Genome-Wide Identification, Characterization, and Expression Analysis of Orphan Genes Within Coriander
Abstract
1. Introduction
2. Results
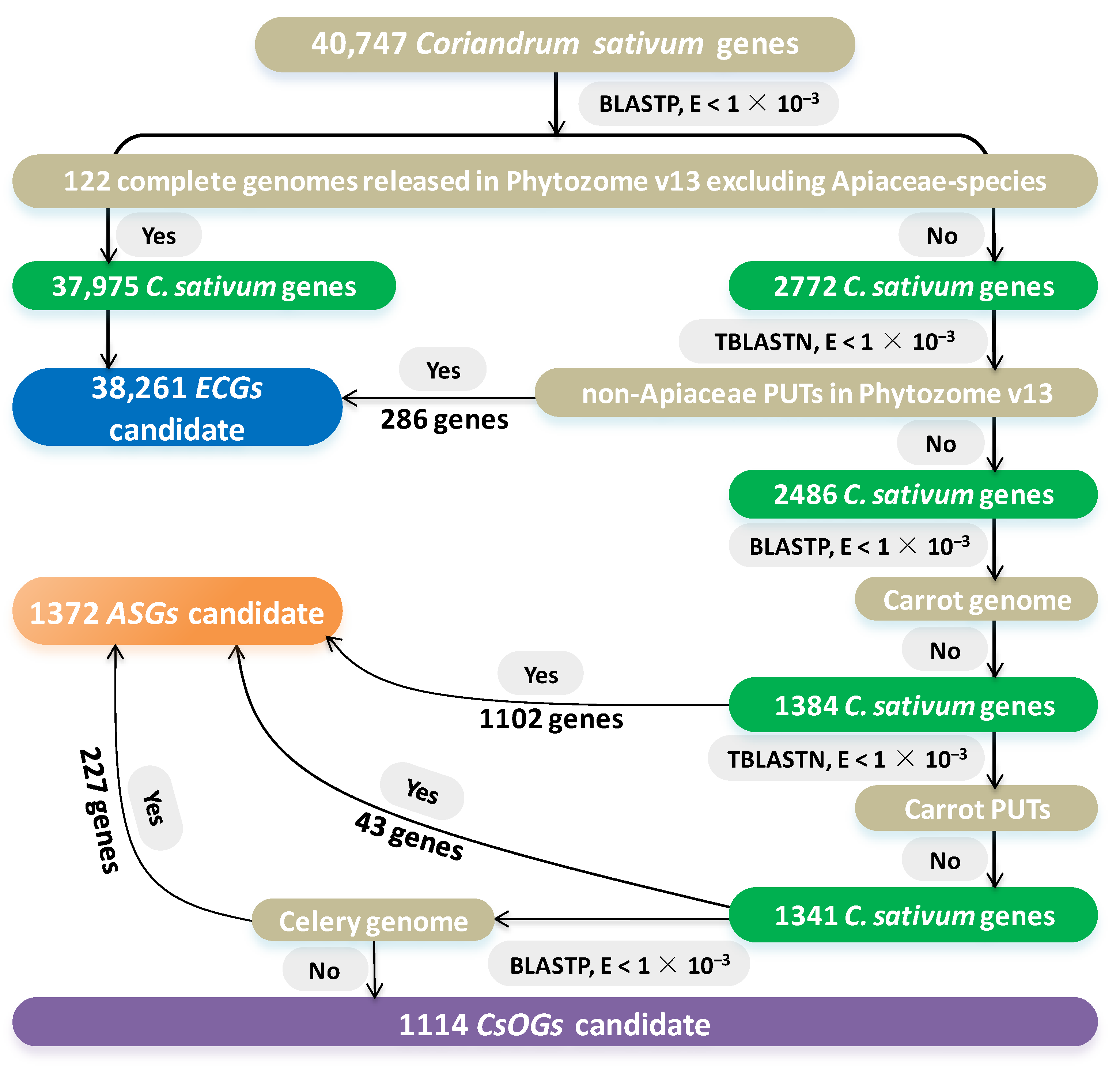
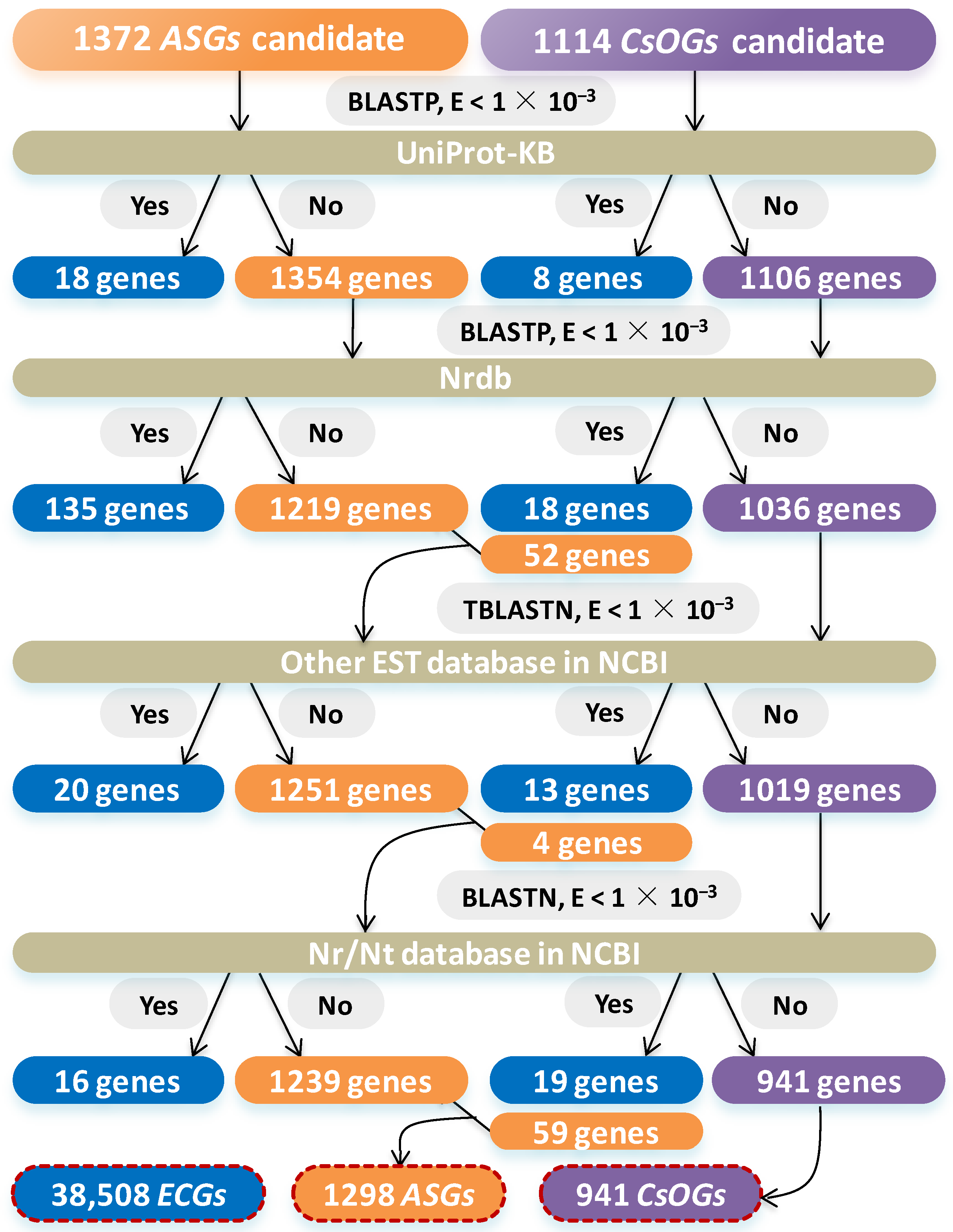
2.1. Identification of CsOGs and ASGs in C. sativum
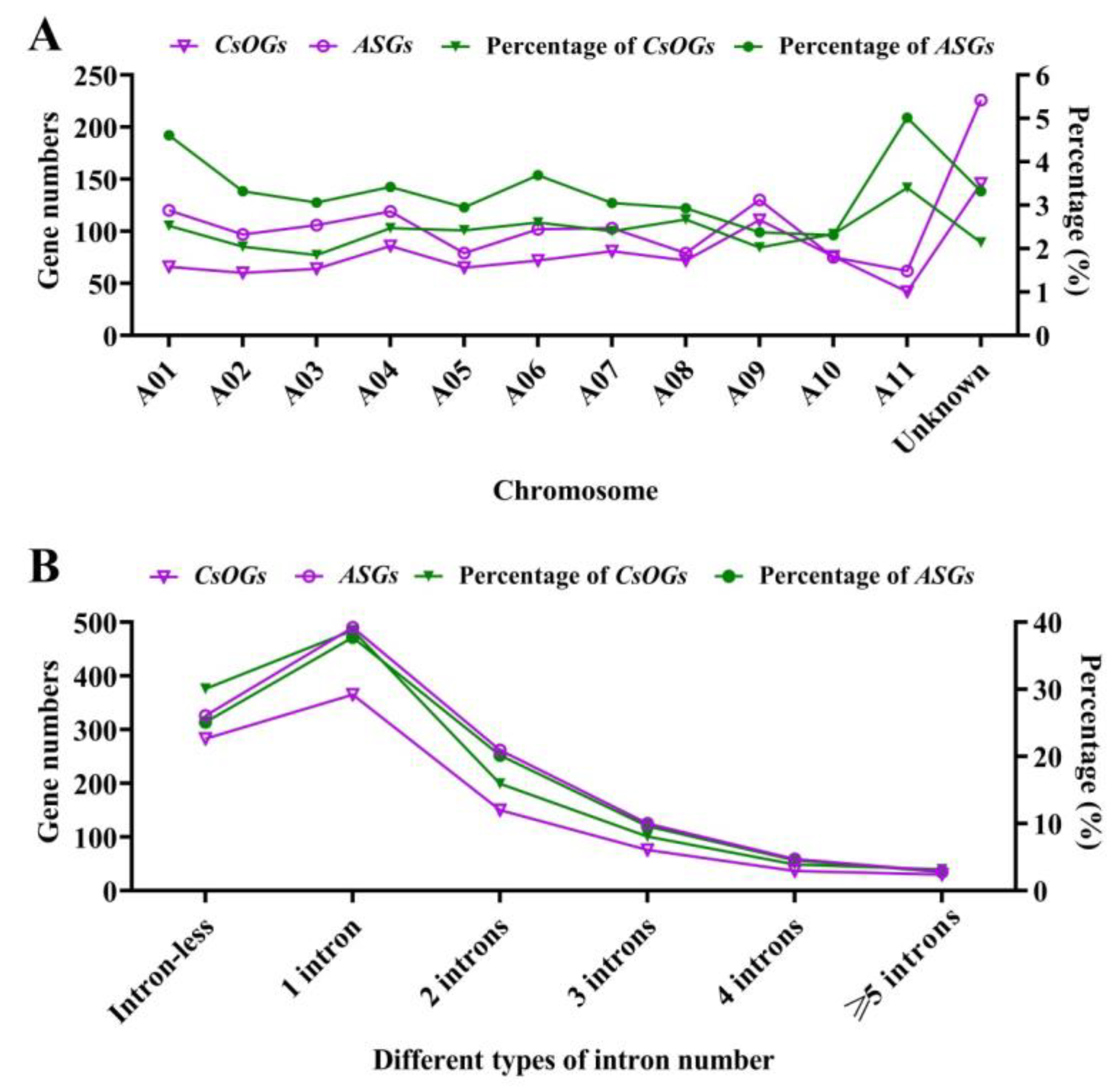
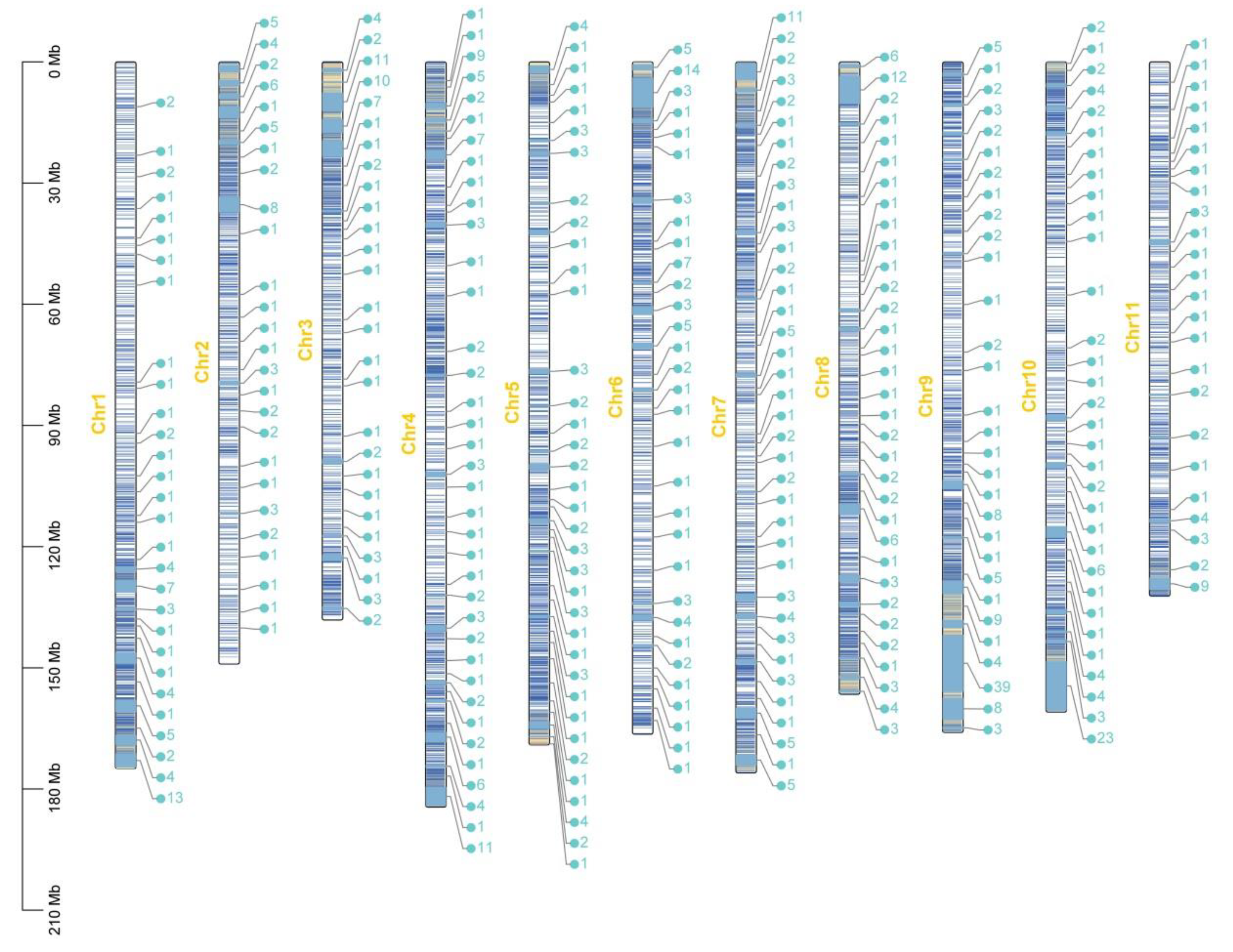
2.2. Genic Features of the CsOGs and ASGs in C. sativum
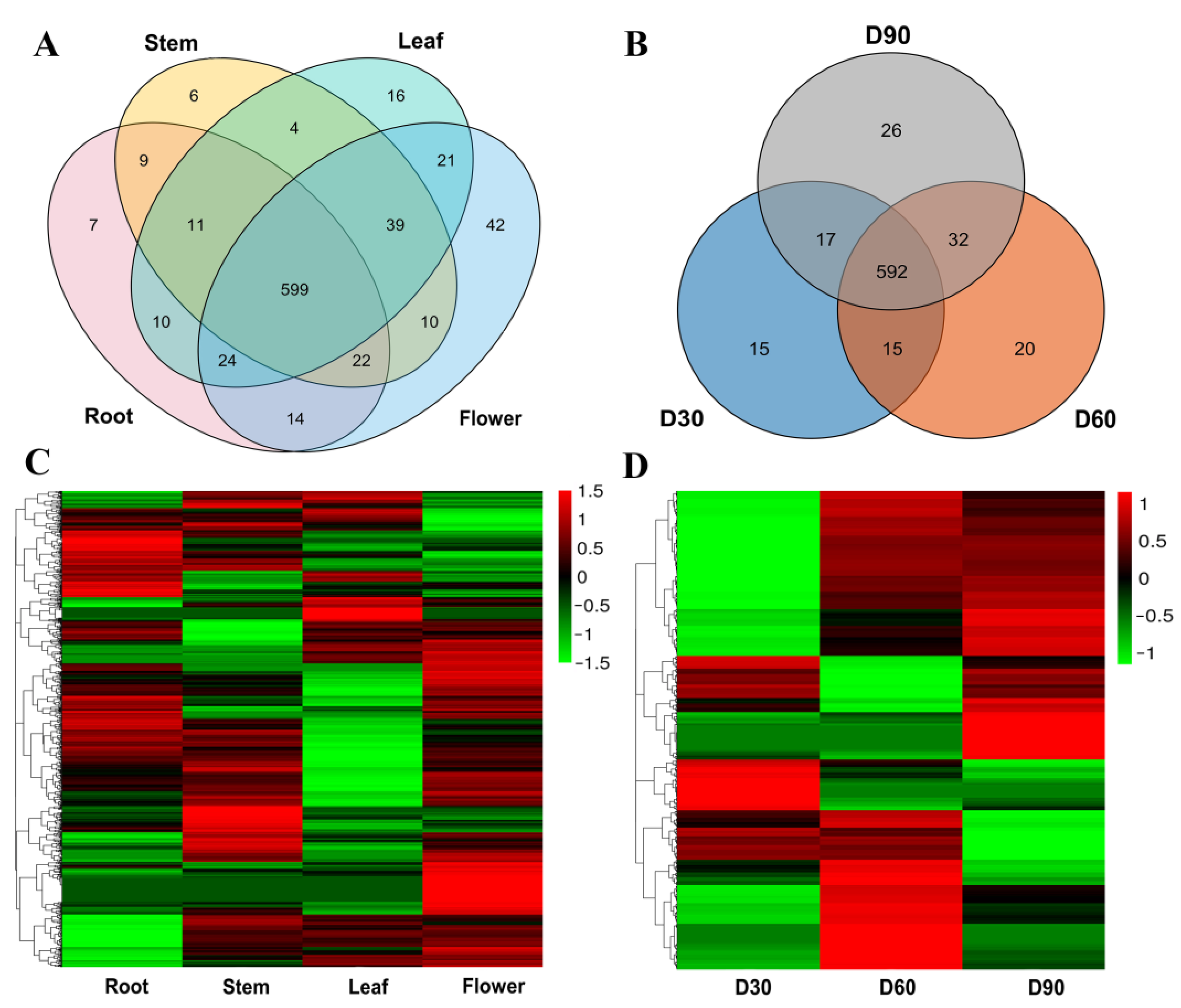
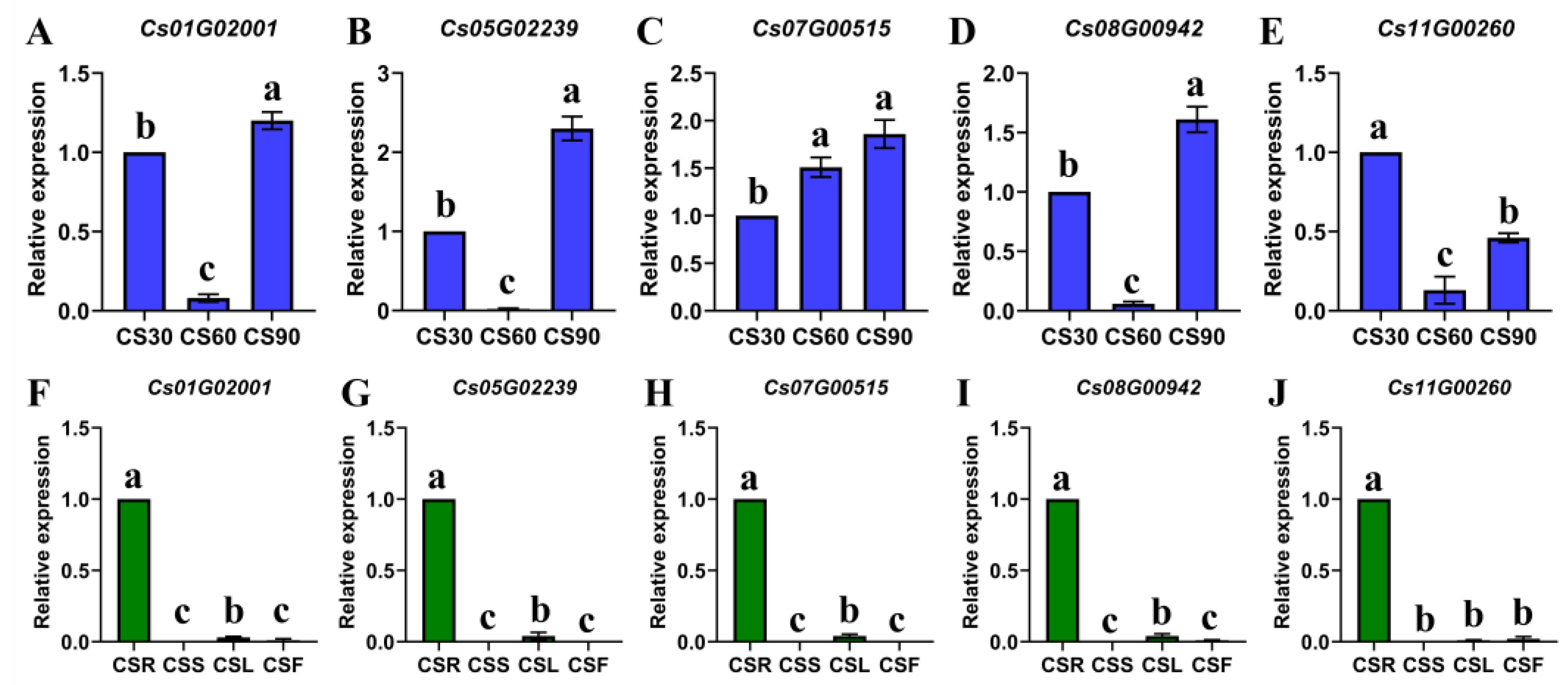
2.3. Expression Patterns During Different Growth Stages and in Different Tissues
2.4. Expression Patterns of CsOGs in C. sativum After Cold Stress
3. Discussion
4. Materials and Methods
4.1. Genome Datasets
4.2. Homology Search
4.3. Genic Features and Physical Mapping
4.4. Gene Functional Annotation and Expression Evidence
4.5. Sampling and Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ASGs | Apiaceae-specific genes |
| CGDB | Coriander Genome Database |
| CsOGs | C. sativum OGs |
| ECGs | Evolutionarily conserved genes |
| ESTs | Expressed sequence tags |
| FPKM | Fragments Per Kilobase of exon model per Million mapped fragments |
| GSA | Genome Sequence Archive |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NCBI | National Center for Biotechnology Information |
| Nrdb | Non-redundant protein database |
| Nr/Nt | Nucleotide collection |
| OGs | Orphan genes |
| PUTs | PlantGDB-generated unique transcripts |
| SUS | Sucrose synthase |
| TEs | Transposable elements |
| TFs | Transcription factors |
| UniProt | Universal protein resource |
| UniProt-KB | UniProt knowledgebase |
References
- Li, L.; Zheng, W.; Zhu, Y.; Ye, H.; Tang, B.; Arendsee, Z.W.; Jones, D.; Li, R.; Ortiz, D.; Zhao, X.; et al. QQS orphan gene regulates carbon and nitrogen partitioning across species via NF-YC interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 14734–14739. [Google Scholar] [CrossRef]
- Jiang, M.; Dong, X.; Lang, H.; Pang, W.; Zhan, Z.; Li, X.; Piao, Z. Mining of Brassica-Specific Genes (BSGs) and Their Induction in Different Developmental Stages and under Plasmodiophora brassicae Stress in Brassica rapa. Int. J. Mol. Sci. 2018, 19, 2064. [Google Scholar] [CrossRef]
- Kaessmann, H. Origins, evolution, and phenotypic impact of new genes. Genome Res. 2010, 20, 1313–1326. [Google Scholar] [CrossRef]
- Donoghue, M.T.; Keshavaiah, C.; Swamidatta, S.H.; Spillane, C. Evolutionary origins of Brassicaceae specific genes in Arabidopsis thaliana. BMC Evol. Biol. 2011, 11, 47–59. [Google Scholar] [CrossRef]
- Arendsee, Z.W.; Li, L.; Wurtele, E.S. Coming of age: Orphan genes in plants. Trends Plant Sci. 2014, 19, 698–708. [Google Scholar] [CrossRef]
- Carvunis, A.-R.; Rolland, T.; Wapinski, I.; Calderwood, M.A.; Yildirim, M.A.; Simonis, N.; Charloteaux, B.; Hidalgo, C.A.; Barbette, J.; Santhanam, B.; et al. Proto-genes and de novo gene birth. Nature 2012, 487, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Wurtele, E.S. How new genes are born. eLife 2020, 9, e55136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, X.; Dong, X.; Zu, Y.; Zhan, Z.; Piao, Z.; Lang, H. Research Advances and Prospects of Orphan Genes in Plants. Front. Plant Sci. 2022, 13, 947129. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wurtele, S.E. The QQS orphan gene of Arabidopsis modulates carbon and nitrogen allocation in soybean. Plant Biotechnol. J. 2015, 13, 177–187. [Google Scholar] [CrossRef]
- Cui, X.; Lv, Y.; Chen, M.; Nikoloski, Z.; Twell, D.; Zhang, D. Young Genes out of the Male: An Insight from Evolutionary Age Analysis of the Pollen Transcriptome. Mol. Plant 2015, 8, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Zhou, Y.; Yang, H.; Hu, Y.; Shi, Y.; Li, L.; Siddique, A.N.; Liu, C.; Zhu, A.; Zhang, C.; et al. Genetic innovations: Transposable element recruitment and de novo formation lead to the birth of orphan genes in the rice genome. J. Syst. Evol. 2019, 59, 341–351. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Hu, Y.; Muñoz-Amatriaín, M.; Luo, J.; Zhou, W.; Wang, B.; Wang, Y.; Wu, X.; Huang, L.; et al. Orphan genes are involved in drought adaptations and ecoclimatic-oriented selections in domesticated cowpea. J. Exp. Bot. 2019, 70, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ding, Q.; Guo, Z.; Zhao, Z.; Wei, L.; Li, Y.; Song, S.; Zheng, H. Identification, characterization and expression analysis of lineage-specific genes within mangrove species Aegiceras corniculatum. Mol. Genet. Genom. 2021, 296, 1235–1247. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, Y.; Tao, Y.; Jia, H.; Ma, Z. Identification, characterization and expression analysis of lineage-specific genes within Triticeae. Genom. 2020, 112, 1343–1350. [Google Scholar] [CrossRef]
- Mukherjee, S.; Panda, A.; Ghosh, T.C. Elucidating evolutionary features and functional implications of orphan genes in Leishmania major. Infect. Genet. Evol. 2015, 32, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Sun, S.; Zhan, Z.; Li, X.; Piao, Z. Chinese cabbage orphan gene BR3 confers bolting resistance to Arabidopsis through the gibberellin pathway. Front. Plant Sci. 2025, 15, 1518962. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Y.; Yang, X.; Li, X.; Lang, H. Brassica rapa orphan gene BR1 delays flowering time in Arabidopsis. Front. Plant Sci. 2023, 14, 1135684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhan, Z.; Li, H.; Dong, X.; Cheng, F.; Piao, Z. Brassica rapa orphan genes largely affect soluble sugar metabolism. Hortic. Res. 2020, 7, 181. [Google Scholar] [CrossRef]
- Zu, Y.; Jiang, M.; Zhan, Z.; Li, X.; Piao, Z. Orphan gene BR2 positively regulates bolting resistance through the vernalization pathway in Chinese cabbage. Hortic. Res. 2024, 11, uhae216. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Chen, S.; Heng, Y.; Chen, Z.; Yang, J.; Zhou, K.; Pei, J.; He, H.; Deng, X.W. Poaceae-specific MS1 encodes a phospholipid-binding protein for male fertility in bread wheat. Proc. Natl. Acad. Sci. USA 2017, 114, 12614–12619. [Google Scholar] [CrossRef]
- Ni, F.; Qi, J.; Hao, Q.; Lyu, B.; Luo, M.C.; Wang, Y.; Chen, F.; Wang, S.; Zhang, C.; Epstein, L. Wheat Ms2 encodes for an orphan protein that confers male sterility in grass species. Nat. Commun. 2017, 8, 15121. [Google Scholar] [CrossRef]
- Pei, Q.; Li, N.; Bai, Y.; Wu, T.; Yang, Q.; Yu, T.; Wang, Z.; Liu, Z.; Li, Q.; Lin, H. Comparative analysis of the TCP gene family in celery, coriander and carrot (family Apiaceae). Veg. Res. 2021, 1, 5. [Google Scholar] [CrossRef]
- Song, X.; Wang, J.; Li, N.; Yu, J.; Meng, F.; Wei, C.; Liu, C.; Chen, W.; Nie, F.; Zhang, Z.; et al. Deciphering the high-quality genome sequence of coriander that causes controversial feelings. Plant Biotechnol. J. 2020, 18, 1444–1456. [Google Scholar] [CrossRef]
- Pei, Q.; Yu, T.; Wu, T.; Yang, Q.; Gong, K.; Zhou, R.; Cui, C.; Yu, Y.; Zhao, W.; Kang, X.; et al. Comprehensive identification and analyses of the Hsf gene family in the whole-genome of three Apiaceae species. Hortic. Plant J. 2021, 7, 457–468. [Google Scholar] [CrossRef]
- Sowmya, R.S.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Effect of amino acids on growth, elemental content, functional groups, and essential oils composition on hydroponically cultivated coriander under different conditions. Ind. Crops Prod. 2023, 197, 116577. [Google Scholar]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Chahed, T.; Kchouk, M.E.; Marzouk, B. Changes on essential oil composition of coriander (Coriandrum sativum L.) fruits during three stages of maturity. Food Chem. 2007, 102, 1131–1134. [Google Scholar] [CrossRef]
- Grosso, C.; Ferraro, V.; Figueiredo, A.C.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M. Supercritical carbon dioxide extraction of volatile oil from Italian coriander seeds. Food Chem. 2008, 111, 197–203. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Hussein, O.S.; Abdelkader, A.F.; Farag, I.A.; Ibrahim, M. Metabolic activities and molecular investigations of the ameliorative impact of some growth biostimulators on chilling-stressed coriander (Coriandrum sativum L.) plant. BMC Plant Biol. 2021, 21, 361. [Google Scholar] [CrossRef]
- Wu, T.; Feng, S.-y.; Yang, Q.-h.; Bhetariya, P.J.; Gong, K.; Cui, C.-l.; Song, J.; Ping, X.-r.; Pei, Q.-y.; Yu, T.; et al. Integration of the metabolome and transcriptome reveals the metabolites and genes related to nutritional and medicinal value in Coriandrum sativum. J. Int. Agric. 2021, 20, 1807–1818. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef]
- Li, M.Y.; Feng, K.; Hou, X.L.; Jiang, Q.; Xiong, A.S. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic. Res. 2020, 7, 223–253. [Google Scholar] [CrossRef]
- Song, X.; Sun, P.; Yuan, J.; Gong, K.; Li, N.; Meng, F.; Zhang, Z.; Li, X.; Hu, J.; Wang, J.; et al. The celery genome sequence reveals sequential paleo-polyploidizations, karyotype evolution and resistance gene reduction in apiales. Plant Biotechnol. J. 2021, 19, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R.; Tsutsui, N.D. Taxonomically restricted genes are associated with the evolution of sociality in the honey bee. BMC Genom. 2011, 12, 164. [Google Scholar] [CrossRef]
- Lin, H.; Moghe, G.; Ouyang, S.; Iezzoni, A.; Shiu, S.H.; Gu, X.; Buell, C.R. Comparative analyses reveal distinct sets of lineage-specific genes within Arabidopsis thaliana. BMC Evol. Biol. 2010, 10, 41. [Google Scholar] [CrossRef]
- Yang, L.; Zou, M.; Fu, B.; He, S. Genome-wide identification, characterization, and expression analysis of lineage-specific genes within zebrafish. BMC Genom. 2013, 14, 65. [Google Scholar] [CrossRef]
- Lin, W.L.; Cai, B.; Cheng, Z.M. Identification and characterization of lineage-specific genes in Populus trichocarpa. Plant Cell Tiss. Organ Cult. 2014, 116, 217–225. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, G.; Hao, B.; Chen, L.; Deng, X.; Xu, Q. Identification, characterization and expression analysis of lineage-specific genes within sweet orange (Citrus sinensis). BMC Genom. 2015, 16, 995. [Google Scholar] [CrossRef]
- Yang, X.; Jawdy, S.; Tschaplinski, T.J.; Tuskan, G.A. Genome-wide identification of lineage-specific genes in Arabidopsis, Oryza and Populus. Genom. 2009, 93, 473–480. [Google Scholar] [CrossRef]
- Zou, M.; Guo, B.; He, S. The Roles and Evolutionary Patterns of Intronless Genes in Deuterostomes. Comp. Funct. Genomics 2011, 2011, 680673. [Google Scholar] [CrossRef] [PubMed]
- Tautz, D.; Domazet-Lošo, T. The evolutionary origin of orphan genes. Nat. Rev. Genet. 2011, 12, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Jianguang, J.; Kahla, A.; Arunachalam, C.; Scofield, S.R.; Bowden, S.; Wallington, E.; Doohan, F.M. TaFROG Encodes a Pooideae Orphan Protein That Interacts with SnRK1 and Enhances Resistance to the Mycotoxigenic Fungus Fusarium graminearum. Plant Physiol. 2015, 169, 2895–2906. [Google Scholar] [CrossRef]
- Jiang, M.; Zhan, Z.; Li, X.; Piao, Z. Construction and evaluation of Brassica rapa orphan genes overexpression library. Front. Plant Sci. 2025, 16, 1532449. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.M.; Pu, X.J.; Zhou, S.Z.; Li, P.; Luo, T.; Chen, Z.X.; Chen, S.L.; Liu, L. Orphan gene PpARDT positively involved in drought tolerance potentially by enhancing ABA response in Physcomitrium (Physcomitrella) patens. Plant Sci. 2022, 319, 111222. [Google Scholar] [CrossRef] [PubMed]
- Luhua, S.; Hegie, A.; Suzuki, N.; Shulaev, E.; Luo, X.; Cenariu, D.; Ma, V.; Kao, S.; Lim, J.; Gunay, M.B. Linking genes of unknown function with abiotic stress responses by high-throughput phenotype screening. Physiol. Plant. 2013, 148, 322–333. [Google Scholar] [CrossRef]
- Bosch, T.C.G.; Augustin, R.; Anton-Erxleben, F.; Fraune, S.; Hemmrich, G.; Zill, H.; Rosenstiel, P.; Jacobs, G.; Schreiber, S.; Leippe, M.; et al. Uncovering the evolutionary history of innate immunity: The simple metazoan Hydra uses epithelial cells for host defence. Dev. Comp. Immunol. 2008, 33, 559–569. [Google Scholar] [CrossRef]
- Guo, W.J.; Li, P.; Ling, J.; Ye, S.P. Significant Comparative Characteristics between Orphan and Nonorphan Genes in the Rice (Oryza sativa L.) Genome. Comp. Funct. Genom. 2007, 2007, 21676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Zhu, S.; Sun, Y.; Wang, C.; Yan, X.; Zhou, Z. Identification and analysis of PAT gene family in three kinds of Apiaceae vegetable crops. Acta Agric. Zhejiangensis 2023, 35, 1315–1327. [Google Scholar]
- Xu, Z.; Huang, S.; Xie, Y.; Wang, S.; Jin, N.; Jin, L.; Tie, J.; Meng, X.; Li, Z.; Lyu, J.; et al. Physiological responses of coriander (Coriandrum sativum L.) to exogenous 2,4-epibrassinolide at different concentrations. BMC Plant Biol. 2023, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Features | 941 CsOGs | 1298 ASGs | 38,508 ECGs |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Protein length | 115.72 (52.80) | 171.00 (102.65) | 371.21 (281.89) |
| CDS length | 350.04 (158.40) | 515.93 (308.00) | 1116.60 (845.67) |
| Gene length | 1778.90 (3229.96) | 1666.62 (2721.17) | 3729.82 (4891.99) |
| Intron length | 646.41 (1385.30) | 583.73 (2157.20) | 536.15 (1305.61) |
| CDS GC (%) | 42.59 (5.54) | 42.79 (5.04) | 42.69 (3.26) |
| Intron GC (%) | 23.44 (16.15) | 24.71 (15.12) | 25.69 (13.20) |
| Gene GC (%) | 37.64 (5.15) | 38.64 (5.04) | 37.22 (3.64) |
| Exons/gene | 2.30 (1.42) | 2.42 (1.37) | 4.98 (4.71) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhu, M.; Lang, H.; Wang, W.; Li, X.; Jiang, M. Genome-Wide Identification, Characterization, and Expression Analysis of Orphan Genes Within Coriander. Plants 2025, 14, 778. https://doi.org/10.3390/plants14050778
Zhang M, Zhu M, Lang H, Wang W, Li X, Jiang M. Genome-Wide Identification, Characterization, and Expression Analysis of Orphan Genes Within Coriander. Plants. 2025; 14(5):778. https://doi.org/10.3390/plants14050778
Chicago/Turabian StyleZhang, Meidi, Mo Zhu, Hong Lang, Weiming Wang, Xiaonan Li, and Mingliang Jiang. 2025. "Genome-Wide Identification, Characterization, and Expression Analysis of Orphan Genes Within Coriander" Plants 14, no. 5: 778. https://doi.org/10.3390/plants14050778
APA StyleZhang, M., Zhu, M., Lang, H., Wang, W., Li, X., & Jiang, M. (2025). Genome-Wide Identification, Characterization, and Expression Analysis of Orphan Genes Within Coriander. Plants, 14(5), 778. https://doi.org/10.3390/plants14050778





