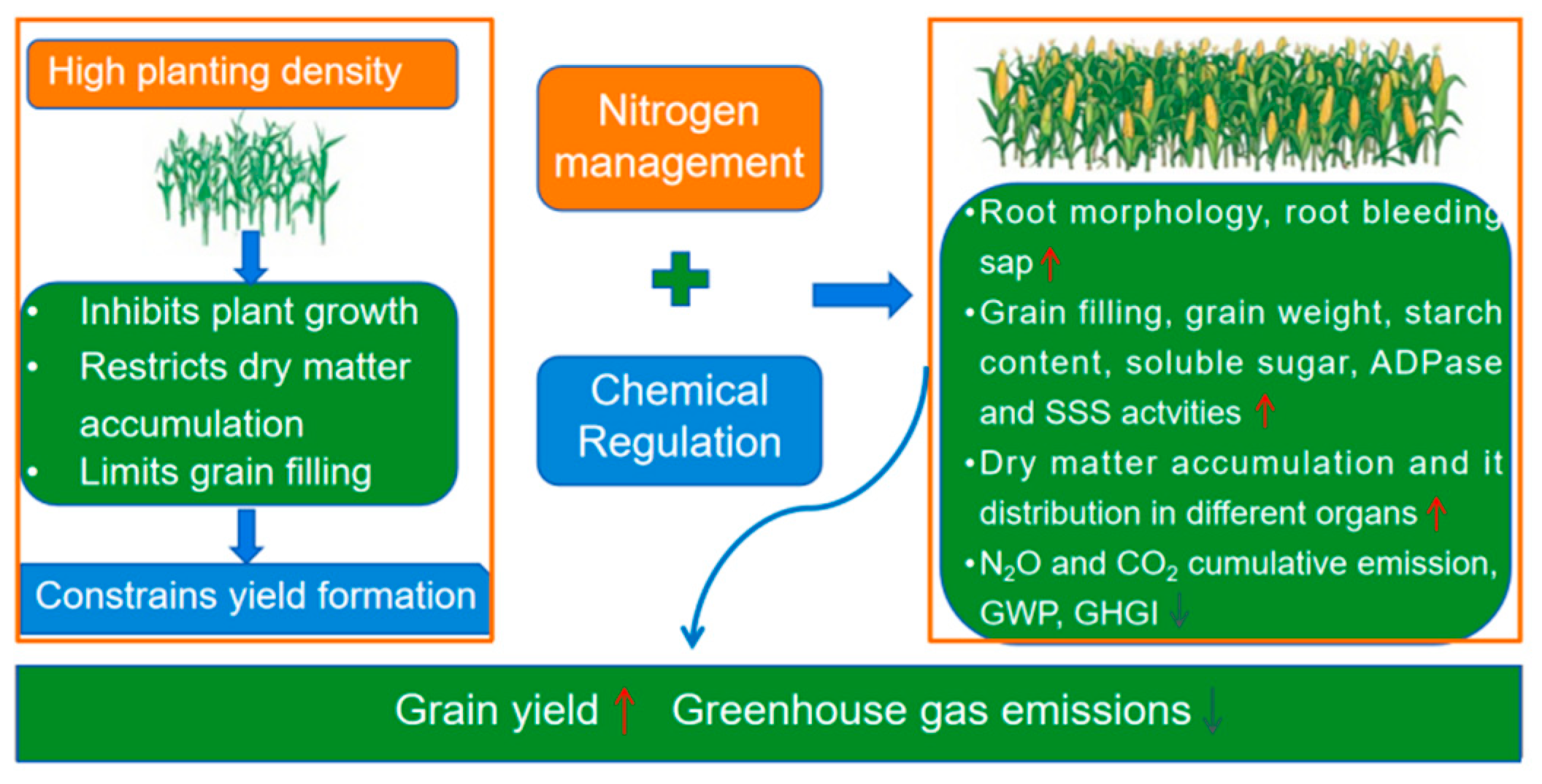
Boosting Maize Yield and Mitigating Greenhouse Gas Emissions Through Synergistic Nitrogen and Chemical Regulation by Optimizing Roots and Developing Grains Under High-Density Planting in Northeast China
Abstract
1. Introduction
2. Results
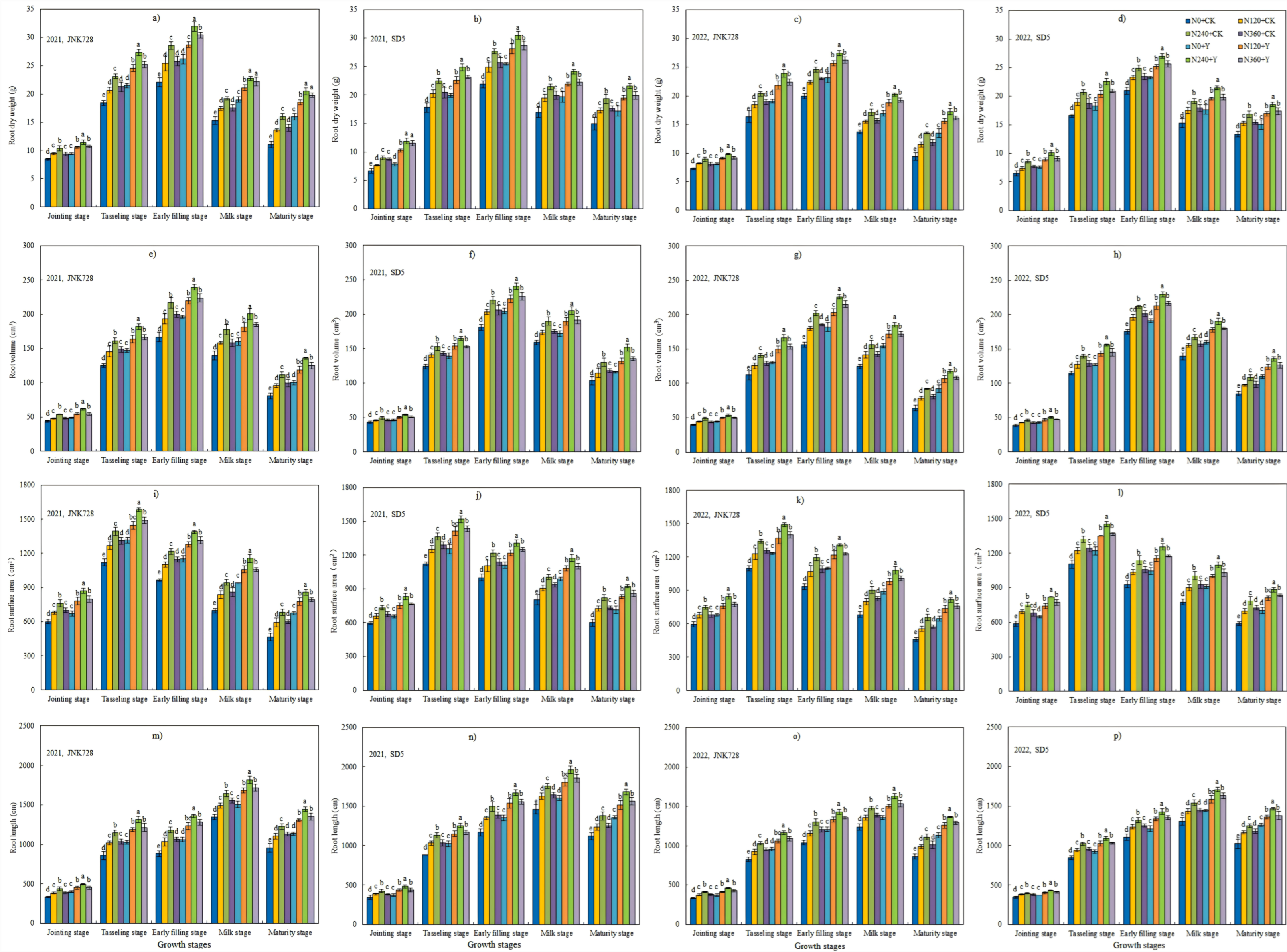
2.1. Root Morphology
2.2. Root Bleeding Sap
2.2.1. Bleeding Sap Rate
2.2.2. Mineral Nutrient and Amino Acid Concentrations in Root Bleeding Sap
2.3. Grain Formation
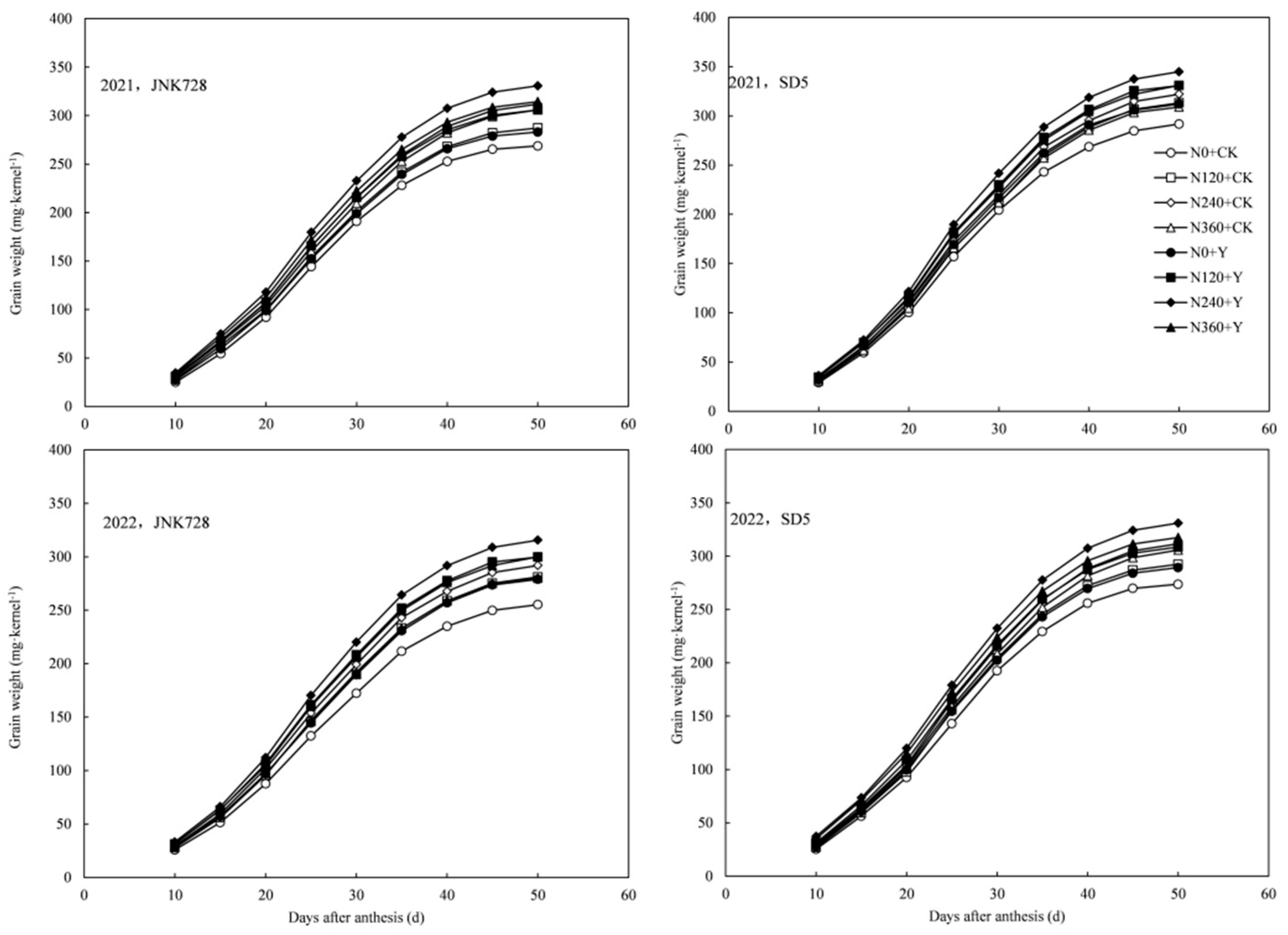
2.3.1. Grain-Filling Parameters
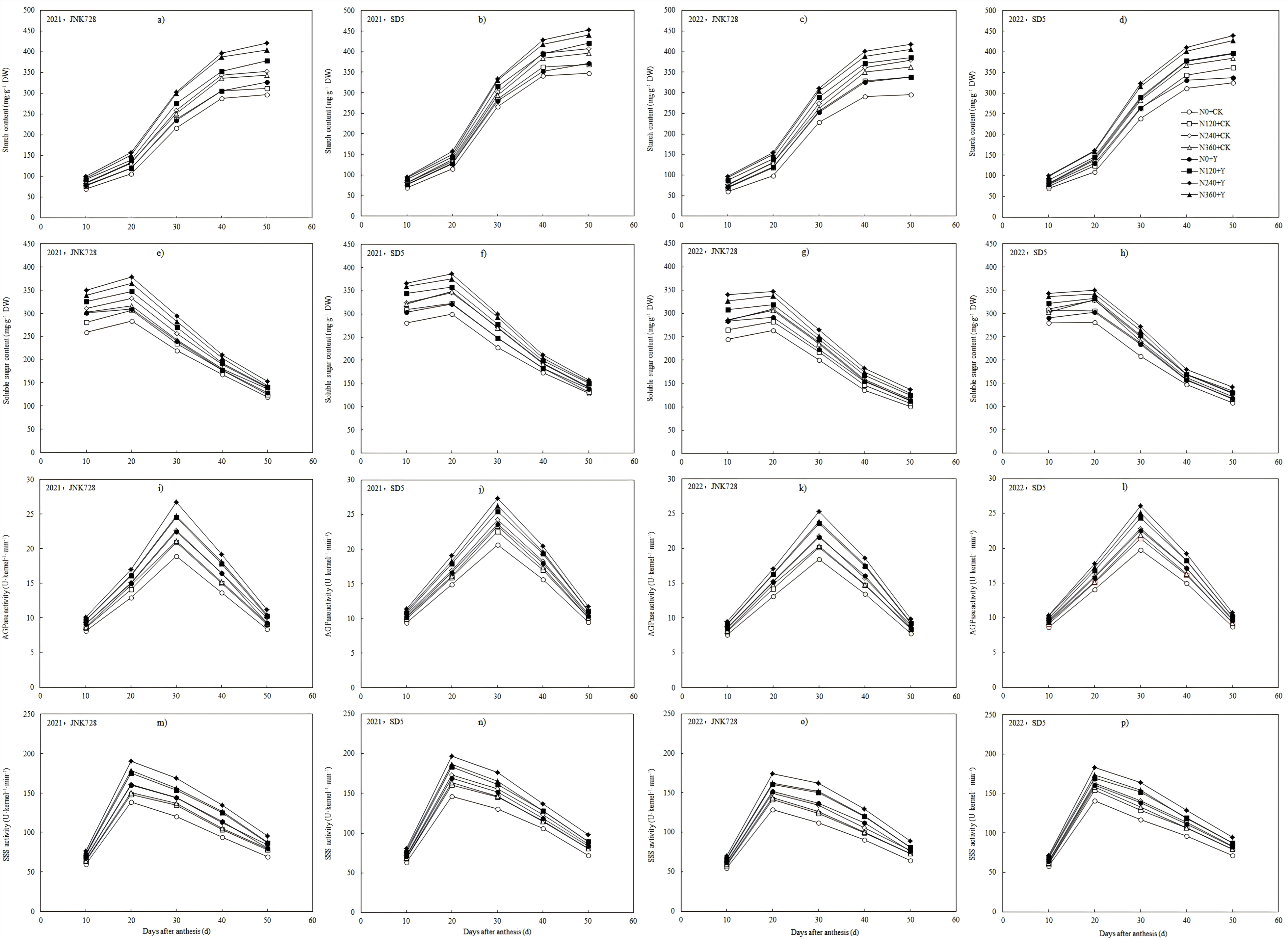
2.3.2. Grain Weight and Starch Content
2.3.3. Soluble Sugar Content in Grain
2.3.4. ADP-Glucose Pyrophosphorylase (AGPase) and Soluble Starch Synthase (SSS) Activities in Grain
2.4. Dry Matter Accumulation
2.4.1. Dry Matter Accumulation per Plant
2.4.2. Dry Matter Distribution in Different Organs at Maturity Stage
2.5. Yield and Greenhouse Gas Emissions
2.5.1. Yield
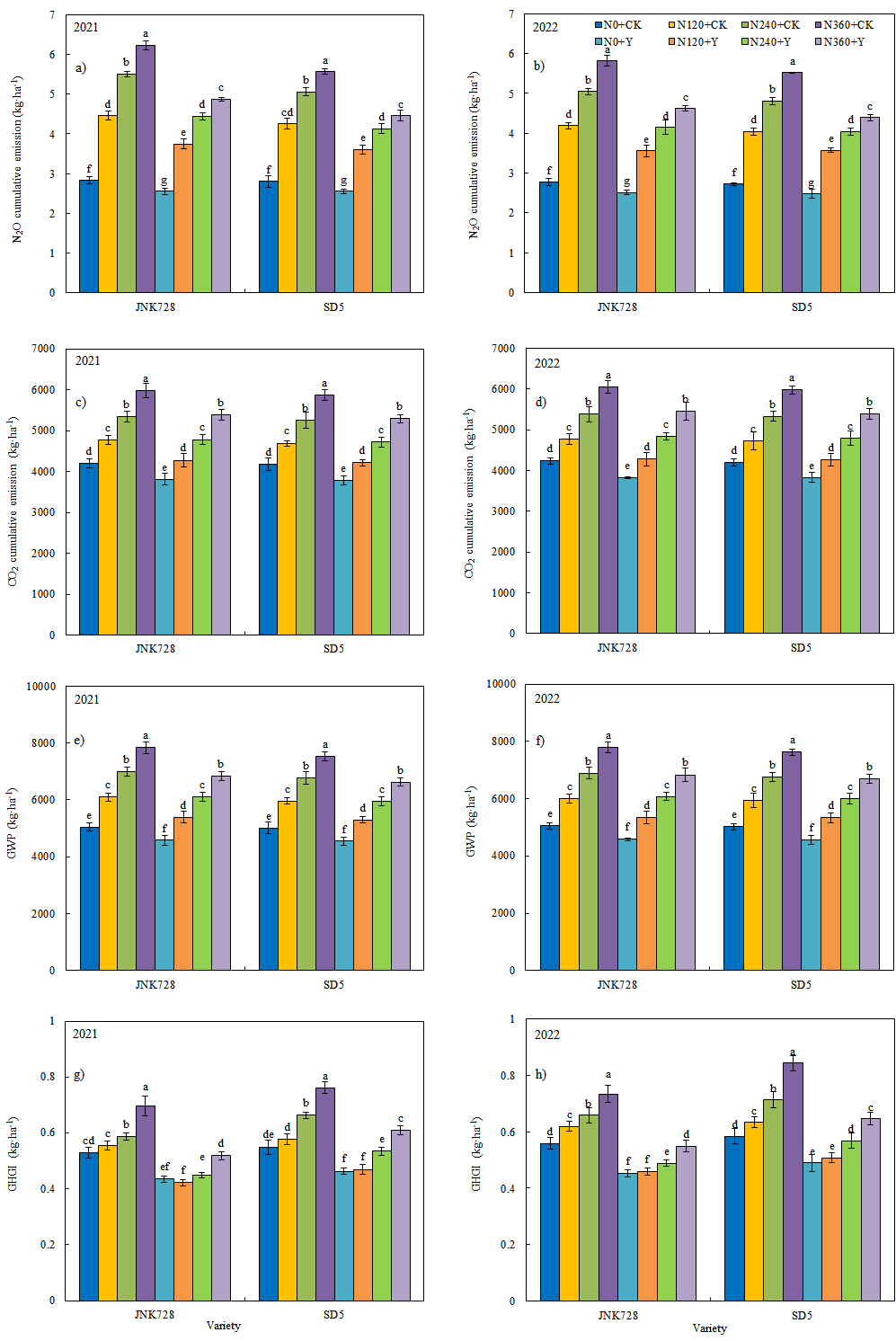
2.5.2. N2O and CO2 Cumulative Emission, Global Warming Potential (GWP), Greenhouse Gas Intensity (GHGI)
3. Discussion
4. Materials and Methods
4.1. Field Sites
4.2. Experimental Design and Field Management
4.3. Sampling and Measurements
4.3.1. Root Morphology and Root Bleeding Sap
4.3.2. Grain Filling, Grain Formation and Yield
4.3.3. Dry Matter Accumulation
4.3.4. Greenhouse Gas Emissions
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGRs | Plant growth regulators |
| DA-6 | Diethyl aminoethyl hexanoate |
| AGPase | ADP-glucose pyrophosphorylase |
| SSS | Soluble starch synthase |
| ADMA | Amount of dry matter per plant after anthesis |
| CPDMA | Contribution proportion of the dry matter after anthesis |
| GWP | Global warming potential |
| GHGI | Greenhouse gas intensity |
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Shen, D.; Wang, K.; Zhou, L.; Fang, L.; Wang, Z.; Fu, J.; Zhang, T.; Liang, Z.; Xie, R.; Ming, B.; et al. Increasing planting density and optimizing irrigation to improve maize yield and water-use efficiency in Northeast China. Agronomy 2024, 14, 400. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Chen, F.; Qin, W.; Yang, H.; Zhao, S.; Xia, J.; Du, X.; Zhu, Y.; Wu, L.; et al. Maize smart-canopy architecture enhances yield at high densities. Nature 2024, 632, 576–584. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, C.; Lv, Y.; Wang, X.; Wang, X.; Zhao, D.; Hu, F.; Wen, X.; Han, J.; Liao, Y. Is it necessary to increase the maize planting density in China? Eur. J. Agron. 2024, 159, 127235. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Wang, G.; Liu, Z.; Sun, W.; Zhang, Y.; Zhang, X. Different responses in root water uptake of summer maize to planting density and nitrogen fertilization. Front. Plant Sci. 2022, 13, 918043. [Google Scholar] [CrossRef]
- Saenz, E.; Ruiz, A.; Sciarresi, C.; King, K.; Baum, M.; Ferela, A.; Danalatos, G.J.N.; Gambin, B.; Kalogeropoulos, G.; Thies, A.; et al. Historical increases in plant density increased vegetative maize biomass while breeding increased reproductive biomass and allocation to ear over stem. Field Crops Res. 2025, 322, 109704. [Google Scholar] [CrossRef]
- Guo, X.; Liu, W.; Yang, Y.; Liu, G.; Ming, B.; Xie, R.; Wang, K.; Li, S.; Hou, P. Optimal nitrogen distribution in maize canopy can synergistically improve maize yield and nitrogen utilization efficiency while reduce environmental risks. Agric. Ecosyst. Environ. 2025, 383, 109540. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Wang, P.; Huang, S. Drip irrigation coupled with appropriate N input increased maize (Zea mays L.) yield and lodging resistance via optimizing root and stem trait. Eur. J. Agron. 2024, 160, 127298. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Li, Y.; Yang, L.; Shi, W.; Liu, Y.; Chang, S.; Hou, F.; Jia, Q. Enhance root-bleeding sap flow and root lodging resistance of maize under a combination of nitrogen strategies and farming practices. Agric. Water Manag. 2019, 224, 105742. [Google Scholar] [CrossRef]
- Fang, H.; Li, Y.; Gu, X.; Chen, P.; Li, Y. Root characteristics, utilization of water and nitrogen, and yield of maize under biodegradable film mulching and nitrogen application. Agric. Water Manag. 2022, 262, 107392. [Google Scholar] [CrossRef]
- Zhao, B.; Tong, L.; Liu, H.; Hao, M.; Zhang, R. Optimizing root morphology is a key to improving maize yield under nitrogen reduction and densification cultivation. Field Crops Res. 2025, 329, 109958. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, Y.; Zhao, M.; Qi, H.; Li, C. Nitrogen supply regulates vascular bundle structure and matter transport characteristics of spring maize under high plant density. Front. Plant Sci. 2021, 11, 602739. [Google Scholar] [CrossRef]
- Swank, J.C.; Below, F.E.; Lambert, R.J.; Hageman, R.H. Interaction of carbon and nitrogen-metabolism in the productivity of maize. Plant Physiol. 1982, 70, 1185–1190. [Google Scholar] [CrossRef]
- Sun, W.; He, Q.; Zhou, G.; Song, Y. Response of grain quality to plant growth dynamics in summer maize as influenced by sowing dates and weather factors. Ind. Crops Prod. 2025, 231, 121210. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, T.; Yu, S.; Zhou, C.; Teng, A.; Lei, L.; Li, F. Optimizing the mulching pattern and nitrogen application rate to improve maize photosynthetic capacity, yield, and nitrogen fertilizer utilization efficiency. Plants 2024, 13, 1241. [Google Scholar] [CrossRef]
- Ren, H.; Xu, S.; Zhang, F.; Sun, M.; Zhang, R. Cultivation and nitrogen management practices effect on soil carbon fractions, greenhouse gas emissions, and maize production under dry-land farming system. Land 2023, 12, 1306. [Google Scholar] [CrossRef]
- Ren, J.; Jiang, Y.; Han, W.; Shi, L.; Zhang, Y.; Liu, G.; Cui, Y.; Du, X.; Gao, Z.; Liang, X. Simultaneous enhancement of maize yield and lodging resistance via delaying plant growth retardant application. Field Crops Res. 2024, 317, 109530. [Google Scholar] [CrossRef]
- Jiang, N.; Zou, T.; Huang, H.; Li, C.; Xia, Y.; Yang, L. Auxin synthesis promotes N metabolism and optimizes root structure enhancing N acquirement in maize (Zea mays L.). Planta 2024, 259, 46. [Google Scholar] [CrossRef]
- Wang, X.; Song, G.; Shah, S.; Ren, H.; Ren, B.; Zhang, J.; Liu, P.; Zhao, B. The potential of EDAH in promoting kernel formation and grain yield in summer maize. Field Crops Res. 2024, 319, 109655. [Google Scholar] [CrossRef]
- Xu, C.; Gao, Y.; Tian, B.; Ren, J.; Meng, Q.; Wang, P. Effects of EDAH, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crops Res. 2017, 200, 71–79. [Google Scholar] [CrossRef]
- Liu, X.; Gu, W.; Li, C.; Li, J.; Wei, S. Effects of nitrogen fertilizer and chemical regulation on spring maize lodging characteristics, grain filling and yield formation under high planting density in Heilongjiang Province, China. J. Integr. Agric. 2021, 20, 511–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Liu, C.; Zhang, M.; Ren, D.; Li, Z.; Zhang, M. Ethephon reduces maize nitrogen uptake but improves nitrogen utilization in Zea mays L. Front. Plant Sci. 2022, 12, 762736. [Google Scholar] [CrossRef]
- Liu, C.; Feng, N.; Zheng, D.; Cui, H.; Sun, F.; Gong, X. Uniconazole and diethyl aminoethyl hexanoate increase soybean pod setting and yield by regulating sucrose and starch content. J. Sci. Food Agric. 2019, 99, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dai, Q.; He, Y.; Yan, L.; Niu, J.; Wang, X.; Xie, Y.; Yu, X.; Tang, W.; Li, H.; et al. Exogenous diethyl aminoethyl hexanoate alleviates the damage caused by low-temperature stress in Phaseolus vulgaris L. seedlings through photosynthetic and antioxidant systems. BMC Plant Biol. 2025, 25, 75. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Evers, J.; Evers, J.; van der Werf, W.; Zhang, W.; Duan, L. Maize yield and quality in response to plant density and application of a novel plant growth regulator. Field Crops Res. 2014, 164, 82–89. [Google Scholar] [CrossRef]
- Sun, N.; Chen, X.; Zhao, H.; Meng, X.; Bian, S. Effects of plant growth regulators and nitrogen management on root lodging resistance and grain yield under high-density maize crops. Agronomy 2022, 12, 2892. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, K.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Effects of integrated agronomic practices management on root growth and development of summer maize. Eur. J. Agron. 2017, 84, 140–151. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Wu, P.; Zhao, X.; Gao, X.; Chen, X. Recovery growth and water use of intercropped maize following wheat harvest in wheat/maize relay strip intercropping. Field Crops Res. 2020, 256, 107924. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Z.; Jiang, H.; Zhang, L. Black film mulching and plant density influencing soil water temperature conditions and maize root growth. Vadose Zone J. 2018, 17, 180104. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, G.; Guo, Q.; Peng, C.; Liu, Y.; Zhang, M.; Li, Z.; Zhou, Y.; Duan, L. Increase in root density induced by coronatine improves maize drought resistance in North China. Crop J. 2023, 11, 278–290. [Google Scholar] [CrossRef]
- Li, R.; Hu, D.; Ren, H.; Yang, Q.; Dong, S.; Zhang, J.; Zhao, B.; Liu, P. How delaying post-silking senescence in lower leaves of maize plants increases carbon and nitrogen accumulation and grain yield. Crop J. 2022, 10, 853–863. [Google Scholar] [CrossRef]
- Yan, S.; Wu, Y.; Fan, J.; Xiang, Y.; Zheng, J.; Guo, J.; Lu, J.; Wu, L.; Qiang, S.; Xiang, Y. Source-sink relationship and yield stability of two maize cultivars in response to water and fertilizer inputs in northwest China. Agric. Water Manag. 2022, 262, 107332. [Google Scholar] [CrossRef]
- Shao, Z.; Zheng, C.; Postma, J.A.; Gao, Q.; Zhang, J. More N fertilizer, more maize, and less alfalfa: Maize benefits from its higher N uptake per unit root length. Front. Plant Sci. 2024, 15, 1338521. [Google Scholar] [CrossRef]
- Gu, L.; Mu, X.; Qi, J.; Tang, B.; Zhen, W.; Xia, L. Nitrogen reduction combined with ETc irrigation maintained summer maize yield and increased water and nitrogen use efficiency. Front. Plant Sci. 2023, 14, 1180734. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, A.; Kageyama, M.; Shinmachi, F.; Schmidhalter, U.; Hasegawa, I. Potential for using plant xylem sap to evaluate inorganic nutrient availability in soil—I. Influence of inorganic nutrients present in the rhizosphere on those in the xylem sap of Luffa cylindrica Roem. Soil Sci. Plant Nutr. 2005, 51, 333–341. [Google Scholar] [CrossRef]
- Guan, D.; Al-Kaisi, M.M.; Zhang, Y.; Duan, L.; Tan, W.; Zhang, M.; Li, Z. Tillage practices affect biomass and grain yield through regulating root growth, root bleeding sap and nutrients uptake in summer maize. Field Crops Res. 2014, 157, 89–97. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, J.; Hong, M.; Ma, M.; Ma, S.; Yuan, Y. Optimizing water and nitrogen supply can regulate the dynamics of dry matter accumulation in maize, thereby promoting dry matter accumulation and increasing yield. Field Crops Res. 2025, 326, 109837. [Google Scholar] [CrossRef]
- Wu, G.; Ling, J.; Liu, Z.; Xu, Y.; Chen, X.; Wen, Y.; Zhou, S. Soil warming and straw return impacts on winter wheat phenology, photosynthesis, root growth, and grain yield in the North China Plain. Field Crops Res. 2022, 283, 108545. [Google Scholar] [CrossRef]
- Zhiipao, R.R.; Pooniya, V.; Kumar, D.; Biswakarma, N.; Bainsla, N.K.; Saikia, N.; Duo, H.; Dorjee, L.; Govindasamy, P.; Lakhena, K.K.; et al. Late-sown stress afflict post-anthesis dry matter and nutrient partitioning and their remobilization in aestivum wheat genotypes. J. Agron. Crop Sci. 2024, 210, e12693. [Google Scholar] [CrossRef]
- Wu, Y.; Bo, Z.; Li, X.; Liu, Q.; Feng, D.; Lan, T.; Kong, F.; Li, Q.; Yuan, J. Nitrogen application affects maize grain filling by regulating grain water relations. J. Integr. Agric. 2022, 21, 977–994. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, C.; Zhang, Y.; Fan, J.; Yan, S.; Zhang, S.; Li, Z.; Fan, J. Nitrogen application and soil mulching improve grain yield of rainfed maize by optimizing source-sink relationship and grain filling process on the Loess Plateau of China. Eur. J. Agron. 2024, 153, 127060. [Google Scholar] [CrossRef]
- Ntanos, D.; Koutroubas, S. Dry matter and N accumulation and translocation for Indica and Japonica rice under Mediterranean conditions. Field Crops Res. 2002, 74, 93–101. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.; Yu, S.; Zhou, C.; Chen, X.; Teng, A.; Lei, L.; Li, F. Modeling spring maize grain filling under film mulching and nitrogen application in a cold and arid environment. Water 2024, 16, 88. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Z.; Song, S.; Zhang, L.; Zhang, Z.; Jia, X. Tillage practices affects the grain filling of inferior kernel of summer maize by regulating soil water content and photosynthetic capacity. Agric. Water Manag. 2021, 245, 106600. [Google Scholar] [CrossRef]
- Li, Q.; Du, L.; Feng, D.; Ren, Y.; Li, Z.; Kong, F.; Yuan, J. Grain-filling characteristics and yield differences of maize cultivars with contrasting nitrogen efficiencies. Crop J. 2021, 8, 990–1001. [Google Scholar] [CrossRef]
- Yu, T.; Xin, Y.; Liu, P. Effects of 6-benzyladenine (6-BA) on the filling process of maize grains placed at different ear positions under high planting density. Plants 2023, 12, 3590. [Google Scholar] [CrossRef]
- Lu, D.; Sun, X.; Yan, F.; Wang, X.; Xu, R.; Lu, W. Effects of high temperature during grain filling under control conditions on the physicochemical properties of waxy maize flour. Carbohydr. Polym. 2013, 98, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Kimura, K.; Arai, Y.; Kawasaki, T.; Shimada, H.; Baba, T. Starch branching enzymes from immature rice seeds. J. Biochem. 1992, 112, 643–651. [Google Scholar] [CrossRef]
- Fontaine, T.; D’ Hulst, C.; Maddelein, M.; Routier, F.; Pepin, T.M.; Decq, A.; Wieruszeski, J.M.; Delrue, B.; Van den Koornhuyse, N.; Bossu, J.P. Toward an understanding of the biogenesis of the starch granule, evidence that Chlamydomonas soluble starch synthase II controls the synthesis of intermediate size glucans of amylopectin. J. Biol. Chem. 1993, 268, 16223–16230. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Li, L.; Xu, X.; Yang, L.; Luo, Z.; Wang, B.; Ma, S.; Fan, Y.; Huang, Z. The effects of short-term exposure to low temperatures during the booting stage on starch synthesis and yields in wheat grain. Front. Plant Sci. 2021, 12, 684784. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, H.; Feng, L.; Chen, M.; Meng, S.; Ye, N.; Zhang, J. Transcriptomic analysis of grain filling in rice inferior grains under moderate soil drying. J. Exp. Bot. 2019, 70, 1597–1611. [Google Scholar] [CrossRef]
- Singh, R.; Juliano, B.O. Free sugars in relation to starch accumulation in developing rice grain. Plant Physiol. 1977, 59, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, C.; Wang, J.; Liu, X.; Zhang, X.; Zhou, J.; Li, X.; Wang, Y.; Dong, G.; Huang, J.; et al. Modified TAL expression in rice plant regulates yield components and grain quality in a N-rate dependent manner. Field Crops Res. 2024, 306, 109219. [Google Scholar] [CrossRef]
- Feng, W.; Xue, W.; Zhao, Z.; Shi, Z.; Wang, W.; Bai, Y.; Wang, H.; Qiu, P.; Xue, J.; Chen, B. Nitrogen fertilizer application rate affects the dynamic metabolism of nitrogen and carbohydrates in kernels of waxy maize. Front. Plant Sci. 2024, 15, 1416397. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Zhou, C.; Xu, K.; Zhu, C.; Ni, C.; Huo, Z.; Dai, Q.; Xu, K. Agronomic and physiological characteristics of high yield and nitrogen use efficient varieties of rice: Comparison between two near-isogenic lines. Food Energy Secur. 2024, 13, e539. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, L.; Cheng, W.; Liu, B.; Li, W.; Wang, F.; Xu, C.; Zhao, X.; Ding, Z.; Zhang, K.; et al. SH1-dependent maize seed development and starch synthesis via modulating carbohydrate flow and osmotic potential balance. BMC Plant Biol. 2020, 20, 264. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, F.; Fan, X.; Fan, J.; Wang, Y.; Zou, H.; Wang, H.; Li, G. Determining irrigation amount and fertilization rate to simultaneously optimize grain yield, grain nitrogen accumulation and economic benefit of drip-fertigated spring maize in northwest China. Agric. Water Manag. 2021, 243, 106440. [Google Scholar] [CrossRef]
- Yu, N.; Alam, S.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Long-term integrated soil-crop system management promoted rhizosphere nitrogen cycling and reduced N2O emission of maize. Field Crops Res. 2024, 319, 109641. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Du, E.; Tang, A.; Zhang, Y.; Wen, Z.; Hao, T.; Pan, Y.; Zhang, L.; Zhao, Y.; et al. Environmental impacts of nitrogen emissions in China and the role of policies in emission reduction. Philos. Trans. R. Soc. A 2020, 378, 20190324. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Z.; Zhang, J.; Gao, Q.; Feng, G.; Abelrahman, A.M.; Chen, Y. Effects of improving nitrogen management on nitrogen utilization, nitrogen balance, and reactive nitrogen losses in a Mollisol with maize monoculture in Northeast China. Environ. Sci. Pollut. Res. 2016, 23, 4576–4584. [Google Scholar] [CrossRef]
- Omonode, R.A.; Halvorson, A.D.; Gagnon, B.; Vyn, T.J. Achieving lower nitrogen balance and higher nitrogen recovery efficiency reduces nitrous oxide emissions in North America’s maize cropping systems. Front. Plant Sci. 2017, 8, 1080. [Google Scholar] [CrossRef]
- Dawar, K.; Sardar, K.; Zaman, M.; Müller, C.; Sanz-Cobena, A.; Khan, A.; Borzouei, A.; Pérez-Castillo, A.G. Effects of the nitrification inhibitor nitrapyrin and the plant growth regulator gibberellic acid on yield-scale nitrous oxide emission in maize fields under hot climatic conditions. Pedosphere 2021, 31, 323–331. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Madramootoo, C.A.; Zhang, T.; Tan, C. Soil nutrients and plant uptake parameters as related to greenhouse gas emissions. J. Environ. Qual. 2022, 51, 1129–1143. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Jia, Z.; Zhang, P. Ridge and furrow rainfall harvesting can significantly reduce N2O emissions from spring maize fields in semiarid regions of China. Soil Tillage Res. 2021, 209, 104971. [Google Scholar] [CrossRef]
- Shao, W.; Wang, H.; Lu, S.; Wang, X.; Huang, J.; Wang, D.; He, C.; Xu, M. Bacterial-mediated nutrient cycling and yield recovery in high-density cassava-maize intercropping systems enhanced by maize straw return. Field Crops Res. 2025, 328, 109915. [Google Scholar] [CrossRef]
- Sun, G.; Meng, Y.; Wang, Y.; Zhao, M.; Wei, S.; Gu, W. Exogenous hemin optimized maize leaf photosynthesis, root development, grain filling, and resource utilization on alleviating cadmium stress under field condition. J. Plant Nutr. Soil Sci. 2022, 22, 631–646. [Google Scholar] [CrossRef]
- Ren, B.; Hu, J.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Spraying exogenous synthetic cytokinin 6-benzyladenine following the waterlogging improves grain growth of waterlogged maize in the field. J. Agron. Crop Sci. 2019, 205, 616–624. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Gao, J.; Ma, D.; He, T.; Hu, S. Effect of subsoiling on the nutritional quality of grains of maize hybrids of different eras. Plants 2024, 13, 1900. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, H.; Zhao, Z.; Zhang, R.; Hu, H.; Zhao, Z.; Yang, D.; Zhu, X.; Guo, W.; Zhu, J.; et al. Effects of elevated ozone concentration on starch and starch synthesis enzymes of Yangmai 16 under fully open-air field conditions. J. Integr. Agric. 2013, 12, 2157–2163. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, C.; van der Werf, W.; Ning, P.; Zhang, Z.; Wan, S.; Zhang, F. Intercropping modulates the accumulation and translocation of dry matter and nitrogen in maize and peanut. Field Crops Res. 2022, 284, 108561. [Google Scholar] [CrossRef]
- Dyer, L.; Oelbermann, M.; Echarte, L. Soil carbon dioxide and nitrous oxide emissions during the growing season from temperate maize-soybean intercrops. J. Plant Nutr. Soil Sci. 2012, 175, 394–400. [Google Scholar] [CrossRef]
- Ahmad, I.; Yan, Z.; Kamran, M.; Ikram, K.; Ghani, M.; Hou, F. Nitrogen management and supplemental irrigation affected greenhouse gas emissions, yield and nutritional quality of fodder maize in an arid region. Agric. Water Manag. 2022, 269, 107650. [Google Scholar] [CrossRef]
- Yang, X.; Lan, Y.; Meng, J.; Chen, W.; Huang, Y.; Cheng, X.; He, T.; Cao, T.; Liu, Z.; Jiang, L.; et al. Effects of maize stover and its derived biochar on greenhouse gases emissions and C-budget of brown earth in Northeast China. Environ. Sci. Pollut. Res. 2017, 24, 8200–8209. [Google Scholar] [CrossRef] [PubMed]



| Treatment | 2021 | 2022 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Jointing Stage | Tasseling Stage | Early Filling Stage | Milk Stage | Maturity Stage | Jointing Stage | Tasseling Stage | Early Filling Stage | Milk Stage | Maturity Stage | |
| Nitrogen application | ||||||||||
| N0 | 2.48 c | 2.55 c | 2.22 c | 1.96 c | 0.92 c | 2.19 c | 2.23 c | 1.97 c | 1.70 c | 0.72 c |
| N120 | 2.79 b | 2.98 b | 2.63 b | 2.23 b | 1.08 b | 2.47 b | 2.64 b | 2.28 b | 1.99 b | 0.80 b |
| N240 | 3.07 a | 3.39 a | 2.98 a | 2.45 a | 1.21 a | 2.81 a | 3.05 a | 2.54 a | 2.23 a | 0.89 a |
| N360 | 2.85 b | 3.07 b | 2.69 b | 2.27 b | 1.11 b | 2.53 b | 2.77 b | 2.35 b | 2.04 b | 0.82 b |
| Chemical regulation | ||||||||||
| CK | 2.66 b | 2.83 b | 2.52 b | 2.21 b | 1.01 b | 2.33 b | 2.51 b | 2.07 b | 1.66 b | 0.73 b |
| PGR | 2.94 a | 3.17 a | 2.74 a | 2.34 a | 1.15 a | 2.67 a | 2.83 a | 2.35 a | 1.92 a | 0.89 a |
| Variety | ||||||||||
| JNK728 | 2.89 a | 3.08 a | 2.70 a | 2.30 a | 1.11 a | 2.63 a | 2.77 a | 2.29 a | 1.86 a | 0.84 a |
| SD5 | 2.71 b | 2.92 b | 2.56 b | 2.16 b | 1.05 b | 2.37 b | 2.57 b | 2.13 b | 1.72 b | 0.78 b |
| Sources of variation | ||||||||||
| V | ** | * | * | ** | ** | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | * | * | * | NS | NS | * | * | * | NS |
| V × C | * | NS | * | * | * | * | * | NS | * | * |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Treatment | Mineral Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Cu | Zn | Ca | Mg | K | P | B | Si | |
| Jointing stage | ||||||||||
| Nitrogen application | ||||||||||
| N0 | 1.16 c | 4.62 c | 0.348 c | 11.27 c | 321.7 c | 302.2 c | 1788 c | 116.6 c | 1.19 c | 49.91 c |
| N120 | 1.31 b | 5.04 b | 0.382 b | 12.14 b | 348.4 b | 320.1 b | 1947 b | 128.3 b | 1.32 b | 53.53 b |
| N240 | 1.54 a | 5.46 a | 0.419 a | 12.91 a | 376.9 a | 339.3 a | 2109 a | 139.7 a | 1.44 a | 57.32 a |
| N360 | 1.47 a | 5.40 a | 0.410 a | 12.73 a | 368.4 a | 322.4 b | 2022 a | 133.3 a | 1.33 b | 56.03 a |
| Chemical regulation | ||||||||||
| CK | 1.25 b | 4.60 b | 0.367 b | 11.60 b | 334.8 b | 302.5 b | 1856 b | 119.3 b | 1.24 b | 51.05 b |
| PGR | 1.49 a | 5.67 a | 0.412 a | 12.93 a | 372.9 a | 339.4 a | 2077 a | 139.6 a | 1.40 a | 57.34 a |
| Variety | ||||||||||
| JNK728 | 1.41 a | 5.29 a | 0.399 a | 12.66 a | 363.9 a | 329.6 a | 2029 a | 133.3 a | 1.36 a | 55.59 a |
| SD5 | 1.33 b | 4.98 b | 0.380 b | 11.87 b | 343.8 b | 312.4 b | 1904 b | 125.6 b | 1.28 b | 52.80 b |
| Sources of variation | ||||||||||
| V | ** | ** | * | ** | ** | ** | ** | ** | ** | * |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | * | NS | NS | * | NS | NS | * | * | NS |
| V × C | NS | NS | * | * | NS | * | ** | * | NS | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Tasseling stage | ||||||||||
| Nitrogen application | ||||||||||
| N0 | 0.627 c | 4.43 c | 0.502 c | 9.79 c | 308.6 c | 291.8 c | 1608 c | 105.4 c | 1.08 c | 45.83 c |
| N120 | 0.755 b | 4.86 b | 0.545 b | 10.57 b | 332.17 b | 312.7 b | 1727 b | 118.0 b | 1.23 b | 49.19 b |
| N240 | 0.880 a | 5.32 a | 0.595 a | 11.32 a | 351.46 a | 330.4 a | 1847 a | 130.8 a | 1.35 a | 52.72 a |
| N360 | 0.875 a | 5.32 a | 0.590 a | 11.33 a | 330.75 b | 320.0 ab | 1805 ab | 122.0 b | 1.26 b | 52.81 a |
| Chemical regulation | ||||||||||
| CK | 0.725 b | 4.71 b | 0.519 b | 10.02 b | 308.9 b | 299.1 b | 1647 b | 110.9 b | 1.15 b | 46.88 b |
| PGR | 0.844 a | 5.25 a | 0.597 a | 11.48 a | 352.6 a | 328.3 a | 1846 a | 127.2 a | 1.30 a | 53.39 a |
| Variety | ||||||||||
| JNK728 | 0.805 a | 5.14 a | 0.573 a | 11.04 a | 340.4 a | 322.3 a | 1795 a | 122.3 a | 1.26 a | 51.75 a |
| SD5 | 0.764 b | 4.82 b | 0.543 b | 10.46 b | 321.1 b | 305.2 b | 1698 b | 115.7 b | 1.20 b | 48.52 b |
| Sources of variation | ||||||||||
| V | * | ** | * | * | ** | * | * | * | * | ** |
| N | ** | ** | ** | * | * | * | * | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | * | * | * | NS | * | * | NS | * | * | NS |
| V × C | NS | * | NS | * | NS | NS | * | NS | NS | * |
| N × C | NS | NS | NS | NS | NS | * | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Milk stage | ||||||||||
| Nitrogen application | ||||||||||
| N0 | 0.501 c | 1.66 c | 0.149 c | 3.94 c | 130.5 c | 31.37 d | 534.1 d | 47.9 c | 0.275 c | 18.25 c |
| N120 | 0.575 b | 1.89 b | 0.163 b | 4.34 b | 147.0 b | 37.76 c | 612.8 c | 54.8 b | 0.310 b | 20.74 b |
| N240 | 0.660 a | 2.27 a | 0.181 a | 4.69 a | 163.3 a | 47.14 a | 698.7 a | 61.2 a | 0.335 a | 23.47 a |
| N360 | 0.655 a | 2.21 a | 0.172 ab | 4.73 a | 157.5 a | 44.63 b | 652.0 b | 56.5 b | 0.285 c | 23.34 a |
| Chemical regulation | ||||||||||
| CK | 0.528 b | 1.77 b | 0.149 b | 4.13 b | 140.7 b | 35.03 b | 580.5 b | 51.7 b | 0.272 b | 19.44 b |
| PGR | 0.668 a | 2.24 a | 0.183 a | 4.71 a | 158.5 a | 45.42 a | 668.4 a | 58.5 a | 0.331 a | 23.46 a |
| Variety | ||||||||||
| JNK728 | 0.613 a | 2.07 a | 0. 171 a | 4.57 a | 153.3 a | 41.33 a | 640.6 a | 56.7 a | 0.309 a | 22.17 a |
| SD5 | 0.583 b | 1.94 b | 0. 162 b | 4.28 b | 145.8 b | 39.12 b | 608.3 b | 53.4 b | 0.293 b | 20.73 b |
| Sources of variation | ||||||||||
| V | * | ** | * | ** | * | * | * | ** | * | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | * | * | * | NS | NS | * | * | * | NS | * |
| V × C | NS | NS | NS | NS | * | * | * | * | NS | NS |
| N × C | NS | NS | * | NS | NS | NS | * | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | * | NS | NS | NS |
| Treatment | Mineral Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Cu | Zn | Ca | Mg | K | P | B | Si | |
| Jointing stage | ||||||||||
| Nitrogen application | ||||||||||
| N0 | 1.45 c | 3.75 c | 0.291 c | 9.54 c | 284.5 c | 256.5 c | 1564 c | 97.5 c | 0.984 c | 43.77 c |
| N120 | 1.68 b | 4.16 b | 0.322 b | 10.51 b | 310.6 b | 279.4 b | 1715 b | 108.9 b | 1.075 b | 47.41 b |
| N240 | 1.82 a | 4.60 a | 0.355 a | 11.53 a | 340.2 a | 302.7 a | 1861 a | 119.4 a | 1.160 a | 51.14 a |
| N360 | 1.79 a | 4.68 a | 0.345 a | 11.24 a | 329.7 a | 281.6 b | 1794 ab | 114.6 a | 1.055 b | 49.93 ab |
| Chemical regulation | ||||||||||
| CK | 1.56 b | 3.82 b | 0.310 b | 10.01 b | 295.5 b | 261.9 b | 1621 b | 102.3 b | 0.995 b | 44.66 b |
| PGR | 1.81 a | 4.77 a | 0.346 a | 11.39 a | 337.0 a | 298.2 a | 1846 a | 118.0 a | 1.142 a | 51.46 a |
| Variety | ||||||||||
| JNK728 | 1.74 a | 4.41 a | 0.338 a | 10.97 a | 324.7 a | 289.3 a | 1780 a | 113.6 a | 1.095 a | 49.64 a |
| SD5 | 1.63 b | 4.18 b | 0.318 b | 10.43 b | 307.8 b | 270.8 b | 1687 b | 106.7 b | 1.042 b | 46.48 b |
| Sources of variation | ||||||||||
| V | ** | * | ** | * | ** | ** | ** | ** | * | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | * | * | NS | * | NS | NS | * | * | NS |
| V × C | * | * | NS | * | NS | * | * | * | NS | * |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Tasseling stage | ||||||||||
| Nitrogen application | ||||||||||
| N0 | 0.413 c | 3.75 c | 0.399 c | 8.42 c | 274.5 c | 260.5 c | 1399 c | 96.5 c | 1.032 c | 40.88 c |
| N120 | 0.505 b | 4.17 b | 0.441 b | 9.12 b | 291.7 b | 280.0 b | 1517 b | 104.6 b | 1.045 b | 43.54 b |
| N240 | 0.646 a | 4.63 a | 0.490 a | 9.75 a | 311.9 a | 297.8 a | 1637 a | 113.9 a | 1.140 a | 46.18 a |
| N360 | 0.634 a | 4.64 a | 0.485 a | 9.66 a | 297.5 ab | 286.9 ab | 1594 a | 106.7 b | 1.075 b | 45.25 ab |
| Chemical regulation | ||||||||||
| CK | 0.513 b | 4.01 b | 0.415 b | 8.65 b | 278.2 b | 267.3 b | 1446 b | 99.6 b | 1.002 b | 41.85 b |
| PGR | 0.586 a | 4.59 a | 0.492 a | 9.82 a | 309.6 a | 295.4 a | 1628 a | 111.3 a | 1.144 a | 46.07 a |
| Variety | ||||||||||
| JNK728 | 0.564 a | 4.41 a | 0.466 a | 9.51 a | 302.4 a | 289.3 a | 1578 a | 108.3 a | 1.105 a | 45.29 a |
| SD5 | 0.535 b | 4.18 b | 0.441 b | 8.96 b | 285.4 b | 273.4 b | 1496 b | 102.6 b | 1.041 b | 42.63 b |
| Sources of variation | ||||||||||
| V | ** | * | ** | * | ** | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** | * |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | * | * | NS | * | * | * | NS | * | * | NS |
| V × C | NS | * | * | NS | NS | * | * | * | NS | * |
| N × C | NS | NS | * | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Milk stage | ||||||||||
| Nitrogen application | ||||||||||
| N0 | 0.331 c | 1.07 c | 0.110 d | 2.64 c | 103.4 c | 16.32 c | 425.6 c | 36.39 c | 0.181 d | 13.34 c |
| N120 | 0.390 b | 1.29 b | 0.126 c | 3.03 b | 115.4 b | 22.24 b | 487.2 b | 41.94 b | 0.213 c | 14.83 b |
| N240 | 0.445 a | 1.64 a | 0.143 a | 3.58 a | 128.8 a | 30.10 a | 552.9 a | 47.73 a | 0.245 a | 16.21 a |
| N360 | 0.405 b | 1.69 a | 0.134 b | 3.56 a | 124.2 a | 28.76 a | 527.7 a | 45.60 a | 0.230 b | 15.73 a |
| Chemical regulation | ||||||||||
| CK | 0.350 b | 1.26 b | 0.118 b | 2.97 b | 110.4 b | 21.13 b | 463.8 b | 40.50 b | 0.198 b | 13.34 b |
| PGR | 0.436 a | 1.58 a | 0.138 a | 3.43 a | 125.5 a | 27.58 a | 532.9 a | 45.33 a | 0.236 a | 16.71 a |
| Variety | ||||||||||
| JNK728 | 0.405 a | 1.47 a | 0.132 a | 3.29 a | 121.5 a | 25.06 a | 511.8 a | 44.05 a | 0.225 a | 15.42 a |
| SD5 | 0.381 b | 1.37 b | 0.124 b | 3.11 b | 114.4 b | 23.65 b | 484.8 b | 41.78 b | 0.210 b | 14.63 b |
| Sources of variation | ||||||||||
| V | ** | ** | ** | ** | ** | ** | ** | * | ** | * |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | * | * | * | NS | NS | * | * | * | NS | * |
| V × C | NS | * | NS | * | * | * | NS | * | * | NS |
| N × C | NS | NS | NS | NS | NS | NS | * | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Treatment | Ser | Glu | Gly | Ala | Val | Lys | Met | Arg | Leu |
|---|---|---|---|---|---|---|---|---|---|
| Jointing stage | |||||||||
| Nitrogen application | |||||||||
| N0 | 475.0 c | 293.6 c | 1.34 c | 14.37 c | 57.78 c | 97.57 c | 5.40 c | 86.09 c | 17.11 c |
| N120 | 514.5 b | 319.6 b | 1.46 b | 15.41 b | 63.60 b | 103.36 b | 5.82 b | 93.99 b | 19.36 b |
| N240 | 556.6 a | 346.3 a | 1.59 a | 16.50 a | 69.24 a | 109.58 a | 6.19 a | 101.88 a | 22.65 a |
| N360 | 544.1 a | 332.0 ab | 1.58 a | 16.13 ab | 66.06 ab | 104.10 b | 6.10 ab | 100.68 a | 21.71 a |
| Chemical regulation | |||||||||
| CK | 496.2 b | 298.8 b | 1.39 b | 14.66 b | 60.72 b | 97.88 b | 5.53 b | 90.45 b | 18.92 b |
| PGR | 548.9 a | 347.0 a | 1.60 a | 16.54 a | 67.62 a | 109.42 a | 6.23 a | 100.87 a | 21.49 a |
| Variety | |||||||||
| JNK728 | 543.9 a | 334.4 a | 1.54 a | 16.09 a | 66.29 a | 107.55 a | 6.06 a | 99.29 a | 20.91 a |
| SD5 | 501.2 b | 311.4 b | 1.45 b | 15.11 b | 62.05 b | 99.75 b | 5.70 b | 92.03 b | 19.50 b |
| Sources of variation | |||||||||
| V | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | * | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × C | NS | NS | NS | NS | NS | * | NS | NS | NS |
| N × C | NS | * | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Tasseling stage | |||||||||
| Nitrogen application | |||||||||
| N0 | 368.8 c | 232.1 c | 1.13 c | 10.44 c | 51.10 c | 73.96 c | 4.24 c | 75.10 c | 14.72 c |
| N120 | 405.4 b | 248.7 b | 1.21 b | 12.57 b | 55.01 b | 81.14 b | 4.75 b | 80.66 b | 15.89 b |
| N240 | 444.7 a | 262.7 a | 1.30 a | 14.66 a | 58.21 a | 88.73 a | 5.26 a | 86.27 a | 17.01 a |
| N360 | 434.6 a | 254.5 ab | 1.30 a | 14.57 a | 54.78 b | 88.57 a | 4.91 b | 84.29 ab | 17.03 a |
| Chemical regulation | |||||||||
| CK | 390.12 b | 233.6 b | 1.16 b | 12.28 b | 51.97 b | 78.45 b | 4.49 b | 77.39 b | 15.34 b |
| PGR | 436.63 a | 265.3 a | 1.31 a | 13.84 a | 57.58 a | 87.75 a | 5.09 a | 85.77 a | 16.98 a |
| Variety | |||||||||
| JNK728 | 427.4 a | 257.3 a | 1.27 a | 13.51 a | 56.36 a | 85.44 a | 4.93 a | 84.22 a | 16.64 a |
| SD5 | 399.4 b | 241.7 b | 1.20 b | 12.62 b | 53.19 b | 80.76 b | 4.65 b | 78.94 b | 15.68 b |
| Sources of variation | |||||||||
| V | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × C | NS | NS | NS | * | NS | NS | NS | NS | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Milk stage | |||||||||
| Nitrogen application | |||||||||
| N0 | 138.0 d | 118.5 c | 0.596 c | 6.39 d | 26.18 c | 44.87 c | 1.94 c | 31.32 c | 4.28 c |
| N120 | 166.1 c | 133.6 b | 0.672 b | 7.33 c | 28.78 b | 49.12 b | 2.23 b | 35.60 b | 4.85 b |
| N240 | 207.3 a | 144.4 a | 0.746 a | 8.36 a | 31.13 a | 54.39 a | 2.55 a | 40.28 a | 5.83 a |
| N360 | 196.3 b | 122.8 c | 0.720 a | 7.80 b | 31.36 a | 51.83 a | 2.53 a | 40.06 a | 5.68 a |
| Chemical regulation | |||||||||
| CK | 164.2 b | 119.3 b | 0.636 b | 6.90 b | 27.15 b | 46.36 b | 2.12 b | 33.86 b | 4.74 b |
| PGR | 189.6 a | 140.3 a | 0.731 a | 8.04 a | 31.58 a | 53.75 a | 2.51 a | 39.77 a | 5.58 a |
| Variety | |||||||||
| JNK728 | 183.6 a | 133.3 a | 0.707 a | 7.71 a | 30.36 a | 51.44 a | 2.84 a | 38.02 a | 5.33 a |
| SD5 | 170.5 b | 126.4 b | 0.660 b | 7.24 b | 28.37 b | 48.67 b | 1.79 b | 35.61 b | 4.99 b |
| Sources of variation | |||||||||
| V | ** | * | ** | ** | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Treatment | Ser | Glu | Gly | Ala | Val | Lys | Met | Arg | Leu |
|---|---|---|---|---|---|---|---|---|---|
| Jointing stage | |||||||||
| Nitrogen application | |||||||||
| N0 | 399.0 d | 292.6 c | 1.25 c | 12.89 d | 55.13 c | 89.96 c | 4.66 c | 81.42 c | 15.92 c |
| N120 | 480.2 c | 329.8 b | 1.41 b | 14.79 c | 60.59 b | 98.48 b | 5.35 b | 93.03 b | 18.04 b |
| N240 | 599 a | 356.4 a | 1.57 a | 16.86 a | 65.56 a | 109.1 a | 6.14 a | 103.84 a | 21.67 a |
| N360 | 567.5 b | 303.2 c | 1.51 a | 15.73 b | 66.05 a | 103.9 a | 6.09 a | 95.86 b | 21.15 a |
| Chemical regulation | |||||||||
| CK | 486.5 b | 301.7 b | 1.36 b | 14.09 b | 58.58 b | 94.95 b | 5.12 b | 87.61 b | 17.81 b |
| PGR | 536.6 a | 339.3 a | 1.51 a | 16.04 a | 65.08 a | 105.75 a | 5.99 a | 99.47 a | 20.58 a |
| Variety | |||||||||
| JNK728 | 524 a | 331.3 a | 1.48 a | 15.51 a | 63.56 a | 103.44 a | 5.73 a | 96.22 a | 19.84 a |
| SD5 | 498.7 b | 309.7 b | 1.39 b | 14.63 b | 60.10 b | 97.26 b | 5.38 b | 90.86 b | 18.55 b |
| Sources of variation | |||||||||
| V | * | ** | ** | ** | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × C | * | NS | NS | NS | NS | NS | NS | NS | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Tasseling stage | |||||||||
| Nitrogen application | |||||||||
| N0 | 368.8 c | 216.7 c | 1.03 c | 11.62 c | 49.99 c | 69.28 c | 3.42 c | 71.61 c | 14.39 c |
| N120 | 396.5 b | 234.8 b | 1.14 b | 12.61 b | 50.63 b | 77.03 b | 4.19 b | 76.27 b | 15.30 b |
| N240 | 421.6 a | 251.0 a | 1.25 a | 13.73 a | 55.23 a | 85.44 a | 5.35 a | 80.88 a | 16.36 a |
| N360 | 406.3 ab | 248.5 a | 1.22 a | 12.85 b | 52.08 b | 85.62 a | 5.27 a | 79.26 ab | 15.61 ab |
| Chemical regulation | |||||||||
| CK | 376.9 b | 224.2 b | 1.11 b | 11.96 b | 48.89 b | 74.94 b | 4.32 b | 73.54 b | 14.65 b |
| PGR | 419.6 a | 251.3 a | 1.21 a | 13.44 a | 55.08 a | 83.75 a | 4.79 a | 80.47 a | 16.18 a |
| Variety | |||||||||
| JNK728 | 408.4 a | 244.3 a | 1.20 a | 13.11 a | 53.56 a | 81.44 a | 4.69 a | 79.02 a | 15.84 a |
| SD5 | 388.2 b | 231.2 b | 1.12 b | 12.30 b | 50.41 b | 77.25 b | 4.42 b | 74.99 b | 14.99 b |
| Sources of variation | |||||||||
| V | * | ** | ** | ** | ** | * | ** | * | ** |
| N | ** | ** | ** | ** | ** | ** | ** | * | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Milk stage | |||||||||
| Nitrogen application | |||||||||
| N0 | 114.5 c | 111.8 c | 0.579 c | 5.82 c | 23.07 d | 36.03 c | 1.78 c | 28.33 c | 4.34 d |
| N120 | 156.0 b | 124.2 b | 0.646 b | 6.68 b | 26.30 c | 43.23 b | 2.09 b | 32.65 b | 5.10 c |
| N240 | 211.1 a | 135.8 a | 0.721 a | 7.88 a | 29.96 a | 55.17 a | 2.39 a | 37.16 a | 5.87 a |
| N360 | 201.7 a | 131.7 a | 0.695 a | 7.84 a | 28.08 b | 56.68 a | 2.17 b | 35.50 a | 5.51 b |
| Chemical regulation | |||||||||
| CK | 154.0 b | 116.4 b | 0.599 b | 6.67 b | 23.62 b | 43.80 b | 1.92 b | 31.35 b | 4.73 b |
| PGR | 187.6 a | 135.3 a | 0.721 a | 7.44 a | 30.08 a | 51.75 a | 2.29 a | 35.47 a | 5.68 a |
| Variety | |||||||||
| JNK728 | 176.4 a | 130.3 a | 0.677 a | 7.28 a | 27.56 a | 49.14 a | 2.17 a | 34.32 a | 5.39 a |
| SD5 | 165.3 | 121.5 | 0.643 | 6.84 | 26.14 | 46.41 | 2.04 | 32.50 | 5.02 |
| Sources of variation | |||||||||
| V | ** | ** | * | ** | * | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| C | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| V × N | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Treatment | 2021 | 2022 | ||||||
|---|---|---|---|---|---|---|---|---|
| Tmax (d) | Vmax (g 100-grain−1 d−1) | Vm (g 100-grain−1 d−1) | P (d) | Tmax (d) | Vmax (g 100-grain−1 d−1) | Vm (g 100-grain−1 d−1) | P (d) | |
| Nitrogen application | ||||||||
| N0 | 28.64 a | 1.032 c | 0.487 c | 43.63 b | 28.37 a | 1.004 c | 0.471 c | 43.85 a |
| N120 | 29.33 a | 1.097 b | 0.519 b | 45.58 ab | 28.86 a | 1.065 b | 0.499 b | 45.18 a |
| N240 | 29.69 a | 1.165 a | 0.554 a | 46.95 a | 29.23 a | 1.129 a | 0.530 a | 45.92 a |
| N360 | 29.51 a | 1.151 ab | 0.545 a | 46.07 a | 29.02 a | 1.112 ab | 0.522 ab | 45.56 a |
| Chemical regulation | ||||||||
| CK | 29.75 a | 1.083 b | 0.511 b | 45.44 a | 29.29 a | 1.049 b | 0.492 b | 45.35 a |
| PGR | 28.84 a | 1.14 a | 0.542 a | 45.68 a | 28.45 a | 1.106 a | 0.519 a | 44.91 a |
| Variety | ||||||||
| JNK728 | 30.42 a | 1.081 b | 0.512 b | 46.32 a | 29.75 a | 1.049 b | 0.493 b | 45.74 a |
| SD5 | 28.17 b | 1.142 a | 0.541 a | 44.80 a | 27.99 b | 1.106 a | 0.518 a | 44.52 a |
| Sources of variation | ||||||||
| V | ** | ** | ** | NS | ** | ** | ** | NS |
| N | NS | ** | ** | * | NS | ** | ** | NS |
| C | NS | ** | ** | NS | NS | ** | ** | NS |
| V × N | NS | NS | * | NS | NS | NS | NS | NS |
| V × C | NS | * | NS | NS | NS | NS | * | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS | NS |
| Treatment | Dry Matter Accumulation per Plant (g plant−1) | ADMA | CPDMA | ||||
|---|---|---|---|---|---|---|---|
| Jointing Stage | Tasseling Stage | Early Filling Stage | Milk Stage | Maturity Stage | (g plant−1) | (%) | |
| Nitrogen application | |||||||
| N0 | 38.6 c | 143.6 c | 186.6 c | 279.0 c | 285.7 c | 142.1 d | 49.7 b |
| N120 | 43.9 b | 155.9 b | 202.0 b | 306.2 b | 330.9 b | 175.0 c | 52.9 a |
| N240 | 48.2 a | 165.6 a | 218.9 a | 330.3 a | 362.8 a | 197.1 a | 54.3 a |
| N360 | 49.7 a | 165.1 a | 217.2 a | 320.6 ab | 350.4 a | 185.3 b | 52.9 a |
| Chemical regulation | |||||||
| CK | 47.0 a | 161.3 a | 208.3 a | 300.3 b | 320.1 b | 158.8 b | 49.6 b |
| PGR | 43.1 b | 151.8 b | 204.0 a | 317.8 a | 344.7 a | 193.0 a | 56.0 a |
| Variety | |||||||
| JNK728 | 45.7 a | 159.4 a | 211.6 a | 318.3 a | 342.6 a | 183.2 a | 53.5 a |
| SD5 | 44.5 a | 153.7 a | 200.6 b | 299.8 b | 322.3 b | 168.6 b | 52.3 a |
| Sources of variation | |||||||
| V | NS | NS | * | ** | ** | ** | NS |
| N | ** | ** | ** | ** | ** | ** | * |
| C | ** | * | NS | * | ** | ** | ** |
| V × N | NS | NS | * | * | NS | NS | NS |
| V × C | NS | NS | * | NS | * | * | NS |
| N × C | NS | NS | NS | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS |
| Treatment | Dry Matter Accumulation per Plant (g plant−1) | ADMA | CPDMA | ||||
|---|---|---|---|---|---|---|---|
| Jointing Stage | Tasseling Stage | Early Filling Stage | Milk Stage | Maturity Stage | (g plant−1) | (%) | |
| Nitrogen application | |||||||
| N0 | 35.1 c | 132.5 c | 174.1 c | 263.5 c | 271.0 c | 138.5 c | 51.1 b |
| N120 | 39.0 b | 139.4 b | 185.7 b | 281.6 b | 303.0 b | 163.5 b | 54.0 a |
| N240 | 42.7 a | 148.1 a | 197.3 a | 299.5 a | 330.6 a | 182.5 a | 55.2 a |
| N360 | 44.3 a | 150.4 a | 196.9 a | 297.3 a | 329.1 a | 178.7 a | 54.3 a |
| Chemical regulation | |||||||
| CK | 46.4 a | 161.9 a | 210.3 a | 277.2 b | 297.0 b | 135.2 b | 45.5 b |
| PGR | 42.1 b | 151.5 b | 204.0 a | 293.8 a | 319.8 a | 168.2 a | 52.6 a |
| Variety | |||||||
| JNK728 | 44.8 a | 159.9 a | 213.0 a | 325.6 a | 320.1 a | 160.1 a | 50.0 a |
| SD5 | 43.7 a | 153.5 a | 201.3 b | 305.1 b | 296.7 b | 143.3 b | 48.3 a |
| Sources of variation | |||||||
| V | NS | NS | ** | ** | ** | ** | NS |
| N | ** | ** | ** | ** | ** | ** | * |
| C | ** | ** | NS | ** | ** | ** | ** |
| V × N | NS | NS | NS | * | NS | NS | NS |
| V × C | NS | NS | * | NS | * | * | NS |
| N × C | NS | NS | * | NS | NS | NS | NS |
| V × N × C | NS | NS | NS | NS | NS | NS | NS |
| Treatment | 2021 | 2022 | ||||||
|---|---|---|---|---|---|---|---|---|
| Yield (kg ha−1) | Ears Number (ears ha−1) | Grains Number per Ear | 1000-Grain Weight (g) | Yield (kg ha−1) | Ears Number (ears ha−1) | Grains Number per Ear | 1000-Grain Weight (g) | |
| Nitrogen application | ||||||||
| N0 | 9748 c | 81,162 a | 415 c | 358 b | 9093 c | 81,161 a | 395 b | 331 b |
| N120 | 11,350 b | 81,280 a | 442 b | 366 ab | 10,129 b | 81,388 a | 427 a | 347 a |
| N240 | 11,724 a | 81,386 a | 457 a | 376 a | 10,560 a | 81,413 a | 433 a | 355 a |
| N360 | 11,305 b | 81,468 a | 461 a | 372 ab | 10,421 a | 81,444 a | 428 a | 351 a |
| Chemical regulation | ||||||||
| CK | 10,409 b | 81,213 a | 425 b | 359 b | 9537 b | 81,334 a | 400 b | 339 b |
| PGR | 11,654 a | 81,434 a | 463 a | 377 a | 10,565 a | 81,368 a | 441 a | 353 a |
| Variety | ||||||||
| JNK728 | 11,729 a | 81,760 a | 511 a | 362 a | 10,618 a | 81,418 a | 479 a | 342 a |
| SD5 | 10,334 b | 80,889 a | 376 b | 374 a | 9484 b | 81,285 a | 362 b | 351 a |
| Sources of variation | ||||||||
| V | ** | NS | ** | NS | ** | NS | ** | NS |
| N | ** | NS | ** | * | ** | NS | ** | ** |
| C | ** | NS | ** | ** | ** | NS | ** | ** |
| V × N | * | NS | NS | NS | ** | NS | NS | NS |
| V × C | * | NS | NS | NS | * | NS | NS | NS |
| N × C | NS | NS | NS | NS | * | NS | NS | NS |
| V × N × C | NS | NS | NS | * | NS | NS | NS | NS |
| Year | Month | Average Temperature (°C) | Total Rainfall (mm) | Average Wind Velocity (m·s−1) |
|---|---|---|---|---|
| 2021 | May | 16.08 | 80.77 | 6.01 |
| June | 20.57 | 79.50 | 5.08 | |
| July | 25.96 | 167.89 | 4.55 | |
| August | 20.96 | 146.81 | 5.57 | |
| September | 16.20 | 63.75 | 4.12 | |
| 2022 | May | 14.97 | 65.02 | 6.21 |
| June | 20.84 | 128.02 | 5.44 | |
| July | 24.63 | 59.44 | 4.67 | |
| August | 20.96 | 187.71 | 4.55 | |
| September | 16.72 | 4.57 | 5.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Meng, Y.; Xie, L.; Hao, Y.; Yu, Y.; Lv, G.; Jiang, Y.; Zhang, Y.; Qian, C.; Gu, W. Boosting Maize Yield and Mitigating Greenhouse Gas Emissions Through Synergistic Nitrogen and Chemical Regulation by Optimizing Roots and Developing Grains Under High-Density Planting in Northeast China. Plants 2025, 14, 3193. https://doi.org/10.3390/plants14203193
Liu X, Meng Y, Xie L, Hao Y, Yu Y, Lv G, Jiang Y, Zhang Y, Qian C, Gu W. Boosting Maize Yield and Mitigating Greenhouse Gas Emissions Through Synergistic Nitrogen and Chemical Regulation by Optimizing Roots and Developing Grains Under High-Density Planting in Northeast China. Plants. 2025; 14(20):3193. https://doi.org/10.3390/plants14203193
Chicago/Turabian StyleLiu, Xiaoming, Yao Meng, Lihua Xie, Yubo Hao, Yang Yu, Guoyi Lv, Yubo Jiang, Yiteng Zhang, Chunrong Qian, and Wanrong Gu. 2025. "Boosting Maize Yield and Mitigating Greenhouse Gas Emissions Through Synergistic Nitrogen and Chemical Regulation by Optimizing Roots and Developing Grains Under High-Density Planting in Northeast China" Plants 14, no. 20: 3193. https://doi.org/10.3390/plants14203193
APA StyleLiu, X., Meng, Y., Xie, L., Hao, Y., Yu, Y., Lv, G., Jiang, Y., Zhang, Y., Qian, C., & Gu, W. (2025). Boosting Maize Yield and Mitigating Greenhouse Gas Emissions Through Synergistic Nitrogen and Chemical Regulation by Optimizing Roots and Developing Grains Under High-Density Planting in Northeast China. Plants, 14(20), 3193. https://doi.org/10.3390/plants14203193






