Exploring the Bioactive Potential and Chemical Profile of Schinus molle Essential Oil: An Integrated In Silico and In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Identification, and Extraction
2.2. Essential Oil Analysis (GC-MS)
2.3. Computational Methods
2.3.1. Density Functional Theory (DFT) Calculation
2.3.2. Molecular Electrostatic Potential (MEP) Surfaces
2.3.3. Pharmacokinetics and Toxicity
2.4. Antioxidant Capacity
2.4.1. Chemical Materials
2.4.2. FRAP Assay
2.4.3. Metal Chelating Activity (Ferrozine)
2.4.4. DPPH Radical Scavenging Assay
2.4.5. ABTS Radical Scavenging Assay
2.5. Antibacterial Activity
2.5.1. Chemical Materials and Antibiotics
2.5.2. Microbial Strains
2.5.3. Microplate Assay
2.6. Cytotoxicity
2.6.1. Cell Line Culture
2.6.2. Crystal Violet Proliferation Assay
2.6.3. Western Blot Analysis
2.7. Toxicity
2.7.1. Maintenance of Caenorhabditis Elegans Culture
2.7.2. Test Preparation
3. Results
3.1. Composition
3.2. Reactivity of SM_EO Components
3.2.1. Frontier Orbitals
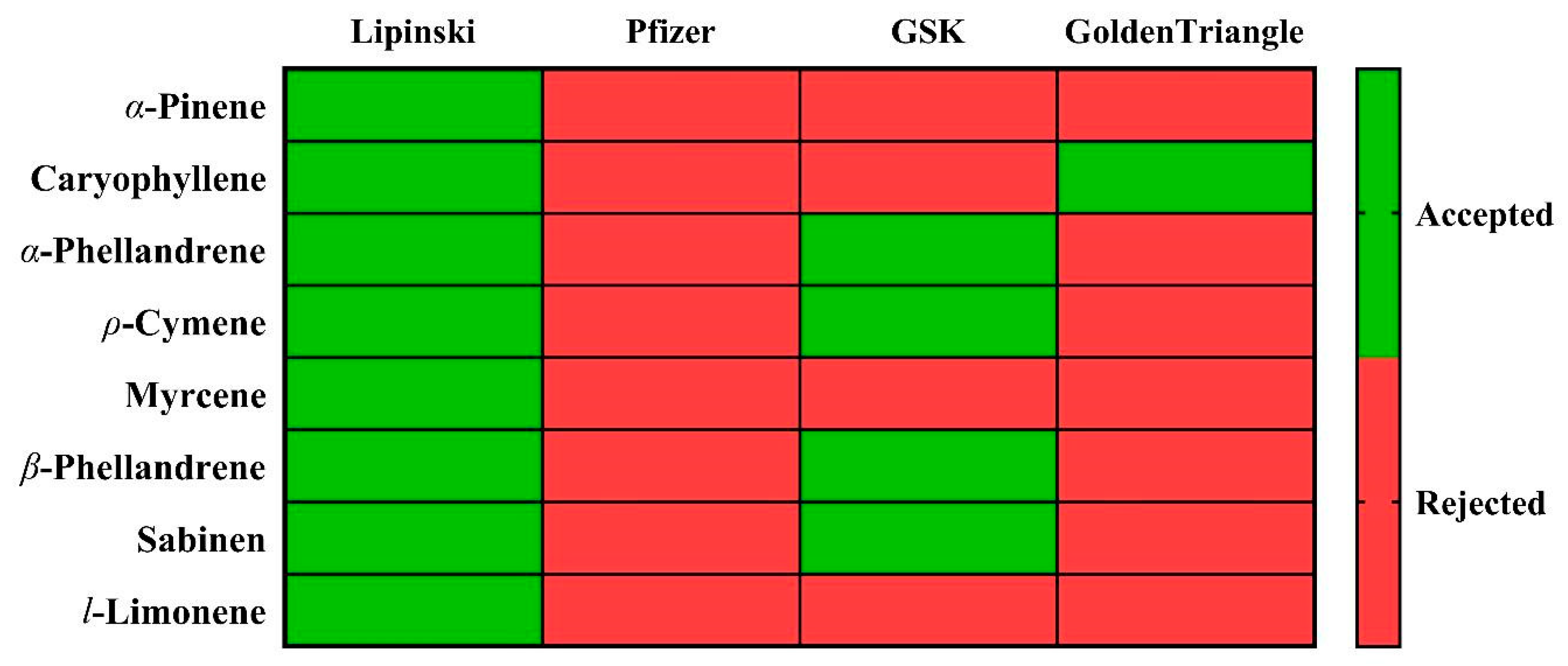
3.2.2. In Silico Pharmacokinetic Prediction
3.3. Antioxidant Activity
3.4. Antibacterial Activity
3.5. Cytotoxicity and Biological Activity
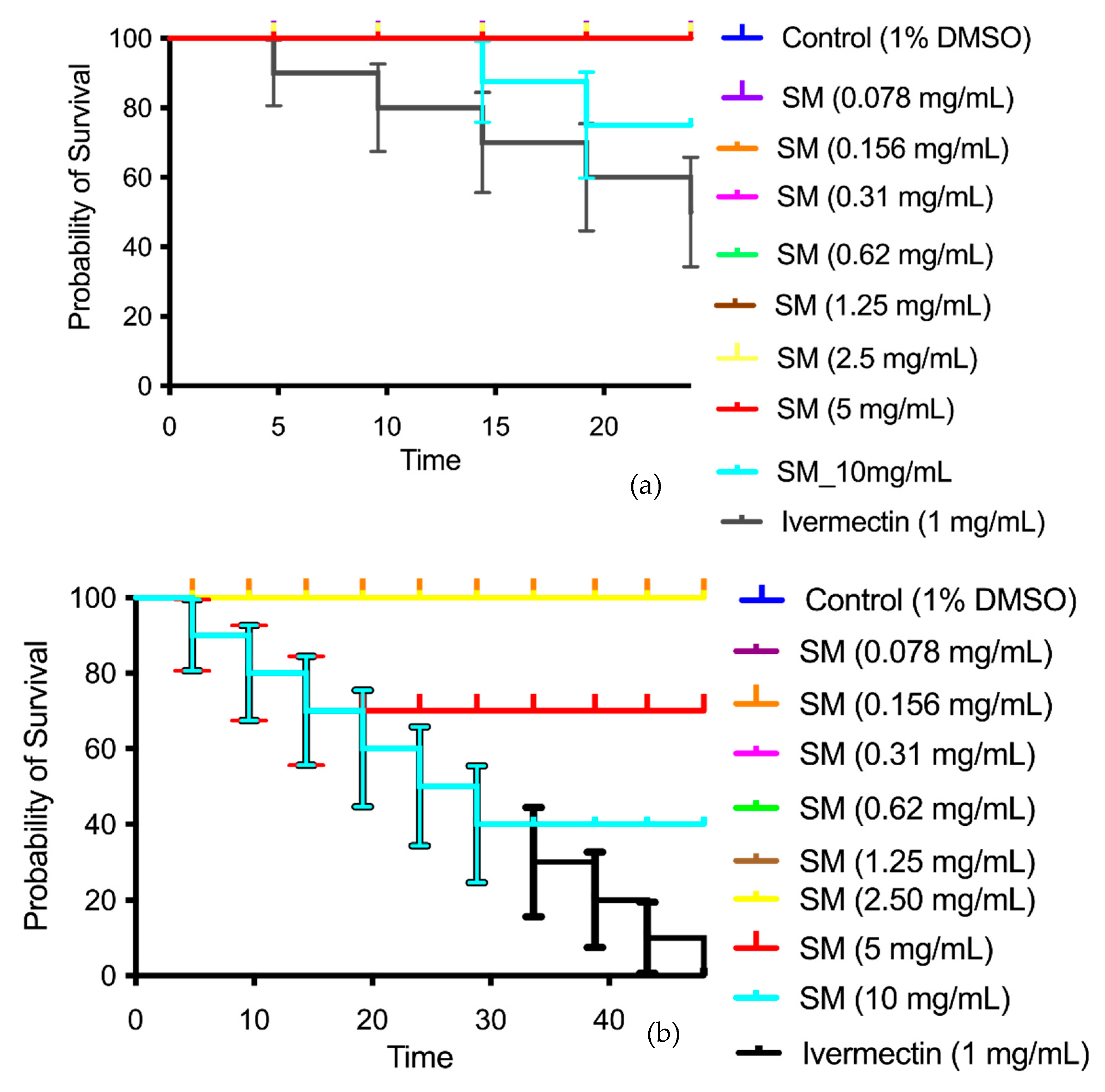
3.6. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rossini, C.; Menéndez, P.; Dellacassa, E.; Moyna, P. Essential Oils from Leaves of Schinus molle and S. lentiscifolius of Uruguayan Origin. J. Essent. Oil Res. 1996, 8, 71–73. [Google Scholar] [CrossRef]
- Kasimala MB, K.B. A review on Brazilian pepper plant: Schinus molle. J. At. Mol. 2012, 2, 6–13. [Google Scholar]
- Madaleno, I.M.; Delatorre-Herrera, J. Medicina popular de Iquique, Tarapacá. Idesia 2013, 31, 67–78. [Google Scholar] [CrossRef][Green Version]
- Marongiu, B.; Porcedda, A.P.S.; Casu, R.; Pierucci, P. Chemical composition of the oil and supercritical CO2 extract of Schinus molle L. Flavour. Fragr. J. 2004, 19, 554–558. [Google Scholar] [CrossRef]
- MINSAL. 2012. Available online: https://ispch.gob.cl/andim/repositorio-publicaciones-isp/repositorio-publicacion/?id_p=306 (accessed on 17 July 2025).
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of Plant Essential Oils and Their Components in Animal Agriculture - in vitro Studies on Antibacterial Mode of Action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Zaitoun, A.A.; Farag, M.A.; Gayed, S.H.; Harraz, F.M. Chemical composition, insecticidal and insect repellent activity of Schinus molle L. leaf and fruit essential oils against Trogoderma granarium and Tribolium castaneum. Nat. Prod. Res. 2010, 24, 226–235. [Google Scholar] [CrossRef]
- Martins Mdo, R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
- Hosni, K.; Jemli, M.; Dziri, S.; M’rabet, Y.; Ennigrou, A.; Sghaier, A.; Casabianca, H.; Vulliet, E.; Brahim, N.B.; Sebei, H. Changes in phytochemical, antimicrobial and free radical scavenging activities of the Peruvian pepper tree (Schinus molle L.) as influenced by fruit maturation. Ind. Crops Prod. 2011, 34, 1622–1628. [Google Scholar] [CrossRef]
- Giuffrida, D.; Martínez, N.; Arrieta-Garay, Y.; Fariña, L.; Boido, E.; Dellacassa, E. Valorisation of Schinus molle fruit as a source of volatile compounds in foods as flavours and fragrances. Food Res. Int. 2020, 133, 109103. [Google Scholar] [CrossRef]
- Zabetakis, I.; Holden, M.A. Strawberry Flavour: Analysis and Biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Ninio, R.; Lewinsohn, E.; Mizrahi, Y.; Sitrit, Y. Quality attributes of stored koubo (Cereus peruvianus (L.) Miller) fruit. Postharvest Biol. Technol. 2003, 30, 273–280. [Google Scholar] [CrossRef]
- Yoshihiko, A.; Sakamoto, M.; Ikeda, Y.; Takana, M. Identification and Characterization of Volatile Components of the Japanese Sour Citrus Fruit Citrus nagato-yuzukichi Tanaka. Biosci. Biotechnol. Biochem. 2008, 72, 1965–1968. [Google Scholar] [CrossRef]
- Camara, B. Biochemistry of fruit ripening: Edited by G.B. Seymour, J.E. Taylor and G.A. Tucker, Chapman & Hall, 1993, 454 pp. £60.00. Plant Sci. 1994, 97, 227. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Kader, A. A Perspective on Postharvest Horticulture (1978–2003). HortScience 2003, 38, 1004–1008. [Google Scholar] [CrossRef]
- Chaudhary, M.K.; Srivastava, A.; Singh, K.K.; Tandon, P.; Joshi, B.D. Computational evaluation on molecular stability, reactivity, and drug potential of frovatriptan from DFT and molecular docking approach. Comput. Theor. Chem. 2020, 1191, 113031. [Google Scholar] [CrossRef]
- Chaudhary, S.C.; Siddiqui, M.S.; Athar, M.; Alam, M.S. d-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Human. Exp. Toxicol. 2012, 31, 798–811. [Google Scholar] [CrossRef]
- Bruna, F.; Fernández, K.; Urrejola, F.; Touma, J.; Navarro, M.; Sepúlveda, B.; Larrazabal-Fuentes, M.; Paredes, A.; Neira, I.; Ferrando, M.; et al. Chemical composition, antioxidant, antimicrobial and antiproliferative activity of Laureliopsis philippiana essential oil of Chile, study in vitro and in silico. Arab. J. Chem. 2022, 15, 104271. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chavez, H.; Enciso-Roca, E.C.; Común-Ventura, P.W.; Hañari-Quispe, R.D.; Figueroa-Salvador, L.; Loyola-Gonzales, E.L.; Pari-Olarte, J.B.; Aljarba, N.H.; Alkahtani, S.; et al. GC-MS Profile, Antioxidant Activity, and In Silico Study of the Essential Oil from Schinus molle L. Leaves in the Presence of Mosquito Juvenile Hormone-Binding Protein (mJHBP) from Aedes aegypti. Biomed. Res. Int. 2022, 2022, 5601531. [Google Scholar] [CrossRef]
- Díaz, C.; Quesada, S.; Brenes, O.; Aguilar, G.; Cicció, J.F. Chemical composition of Schinus molle essential oil and its cytotoxic activity on tumour cell lines. Nat. Product. Res. 2008, 22, 1521–1534. [Google Scholar] [CrossRef]
- Turchetti, G.; Garzoli, S.; Laghezza Masci, V.; Sabia, C.; Iseppi, R.; Giacomello, P.; Tiezzi, A.; Ovidi, E. Antimicrobial Testing of Schinus molle (L.) Leaf Extracts and Fractions Followed by GC-MS Investigation of Biological Active Fractions. Molecules 2020, 25, 1977. [Google Scholar] [CrossRef] [PubMed]
- do Prado, A.C.; Garces, H.G.; Bagagli, E.; Rall, V.L.M.; Furlanetto, A.; Fernandes Junior, A.; Furtado, F.B. Schinus molle essential oil as a potential source of bioactive compounds: Antifungal and antibacterial properties. J. Appl. Microbiol. 2019, 126, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Eryigit, T.; Yildirim, B.; Ekici, K.; Çirka, M. Chemical Composition, Antimicrobial and Antioxidant Properties of Schinus molle L. Essential Oil from Turkey. J. Essent. Oil Bear. Plants 2017, 20, 570–577. [Google Scholar] [CrossRef]
- Duarte, J.A.; Zambrano, L.A.B.; Quintana, L.D.; Rocha, M.B.; Schmitt, E.G.; Boligon, A.A.; Anraku de Campos, M.M.; de Oliveira, L.F.S.; Machado, M.M. Immunotoxicological Evaluation of Schinus molle L. (Anacardiaceae) Essential Oil in Lymphocytes and Macrophages. Evid. Based Complement. Altern. Med. 2018, 2018, 6541583. [Google Scholar] [CrossRef]
- Lanzerstorfer, P.; Sandner, G.; Pitsch, J.; Mascher, B.; Aumiller, T.; Weghuber, J. Acute, reproductive, and developmental toxicity of essential oils assessed with alternative in vitro and in vivo systems. Arch. Toxicol. 2021, 95, 673–691. [Google Scholar] [CrossRef]
- Avello Lorca, M.; López Canales, C.; Gatica Valenzuela, C.; Bustos Concha, E.; Brieva Chait, A.; Pastene Navarrete, E.; Bittner Berner, M. Efectos antimicrobianos de extractos de plantas chilenas de las familias Lauraceae y Atherospermataceae. Rev. Cuba. Plantas Med. 2012, 17, 73–83. [Google Scholar]
- Volpini-Klein, A.; Lima-Junior, S.; Cardoso, C.; Cabral, M.R.; Louro, G.; Coutinho, E.; Jesus, D.; Junior, D.; Simionatto, E. Chemical Composition of Essential Oils from Leaves and Fruits of Schinus molle Obtained by Different Extraction Methods (Hydrodistillation, Fractional Hydrodistillation and Steam Distillation) and Seasonal Variations. J. Essent. Oil Bear. Plants 2021, 24, 228–242. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol. Stream 2007, 16, 65–120. [Google Scholar]
- Stewart, J. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2008, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Pino-Rios, R.; Yañez, O.; Inostroza, D.; Ruiz, L.; Cardenas, C.; Fuentealba, P.; Tiznado, W. Proposal of a simple and effective local reactivity descriptor through a topological analysis of an orbital-weighted fukui function. J. Comput. Chem. 2017, 38, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA 1986, 83, 8440–8441. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Pearson, R.G. Applications of DFT. In Chemical Hardness; Wiley: Hoboken, NJ, USA, 1997; pp. 59–97. [Google Scholar]
- Pearson, R.G. The Principle of Maximum Hardness. In Chemical Hardness; Wiley: Hoboken, NJ, USA, 1997; pp. 99–124. [Google Scholar]
- Gázquez, J.L.; Cedillo, A.; Vela, A. Electrodonating and Electroaccepting Powers. J. Phys. Chem. A 2007, 111, 1966–1970. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Chakraborty, A.; Giri, S. Net Electrophilicity. J. Phys. Chem. A 2009, 113, 10068–10074. [Google Scholar] [CrossRef]
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. WIREs Comput. Mol. Sci. 2022, 12, e1601. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Sudan, R.; Bhagat, M.; Gupta, S.; Singh, J.; Koul, A. Iron (FeII) chelation, ferric reducing antioxidant power, and immune modulating potential of Arisaema jacquemontii (Himalayan Cobra Lily). Biomed. Res. Int. 2014, 2014, 179865. [Google Scholar] [CrossRef]
- Touma, J.; Navarro, M.; Sepúlveda, B.; Pavon, A.; Corsini, G.; Fernández, K.; Quezada, C.; Torres, A.; Larrazabal-Fuentes, M.J.; Paredes, A.; et al. The Chemical Compositions of Essential Oils Derived from Cryptocarya alba and Laurelia sempervirens Possess Antioxidant, Antibacterial and Antitumoral Activity Potential. Molecules 2020, 25, 5600. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Larrazabal-Fuentes, M.; Palma, J.; Paredes, A.; Mercado, A.; Neira, I.; Lizama, C.; Sepulveda, B.; Bravo, J. Chemical composition, antioxidant capacity, toxicity and antibacterial activity of the essential oils from Acantholippia deserticola (Phil.) Moldenke (Rica rica) and Artemisia copa Phil. (Copa copa) extracted by microwave-assisted hydrodistillation. Ind. Crops Prod. 2019, 142, 111830. [Google Scholar] [CrossRef]
- CLSI Document M02-A11; Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard. CLSI: Wayne, PA, USA, 2012.
- Akman, F.; Demirpolat, A.; Kazachenko, A.S.; Kazachenko, A.S.; Issaoui, N.; Al-Dossary, O. Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri. Molecules 2023, 28, 2684. [Google Scholar] [CrossRef]
- Nenadis, N.; Pyrka, I.; Tsimidou, M.Z. The Contribution of Theoretical Prediction Studies to the Antioxidant Activity Assessment of the Bioactive Secoiridoids Encountered in Olive Tree Products and By-Products. Molecules 2023, 28, 2267. [Google Scholar] [CrossRef]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Spiegel, M. Current Trends in Computational Quantum Chemistry Studies on Antioxidant Radical Scavenging Activity. J. Chem. Inf. Model. 2022, 62, 2639–2658. [Google Scholar] [CrossRef]
- Srivastava, R. Physicochemical, antioxidant properties of carotenoids and its optoelectronic and interaction studies with chlorophyll pigments. Sci. Rep. 2021, 11, 18365. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.; Domingo, L.R.; Andrés, J.; Pérez, P.; Tapia, O. Nonlocal (Pair Site) Reactivity from Second-Order Static Density Response Function: Gas- and Solution-Phase Reactivity of the Acetaldehyde Enolate as a Test Case. J. Phys. Chem. A 1999, 103, 1367–1375. [Google Scholar] [CrossRef]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; Decrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Gleeson, M.P. Generation of a Set of Simple, Interpretable ADMET Rules of Thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Johnson, T.W.; Dress, K.R.; Edwards, M. Using the Golden Triangle to optimize clearance and oral absorption. Bioorganic Med. Chem. Lett. 2009, 19, 5560–5564. [Google Scholar] [CrossRef]
- Abderrahim, A.; Belhamel, K.; Chalard, P.; Figuérédo, G. Correlation between chemical composition and antioxidant activity of the essential oils from leaves and berries of Schinus molle L. growing in two areas of Bejaia (Algeria). J. Food Meas. Charact. 2018, 12, 1123–1134. [Google Scholar] [CrossRef]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef] [PubMed]
- Belhamel, K.; Abderrahim, A.; Ludwig, R. Chemical composition and antibacterial activity of the essential oil of Schinus molle L. grown in Algeria. Int. J. Essent. Oil Ther. 2008, 2, 175–177. [Google Scholar] [CrossRef]
- Zahed, N.; Hosni, K.; Brahim, N.; Kallel, M.; Sebei, H. Allelopathic effect of Schinus molle essential oils on wheat germination. Acta Physiol. Plant. 2010, 32, 1221–1227. [Google Scholar] [CrossRef]
- Ghavam, M. Relationships of irrigation water and soil physical and chemical characteristics with yield, chemical composition and antimicrobial activity of Damask rose essential oil. PLoS ONE 2021, 16, e0249363. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Erukainure, O.; Kayode, F.; Adeyoju, O.; Adenekan, S.; Asieba, G.; Ajayi, A.; Adegbola, M.; Sarum, B. Antioxidant and chemical properties of essential oil extracted from blend of selected spices. J. Coast. Life Med. 2015, 3, 575–578. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Navarro, M.; Urrejola, F.; Espinoza, M.; Silva, S.; González, S.; Utreras, D.; Fernández, K.; Bravo, J. Biological activity of the essential oil of Drimys winteri. Front. Chem. 2024, 12, 1321300. [Google Scholar] [CrossRef]
- Guala, M.S.; Elder, H.V.; Perez, G.; Chiesa, A. Evaluación del Poder Antioxidante de Fracciones de Aceite Esencial Crudo de Schinus molle L. obtenidas por Destilación al Vacío. Inf. Tecnológica 2009, 20, 83–88. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Aruna, T.; Hemalatha, G.; Kumutha, K.; Kanchana, S.; Sampathrajan, V. Physicochemical, antioxidant and antimicrobial properties of citrus peel essential oils. J. Appl. Nat. Sci. 2022, 14, 640–646. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kazyoba, P.; Viljoen, A. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Product. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Rocha, P.M.d.M.; Rodilla, J.M.; Díez, D.; Elder, H.; Guala, M.S.; Silva, L.A.; Pombo, E.B. Synergistic Antibacterial Activity of the Essential Oil of Aguaribay (Schinus molle L.). Molecules 2012, 17, 12023–12036. [Google Scholar] [CrossRef]
- Gundidza, M. Antimicrobial activity of essential oil from Schinus molle Linn. Cent. Afr. J. Med. 1993, 39, 231–234. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- de Vasconcelos, C.B.J.; de Carvalho, F.O.; de Vasconcelos, C.M.D.; Calixto, F.A.F.; Santana, H.S.R.; Almeida, I.B.; de Aquino, L.A.G.; de Souza Araújo, A.A.; Serafini, M.R. Mechanism of Action of Limonene in Tumor Cells: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2021, 27, 2956–2965. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Hussein, S.Z.; MalAllah, M.Q.; Abdulamir, A.S.; Abu Bakar, F. A High-throughput Quantitative Expression Analysis of Cancer-related Genes in Human HepG2 Cells in Response to Limonene, a Potential Anticancer Agent. Curr. Cancer Drug Targets 2018, 18, 807–815. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Poliseno, L.; Mariani, L.; Collecchi, P.; Piras, A.; Zaccaro, L.; Rainaldi, G. Bcl2-negative MCF7 cells overexpress p53: Implications for the cell cycle and sensitivity to cytotoxic drugs. Cancer Chemother. Pharmacol. 2002, 50, 127–130. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Fuentes, C.; Verdú, S.; Fuentes, A.; Ruiz, M.J.; Barat, J.M. Effects of essential oil components exposure on biological parameters of Caenorhabditis elegans. Food Chem. Toxicol. 2022, 159, 112763. [Google Scholar] [CrossRef]
- Wu, T.; Xu, H.; Liang, X.; Tang, M. Caenorhabditis elegans as a complete model organism for biosafety assessments of nanoparticles. Chemosphere 2019, 221, 708–726. [Google Scholar] [CrossRef]
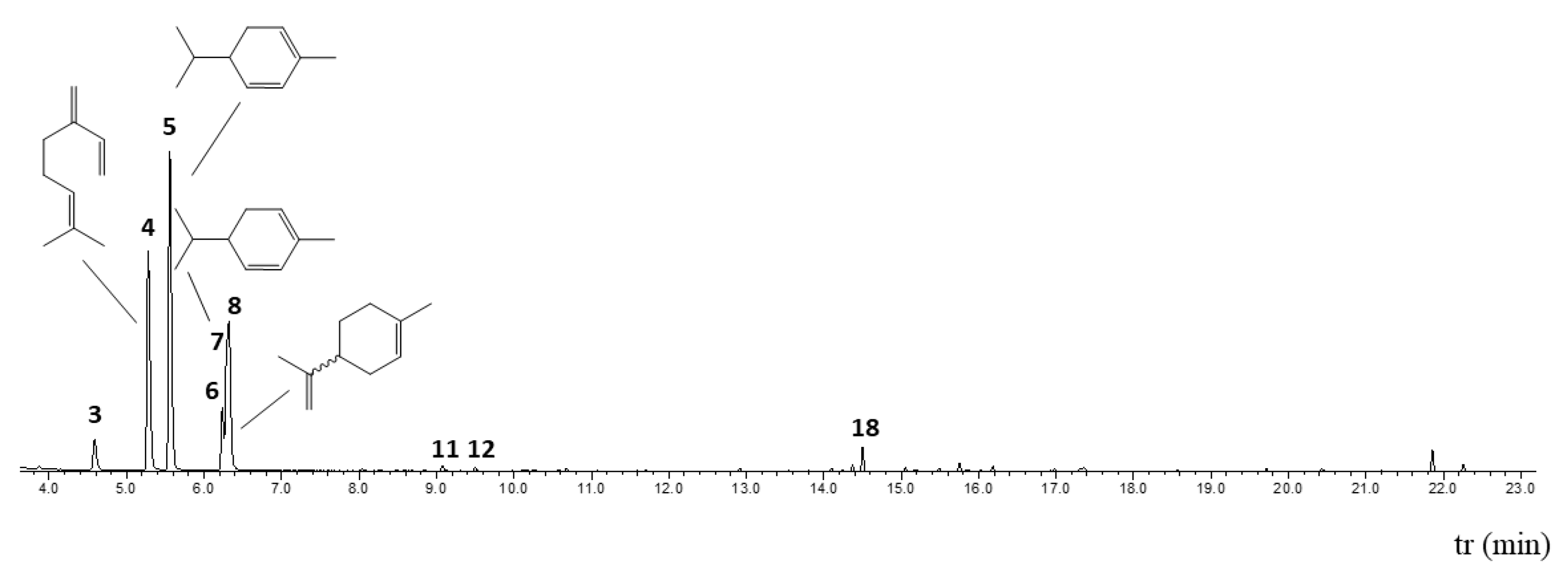





| Composition | |||||||
|---|---|---|---|---|---|---|---|
| N° | tr (min) | R.I. e | R.I. t | Area (%) | Compounds | Id | CAS |
| 1 | 3.545 | 930 | 931 | 4.80 | α-pinene | GC-MS, R.I. | 80-56-8 |
| 2 | 3.873 | 942 | 946 | 0.16 | camphene | GC-MS, R.I. | 79-92-5 |
| 3 | 4.586 | 967 | 969 | 3.52 | sabinene | GC-MS, R.I. | 3387-41-5 |
| 4 | 5.279 | 991 | 991 | 23.87 | β-myrcene | GC-MS, R.I. | 123-35-3 |
| 5 | 5.557 | 1001 | 1000 | 34.02 | α-phellandrene | GC-MS, R.I. | 99-83-2 |
| 6 | 6.236 | 1025 | 1026 | 5.36 | p-cymene | GC-MS, R.I. | 99-87-6 |
| 7 | 6.305 | 1028 | 1027 | 7.01 | β-phellandrene | GC-MS, R.I. | 555-10-2 |
| 8 | 6.320 | 1028 | 1028 | 13.99 | limonene 1 | GC-MS, R.I. | |
| 9 | 7.246 | 1061 | 1062 | 0.06 | γ-terpinene | GC-MS, R.I. | 99-85-4 |
| 10 | 8.034 | 1089 | 1088 | 0.14 | terpinolene | GC-MS, R.I. | 586-62-9 |
| 11 | 9.075 | 1132 | 1129 | 0.41 | octanoic acid, methyl ester | GC-MS, R.I. | 111-11-5 |
| 12 | 9.498 | 1151 | 1145 | 0.22 | limonene oxide, cis | GC-MS, R.I. | 13837-75-7 |
| 13 | 10.111 | 1178 | 1177 | 0.05 | terpinen-4-ol | GC-MS, R.I. | 562-74-3 |
| 14 | 10.681 | 1204 | 1202 | 0.19 | α-phellandrene epoxide | GC-MS, R.I. | 288393-04-4 |
| 15 | 13.798 | 1377 | 1377 | 0.04 | α-copaene | GC-MS, R.I. | 3856-25-5 |
| 16 | 14.102 | 1394 | 1387 | 0.13 | (E)-β-elemene | GC-MS, R.I. | 33880-83-0 |
| 17 | 14.371 | 1410 | 1410 | 0.42 | 1H-cycloprop[e]azulene, 1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl | GC-MS, R.I. | 489-40-7 |
| 18 | 14.502 | 1418 | 1418 | 1.77 | β-caryophyllene | GC-MS, R.I. | 87-44-5 |
| 19 | 15.054 | 1454 | 1455 | 0.27 | α-caryophyllene | GC-MS, R.I. | 6753-98-6 |
| 20 | 15.178 | 1462 | 1462 | 0.09 | (Z,E)-α-farnesene | GC-MS, R.I. | 502-61-4 |
| 21 | 15.494 | 1482 | IRNR | 0.17 | α-amorphene | GC-MS, R.I. | 483-75-0 |
| 22 | 15.752 | 1499 | 1498 | 0.64 | bicyclogermacrene | GC-MS, R.I. | 67650-90-2 |
| 23 | 15.841 | 1504 | 1504 | 0.07 | α-muurolene | GC-MS, R.I. | 10208-80-7 |
| 24 | 16.184 | 1527 | 1523 | 0.34 | δ-cadinene | GC-MS, R.I. | 483-76-1 |
| 25 | 16.976 | 1579 | 1577 | 0.10 | caryophyllene oxide | GC-MS, R.I. | 1139-30-6 |
| Oil | IC50 ABTS a | IC50 DPPH a | IC50 Ferrozine a | FRAP b |
|---|---|---|---|---|
| SM_EO | 62.07 ± 1.25 | 48.89 ± 0.82 | 145.06 ± 1.54 | 21.45 ± 1.56 |
| Trolox | 26.48 ± 1.60 | 27 ± 0.51 | ---- | ---- |
| EDTA | ---- | ---- | 12 ± 0.18 | ---- |
| Microbial Strain | SM_EO MIC (μg·μL−1) | α-Phellandrene MIC (μg·μL−1) | Limonene MIC (μg·μL−1) |
|---|---|---|---|
| B. cereus * | 2.0 | 34 | 67.4 |
| S. flexneri * | 32.5 | 8.5 | 33.7 |
| Y. enterocolitica | 32.5 | 34 | 33.7 |
| S. enteritidis | 32.5 | 68 | 67.4 |
| S. sonnei * | 32.5 | 68 | 67.4 |
| S. typhimurium | 32.5 | 68 | 67.4 |
| C. striatum * | 65 | - | - |
| E. coli | 65 | 68 | 67.4 |
| E. faecalis | 65 | 136 | - |
| S. epidermidis | 65 | 136 | 134.7 |
| S. paratyphi * | 65 | 68 | 134.7 |
| S. sciuri | 65 | 68 | 67.4 |
| L. monocytogenes | 130 | - | 134.7 |
| S. aureus | 130 | 68 | - |
| S. boydii * | 130 | - | 16.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oses, R.; Ferrando, M.; Bruna, F.; Retamales, P.; Navarro, M.; Fernández, K.; Vera, W.; Larrazábal, M.J.; Neira, I.; Paredes, A.; et al. Exploring the Bioactive Potential and Chemical Profile of Schinus molle Essential Oil: An Integrated In Silico and In Vitro Evaluation. Plants 2025, 14, 2449. https://doi.org/10.3390/plants14152449
Oses R, Ferrando M, Bruna F, Retamales P, Navarro M, Fernández K, Vera W, Larrazábal MJ, Neira I, Paredes A, et al. Exploring the Bioactive Potential and Chemical Profile of Schinus molle Essential Oil: An Integrated In Silico and In Vitro Evaluation. Plants. 2025; 14(15):2449. https://doi.org/10.3390/plants14152449
Chicago/Turabian StyleOses, Rómulo, Matías Ferrando, Flavia Bruna, Patricio Retamales, Myriam Navarro, Katia Fernández, Waleska Vera, María José Larrazábal, Iván Neira, Adrián Paredes, and et al. 2025. "Exploring the Bioactive Potential and Chemical Profile of Schinus molle Essential Oil: An Integrated In Silico and In Vitro Evaluation" Plants 14, no. 15: 2449. https://doi.org/10.3390/plants14152449
APA StyleOses, R., Ferrando, M., Bruna, F., Retamales, P., Navarro, M., Fernández, K., Vera, W., Larrazábal, M. J., Neira, I., Paredes, A., Osorio, M., Yáñez, O., Jacobs, M., & Bravo, J. (2025). Exploring the Bioactive Potential and Chemical Profile of Schinus molle Essential Oil: An Integrated In Silico and In Vitro Evaluation. Plants, 14(15), 2449. https://doi.org/10.3390/plants14152449







