of maize stover by an anecic earthworm Amynthas hupeiensis. Polym. Degrad. Stabil. 2015, 120, 169–177. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, Q.; Xu, L.; Li, R.; Luo, X.; Zhang, X.; Tong, J. Earthworms modify microbial community structure and accelerate maize stover decomposition during vermicomposting. Environ. Sci. Pollut. Res. 2015, 22, 17161–17170. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.; Velmourougane, K. Chemical and microbiological changes during vermicomposting of coffee pulp using exotic (Eudrilus eugeniae) and native earthworm (Perionyx ceylanesis) species. Biodegradation 2011, 22, 497–507. [Google Scholar] [CrossRef]
- Reyes-Pérez, J.J.; Tipán-Torres, H.C.; Llerena-Ramos, L.T.; Hernandez-Montiel, L.G.; Rivas-Garcia, T. Silicon increased the growth, productivity, and nutraceutical quality of tomato (Solanum lycopersicum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13155. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xu, W.; Cheng, J.; Liu, J.; Pei, W.; Wang, J.; Chuang, S. Amino acid fertilizer strengthens its effect on crop yield and quality by recruiting beneficial rhizosphere microbes. J. Sci. Food Agric. 2023, 103, 5970–5980. [Google Scholar] [CrossRef]
- Liu, S.; An, X.; Xu, C.; He, D.; Li, X.; Chen, C.; Guo, B.; Xu, D.; Huang, J. Integrative transcriptomic-physiological analysis deciphers nitrogen-mediated carbon reallocation balancing growth and flavonoid metabolism in Epimedium pubescens. Front. Plant Sci. 2025, 16, 1539445. [Google Scholar] [CrossRef]
- Dong, H.; Li, F.; Xuan, X.; Ahiakpa, J.K.; Tao, J.; Zhang, X.; Ge, P.; Wang, Y.; Gai, W.; Zhang, Y. The genetic basis and improvement of photosynthesis in tomato. Hortic. Plant J. 2025, 11, 69–84. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, S. Lycopene-a bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.M.; Haque, A.N.A. Impact of foliar application of zinc and zinc oxide nanoparticles on growth, yield, nutrient uptake and quality of tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, L.; Zhong, H.; Cai, H.; Xu, J.; Li, Z. Effects of irrigation, fertilization, and aeration coupling on yield and quality of greenhouse tomatoes. Agric. Water Manag. 2024, 299, 108893. [Google Scholar] [CrossRef]
- Becerra, L.D.; Ruiz-Pardo, R.Y.; Vaillant, F.; Zuluaga, M.V.; Boulanger, R.; Santander, M.; Escobar, S. Modulating fine flavor cocoa attributes: Impact of seed-to-bean transformation under controlled conditions on metabolite, volatile and sensory profiles. Food Res. Int. 2024, 196, 115109. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.; Lyu, H.; Chen, J.; Cao, X.; Du, R.; Ma, L.; Wang, N.; Zhu, Z.; Rao, J.; Wang, J.; et al. Releasing a sugar brake generates sweeter tomato without yield penalty. Nature 2024, 635, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Dou, X.; Liao, D.; Li, K.; An, C.; Li, G.; Dong, Z. Microbial fertilizers improve soil quality and crop yield in coastal saline soils by regulating soil bacterial and fungal community structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Jiang, M.; Dong, C.; Bian, W.; Zhang, W.; Wang, Y. Effects of different fertilization practices on maize yield, soil nutrients, soil moisture, and water use efficiency in northern China based on a meta-analysis. Sci. Rep. 2024, 14, 6480. [Google Scholar] [CrossRef]
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: A review. Pedosphere 2023, 33, 385–406. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Q.; Guo, X.; Ali, I.; Li, F.; Lin, S.; Liu, D. Effects of biochar and organic-inorganic fertilizer on pomelo orchard soil properties, enzyme activities, and microbial community structure. Front. Microbiol. 2022, 13, 980241. [Google Scholar] [CrossRef]
- Balota, E.L.; Chaves, J.C.D. Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. Rev. Bras. Ciênc. Solo 2010, 34, 1573–1583. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Bharti, P.; Das, A.; Kumar, S.; Rakshit, R. Assessment of soil specific enzyme activities in aggregates size fractions: A case study from subtropical Agro-ecosystem. Eurasian Soil Sci. 2024, 57, 646–656. [Google Scholar] [CrossRef]
- Singh, A.; Kar, A.K.; Singh, D.; Verma, R.; Shraogi, N.; Zehra, A.; Gautam, K.; Anbumani, S.; Ghosh, D.; Patnaik, S. pH-responsive eco-friendly chitosan modified cenosphere/alginate composite hydrogel beads as carrier for controlled release of Imidacloprid towards sustainable pest control. Chem. Eng. J. 2022, 427, 131215. [Google Scholar] [CrossRef]
- Ai, S.; Zhang, Z.; Wang, X.; Meng, X.; Gao, Q.; Liu, Z.; Yang, F.; Cheng, K. Coupling Mechanism of Methane Production and Carbon Fixation Regulated by Artificial Humic Acid in Paddy Soils: Insights into Microbial Communities and Metabolic Pathways. Environ. Sci. Technol. 2025, 59, 15777–15791. [Google Scholar] [CrossRef] [PubMed]
- Wadood, A.; Hameed, A.; Akram, S.; Ghaffar, M. Unraveling the impact of water deficit stress on nutritional quality and defense response of tomato genotypes. Front. Plant Sci. 2024, 15, 1403895. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Obadi, A.; Alharbi, A.; Alomran, A.; Alghamdi, A.G.; Louki, I.; Alkhasha, A.; Alqardaeai, T. Enhancement in tomato yield and quality using biochar amendments in greenhouse under salinity and drought stress. Plants 2024, 13, 1634. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Wang, H.; Jia, X.; Song, Q.; Chen, T.; Chen, Z. Effects of harvest ripeness on storage quality of tomato fruits. Emir. J. Food Agric. 2023, 35, 978–987. [Google Scholar]
- Agbna, G.H.; Dongli, S.; Zhipeng, L.; Elshaikh, N.A.; Guangcheng, S.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Ou, X.; Liu, D.; Liu, A.; Liu, H.; Chen, R.; Zhang, Y. Effects of nutrient solution management modes on fruit production and quality of tomatoes grown in extremely root restriction. Sci. Hortic. 2023, 321, 112366. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shen, L. Effects of soluble organic fertilizer combined with inorganic fertilizer on greenhouse tomatoes with different irrigation techniques. Agriculture 2024, 14, 313. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzyme and Its Study Method; China Agriculture Press: Beijing, China, 1986; pp. 80–125. [Google Scholar]
- Gong, X.; Liu, C.; Li, J.; Luo, Y.; Yang, Q.; Zhang, W.; Yang, P.; Feng, B. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Tillage Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, N.; Wang, H.; Li, J.; Zhong, H.; Dong, H.; Zeng, Z.; Zong, C. Variations in the diversity of the soil microbial community and structure under various categories of degraded wetland in Sanjiang Plain, northeastern China. Land Degrad. Dev. 2021, 32, 2143–2156. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Chen, J.; Arafat, Y.; Wu, L.; Xiao, Z.; Li, Q.; Khan, M.A.; Khan, M.U.; Lin, S.; Lin, W. Shifts in soil microbial community, soil enzymes and crop yield under peanut/maize intercropping with reduced nitrogen levels. Appl. Soil Ecol. 2018, 124, 327–334. [Google Scholar] [CrossRef]
- Wan, L.J.; Tian, Y.; He, M.; Zheng, Y.Q.; Lyu, Q.; Xie, R.J.; Ma, Y.Y.; Deng, L.; Yi, S.L. Effects of chemical fertilizer combined with organic fertilizer application on soil properties, citrus growth physiology, and yield. Agriculture 2021, 11, 1207. [Google Scholar] [CrossRef]


| Variety | Treatment | SOM (g·kg−1) | pH | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| DRK0568 | CK | 44.68 ± 1.00 b | 7.98 ± 0.02 d | 2.32 ± 0.20 b | 2.08 ± 0.15 ab | 19.47 ± 0.85 a | 318.28 ± 2.85 b | 95.54 ± 0.04 e | 245.25 ± 1.25 d |
| T1 | 53.09 ± 0.58 a | 7.97 ± 0.02 d | 2.86 ± 0.02 a | 2.18 ± 0.15 a | 20.45 ± 0.38 a | 365.16 ± 11.58 a | 98.27 ± 0.09 d | 285.25 ± 0.25 a | |
| T2 | 52.42 ± 2.00 a | 8.12 ± 0.02 a | 2.89 ± 0.01 a | 1.99 ± 0.08 ab | 20.37 ± 0.45 a | 366.79 ± 7.35 a | 104.00 ± 0.09 a | 238.50 ± 0.50 e | |
| T3 | 52.14 ± 1.00 a | 8.06 ± 0.01 c | 2.75 ± 0.06 a | 1.89 ± 0.06 b | 16.55 ± 1.14 b | 358.18 ± 7.86 a | 98.45 ± 0.09 c | 240.50 ± 10 e | |
| T4 | 46.08 ± 2.00 b | 8.08 ± 0.01 bc | 2.81 ± 0.02 a | 2.09 ± 0.01 ab | 19.16 ± 1.00 a | 345.41 ± 12.70 a | 99.12 ± 0.04 b | 264.00 ± 2.00 b | |
| T5 | 50.24 ± 0.09 a | 8.09 ± 0.01 b | 2.4 ± 0.01 b | 2.20 ± 0.16 a | 21.10 ± 0.15 a | 321.83 ± 11.61 b | 94.33 ± 0.09 f | 252.50 ± 1.00 c | |
| Sangfen 180 | CK | 38.90 ± 3.03 c | 8.41 ± 0.02 b | 1.19 ± 0.12 e | 1.47 ± 0.02 f | 16.99 ± 0.05 c | 225.04 ± 4.36 f | 87.35 ± 1.73 c | 209.50 ± 3.77 d |
| T1 | 46.25 ± 1.00 b | 8.26 ± 0.02 c | 2.33 ± 0.10 b | 1.93 ± 0.02 b | 14.43 ± 0.10 d | 359.54 ± 2.66 a | 95.16 ± 1.99 b | 213.42 ± 5.51 d | |
| T2 | 43.78 ± 3.00 bc | 8.21 ± 0.02 d | 1.96 ± 0.14 c | 1.86 ± 0.01 c | 17.54 ± 0.10 b | 335.36 ± 3.55 c | 94.17 ± 3.10 b | 240.75 ± 3.54 b | |
| T3 | 52.74 ± 0.91 a | 8.23 ± 0.01 d | 2.50 ± 0.01 a | 1.79 ± 0.01 d | 16.76 ± 0.10 c | 344.89 ± 4.49 b | 99.97 ± 0.09 a | 223.25 ± 2.84 c | |
| T4 | 39.72 ± 4.00 c | 8.5 ± 0.01 a | 1.42 ± 0.06 d | 1.57 ± 0.01 e | 18.01 ± 0.49 a | 258.19 ± 6.13 e | 81.08 ± 1.05 d | 253.33 ± 7.37 a | |
| T5 | 43.65 ± 0.92 bc | 8.01 ± 0.01 e | 2.26 ± 0.04 b | 1.99 ± 0.01 a | 18.40 ± 0.10 a | 311.06 ± 5.98 d | 99.59 ± 0.71 a | 240.00 ± 1.5 b |
| Variety | Treatment | Soil Urease Activity (µg·d−1·g−1) | Soil Catalase Activity (µg·h−1·g−1) | Soil Sucrase Activity (mg·d−1·g−1) | Soil Alkaline Phosphatase Activity (µg·h−1·g−1) |
|---|---|---|---|---|---|
| DRK0568 | CK | 115.13 ± 3.24 c | 1.32 ± 0.10 de | 7.19 ± 0.13 c | 71.58 ± 0.37 b |
| T1 | 165.26 ± 4.69 b | 1.51 ± 0.18 d | 7.96 ± 0.05 a | 77.75 ± 0.94 a | |
| T2 | 184.58 ± 5.95 a | 3.42 ± 0.11 b | 7.79 ± 0.06 b | 76.16 ± 0.58 a | |
| T3 | 160.94 ± 6.11 b | 5.45 ± 0.18 a | 7.64 ± 0.06 b | 76.98 ± 0.10 a | |
| T4 | 162.16 ± 1.76 b | 1.10 ± 0.06 e | 7.73 ± 0.10 b | 77.23 ± 1.10 a | |
| T5 | 116.08 ± 0.80 c | 1.93 ± 0.05 c | 7.63 ± 0.07 b | 72.63 ± 1.22 b | |
| Sangfen 180 | CK | 113.01 ± 5.11 e | 1.59 ± 0.02 e | 7.57 ± 0.10 c | 65.02 ± 0.10 e |
| T1 | 114.72 ± 3.98 e | 2.87 ± 0.07 d | 7.91 ± 0.04 b | 77.51 ± 1.10 b | |
| T2 | 173.35 ± 1.50 b | 3.22 ± 0.07 c | 7.81 ± 0.20 b | 74.37 ± 0.66 c | |
| T3 | 153.40 ± 1.56 d | 4.51 ± 0.15 a | 7.83 ± 0.16 b | 85.56 ± 0.79 a | |
| T4 | 184.07 ± 3.75 a | 4.17 ± 0.11 b | 7.87 ± 0.06 b | 62.72 ± 1.59 f | |
| T5 | 160.32 ± 0.72 c | 1.13 ± 0.12 f | 8.17 ± 0.05 a | 69.58 ± 0.13 d |
| Treatment | Name | Form | Dosage (Hectare) |
|---|---|---|---|
| CK | Water | - | - |
| T1 | Mineral-sourced potassium fulvate fertiliser | Powder | 15 kg |
| T2 | Humic acid water-soluble fertiliser | Powder | 30 kg |
| T3 | Water-soluble NPK fertiliser with silicon | Agent | 15 L |
| T4 | Type II algal polysaccharide fertiliser additive | Agent | 15 L |
| T5 | Peptide-enriched fish protein organic liquid fertiliser | Agent | 30 L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhang, K.; Zhang, W.; Zhang, X.; Cheng, M.; Bao, R.; Zhang, M. Effects of Synergistic Regulation of Functional Fertilisers and Vermicompost on Soil Fertility and the Growth and Quality of Two Tomato Varieties. Plants 2025, 14, 3224. https://doi.org/10.3390/plants14203224
Zhang T, Zhang K, Zhang W, Zhang X, Cheng M, Bao R, Zhang M. Effects of Synergistic Regulation of Functional Fertilisers and Vermicompost on Soil Fertility and the Growth and Quality of Two Tomato Varieties. Plants. 2025; 14(20):3224. https://doi.org/10.3390/plants14203224
Chicago/Turabian StyleZhang, Tianmi, Kangjie Zhang, Wenhao Zhang, Xuefeng Zhang, Mengyao Cheng, Ruilong Bao, and Mingke Zhang. 2025. "Effects of Synergistic Regulation of Functional Fertilisers and Vermicompost on Soil Fertility and the Growth and Quality of Two Tomato Varieties" Plants 14, no. 20: 3224. https://doi.org/10.3390/plants14203224
APA StyleZhang, T., Zhang, K., Zhang, W., Zhang, X., Cheng, M., Bao, R., & Zhang, M. (2025). Effects of Synergistic Regulation of Functional Fertilisers and Vermicompost on Soil Fertility and the Growth and Quality of Two Tomato Varieties. Plants, 14(20), 3224. https://doi.org/10.3390/plants14203224




