Application of Municipal Biowaste-Derived Products in Tomato Cultivation for Enhanced Fruit Quality Attributes and Nutritional Profile
Abstract
1. Introduction
2. Results
2.1. Soil Physicochemical Parameters and Leachates Characterization
2.2. Plant Growth, Yield, and Physiological Parameters
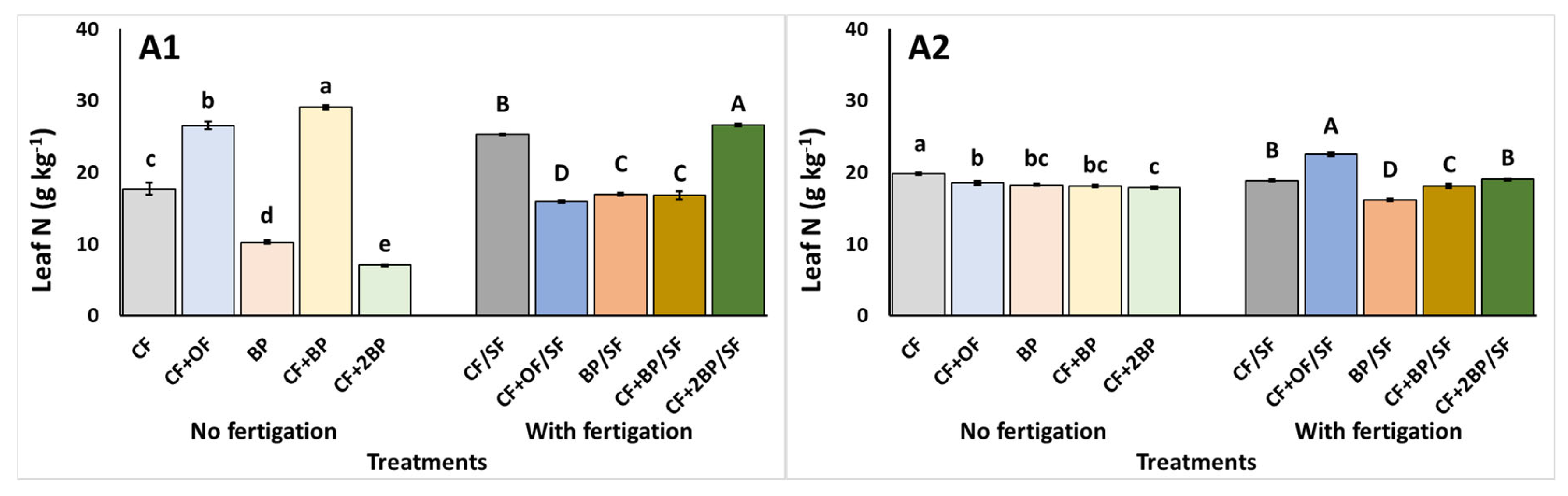
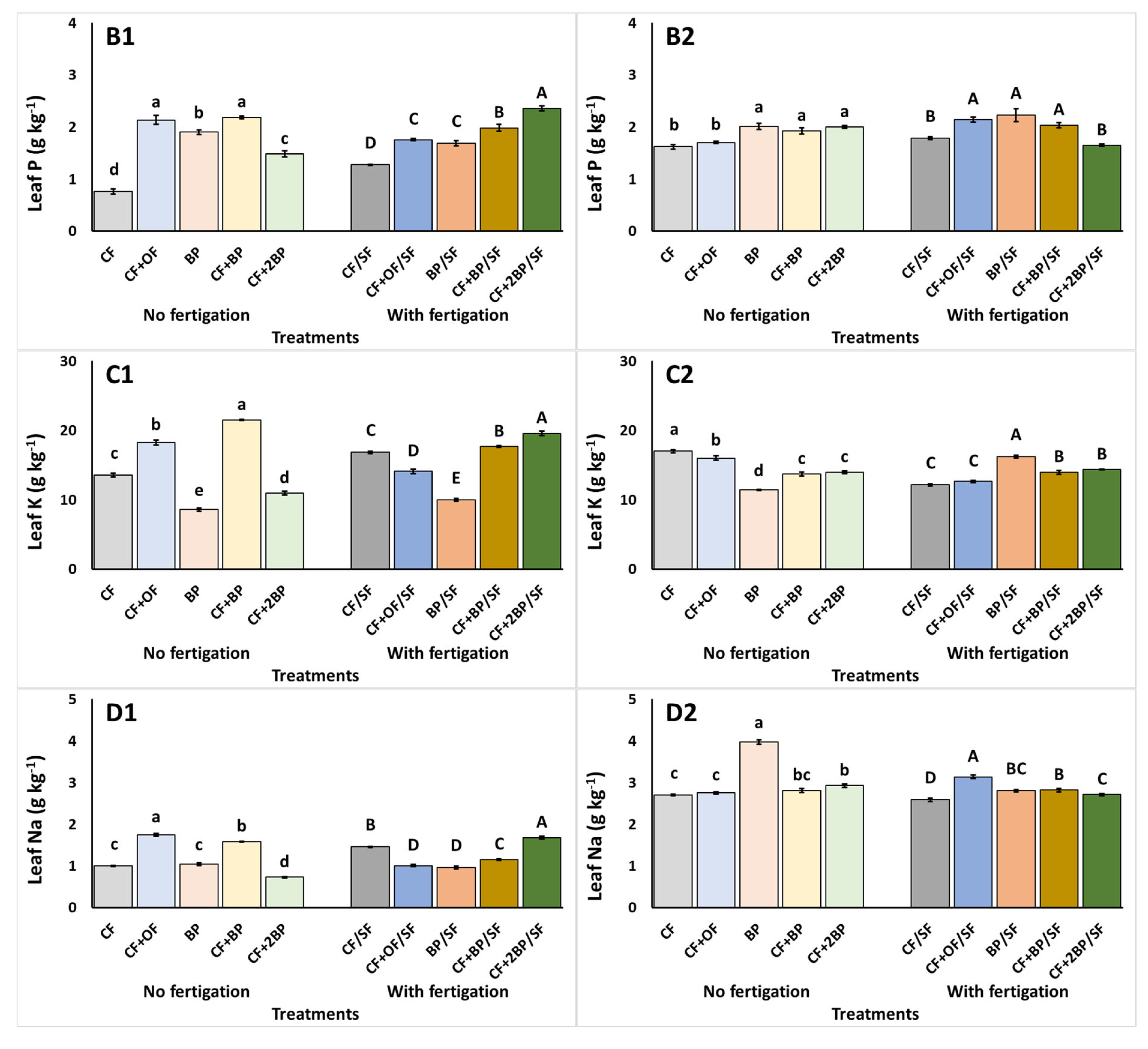
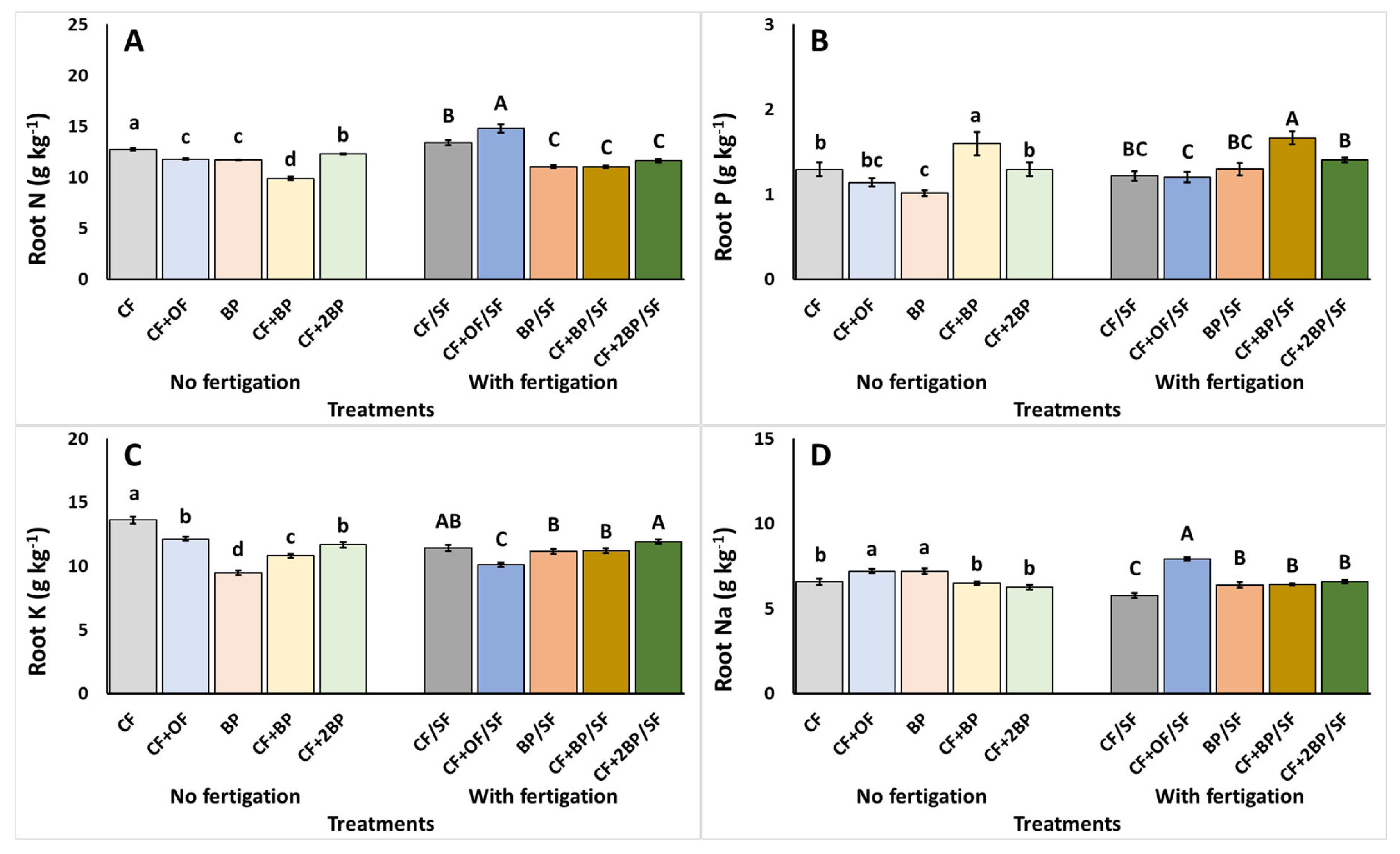
2.3. Plant Tissue Nutrient Content
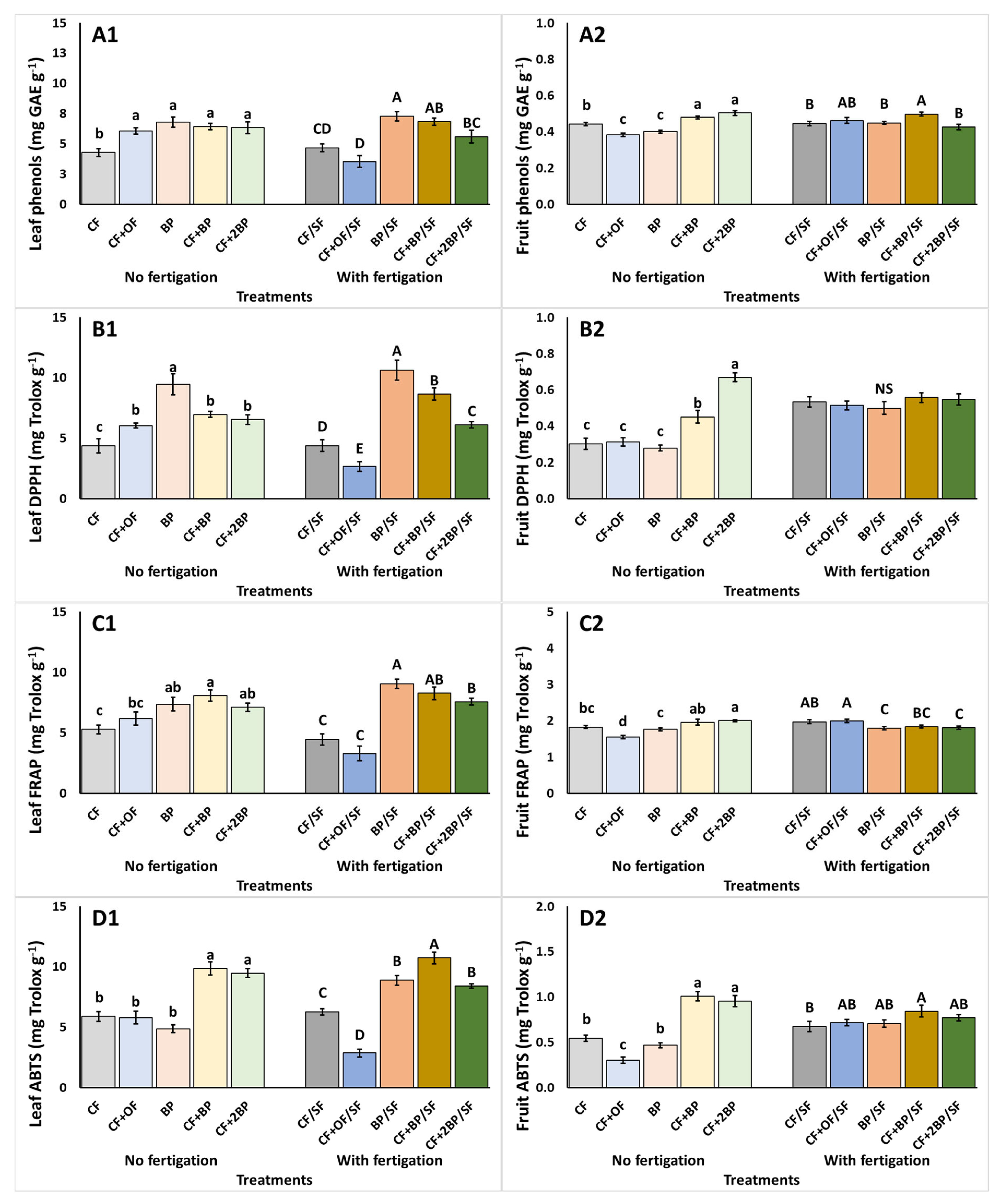
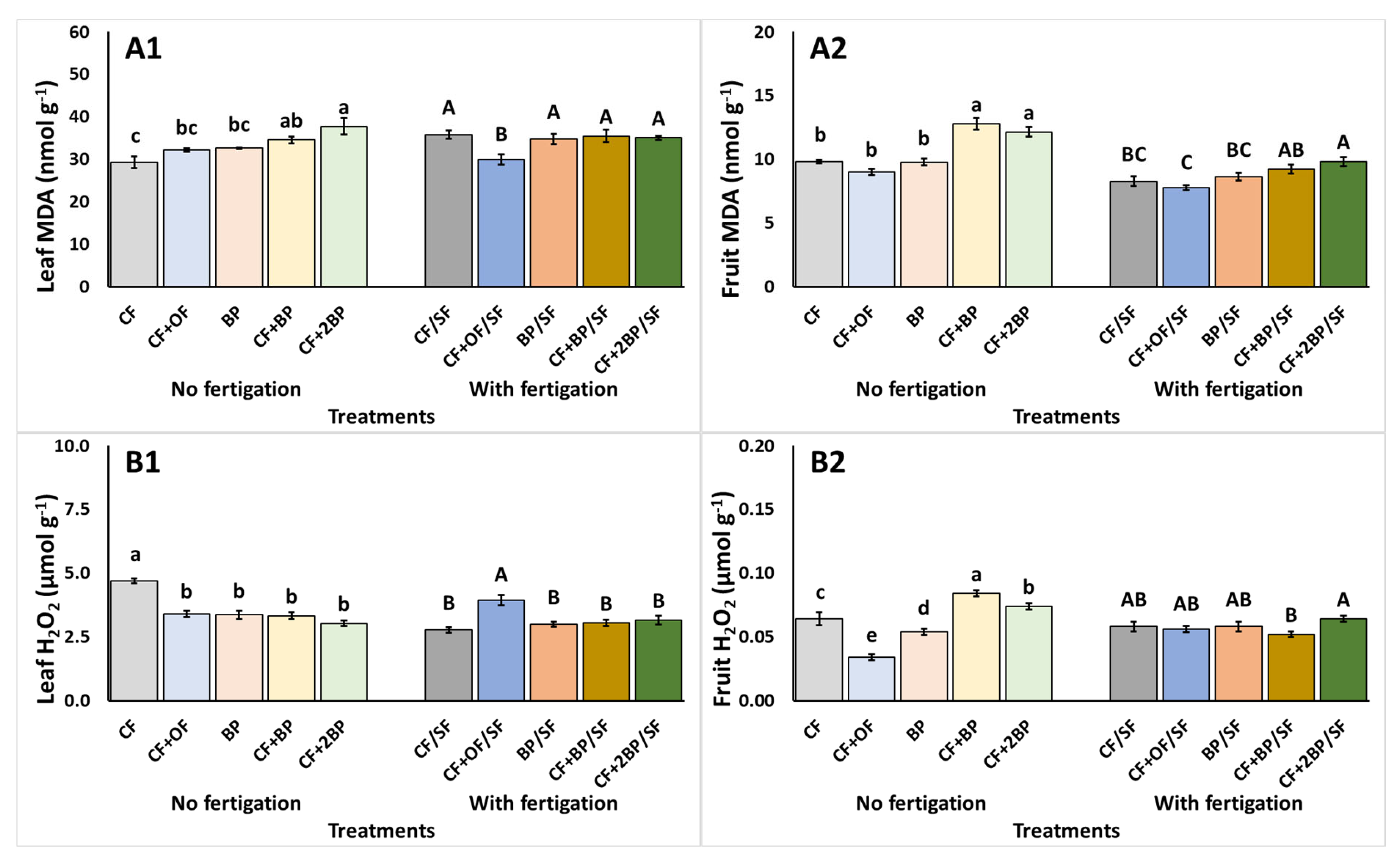
2.4. Total Phenols Content, Antioxidant Activity, and Stress Indicators
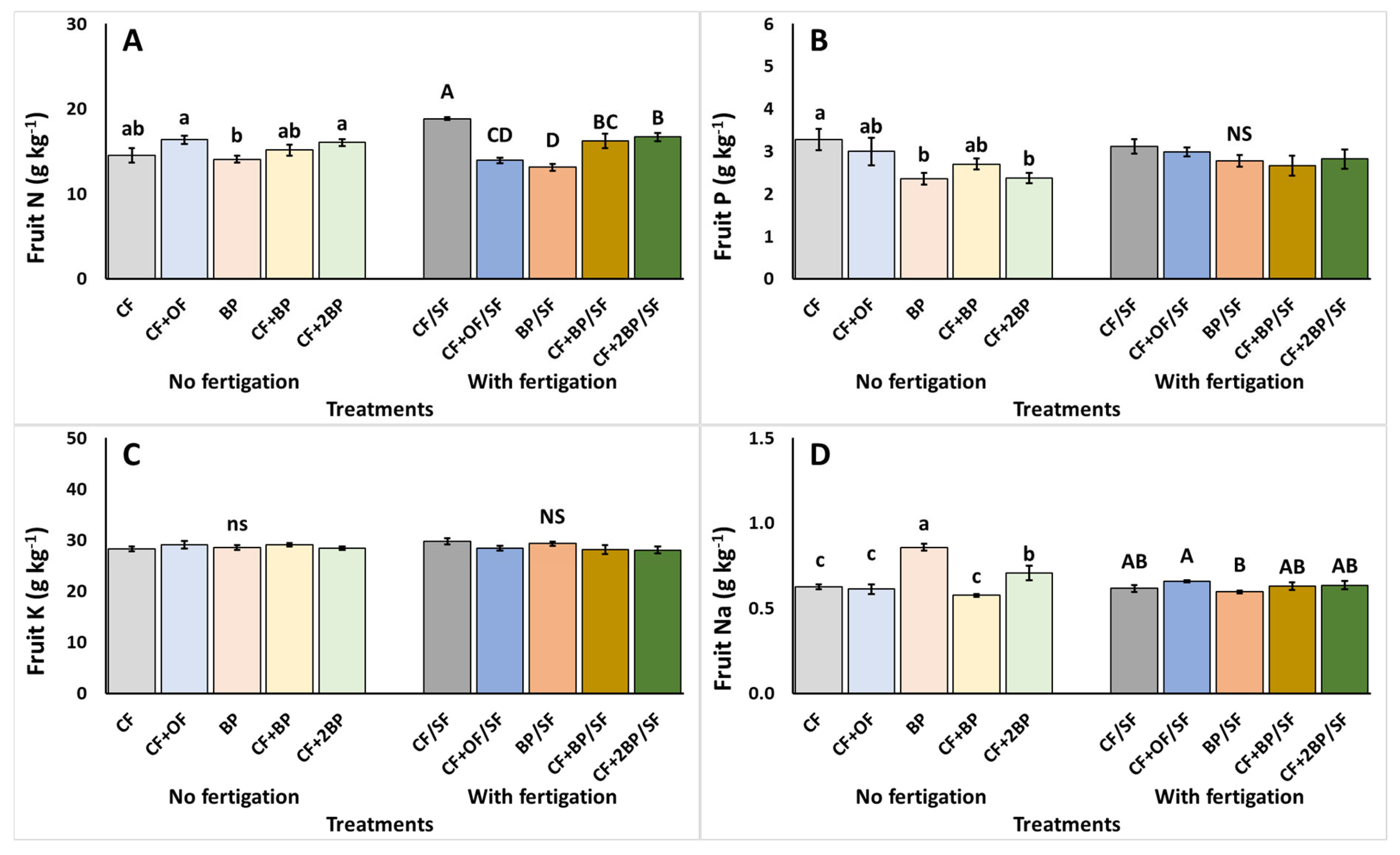
2.5. Fruit Quality Assessment and Macroscopic Evaluation
3. Discussion
3.1. Soil Physicochemical Parameters and Leachates Characterization
3.2. Plant Growth, Yield, and Physiological Parameters
3.3. Plant Tissue Nutrient Content
3.4. Total Phenols Content, Antioxidant Activity, and Stress Indicators
3.5. Fruit Quality Assessment and Macroscopic Evaluation
3.6. Final Considerations and Future Perspectives
4. Materials and Methods
4.1. Plant Material and Experimental Site
4.2. Soil Physicochemical Parameters and Leachates Characterization
4.3. Plant Growth and Physiological Parameters
4.4. Produce Harvesting
4.5. Plant Tissue Nutrient Content
4.6. Leaf and Fruit Total Phenols Content, Antioxidant Activity, and Stress Indicators
4.7. Fruit Quality Assessment and Macroscopic Evaluation
4.8. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fascella, G.; Montoneri, E.; Ginepro, M.; Francavilla, M. Effect of urban biowaste derived soluble substances on growth, photosynthesis and ornamental value of Euphorbia x lomi. Sci. Hortic. 2015, 197, 90–98. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yen, H.W.; Nomanbhay, S.; Ho, Y.C.; Show, P.L. Transformation of biomass waste into sustainable organic fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Rossi, G.; Beni, C.; Benedetti, A.; Felici, B.; Neri, U. Effect of Mineral or OFMSW Digestate Fertilization on Ryegrass and Nitrogen Leaching. Agronomy 2023, 13, 1316. [Google Scholar] [CrossRef]
- Montoneri, E. Municipal Waste Treatment, Technological Scale up and Commercial Exploitation: The Case of Bio-waste Lignin to Soluble Lignin-like Polymers. In Food Waste Reduction and Valorisation; Springer International Publishing: Cham, Switzerland, 2017; pp. 79–120. [Google Scholar]
- Fascella, G.; Montoneri, E.; Francavilla, M. Biowaste versus fossil sourced auxiliaries for plant cultivation: The Lantana case study. J. Clean. Prod. 2018, 185, 322–330. [Google Scholar] [CrossRef]
- Rezapour, S.; Samadi, A.; Kalavrouziotis, I.K.; Ghaemian, N. Impact of the uncontrolled leakage of leachate from a municipal solid waste landfill on soil in a cultivated-calcareous environment. Waste Manag. 2018, 82, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Ji, J.; Lan, X.; Drosos, M.; Liu, X.; Lv, Z.; Liu, Y.; Cheng, Z.; Zhou, W. Organic Fertilizers as Partial Substitutes for Chemical Fertilizers Enhance Nitrogen Immobilization and Optimize Nitrogen Fate in Paddy Soils. Agriculture 2024, 14, 2300. [Google Scholar] [CrossRef]
- Penuelas, J.; Coello, F.; Sardans, J. A better use of fertilizers is needed for global food security and environmental sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Aslam, S.; Nazir, A. Valorizing Combustible and Compostable Fractions of Municipal Solid Waste to Biochar and Compost as an Alternative to Chemical Fertilizer for Improving Soil Health and Sunflower Yield. Agronomy 2024, 14, 1449. [Google Scholar] [CrossRef]
- Holka, M.; Kowalska, J.; Jakubowska, M. Reducing Carbon Footprint of Agriculture—Can Organic Farming Help to Mitigate Climate Change? Agriculture 2022, 12, 1383. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Gorazda, K.; Kulczycka, J.; Kominko, H.; Moustakas, K.; Witek-Krowiak, A. Practical aspects of biowastes conversion to fertilizers. Biomass Convers. Biorefinery 2024, 14, 1515–1533. [Google Scholar] [CrossRef]
- Badagliacca, G.; Testa, G.; La Malfa, S.G.; Cafaro, V.; Lo Presti, E.; Monti, M. Organic Fertilizers and Bio-Waste for Sustainable Soil Management to Support Crops and Control Greenhouse Gas Emissions in Mediterranean Agroecosystems: A Review. Horticulturae 2024, 10, 427. [Google Scholar] [CrossRef]
- Fragalà, F.; Puglisi, I.; Padoan, E.; Montoneri, E.; Stevanato, P.; Gomez, J.M.; Herrero, N.; La Bella, E.; Salvagno, E.; Baglieri, A. Effect of municipal biowaste derived biostimulant on nitrogen fate in the plant-soil system during lettuce cultivation. Sci. Rep. 2023, 13, 7944. [Google Scholar] [CrossRef]
- Ferreira, E.T.; Barrochelo, S.C.; De Paula De Melo, S.; Araujo, T.; Xavier, A.C.C.; Cechin, I.; Da Silva, G.H.R. Biofertilizers from wastewater treatment as a potential source of mineral nutrients for growth of amaranth plants. PLoS ONE 2023, 18, e0295624. [Google Scholar] [CrossRef]
- Sortino, O.; Montoneri, E.; Patanè, C.; Rosato, R.; Tabasso, S.; Ginepro, M. Benefits for agriculture and the environment from urban waste. Sci. Total Environ. 2014, 487, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Fragalà, F.; Castello, I.; Puglisi, I.; Padoan, E.; Baglieri, A.; Montoneri, E.; Vitale, A. New insights into municipal biowaste derived products as promoters of seed germination and potential antifungal compounds for sustainable agriculture. Chem. Biol. Technol. Agric. 2022, 9, 69. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Montoneri, E.; Baglieri, A.; Fascella, G. Biostimulant Effects of Waste Derived Biobased Products in the Cultivation of Ornamental and Food Plants. Agriculture 2022, 12, 994. [Google Scholar] [CrossRef]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.W.; Ramadass, K.; Vinu, A.; Kirkham, M.B.; Bolan, N.S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef]
- Sortino, O.; Dipasquale, M.; Montoneri, E.; Tomasso, L.; Perrone, D.G.; Vindrola, D.; Negre, M.; Piccone, G. Refuse derived soluble bio-organics enhancing tomato plant growth and productivity. Waste Manag. 2012, 32, 1792–1801. [Google Scholar] [CrossRef]
- Sortino, O.; Dipasquale, M.; Montoneri, E.; Tomasso, L.; Avetta, P.; Bianco Prevot, A. 90% Yield Increase of Red Pepper With Unexpectedly Low Doses of Compost Soluble Substances. Agron. Sustain. Dev. 2013, 33, 433–441. [Google Scholar] [CrossRef]
- Baglieri, A.; Cadili, V.; Mozzetti Monterumici, C.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Massa, D.; Lenzi, A.; Montoneri, E.; Ginepro, M.; Prisa, D.; Burchi, G. Plant response to biowaste soluble hydrolysates in hibiscus grown under limiting nutrient availability. J. Plant Nutr. 2018, 41, 396–409. [Google Scholar] [CrossRef]
- Fascella, G.; Montoneri, E.; Rouphael, Y. Biowaste-Derived Humic-like Substances Improve Growth and Quality of Orange Jasmine (Murraya paniculata L. Jacq.) Plants in Soilless Potted Culture. Resources 2021, 10, 80. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Han, N.; Li, X.; Bai, R.; Magaña, J.; Shimizu, N. By-Product from Livestock Waste Recovery System Used as Fertilizer: Bioactive Compounds and Antioxidant Activity of Tomato Fruit as Affected by Fertilization under Field and Greenhouse Conditions. Fermentation 2023, 9, 714. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Tolisano, C.; Del Buono, D. Biobased: Biostimulants and biogenic nanoparticles enter the scene. Sci. Total Environ. 2023, 885, 163912. [Google Scholar] [CrossRef]
- Bian, H.; Li, C.; Zhu, J.; Xu, L.; Li, M.; Zheng, S.; He, N. Soil Moisture Affects the Rapid Response of Microbes to Labile Organic C Addition. Front. Ecol. Evol. 2022, 10, 857185. [Google Scholar] [CrossRef]
- Stegarescu, G.; Escuer-Gatius, J.; Soosaar, K.; Kauer, K.; Tõnutare, T.; Astover, A.; Reintam, E. Effect of crop residue decomposition on soil aggregate stability. Agriculture 2020, 10, 527. [Google Scholar] [CrossRef]
- Massa, D.; Prisa, D.; Montoneri, E.; Battaglini, D.; Ginepro, M.; Negre, M.; Burchi, G. Application of municipal biowaste derived products in Hibiscus cultivation: Effect on leaf gaseous exchange activity, and plant biomass accumulation and quality. Sci. Hortic. 2016, 205, 59–69. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem. Biol. Technol. Agric. 2015, 2, 5. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadili, V.; Tambone, F.; Gennari, M.; Nardi, S. Humic-like substances from agro-industrial residues affect growth and nitrogen assimilation in maize (Zea mays L.) plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Barzee, T.J.; Edalati, A.; El-Mashad, H.; Wang, D.; Scow, K.; Zhang, R. Digestate Biofertilizers Support Similar or Higher Tomato Yields and Quality Than Mineral Fertilizer in a Subsurface Drip Fertigation System. Front. Sustain. Food Syst. 2019, 3, 58. [Google Scholar] [CrossRef]
- Bilalis, D.; Krokida, M.; Roussis, I.; Papastylianou, P.; Travlos, I.; Cheimona, N.; Dede, A. Effects of organic and inorganic fertilization on yield and quality of processing tomato (Lycopersicon esculentum Mill.). Folia Hortic. 2018, 30, 321–332. [Google Scholar] [CrossRef]
- Savarese, C.; Cozzolino, V.; Verrillo, M.; Vinci, G.; De Martino, A.; Scopa, A.; Piccolo, A. Combination of humic biostimulants with a microbial inoculum improves lettuce productivity, nutrient uptake, and primary and secondary metabolism. Plant Soil 2022, 481, 285–314. [Google Scholar] [CrossRef]
- Zhang, X.; Taylor, Z.; Goatley, M.; Booth, J.; Brown, I.; Kosiarski, K. Seaweed Extract-based Biostimulant Impacts on Nitrate Reductase Activity and Root Viability of Ultradwarf Bermudagrass Subjected to Heat and Drought Stress. HortScience 2022, 57, 1328–1333. [Google Scholar] [CrossRef]
- Ragályi, P.; Szécsy, O.; Uzinger, N.; Magyar, M.; Szabó, A.; Rékási, M. Factors Influencing the Impact of Anaerobic Digestates on Soil Properties. Soil Syst. 2025, 9, 78. [Google Scholar] [CrossRef]
- Hallat-Sanchez, J.; Smith, J.; Norton, G.J. Impact of Adding Anaerobic Digestate to Soil and Consequences on Crop Performance. Agronomy 2023, 13, 2889. [Google Scholar] [CrossRef]
- Santos, M.d.C.; Cavalcanti, M.T.; Pessoa, L.N.; Silva, Z.G.d.; da Silva, A.M.; Souza, T.; Henschel, J.M.; Pereira, E.M.; Diniz Neto, M.A.; Diniz, B.L.M.T. Exploring the Impact of Humic Biostimulants on Cassava Yield and Nutrition in Northeast Brazil. Sustainability 2024, 16, 4088. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Lu, Z.; Meng, F.; Cong, R.; Li, X.; Ren, T.; Lu, J. Imbalance between nitrogen and potassium fertilization influences potassium deficiency symptoms in winter oilseed rape (Brassica napus L.) leaves. Crop J. 2022, 10, 565–576. [Google Scholar] [CrossRef]
- Weimers, K.; Bergstrand, K.J.; Hultberg, M.; Asp, H. Liquid Anaerobic Digestate as Sole Nutrient Source in Soilless Horticulture—Or Spiked With Mineral Nutrients for Improved Plant Growth. Front. Plant Sci. 2022, 13, 770179. [Google Scholar] [CrossRef] [PubMed]
- Foughar, M.; Arrobas, M.; Rodrigues, M.Â. Mealworm Larvae Frass Exhibits a Plant Biostimulant Effect on Lettuce, Boosting Productivity beyond Just Nutrient Release or Improved Soil Properties. Horticulturae 2024, 10, 711. [Google Scholar] [CrossRef]
- Sobhi, M.; Elsamahy, T.; Zakaria, E.; Gaballah, M.S.; Zhu, F.; Hu, X.; Zhou, C.; Guo, J.; Huo, S.; Dong, R. Characteristics, limitations and global regulations in the use of biogas digestate as fertilizer: A comprehensive overview. Sci. Total Environ. 2024, 957, 177855. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, Y.; Cheng, K.; Wang, L.; Dong, W.; Liu, Z.; Yang, F. Artificial humic acid mediated migration of phosphorus in soil: Experiment and modelling. Catena 2024, 238, 107896. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; ISBN 9780123849052. [Google Scholar]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT-Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Laužikė, K.; Gudžinskaitė, I.; Jankauskienė, J. After-Effect of Biogas Digestate Used for Growing Seedlings on the Antioxidant System of Tomato (Solanum lycopersicum) Fruits. Appl. Sci. 2025, 15, 2805. [Google Scholar] [CrossRef]
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Alan, O.; Budak, B.; Sen, F.; Ongun, A.R.; Tepecik, M.; Ata, S. Solid and liquid digestate generated from biogas production as a fertilizer source in processing tomato yield, quality and some health-related compounds. J. Agric. Sci. 2025, 163, 55–70. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Wang, J.; Zhang, A.; Deng, X. Estimation of Malondialdehyde Content in Medicago truncatula under Salt Stress Based on Multi-Order Spectral Transformation Characteristics. Remote Sens. 2024, 16, 4049. [Google Scholar] [CrossRef]
- De Hita, D.; Fuentes, M.; Fernández, V.; Zamarreño, A.M.; Olaetxea, M.; García-Mina, J.M. Discriminating the Short-Term Action of Root and Foliar Application of Humic Acids on Plant Growth: Emerging Role of Jasmonic Acid. Front. Plant Sci. 2020, 11, 493. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Mallamaci, C.; Attinà, E.; Muscolo, A. Using digestate as fertilizer for a sustainable tomato cultivation. Sustainability 2021, 13, 1574. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Morra, L.; Cozzolino, E.; Salluzzo, A.; Modestia, F.; Bilotto, M.; Baiano, S.; Del Piano, L. Plant growth, yields and fruit quality of processing tomato (Solanum lycopersicon L.) as affected by the combination of biodegradable mulching and digestate. Agronomy 2021, 11, 100. [Google Scholar] [CrossRef]
- Jindřichová, B.; Burketová, L.; Montoneri, E.; Francavilla, M. Biowaste-derived hydrolysates as plant disease suppressants for oilseed rape. J. Clean. Prod. 2018, 183, 335–342. [Google Scholar] [CrossRef]
- Fragalà, F.; Salvagno, E.; La Bella, E.; Saccone, R.; Padoan, E.; Montoneri, E.; Miccichè, J.; Ferrarello, D.; Baglieri, A.; Puglisi, I. Enhancing Lettuce Yield through Innovative Foliar Spray of Biopolymers Derived from Municipal Biowastes. Plants 2024, 13, 1664. [Google Scholar] [CrossRef]
- Litskas, V.; Ledo, A.; Lawrence, P.; Chrysargyris, A.; Giannopoulos, G.; Heathcote, R.; Hastings, A.; Tzortzakis, N.; Stavrinides, M. Use of Winery and Animal Waste as Fertilizers to Achieve Climate Neutrality in Non-Irrigated Viticulture. Agronomy 2022, 12, 2375. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Saridakis, C.; Chrysargyris, A. Treated wastewater and fertigation applied for greenhouse tomato cultivation grown in municipal solid waste compost and soil mixtures. Sustainability 2020, 12, 4287. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Drouza, C.; Tzortzakis, N. Optimization of potassium fertilization/nutrition for growth, physiological development, essential oil composition and antioxidant activity of Lavandula angustifolia Mill. J. Soil Sci. Plant Nutr. 2017, 17, 291–306. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Xylia, P.; Chrysargyris, A. Sage essential oil improves the effectiveness of Aloe vera gel on postharvest quality of tomato fruit. Agronomy 2019, 9, 635. [Google Scholar] [CrossRef]
- Xylia, P.; Ioannou, I.; Chrysargyris, A.; Stavrinides, M.C.; Tzortzakis, N. Quality attributes and storage of tomato fruits as affected by an eco-friendly, essential oil-based product. Plants 2021, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
| pH | EC | Organic Matter | CaCO3 | N | P | K | Na | |
|---|---|---|---|---|---|---|---|---|
| CF | 7.73 ± 0.03 cd | 2.71 ± 0.08 b | 1.11 ± 0.07 a | 30.98 ± 0.53 a | 0.66 ± 0.04 a | 0.022 ± 0.004 a | 0.18 ± 0.00 a | 0.55 ± 0.00 c |
| CF + OF | 7.90 ± 0.01 b | 2.56 ± 0.07 b | 1.04 ± 0.02 ab | 28.33 ± 0.41 c | 0.66 ± 0.04 a | 0.019 ± 0.000 a | 0.16 ± 0.00 b | 0.55 ± 0.01 c |
| BP | 7.97 ± 0.02 a | 2.32 ± 0.08 c | 1.06 ± 0.02 ab | 29.90 ± 0.36 ab | 0.69 ± 0.04 a | 0.009 ± 0.001 b | 0.19 ± 0.00 a | 0.48 ± 0.01 d |
| CF + BP | 7.71 ± 0.01 d | 3.29 ± 0.10 a | 0.95 ± 0.02 b | 29.14 ± 0.39 bc | 0.67 ± 0.02 a | 0.018 ± 0.001 a | 0.17 ± 0.00 b | 0.59 ± 0.01 b |
| CF + 2 BP | 7.77 ± 0.01 c | 3.13 ± 0.07 a | 0.95 ± 0.05 b | 28.76 ± 0.39 bc | 0.73 ± 0.05 a | 0.018 ± 0.002 a | 0.19 ± 0.00 a | 0.62 ± 0.01 a |
| CF/SF | 7.86 ± 0.02 C | 2.28 ± 0.03 C | 1.05 ± 0.01 A | 29.49 ± 0.13 AB | 0.72 ± 0.01 AB | 0.025 ± 0.001 AB | 0.17 ± 0.00 B | 0.57 ± 0.01 B |
| CF + OF/SF | 7.90 ± 0.01 B | 2.44 ± 0.05 B | 1.06 ± 0.05 A | 29.61 ± 0.46 AB | 0.63 ± 0.01 C | 0.030 ± 0.001 AB | 0.18 ± 0.00 A | 0.54 ± 0.01 C |
| BP/SF | 7.89 ± 0.00 BC | 2.07 ± 0.05 D | 1.04 ± 0.04 A | 29.90 ± 0.45 AB | 0.68 ± 0.01 BC | 0.023 ± 0.001 B | 0.18 ± 0.00 A | 0.55 ± 0.01 C |
| CF + BP/SF | 7.73 ± 0.00 D | 3.24 ± 0.04 A | 0.82 ± 0.07 B | 30.24 ± 0.51 A | 0.67 ± 0.02 BC | 0.033 ± 0.006 A | 0.16 ± 0.00 C | 0.63 ± 0.00 A |
| CF + 2BP/SF | 8.04 ± 0.02 A | 1.91 ± 0.07 E | 1.01 ± 0.05 A | 28.88 ± 0.23 B | 0.78 ± 0.04 A | 0.033 ± 0.001 A | 0.18 ± 0.00 A | 0.59 ± 0.01 B |
| Intermediate Stage | ||||||
| Plant Height | Leaf Number | Base Stem Thickness | Mid Stem Thickness | SPAD | Fv/Fm | |
| CF | 108.63 ± 3.75 b | 15.25 ± 0.41 ab | 10.76 ± 0.27 a | n.m. | 101.76 ± 8.02 a | 0.76 ± 0.01 a |
| CF + OF | 113.88 ± 1.09 ab | 16.25 ± 0.49 ab | 10.55 ± 0.41 a | n.m. | 108.41 ± 4.20 a | 0.77 ± 0.01 a |
| BP | 111.50 ± 3.00 ab | 15.00 ± 0.50 b | 9.52 ± 0.13 b | n.m. | 93.08 ± 9.38 a | 0.76 ± 0.01 a |
| CF + BP | 119.13 ± 1.09 a | 16.50 ± 0.19 a | 9.45 ± 0.23 b | n.m. | 94.41 ± 5.29 a | 0.76 ± 0.01 a |
| CF + 2 BP | 106.75 ± 4.91 b | 15.50 ± 0.63 ab | 10.56 ± 0.23 a | n.m. | 100.44 ± 4.59 a | 0.74 ± 0.01 a |
| CF/SF | 117.88 ± 1.79 A | 16.25 ± 0.25 A | 10.87 ± 0.37 AB | n.m. | 110.39 ± 4.53 AB | 0.77 ± 0.01 A |
| CF + OF/SF | 109.67 ± 2.91 B | 15.78 ± 0.32 AB | 10.47 ± 0.31 B | n.m. | 114.58 ± 3.65 A | 0.79 ± 0.01 A |
| BP/SF | 114.22 ± 3.20 AB | 15.00 ± 0.65 B | 10.00 ± 0.31 B | n.m. | 96.06 ± 9.25 B | 0.75 ± 0.01 A |
| CF + BP/SF | 117.56 ± 1.02 A | 16.67 ± 0.29 A | 10.72 ± 0.33 B | n.m. | 99.34 ± 4.77 AB | 0.76 ± 0.01 A |
| CF + 2BP/SF | 118.22 ± 1.94 A | 16.33 ± 0.41 A | 11.73 ± 0.24 A | n.m. | 92.53 ± 5.38 B | 0.76 ± 0.01 A |
| Final Stage | ||||||
| Plant Height | Leaf Number | Base Stem Thickness | Mid Stem Thickness | SPAD | Fv/Fm | |
| CF | 195.63 ± 7.78 a | 18.88 ± 1.13 a | 13.03 ± 0.57 a | 6.61 ± 0.48 a | 65.36 ± 5.40 a | 0.73 ± 0.01 a |
| CF + OF | 199.00 ± 15.01 a | 18.00 ± 1.89 a | 12.29 ± 0.66 a | 6.22 ± 0.41 ab | 52.69 ± 6.68 a | 0.70 ± 0.02 a |
| BP | 185.13 ± 6.00 a | 15.88 ± 0.72 a | 10.48 ± 0.32 b | 5.07 ± 0.30 b | 58.21 ± 5.89 a | 0.71 ± 0.01 a |
| CF + BP | 188.88 ± 5.42 a | 17.00 ± 0.85 a | 11.63 ± 0.40 ab | 5.83 ± 0.26 ab | 59.96 ± 5.42 a | 0.73 ± 0.02 a |
| CF + 2BP | 174.13 ± 10.94 a | 16.50 ± 0.82 a | 12.16 ± 0.41 a | 5.85 ± 0.51 ab | 65.78 ± 6.31 a | 0.71 ± 0.01 a |
| CF/SF | 195.13 ± 9.25 A | 17.38 ± 1.16 A | 12.16 ± 0.16 A | 5.86 ± 0.36 | 67.35 ± 4.85 A | 0.75 ± 0.01 A |
| CF + OF/SF | 188.33 ± 4.17 A | 18.44 ± 0.60 A | 11.76 ± 0.34 AB | 5.72 ± 0.44 | 64.53 ± 4.60 A | 0.72 ± 0.02 AB |
| BP/SF | 186.88 ± 5.80 A | 16.75 ± 0.73 A | 10.85 ± 0.37 B | 5.42 ± 0.67 | 57.48 ± 2.97 A | 0.71 ± 0.01 AB |
| CF + BP/SF | 190.11 ± 6.03 A | 17.89 ± 0.95 A | 12.26 ± 0.46 A | 5.73 ± 0.27 | 60.08 ± 4.08 A | 0.70 ± 0.02 B |
| CF + 2BP/SF | 200.33 ± 7.32 A | 17.44 ± 0.94 A | 12.24 ± 0.30 A | 6.26 ± 0.70 | 69.21 ± 6.07 A | 0.72 ± 0.01 AB |
| Leaves FW | Stem FW | Plant FW | Leaf DM | Root DM | |
|---|---|---|---|---|---|
| CF | 542.51 ± 80.95 ab | 244.84 ± 34.23 ab | 2.36 ± 0.35 a | 18.41 ± 0.14 ab | 12.87 ± 0.44 ab |
| CF + OF | 627.50 ± 130.34 a | 277.60 ± 46.76 a | 2.74 ± 0.59 a | 17.72 ± 0.13 b | 12.12 ± 0.27 b |
| BP | 346.70 ± 51.49 b | 160.89 ± 15.61 b | 1.75 ± 0.29 a | 18.81 ± 0.16 ab | 14.16 ± 0.35 a |
| CF + BP | 452.13 ± 7.07 ab | 202.97 ± 6.35 ab | 1.85 ± 0.07 a | 19.10 ± 0.46 ab | 11.83 ± 0.47 b |
| CF + 2BP | 409.87 ± 45.45 ab | 194.87 ± 22.42 ab | 1.78 ± 0.14 a | 20.29 ± 1.00 a | 12.55 ± 0.61 b |
| CF/SF | 548.77 ± 50.04 AB | 240.13 ± 19.10 AB | 2.27 ± 0.21 AB | 19.12 ± 0.42 A | 13.17 ± 0.31 A |
| CF + OF/SF | 481.74 ± 34.70 AB | 207.03 ± 12.31 B | 1.93 ± 0.16 AB | 18.11 ± 0.25 B | 14.36 ± 0.97 A |
| BP/SF | 437.74 ± 48.51 B | 205.95 ± 21.36 B | 1.77 ± 0.07 B | 19.09 ± 0.00 A | 14.85 ± 0.69 A |
| CF + BP/SF | 517.06 ± 34.77 AB | 226.59 ± 13.43 AB | 1.98 ± 0.11 AB | 18.53 ± 0.19 AB | 13.55 ± 0.77 A |
| CF + 2BP/SF | 595.41 ± 59.27 A | 267.58 ± 25.07 A | 2.52 ± 0.31 A | 18.49 ± 0.19 AB | 15.32 ± 0.72 A |
| Fruit No. | Mean Fruit FW | Total Yield FW | Marketable Yield | |
|---|---|---|---|---|
| CF | 11.57 ± 1.45 a | 92.29 ± 9.10 a | 1.58 ± 0.24 a | 1.09 ± 0.21 a |
| CF + OF | 12.14 ± 1.72 a | 101.42 ± 12.24 a | 1.83 ± 0.41 a | 1.35 ± 0.36 a |
| BP | 10.00 ± 1.53 a | 89.61 ± 7.27 a | 1.24 ± 0.23 a | 0.91 ± 0.18 a |
| CF + BP | 9.14 ± 1.61 a | 78.03 ± 6.11 a | 1.20 ± 0.08 a | 0.72 ± 0.14 a |
| CF + 2BP | 8.43 ± 1.25 a | 86.03 ± 5.18 a | 1.18 ± 0.12 a | 0.74 ± 0.14 a |
| CF/SF | 11.43 ± 1.13 AB | 84.21 ± 7.07 A | 1.47 ± 0.15 AB | 0.96 ± 0.13 A |
| CF + OF/SF | 8.50 ± 1.10 B | 88.75 ± 8.52 A | 1.24 ± 0.16 AB | 0.78 ± 0.14 A |
| BP/SF | 8.00 ± 0.85 B | 77.48 ± 5.07 A | 1.13 ± 0.06 B | 0.64 ± 0.11 A |
| CF + BP/SF | 9.57 ± 0.87 AB | 81.42 ± 6.78 A | 1.27 ± 0.09 AB | 0.79 ± 0.10 A |
| CF + 2BP/SF | 12.25 ± 1.54 A | 86.70 ± 7.99 A | 1.65 ± 0.24 A | 1.09 ± 0.21 A |
| Firmness | TSS | TA | TSS/TA | Lycopene | β-Carotene | Ascorbic Acid | Marketability | Aroma | Appearance | |
|---|---|---|---|---|---|---|---|---|---|---|
| CF | 0.59 ± 0.07 a | 5.11 ± 0.32 bc | 4.62 ± 0.58 a | 1.17 ± 0.10 a | 2.46 ± 0.19 b | 1.06 ± 0.03 bc | 20.02 ± 0.79 a | 6.30 ± 0.23 a | 7.15 ± 0.10 a | 7.07 ± 0.11 ab |
| CF + OF | 0.68 ± 0.07 a | 4.73 ± 0.18 bc | 4.77 ± 0.59 a | 1.05 ± 0.09 a | 2.74 ± 0.12 ab | 1.11 ± 0.05 abc | 16.04 ± 0.75 b | 5.93 ± 0.41 a | 6.93 ± 0.22 ab | 7.41 ± 0.21 a |
| BP | 0.57 ± 0.09 a | 4.51 ± 0.14 c | 4.50 ± 0.79 a | 1.11 ± 0.11 a | 2.26 ± 0.17 b | 1.03 ± 0.03 c | 17.76 ± 0.49 ab | 6.33 ± 0.41 a | 6.33 ± 0.14 c | 6.78 ± 0.30 b |
| CF + BP | 0.76 ± 0.13 a | 5.89 ± 0.11 a | 5.19 ± 0.66 a | 1.26 ± 0.17 a | 3.13 ± 0.22 a | 1.18 ± 0.03 ab | 16.35 ± 1.33 b | 6.11 ± 0.26 a | 6.67 ± 0.17 bc | 6.85 ± 0.17 ab |
| CF + 2BP | 0.63 ± 0.09 a | 5.30 ± 0.19 b | 5.36 ± 0.75 a | 1.11 ± 0.15 a | 2.60 ± 0.14 b | 1.24 ± 0.07 a | 19.27 ± 0.63 a | 5.41 ± 0.24 a | 6.52 ± 0.11 bc | 6.78 ± 0.14 b |
| CF/SF | 0.73 ± 0.10 AB | 5.71 ± 0.22 A | 5.81 ± 0.62 A | 1.05 ± 0.12 A | 2.80 ± 0.18 A | 1.19 ± 0.03 A | 20.42 ± 0.77 B | 5.59 ± 0.31 A | 6.74 ± 0.25 A | 6.63 ± 0.18 A |
| CF + OF/SF | 0.52 ± 0.05 B | 4.84 ± 0.10 B | 4.76 ± 0.62 A | 1.10 ± 0.11 A | 2.32 ± 0.08 A | 1.20 ± 0.04 A | 21.35 ± 0.34 B | 6.41 ± 0.43 A | 6.22 ± 0.12 B | 6.82 ± 0.17 A |
| BP/SF | 0.64 ± 0.09 AB | 4.90 ± 0.30 B | 4.75 ± 0.52 A | 1.07 ± 0.07 A | 2.45 ± 0.24 A | 1.16 ± 0.03 A | 20.50 ± 0.28 B | 6.52 ± 0.34 A | 6.48 ± 0.13 AB | 6.96 ± 0.15 A |
| CF + BP/SF | 0.89 ± 0.13 A | 5.91 ± 0.19 A | 5.14 ± 0.41 A | 1.20 ± 0.11 A | 2.83 ± 0.27 A | 1.10 ± 0.03 A | 23.88 ± 0.57 A | 6.33 ± 0.34 A | 6.63 ± 0.07 AB | 7.15 ± 0.16 A |
| CF + 2BP/SF | 0.54 ± 0.08 B | 5.43 ± 0.25 AB | 4.40 ± 0.62 A | 1.34 ± 0.15 A | 2.62 ± 0.15 A | 1.15 ± 0.03 A | 23.09 ± 0.78 A | 5.96 ± 0.24 A | 6.67 ± 0.10 AB | 6.93 ± 0.22 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neofytou, G.; Chrysargyris, A.; Christodoulou, M.; Montoneri, E.; Koutinas, M.; Tzortzakis, N. Application of Municipal Biowaste-Derived Products in Tomato Cultivation for Enhanced Fruit Quality Attributes and Nutritional Profile. Plants 2025, 14, 3212. https://doi.org/10.3390/plants14203212
Neofytou G, Chrysargyris A, Christodoulou M, Montoneri E, Koutinas M, Tzortzakis N. Application of Municipal Biowaste-Derived Products in Tomato Cultivation for Enhanced Fruit Quality Attributes and Nutritional Profile. Plants. 2025; 14(20):3212. https://doi.org/10.3390/plants14203212
Chicago/Turabian StyleNeofytou, Giannis, Antonios Chrysargyris, Marianna Christodoulou, Enzo Montoneri, Michalis Koutinas, and Nikolaos Tzortzakis. 2025. "Application of Municipal Biowaste-Derived Products in Tomato Cultivation for Enhanced Fruit Quality Attributes and Nutritional Profile" Plants 14, no. 20: 3212. https://doi.org/10.3390/plants14203212
APA StyleNeofytou, G., Chrysargyris, A., Christodoulou, M., Montoneri, E., Koutinas, M., & Tzortzakis, N. (2025). Application of Municipal Biowaste-Derived Products in Tomato Cultivation for Enhanced Fruit Quality Attributes and Nutritional Profile. Plants, 14(20), 3212. https://doi.org/10.3390/plants14203212









