The PSRP2/4 Proteins Promote Viral Infection by Interacting with the VPg Protein of TuMV
Abstract
1. Introduction
2. Results
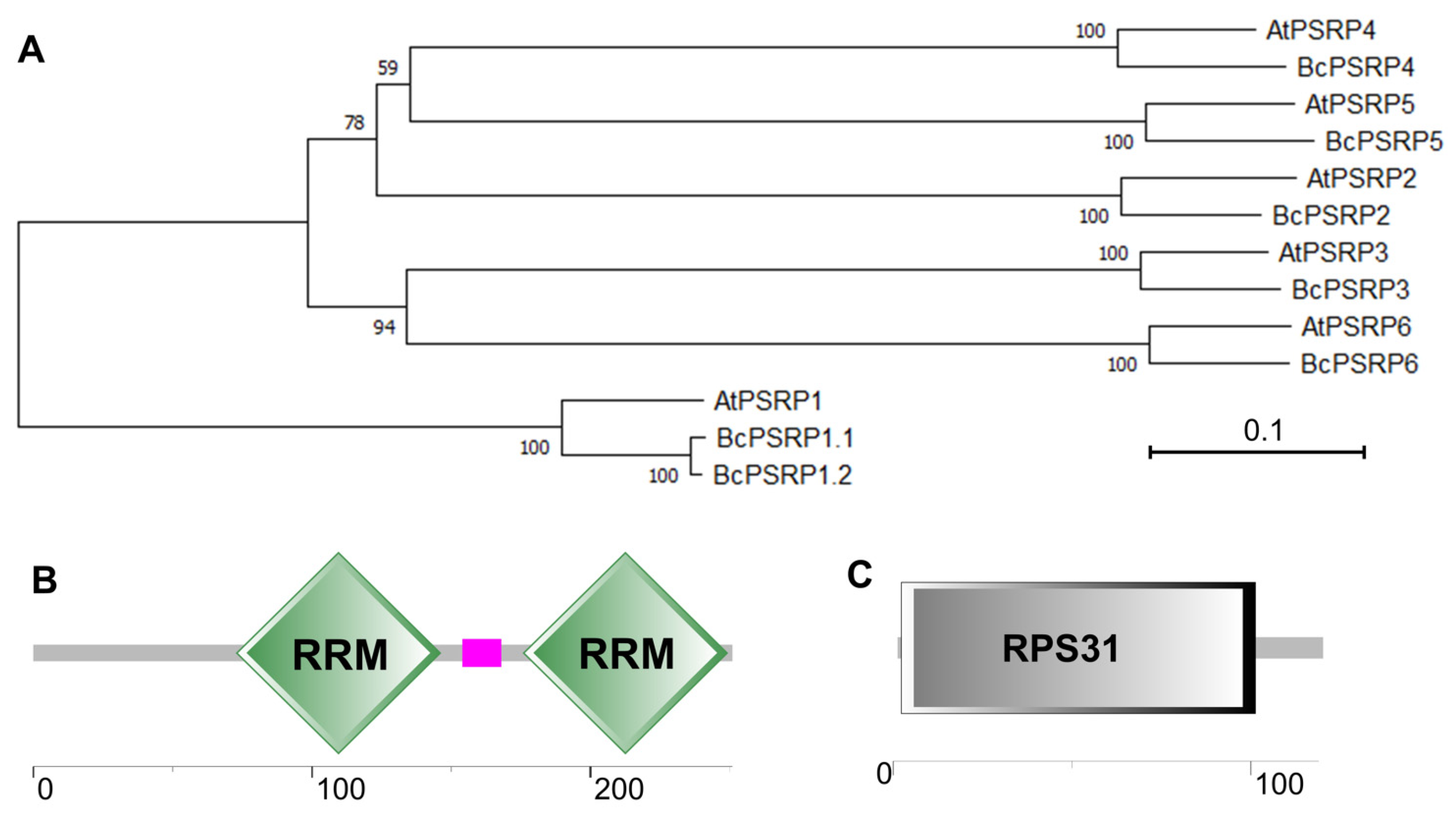
2.1. The Identification of BcPSRPs in Non-Heading Chinese Cabbage
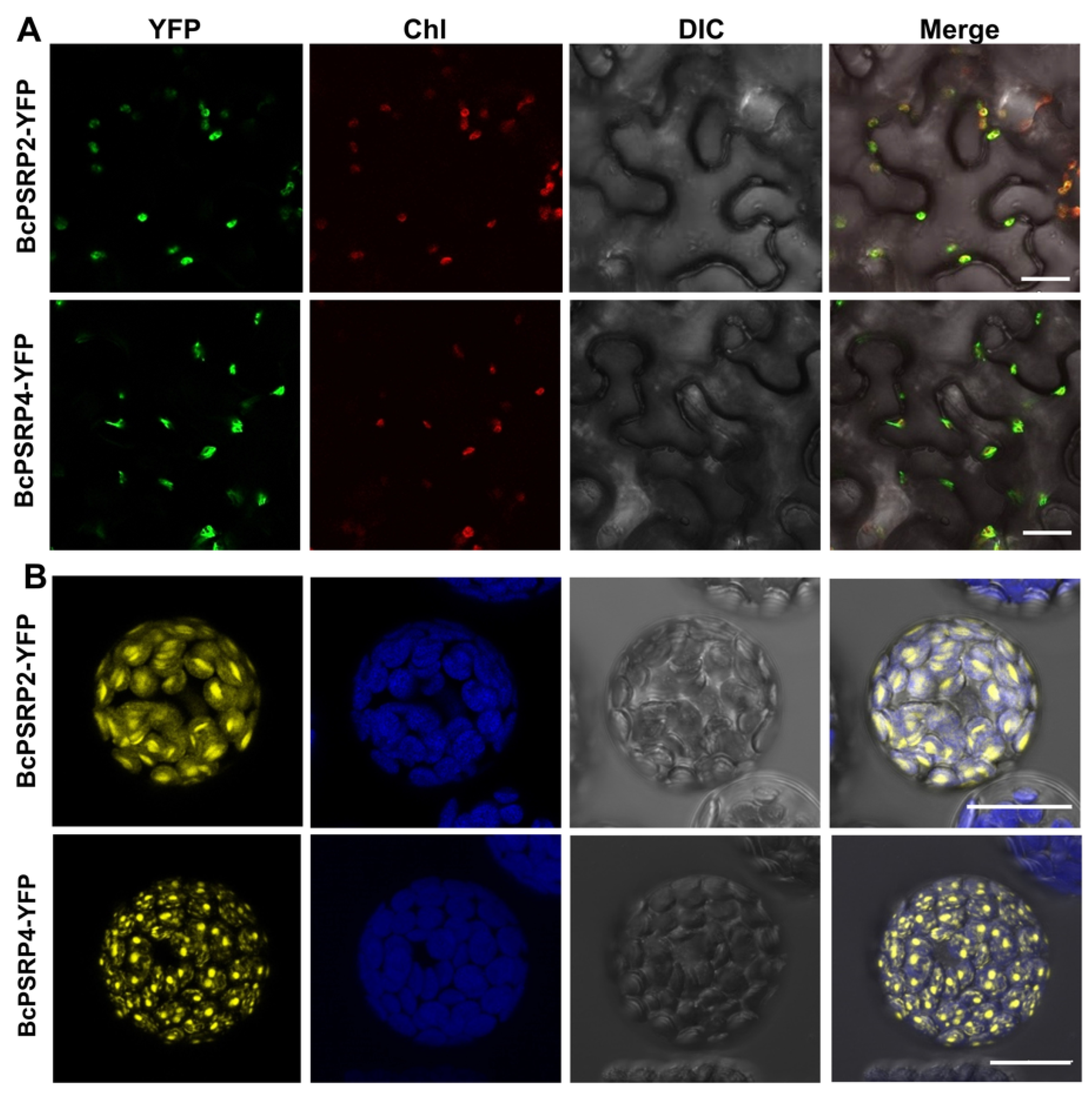
2.2. BcPSRP2/4 Were Localized to Chloroplasts
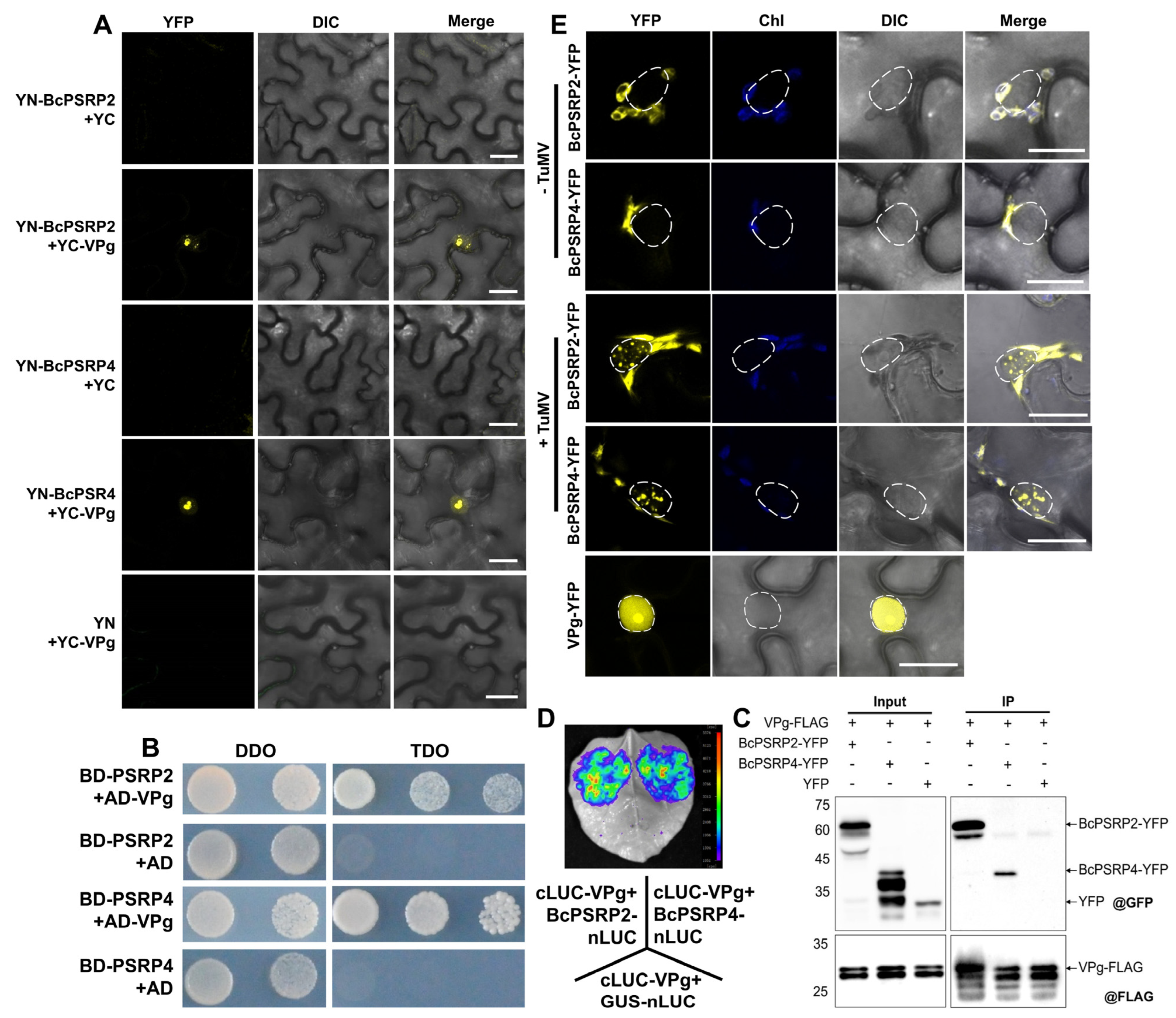
2.3. BcPSRP2/4 Interacts with TuMV VPg and Enters the Nucleus upon Viral Infection
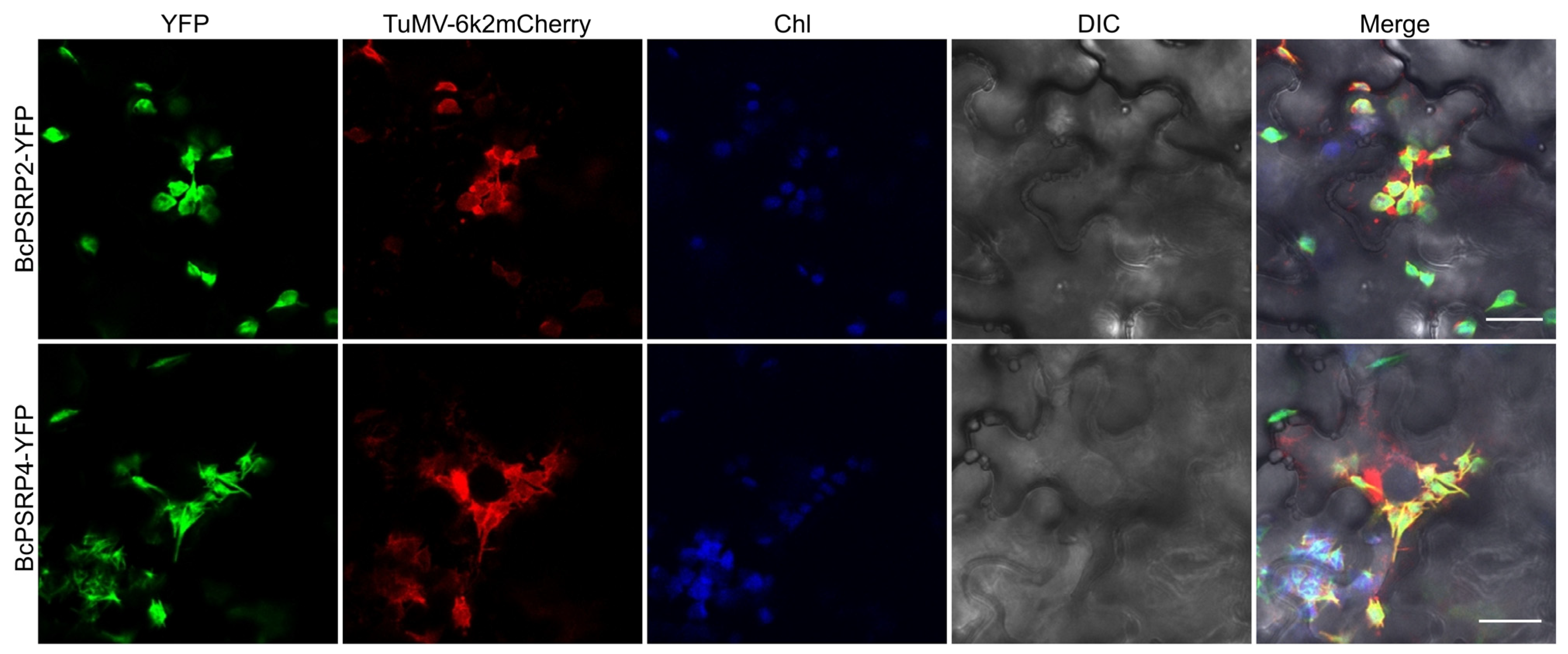
2.4. BcPSRP2/4 Partially Co-Localized with the TuMV Viral Replication Complex
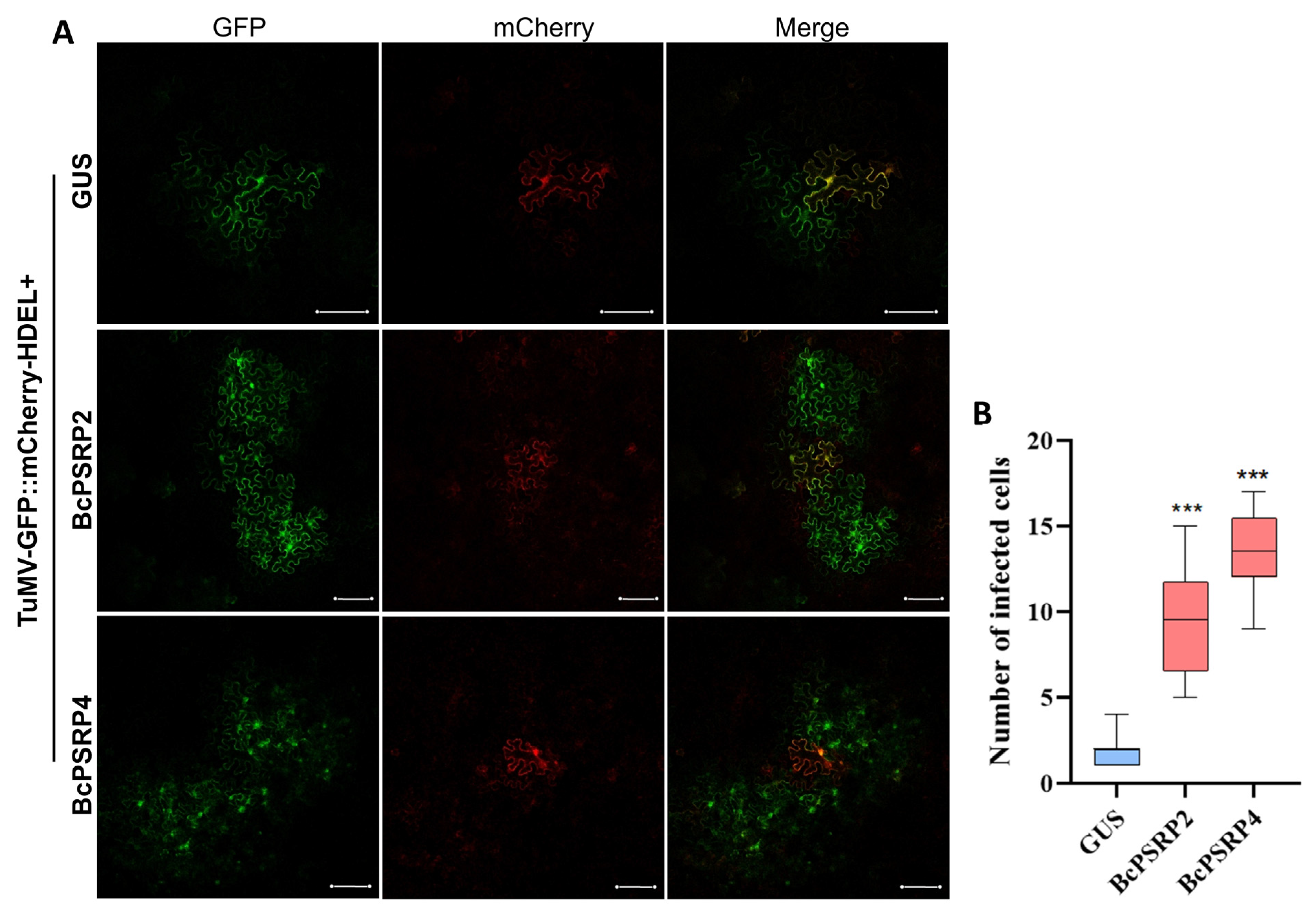
2.5. Overexpression of PSRP2/4 Promotes TuMV Intercellular Movement
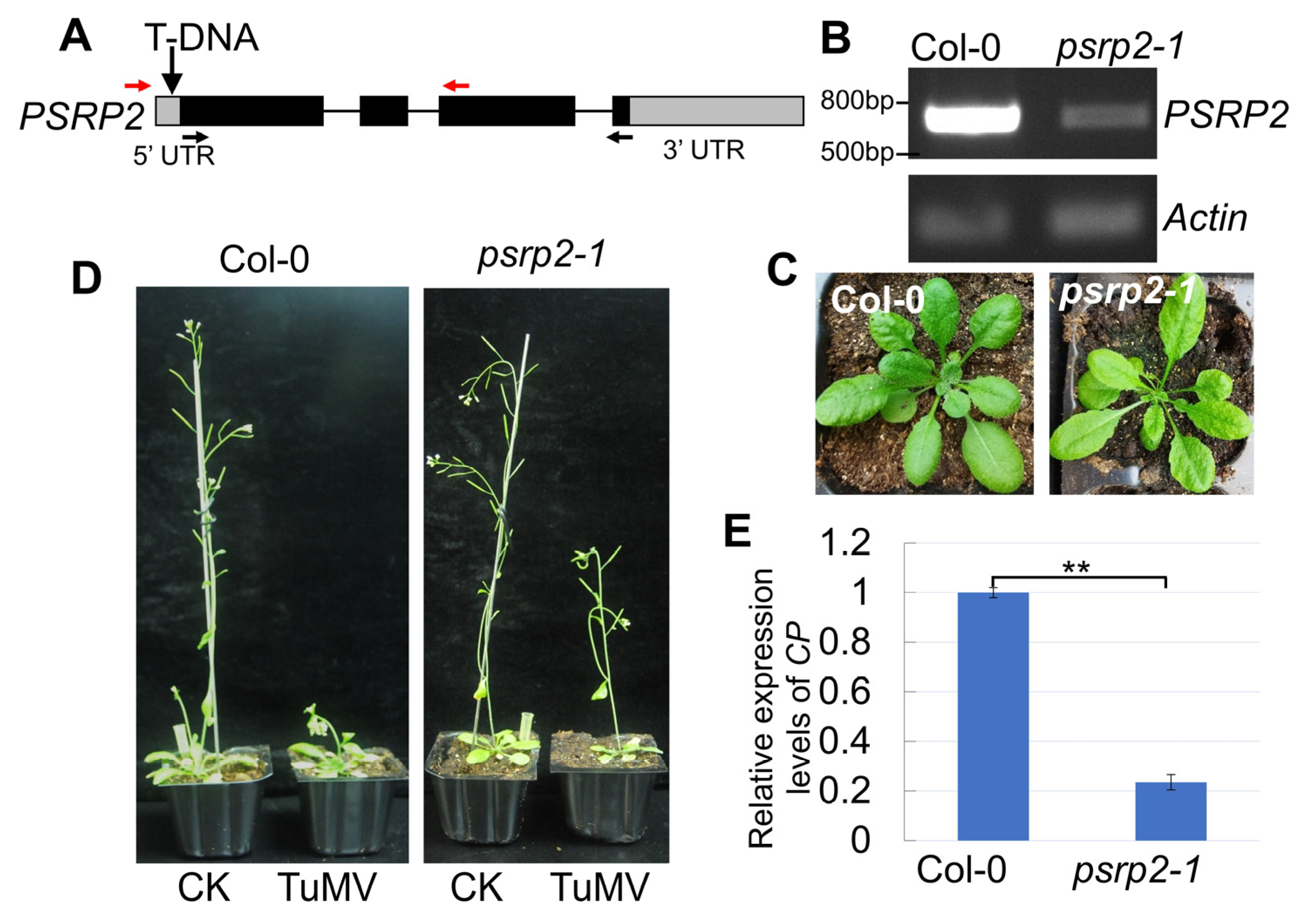
2.6. Down-Regulation of PSRP2 Is Associated with Milder Disease Symptoms in Arabidopsis
2.7. Silencing BcPSRP2/4 Resulted in Milder Symptoms upon TuMV Infection in Brassica rapa
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and Viral Inoculation
4.2. Genome-Wide Characterization of the BcPSRPs
4.3. Subcellular Localization and Bimolecular Fluorescence Complementation (BiFC)
4.4. Cell-to-Cell Movement Assay
4.5. Yeast Two-Hybrid (Y2H) Assay
4.6. Co-Immunoprecipitation (Co-IP) Assay
4.7. Luciferase Complementation Assay (LCA)
4.8. Genetic Identification of Mutant Line
4.9. Virus-Induced Gene Silencing (VIGS)
4.10. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gardner, M. Turnip Mosaic. J. Agric. Res. 1921, 22, 123–124. [Google Scholar]
- Li, G.L.; Lv, H.H.; Zhang, S.J.; Zhang, S.F.; Li, F.; Zhang, H.; Qian, W.; Fang, Z.Y.; Sun, R.F. TuMV management for Brassica crops through host resistance: Retrospect and prospects. Plant Pathol. 2019, 68, 1035–1044. [Google Scholar] [CrossRef]
- Wu, G.; Fang, X.; Yu, T.; Chen, J.; Yan, F. Turnip mosaic virus pathogenesis and host resistance mechanisms in Brassica. Hortic. Plant. J. 2024, 10, 947–960. [Google Scholar] [CrossRef]
- Walsh, J.A.; Jenner, C.E. Turnip mosaic virus and the quest for durable resistance. Mol. Plant Pathol. 2002, 3, 289–300. [Google Scholar] [CrossRef]
- Carrington, J.C.; Kasschau, K.D.; Mahajan, S.K.; Schaad, M.C. Cell-to-cell and long-distance transport of viruses in plants. Plant Cell 1996, 8, 1669. [Google Scholar] [CrossRef]
- Jones, R.A.; Barbetti, M.J. Influence of climate change on plant disease infections and epidemics caused by viruses and bacteria. CABI Rev. 2012, 1–33. [Google Scholar] [CrossRef]
- Wang, A. Dissecting the molecular network of virus-plant interactions: The complex roles of host factors. Annu. Rev. Phytopathol. 2015, 53, 45–66. [Google Scholar] [CrossRef]
- Sanfaçon, H. Plant translation factors and virus resistance. Viruses 2015, 7, 3392–3419. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Bernards, M.A.; Wang, A. Manipulation of the cellular membrane-cytoskeleton network for RNA virus replication and movement in plants. Viruses 2023, 15, 744. [Google Scholar] [CrossRef]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the languages of the chloroplast: Retrograde signaling and beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, H.; Cui, X.; Wang, A. The altered photosynthetic machinery during compatible virus infection. Curr. Opin. Virol. 2016, 17, 19–24. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan horse in plant–virus interaction. Mol. Plant Pathol. 2018, 19, 504–518. [Google Scholar] [CrossRef]
- Littlejohn, G.R.; Breen, S.; Smirnoff, N.; Grant, M. Chloroplast immunity illuminated. New Phytol. 2021, 229, 3088–3107. [Google Scholar] [CrossRef]
- Cheng, S.F.; Huang, Y.P.; Chen, L.H.; Hsu, Y.H.; Tsai, C.H. Chloroplast phosphoglycerate kinase is involved in the targeting of Bamboo mosaic virus to chloroplasts in Nicotiana benthamiana plants. Plant Physiol. 2013, 163, 1598–1608. [Google Scholar] [CrossRef]
- Geng, C.; Yan, Z.Y.; Cheng, D.J.; Liu, J.; Tian, Y.P.; Zhu, C.X.; Wang, H.Y.; Li, X.D. Tobacco vein banding mosaic virus 6K2 protein hijacks NbPsbO1 for virus replication. Sci. Rep. 2017, 7, 43455. [Google Scholar] [CrossRef]
- Qin, L.; Hongjun, L.; Liu, P.; Jiang, L.; Cheng, X.; Li, F.; Shen, W.; Qiu, W.; Dai, Z.; Cui, H. Rubisco small subunit (RbCS) is co-opted by potyvirids as the scaffold protein in assembling a complex for viral intercellular movement. PLoS Path. 2024, 20, e1012064. [Google Scholar] [CrossRef]
- Bwalya, J.; Kim, K.H. The crucial role of chloroplast-related proteins in viral genome replication and host defense against positive-sense single-stranded RNA viruses. Plant Pathol. J. 2023, 39, 28. [Google Scholar] [CrossRef]
- Bwalya, J.; Widyasari, K.; Völz, R.; Kim, K.H. Chloroplast-related host proteins interact with NIb and NIa-Pro of soybeans mosaic virus and induce resistance in the susceptible cultivar. Virus Res. 2023, 336, 199205. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Subramanian, A.R. The plastid ribosomal proteins: Identification of all the proteins in the 50 S subunit of an organelle ribosome (chloroplast). J. Biol. Chem. 2000, 275, 28466–28482. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lee, K.; Gu, L.; Kim, J.I.; Kang, H. Functional characterization of a plastid-specific ribosomal protein PSRP2 in Arabidopsis thaliana under abiotic stress conditions. Plant Physiol. Biochem. 2013, 73, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.R.; Dönhöfer, A.; Barat, C.; Marquez, V.; Datta, P.P.; Fucini, P.; Wilson, D.N.; Agrawal, R.K. PSRP1 is not a ribosomal protein, but a ribosome-binding factor that is recycled by the ribosome-recycling factor (RRF) and elongation factor G (EF-G) 2. J. Biol. Chem. 2010, 285, 4006–4014. [Google Scholar] [CrossRef]
- Tiller, N.; Weingartner, M.; Thiele, W.; Maximova, E.; Schöttler, M.A.; Bock, R. The plastid-specific ribosomal proteins of Arabidopsis thaliana can be divided into non-essential proteins and genuine ribosomal proteins. Plant J. 2012, 69, 302–316. [Google Scholar] [CrossRef]
- Zhao, J.P.; Zhang, X.; Hong, Y.G.; Liu, Y.L. Chloroplast in plant-virus interaction. Front. Microbiol. 2016, 7, 1565. [Google Scholar] [CrossRef]
- Maris, C.; Dominguez, C.; Allain, F.H.T. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005, 272, 2118–2131. [Google Scholar] [CrossRef]
- Lacombe, T.; García-Gómez, J.J.; De La Cruz, J.; Roser, D.; Hurt, E.; Linder, P.; Kressler, D. Linear ubiquitin fusion to Rps31 and its subsequent cleavage are required for the efficient production and functional integrity of 40S ribosomal subunits. Mol. Microbiol. 2009, 72, 69–84. [Google Scholar] [CrossRef]
- Pfalz, J.; Holtzegel, U.; Barkan, A.; Weisheit, W.; Mittag, M.; Pfannschmidt, T. Zmp TAC 12 binds single-stranded nucleic acids and is essential for accumulation of the plastid-encoded polymerase complex in maize. New Phytol. 2015, 206, 1024–1037. [Google Scholar] [CrossRef]
- Wei, T.; Huang, T.S.; McNeil, J.; Laliberté, J.F.; Hong, J.; Nelson, R.S.; Wang, A. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J. Virol. 2010, 84, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Laliberté, J.F. The genome-linked protein VPg of plant viruses—A protein with many partners. Curr. Opin. Virol. 2011, 1, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; He, R.; Bernards, M.A.; Wang, A. The cis-expression of the coat protein of turnip mosaic virus is essential for viral intercellular movement in plants. Mol. Plant Pathol. 2020, 21, 1194–1211. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, Y.; Bernards, M.A.; Wang, A. Turnip mosaic virus selectively subverts a PR-5 thaumatin-like, plasmodesmal protein to promote viral infection. New Phytol. 2025, 245, 299–317. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, C.; Hou, X.; Sanfaçon, H.; Wang, A. The SNARE protein Syp71 is essential for turnip mosaic virus infection by mediating fusion of virus-induced vesicles with chloroplasts. PLoS Path. 2013, 9, e1003378. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, K.Q.; Pan, H.Y.; Liu, J.L. Chloroplast: The emerging battlefield in plant–microbe interactions. Front. Plant. Sci. 2021, 12, 637853. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Q.; Zhang, H.; Jia, Q.; Hong, Y.; Liu, Y. The rubisco small subunit is involved in tobamovirus movement and Tm-22-mediated extreme resistance. Plant Physiol. 2013, 161, 374–383. [Google Scholar] [CrossRef]
- Krenz, B.; Jeske, H.; Kleinow, T. The induction of stromule formation by a plant DNA-virus in epidermal leaf tissues suggests a novel intra-and intercellular macromolecular trafficking route. Front. Plant. Sci. 2012, 3, 291. [Google Scholar] [CrossRef]
- Krenz, B.; Windeisen, V.; Wege, C.; Jeske, H.; Kleinow, T. A plastid-targeted heat shock cognate 70 kDa protein interacts with the abutilon mosaic virus movement protein. Virology 2010, 401, 6–17. [Google Scholar] [CrossRef]
- Cheng, D.J.; Xu, X.J.; Yan, Z.Y.; Tettey, C.K.; Fang, L.; Yang, G.L.; Geng, C.; Tian, Y.P.; Li, X.D. The chloroplast ribosomal protein large subunit 1 interacts with viral polymerase and promotes virus infection. Plant Physiol. 2021, 187, 174–186. [Google Scholar] [CrossRef]
- Li, J.; Feng, H.; Liu, S.; Liu, P.; Chen, X.; Yang, J.; He, L.; Yang, J.; Chen, J. Phosphorylated viral protein evades plant immunity through interfering the function of RNA-binding protein. PLoS Path. 2022, 18, e1010412. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Changwei, Z.; Tang, J.; Li, Y.; Wang, Z.; Jiang, D.; Hou, X. Genome-wide analysis and identification of TIR-NBS-LRR genes in Chinese cabbage (Brassica rapa ssp. pekinensis) reveal expression patterns to TuMV infection. Physiol. Mol. Plant Pathol. 2015, 90, 89–97. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.F.; Ma, L.M.; Liu, T.K.; Zhang, C.W.; Xiao, D.; Zheng, H.K.; Chen, F.; Hou, X.L. A chromosome-level reference genome of non-heading Chinese cabbage [Brassica campestris (syn. Brassica rapa) ssp. chinensis]. Hortic. Res. 2020, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Lu, Q.; Zhang, L.; Kohalmi, S.E.; Cui, Y.H. Detection of protein interactions in plant using a gateway compatible bimolecular fluorescence complementation (BiFC) system. JOVE-J. Vis. Exp. 2011, 16, 3473. [Google Scholar] [CrossRef]
- Wu, G.; Cui, X.; Chen, H.; Renaud, J.B.; Yu, K.; Chen, X.; Wang, A. Dynamin-like proteins of endocytosis in plants are coopted by potyviruses to enhance virus infection. J. Virol. 2018, 92, e01320-18. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Lyu, S.; Mao, Y.; Yu, F.; Liu, S.; Fang, Y.; Deng, S. Arabidopsis CIRP1 E3 ligase modulates drought and oxidative stress tolerance and reactive oxygen species homeostasis by directly degrading catalases. J. Integr. Plant Biol. 2025, 67, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, X.D.; Wang, Q.; Gao, L.W.; Yang, Y.; Xiao, D.; Liu, T.K.; Li, Y.; Hou, X.L.; Zhang, C.W. Efficient virus-induced gene silencing in Brassica rapa using a turnip yellow mosaic virus vector. Biol. Plant. 2018, 62, 826–834. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, S.; Lu, W.; Zhang, C.; Wang, W.; Yuan, M.; E, L.; Liu, T.; Deng, S. The PSRP2/4 Proteins Promote Viral Infection by Interacting with the VPg Protein of TuMV. Plants 2025, 14, 3211. https://doi.org/10.3390/plants14203211
Lyu S, Lu W, Zhang C, Wang W, Yuan M, E L, Liu T, Deng S. The PSRP2/4 Proteins Promote Viral Infection by Interacting with the VPg Protein of TuMV. Plants. 2025; 14(20):3211. https://doi.org/10.3390/plants14203211
Chicago/Turabian StyleLyu, Shanwu, Wenjun Lu, Changwei Zhang, Wenlong Wang, Mengguo Yuan, Liu E, Tingting Liu, and Shulin Deng. 2025. "The PSRP2/4 Proteins Promote Viral Infection by Interacting with the VPg Protein of TuMV" Plants 14, no. 20: 3211. https://doi.org/10.3390/plants14203211
APA StyleLyu, S., Lu, W., Zhang, C., Wang, W., Yuan, M., E, L., Liu, T., & Deng, S. (2025). The PSRP2/4 Proteins Promote Viral Infection by Interacting with the VPg Protein of TuMV. Plants, 14(20), 3211. https://doi.org/10.3390/plants14203211





