Assessment of Phytochemical Composition and Antifungal Activity of Micropropagated Drymis winteri Plants
Abstract
1. Introduction
2. Results
2.1. Propagation of Plantlets and Callus Induction in D. winteri
2.2. Callus Induction
2.3. Calli Histological Analysis
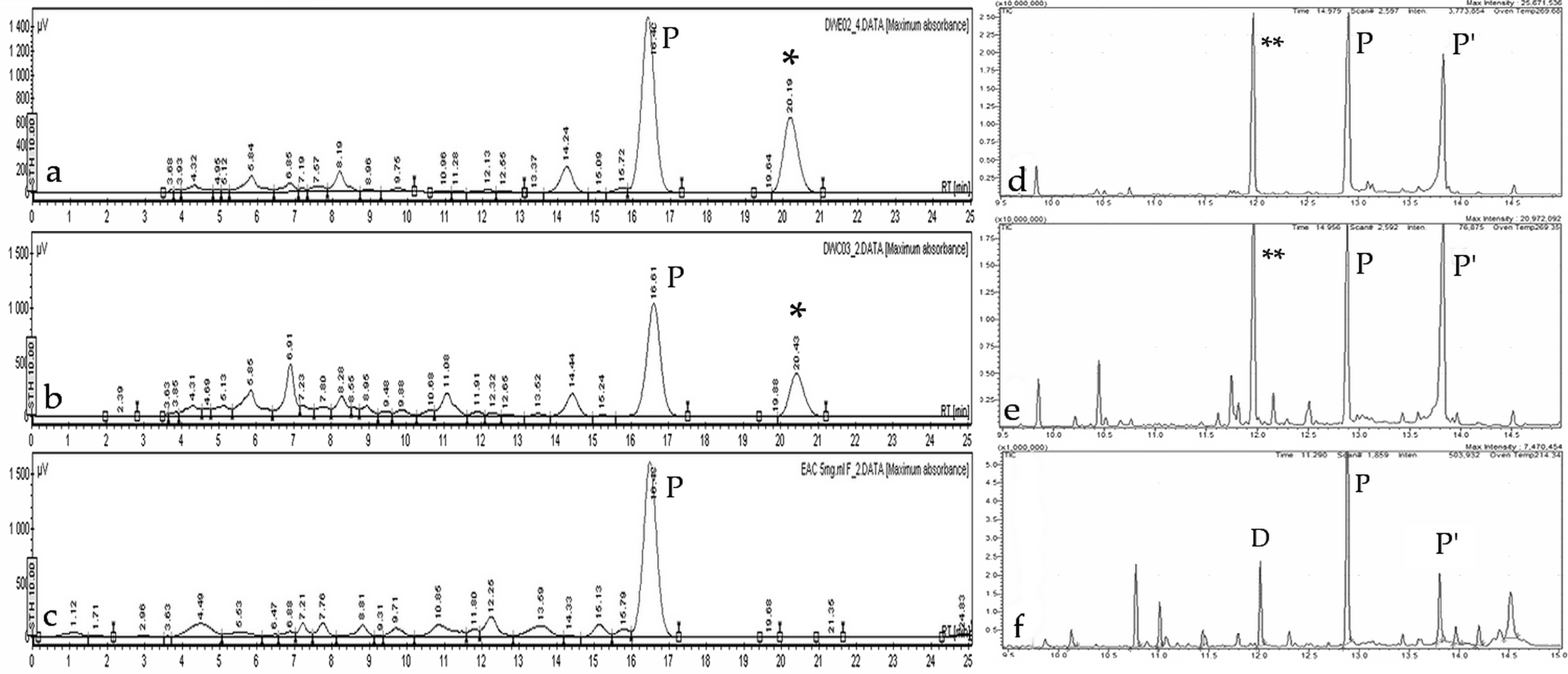
2.4. Analysis of In Vitro Plant and Callus Crude Extracts
2.4.1. Phytochemical Analysis
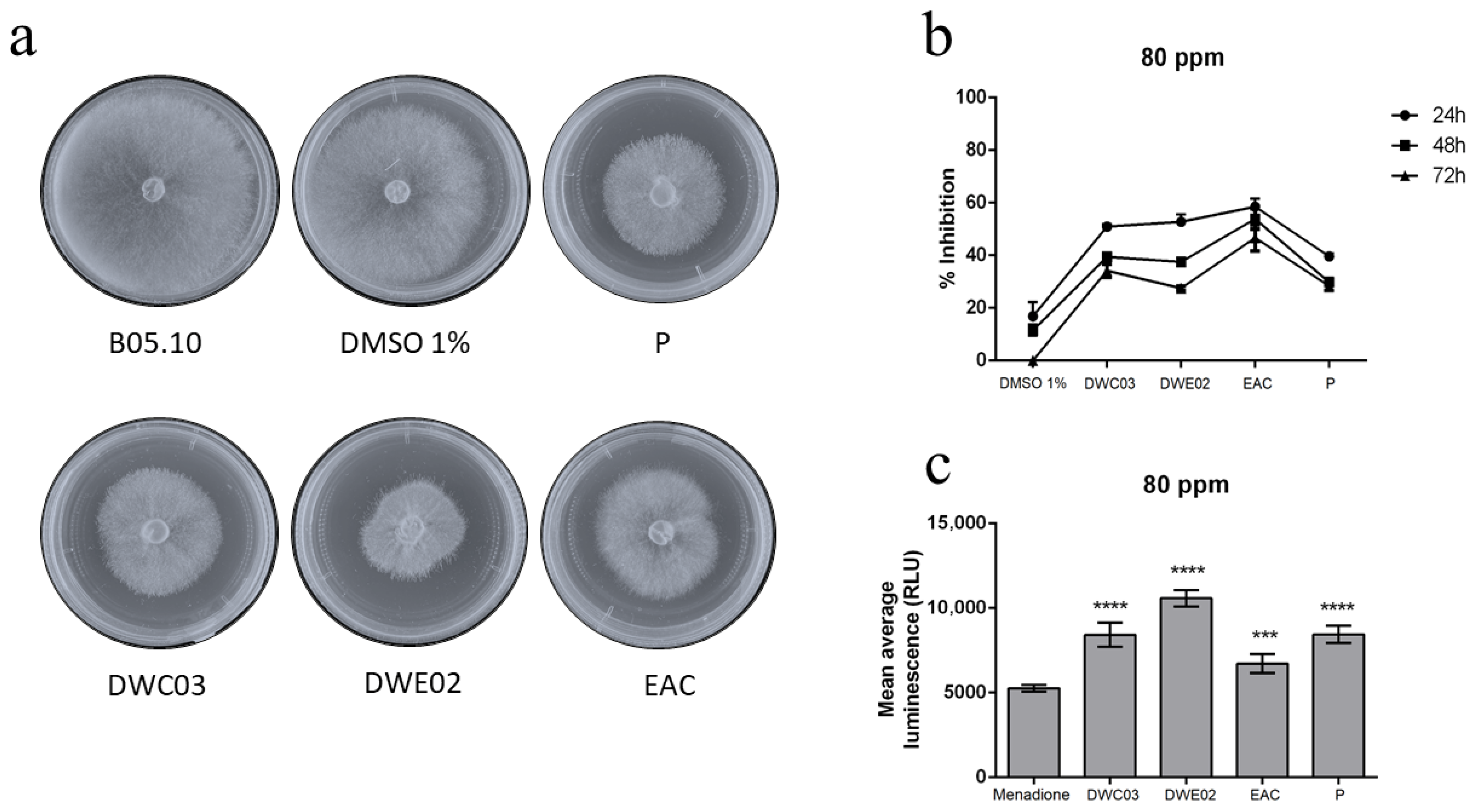
2.4.2. Antifungal Activity of Crude Extracts
2.5. Gene Expression Analysis in B. cinerea
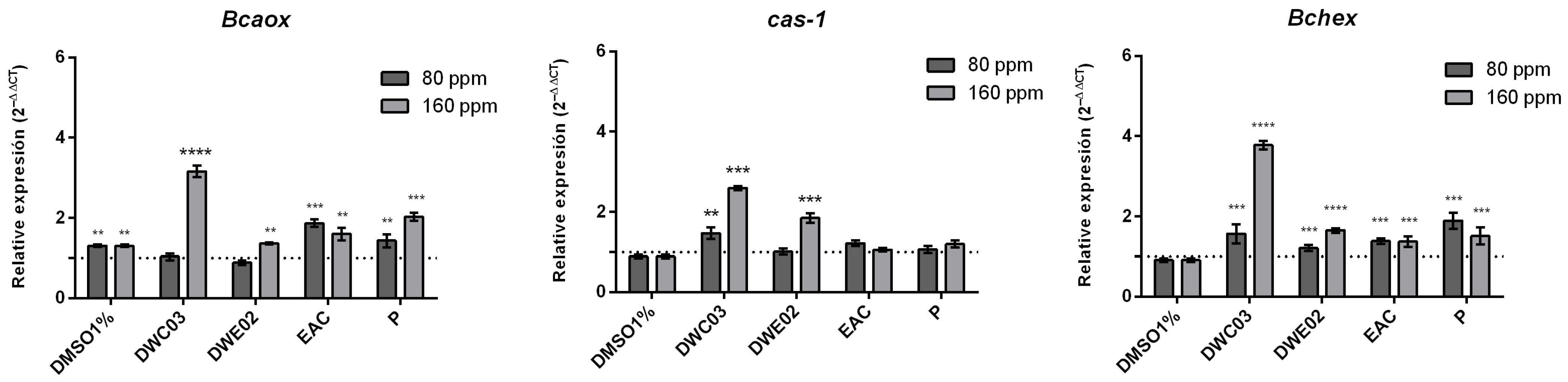
3. Discussion
4. Materials and Methods
4.1. Plant Material and Micropropagation
4.2. Callus Induction and Morpho-Histological Characterization
4.3. Extraction and Analytical Assays
4.4. Antifungal Properties Against Botrytis Cinerea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAP | 6-benzylaminopurine |
| KIN | Kinetin (6-furfurylaminopurine) |
| NAA | 1-naphthaleneacetic acid |
| PIC | Picloram (4-Amino-3,5,6-trichloro-2-pyridinecarboxylic acid) |
| TDZ | thidiazuron |
| Z | zeatin |
| GA3 | gibberellic acid |
| ROS | reactive oxygen species |
| WPM | Woody Plant Medium |
| MS | Murashige and Skoog Medium |
| DWC03 | Basal callus extract |
| DWE02 | In vitro propagated plant extract |
| EAC | Bark extract |
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Jimenez-Reyes, M.F.; Carrasco, H.; Olea, A.F.; Silva Moreno, E. Natural compounds: A sustainable alternative to the phytopathogens control. J. Chil. Chem. Soc. 2019, 64, 4459–4465. [Google Scholar] [CrossRef]
- Kim, S.; Lim, S.-W.; Choi, J. Drug discovery inspired by bioactive small molecules from nature. Anim. Cells Syst. 2022, 26, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Introduction: Biochemistry, Physiology and Ecological Functions of Secondary Metabolites. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism; Wiley-Blackwell: Hoboke, NJ, USA, 2010; pp. 1–19. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef]
- Kabtni, S.; Sdouga, D.; Bettaib Rebey, I.; Save, M.; Trifi-Farah, N.; Fauconnier, M.-L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- Arrieche, D.; Olea, A.F.; Jara-Gutiérrez, C.; Villena, J.; Pardo-Baeza, J.; García-Davis, S.; Viteri, R.; Taborga, L.; Carrasco, H. Ethanolic Extract from Fruits of Pintoa chilensis, a Chilean Extremophile Plant. Assessment of Antioxidant Activity and In Vitro Cytotoxicity. Plants 2024, 13, 1409. [Google Scholar] [CrossRef] [PubMed]
- Bosio, C.; Tomasoni, G.; Martinez, R.; Olea, A.F.; Carrasco, H.; Villena, J. Cytotoxic and apoptotic effects of leptocarpin, a plant-derived sesquiterpene lactone, on human cancer cell lines. Chem. Biol. Inter. 2015, 242, 415–421. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Delporte, C.; Barra, G.V.; Cazar Ramirez, M.E.; López, M.D.; Ramírez-Alarcón, K.; Cruz-Martins, N.; et al. Ethnopharmacology, Phytochemistry and Biological Activities of Native Chilean Plants. Curr. Pharm. Des. 2021, 27, 953–970. [Google Scholar] [CrossRef]
- Muñoz, O.; Tapia-Merino, J.; Nevermann, W.; San-Martín, A. Phytochemistry and biological properties of Drimys winteri JR et G. Forster var chilensis(DC) A. Bol. Latinoam. Caribe Plant Med. Aromat. 2021, 20, 443–462. [Google Scholar] [CrossRef]
- Torres-Ossandón, M.J.; Vega-Gálvez, A.; Salas, C.E.; Rubio, J.; Silva-Moreno, E.; Castillo, L. Antifungal activity of proteolytic fraction (P1G10) from (Vasconcellea cundinamarcensis) latex inhibit cell growth and cell wall integrity in Botrytis cinerea. Int. J. Food Microbiol. 2019, 289, 7–16. [Google Scholar] [CrossRef]
- Carrasco, H.; Robles-Kelly, C.; Rubio, J.; Olea, A.F.; Martínez, R.; Silva-Moreno, E. Antifungal effect of polygodial on Botrytis cinerea, a fungal pathogen affecting table grapes. Int. J. Mol. Sci. 2017, 18, 2251. [Google Scholar] [CrossRef] [PubMed]
- Robles-Kelly, C.; Rubio, J.; Thomas, M.; Sedan, C.; Martinez, R.; Olea, A.F.; Carrasco, H.; Taborga, L.; Silva-Moreno, E. Effect of drimenol and synthetic derivatives on growth and germination of Botrytis cinerea: Evaluation of possible mechanism of action. Pestic. Biochem. Physiol. 2017, 141, 50–56. [Google Scholar] [CrossRef]
- López, N.; Arrieche, D.; Soto, M.; Llovera, L.; Pérez, Y.; Taborga, L.; Carrasco, H. Two new drimane sesquiterpenes isolated from Drimys winteri (Canelo). Antifungal activity and molecular docking effect of winterdial against Botrytis cinerea. Nat. Prod. Res. 2025, 1–8. [Google Scholar] [CrossRef]
- Muñoz-Concha, D.; Vogel, H.; Yunes, R.; Ramilic, I.; Bresciani, L.; Malheiros, A. Presence of polygodial and drimenol in Drimys populations from Chile. Biochem. Syst. Ecol. 2007, 35, 434–438. [Google Scholar] [CrossRef]
- Jones, M.; Fish, N.; Lindsey, K. Plant Tissue Culture. In New Nucleic Acid Techniques; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1988; Volume 4, pp. 499–517. [Google Scholar]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Jordan, M. Morphogenic Response and in vitro Regeneration of Canelo (Drimys Winteri J.R. Et Forster), a Forest Species Used in Chilean Traditional Medicine. Acta Hortic. 1999, 502, 289–294. [Google Scholar] [CrossRef]
- Jordan, M.; Cortes, I. Shoot organogenesis in tissue culture of Drimys Winteri. Plant Sci. Lett. 1981, 23, 177–180. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.; Lee, S.H. Antifungal mechanism of polygodial. J. Agric. Food Chem. 2001, 49, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, J.; Jamieson, P.A.; Zhang, C. Alternative Oxidase Is Involved in the Pathogenicity, Development, and Oxygen Stress Response of Botrytis cinerea. Phytopathology 2019, 109, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, C.; Riquelme, J.; Valenzuela, P.; Hahn, M.; Silva Moreno, E. Bchex virulence gene of Botrytis cinerea: Characterization and functional analysis. J. Gen. Plant Pathol. 2011, 77, 230–238. [Google Scholar] [CrossRef]
- Hoeberichts, F.A.; ten Have, A.; Woltering, E.J. A tomato metacaspase gene is upregulated during programmed cell death in Botrytis cinerea-infected leaves. Planta 2003, 217, 517–522. [Google Scholar] [CrossRef]
- Shlezinger, N.; Minz, A.; Gur, Y.; Hatam, I.; Dagdas, Y.F.; Talbot, N.J.; Sharon, A. Anti-Apoptotic Machinery Protects the Necrotrophic Fungus Botrytis cinerea from Host-Induced Apoptotic-Like Cell Death during Plant Infection. PLoS Pathog. 2011, 7, e1002185. [Google Scholar] [CrossRef]
- Calderón, C.E.; Rotem, N.; Harris, R.; Vela-Corcía, D.; Levy, M. Pseudozyma aphidis activates reactive oxygen species production, programmed cell death and morphological alterations in the necrotrophic fungus Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.A.; Robson, G.D. Oxidative and amphotericin B-mediated cell death in the opportunistic pathogen Aspergillus fumigatus is associated with an apoptotic-like phenotype. Microbiology 2004, 150 Pt 6, 1937–1945. [Google Scholar] [CrossRef]
- Cheng, J.; Park, T.S.; Chio, L.C.; Fischl, A.S.; Ye, X.S. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol. Cell. Biol. 2003, 23, 163–177. [Google Scholar] [CrossRef]
- Amirav, A.; Fialkov, A.; Margolin-Eren, K.; Neumark, B.; Elkabets, O.; Tsizin, S.; Gordin, A.; Alon, T. Gas Chromatography–Mass Spectrometry (GC–MS) with Cold Electron Ionization (EI): Bridging the Gap Between GC–MS and LC–MS. Current Trends in Mass Spectrometry, supplement to LCGC North America. Lc Gc N. Am. 2020, 18, 5–15. [Google Scholar]
- Tarakhovskaya, E.; Marcillo, A.; Davis, C.; Milkovska-Stamenova, S.; Hutschenreuther, A.; Birkemeyer, C. Matrix Effects in GC–MS Profiling of Common Metabolites after Trimethylsilyl Derivatization. Molecules 2023, 28, 2653. [Google Scholar] [CrossRef]
- Paz, C.; Viscardi, S.; Iturra, A.; Marin, V.; Miranda, F.; Barra, P.J.; Mendez, I.; Duran, P. Antifungal Effects of Drimane Sesquiterpenoids Isolated from Drimys winteri against Gaeumannomyces graminis var. tritici. Appl. Environ. Microbiol. 2020, 86, e01834-20. [Google Scholar] [CrossRef]
- Cárcamo-Oyarce, G.; Silva, M.; Becerra, J.; Urrutia, H.; Sossa, K.; Paz, C. Inhibition of quorum sensing by drimane lactones from chilean Flora. J. Chil. Chem. Soc. 2014, 59, 2622–2624. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jangid, V.K.; Senthil-Kumar, M.; Chandran, D.; Sinharoy, S. Callus induction and efficient in vitro plant regeneration protocol for Chickpea. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 156, 21. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique. Nature 1941, 147, 222. [Google Scholar] [CrossRef]
- Novikov, A.; Sup-Novikova, M. Modified staining protocol with Safranin O and Astra Blue for the plant histology. Plant Introd. 2021, 89/90, 110–113. [Google Scholar] [CrossRef]
- Cechinel, V.; Schlemper, V.; Santos, A.R.S.; Pinheiro, T.R.; Yunes, R.A.; Mendes, G.L.; Calixto, J.B.; le Menache, F. Isolation and identification of active compounds from Drimys winteri barks. J. Ethnopharmacol. 1998, 62, 223–227. [Google Scholar] [CrossRef]
- Malheiros, A.; Cechinel, V.; Scmitt, C.; Santos, A.; Scheidt, C.; Calixto, J.; Delle Monache, F.; Yunes, R. A sesquiterpene drimane with antinociceptive activity from Drimys winteri bark. Phytochemistry 2001, 57, 103–107. [Google Scholar] [CrossRef]
- Olea, A.F.; Rubio, J.; Sedan, C.; Carvajal, D.; Nuñez, M.; Espinoza, L.; Llovera, L.; Nuñez, G.; Taborga, L.; Carrasco, H. Antifungal Activity of 2-Allylphenol Derivatives on the Botrytis cinerea Strain: Assessment of Possible Action Mechanism. Int. J. Mol. Sci. 2023, 24, 6530. [Google Scholar] [CrossRef] [PubMed]
- Kelts, J.L.; Cali, J.J.; Duellman, S.J.; Shultz, J. Altered cytotoxicity of ROS-inducing compounds by sodium pyruvate in cell culture medium depends on the location of ROS generation. Springerplus 2015, 4, 269. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, L.; Du, C. Fungal Apoptosis-Related Proteins. Microorganisms 2024, 12, 2289. [Google Scholar] [CrossRef]


| Woody Plant Medium (WPM) | With Vitamins | Without Vitamins | |||
|---|---|---|---|---|---|
| I 2 | P | E | R | ||
| Hormones 1 (μM) | NAA | 0.55 | 0.28 | 0.55 | 5.49 |
| BAP | - | 3.55 | 3.55 | - | |
| KIN | 4.65 | - | - | - | |
| GA3 | 0.15 | - | - | - | |
| Others (mg/L) | Ascorbic acid | 100 | 50 | 50 | - |
| Citric acid | 100 | 50 | 50 | - | |
| Adenine sulfate | - | 40 | - | ||
| Media Formulation | Friable and Compact Calluses | Leaf or Root Organorganogenesisgenesis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS | WPM | |||||||||
| I 1 | II | III | IV | V | VI | VI(1) | VI(1) | VI(2) | VII | |
| Vegetal material | L | S | L | L | L | L | S | L | IS | IS |
| N° of used explants | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| N° of generated calluses | 20 | 6 | 8 | 32 | 30 | 12 | 24 | 16 | 0 | 0 |
| N° of eliminated calluses | 20 | 34 | 32 | 0 | 0 | 28 | 16 | 24 | 40 | 40 |
| CIF (%) | 50 | 15 | 20 | 80 | 75 | 30 | 60 | 40 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, J.; Robles-Kelly, C.; Silva-Moreno, E.; Carrasco, H.; Olea, A.F. Assessment of Phytochemical Composition and Antifungal Activity of Micropropagated Drymis winteri Plants. Plants 2025, 14, 3215. https://doi.org/10.3390/plants14203215
Rubio J, Robles-Kelly C, Silva-Moreno E, Carrasco H, Olea AF. Assessment of Phytochemical Composition and Antifungal Activity of Micropropagated Drymis winteri Plants. Plants. 2025; 14(20):3215. https://doi.org/10.3390/plants14203215
Chicago/Turabian StyleRubio, Julia, Christian Robles-Kelly, Evelyn Silva-Moreno, Héctor Carrasco, and Andrés F. Olea. 2025. "Assessment of Phytochemical Composition and Antifungal Activity of Micropropagated Drymis winteri Plants" Plants 14, no. 20: 3215. https://doi.org/10.3390/plants14203215
APA StyleRubio, J., Robles-Kelly, C., Silva-Moreno, E., Carrasco, H., & Olea, A. F. (2025). Assessment of Phytochemical Composition and Antifungal Activity of Micropropagated Drymis winteri Plants. Plants, 14(20), 3215. https://doi.org/10.3390/plants14203215






