Effect of Nitric Oxide on Adventitious Root Development from Cuttings of Sweetpotato and Associated Biochemical Changes
Abstract
1. Introduction
2. Results
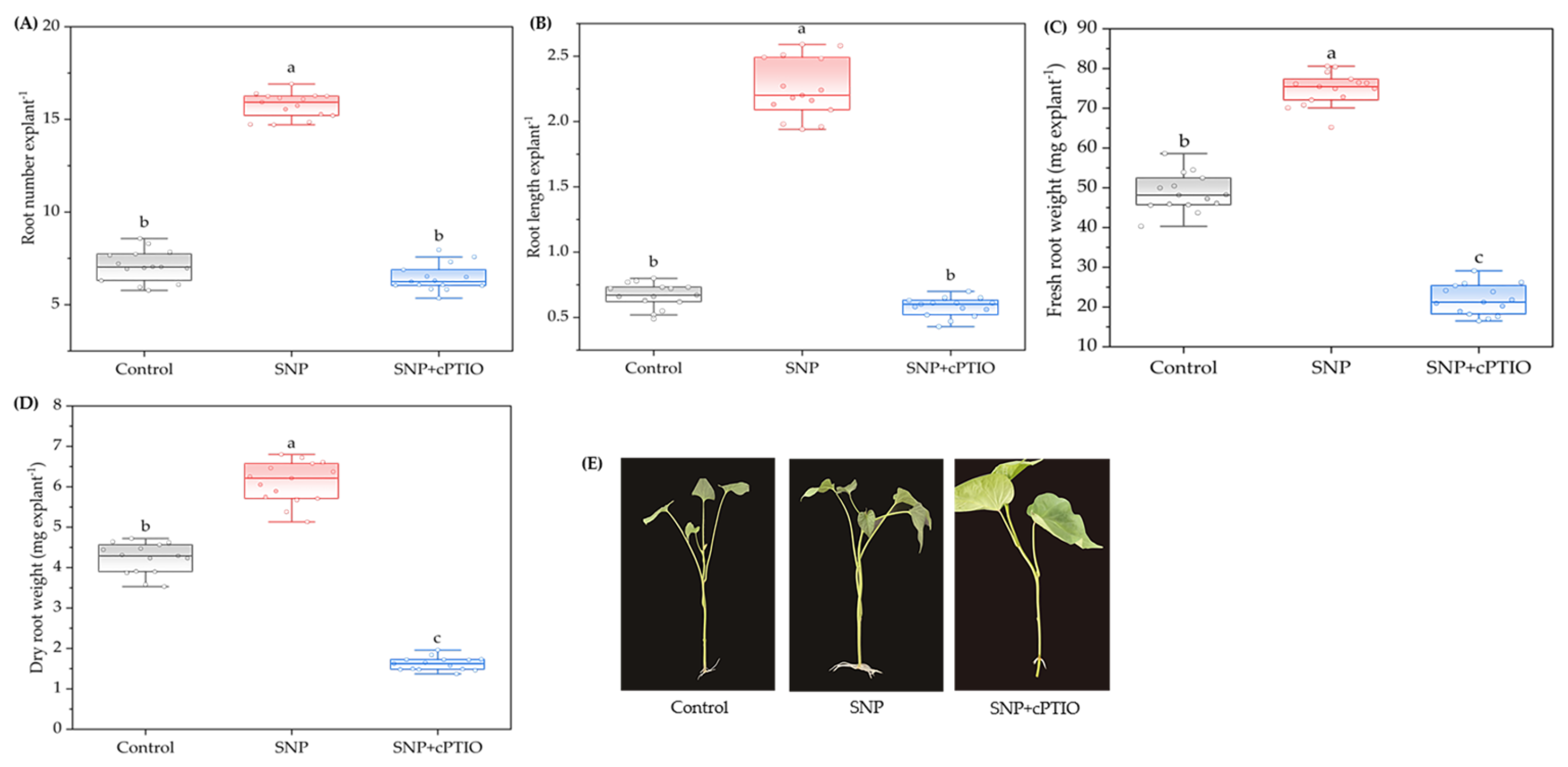
2.1. Effects of Exogenous SNP on Adventitious Root Development
2.2. Effects of NO Scavenger cPTIO on Adventitious Root Development
2.3. Changes in Soluble Sugar, Soluble Protein, and Starch Contents in Cuttings During Adventitious Rooting
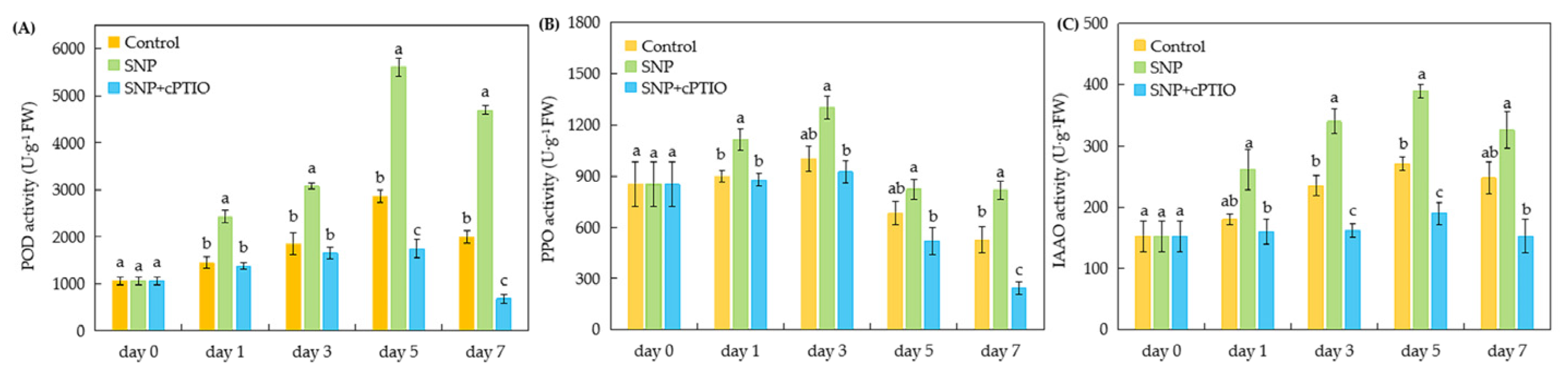
2.4. Changes in Rooting-Related Enzyme Activities in Cuttings During Adventitious Rooting
2.5. Changes in Chlorophyll Content, Fluorescence Parameters, and PSII Reaction Center Parameters in Cuttings During Adventitious Rooting
2.6. Correlation Analysis Between Morphological Characteristics and Physiological Indicators
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Chemicals and Treatments
4.3. Morphological Detection of Adventitious Roots
4.4. Measurement of Soluble Sugar, Soluble Protein, and Starch
4.5. Measurement of Rooting-Related Enzyme Activities
4.6. Measurement of Chlorophyll Content
4.7. Measurement of Chlorophyll Fluorescence
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NO | Nitric oxide |
| SNP | Sodium nitroprusside |
| cPTIO | 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| IAAO | Indole acetic acid oxidase |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| Chl (a + b) | Chlorophyll (a + b) |
| Fo Fm | Initial fluorescence Maximum fluorescence |
| Fv/Fm | Maximum photochemical efficiency of photosystem II |
| ABS/RC | Absorbed light energy |
| TRo/RC | Trapped energy flux |
| ETo/RC | Electron transport flux |
References
- Suparno, A.; Prabawardani, S.; Pattikawa, A. The Nutritional Value of Sweet Potato Tubers [Ipomoea batatas (L.) Lamb.] Consumed by Infants and Children of Dani Tribe in Kurulu District, Baliem-Jayawijaya. J. Agric. Sci. 2016, 8, 64. [Google Scholar] [CrossRef]
- Rosero, A.; Pastrana, I.; Martínez, R.; Perez, J.L.; Espitia, L.; Araujo, H.; Belalcazar, J.; Granda, L.; Jaramillo, A.; Gallego-Castill, S. Nutritional Value and Consumer Perception of Biofortified Sweet Potato Varieties. Ann. Agric. Sci. 2022, 67, 79–89. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Cao, Q.; Ma, D. Current Status and Future Prospective of Sweetpotato Production and Seed Industry in China. Sci. Agric. Sin. 2021, 54, 483–492. [Google Scholar]
- Kurniasari, F.N.; Susetyowati, S.; Hardianti, M.S.; Cempaka, A.R. Nutritional Value and Physical Quality of Oral Nutritional Supplements Made from Purple Sweet Potatoes to Treat Malnutrition in Patients with Cancer. Curr. Nutr. Food Sci. 2024, 20, 262–270. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What Makes Adventitious Roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef]
- Adem, M.; Sharma, L.; Shekhawat, G.S.; Šafranek, M.; Jásik, J. Auxin Signaling, Transport, and Regulation during Adventitious Root Formation. Curr. Plant Biol. 2024, 40, 100385. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Forde, B.G. Quantitative Analysis of Lateral Root Development: Pitfalls and How to Avoid Them. Plant Cell 2012, 24, 4–14. [Google Scholar] [CrossRef]
- Khan, M.N.; Alamri, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedi, B.; Ali, H.M.; Singh, V.P.; Siddiqui, M.H. Effect of Nitric Oxide on Seed Germination and Seedling Development of Tomato under Chromium Toxicity. J. Plant Growth Regul. 2020, 40, 2358–2370. [Google Scholar] [CrossRef]
- Li, F.; Ma, Y.; Yi, Y.; Ren, M.; Li, L.; Chen, Y.; Li, A.; Han, S.; Tang, H.; Jia, H.; et al. Nitric Oxide Induces S-nitrosylation of CESA1 and CESA9 and Increases Cellulose Content in Arabidopsis Hypocotyls. Plant Physiol. Biochem. 2023, 196, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Zhu, Y.; Niu, L.; Jin, X.; Xu, Q.; Liao, W. Effect of Exogenous Nitric Oxide on Vegetative and Reproductive Growth of Oriental Lily ‘Siberia’. Hortic. Environ. Biotechnol. 2015, 56, 677–686. [Google Scholar] [CrossRef]
- Wei, Q.; Yan, Z.; Xiong, Y.; Fang, Z. Altered Expression of OsAAP3 Influences Rice Lesion Mimic and Leaf Senescence by Regulating Arginine Transport and Nitric Oxide Pathway. Int. J. Mol. Sci. 2021, 22, 2181. [Google Scholar] [CrossRef]
- Kaya, C.; Uğurlar, F.; Seth, C.S. Sodium Nitroprusside Modulates Oxidative and Nitrosative Processes in Lycopersicum esculentum L. under Drought Stress. Plant Cell Rep. 2024, 43, 152. [Google Scholar] [CrossRef] [PubMed]
- Chegeni, M.M.; Jafarinia, M.; Ghotbi-Ravandi, A.A. Exogenous Nitric Oxide Enhances Salt Tolerance in Tomato (Lycopersicon esculentum Mill.) by Improving Photosynthetic Performance and Modulating the Expression of Photosystem II Genes. Hortic. Environ. Biotechnol. 2024, 65, 913–922. [Google Scholar] [CrossRef]
- Nawaz, M.; Saleem, M.H.; Khalid, M.R.; Ali, B.; Fahad, S. Nitric Oxide Reduces Cadmium Uptake in Wheat (Triticum aestivum L.) by Modulating Growth, Mineral Uptake, Yield Attributes, and Antioxidant Profile. Environ. Sci. Pollut. Res. Int. 2024, 31, 9844–9856. [Google Scholar] [CrossRef] [PubMed]
- Ravazzolo, L.; Trevisan, S.; Iori, S.; Forestan, C.; Malagoli, M.; Quaggiotti, S. Nitrate Regulates Maize Root Transcriptome through Nitric Oxide Dependent and Independent Mechanisms. Int. J. Mol. Sci. 2021, 22, 9527. [Google Scholar] [CrossRef]
- Li, C.; Huang, D.; Wang, C.; Wang, N.; Yao, Y.; Li, W.; Liao, W. NO is Involved in H2-induced Adventitious Rooting in Cucumber by Regulating the Expression and Interaction of Plasma Membrane H+-ATPase and 14-3-3. Planta 2020, 252, 9. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Z.; Shang, X.; Zhang, X.; Ma, L.; Bian, Y.; Wang, D.; Liu, W. Involvement of GA3-oxidase in Inhibitory Effect of Nitric Oxide on Primary Root Growth in Arabidopsis. Plant Biol. 2023, 26, 117–125. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, M.; Huang, G.; Yu, J. Ca2+ and CaM are Involved in NO- and H2O2-induced Adventitious Root Development in Marigold. J. Plant Growth Regul. 2012, 31, 253–264. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Is Nitric Oxide Toxic or Protective? Trends Plant Sci. 1999, 4, 299–300. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Nitric Oxide is Required for Root Organogenesis. Plant Physiol. 2002, 129, 954–956. [Google Scholar] [CrossRef]
- Liao, W.; Huang, G.; Yu, J.; Zhang, M. Nitric Oxide and Hydrogen Peroxide Alleviate Drought Stress in Marigold Explants and Promote Its Adventitious Root Development. Plant Physiol. Biochem. 2012, 58, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Li, H.; Zhong, Y.; Shen, Z.; Ma, D.; Gao, C.; Liu, Y.; Wang, W.; Zhang, J.; You, Y.; et al. Transcriptomic Analyses Reveal the Effect of Nitric Oxide on the Lateral Root Development and Growth of Mangrove Plant Kandelia Obovata. Plant Soil 2022, 472, 543–564. [Google Scholar] [CrossRef]
- Böhm, F.M.L.Z.; Ferrarese, M.D.L.L.; Zanardo, D.I.L.; Magalhaes, J.R.; Ferrarese-Filho, O. Nitric Oxide Affecting Root Growth, Lignification and Related Enzymes in Soybean Seedlings. Acta Physiol. Plant. 2010, 32, 1039–1046. [Google Scholar] [CrossRef]
- Liao, W.; Xiao, H.; Zhang, M. Role and Relationship of Nitric Oxide and Hydrogen Peroxide in Adventitious Root Development of Marigold. Acta Physiol. Plant. 2009, 31, 1279–1289. [Google Scholar] [CrossRef]
- Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Function of Nitric Oxide and Superoxide Anion in the Adventitious Root Development and Antioxidant Defence in Panax ginseng. Plant Cell Rep. 2008, 27, 563–573. [Google Scholar] [CrossRef]
- Qi, F.; Xiang, Z.; Kou, N.; Cui, W.; Xu, D.; Wang, R.; Zhu, D.; Shen, W. Nitric Oxide is Involved in Methane-Induced Adventitious Root Formation in Cucumber. Physiol. Plant. 2017, 159, 366–377. [Google Scholar] [CrossRef]
- Jin, X.; Liao, W.; Yu, J.; Ren, P.; Dawuda, M.M.; Wang, M.; Niu, L.; Li, X.; Xu, X. Nitric Oxide is Involved in Ethylene-induced Adventitious Rooting in Marigold (Tagetes erecta L.). Can. J. Plant. Sci. 2017, 97, 620–631. [Google Scholar]
- Dick, J.M.P.; Leakey, R.R.B.; McBeath, C.; Harvey, F.; Smith, R.I.; Woods, C. Influence of Nutrient Application Rate on Growth and Rooting Potential of the West African Hardwood Triplochiton scleroxylon. Tree Physiol. 2004, 24, 35–44. [Google Scholar] [CrossRef]
- Ribeiro, C.L.; Silva, C.M.; Drost, D.R.; Novaes, E.; Novaes, C.R.D.B.; Dervinis, C.; Kirst, M. Integration of Genetic, Genomic and Transcriptomic Information Identifies Putative Regulators of Adventitious Root Formation in Populus. BMC Plant Biol. 2016, 16, 66. [Google Scholar] [CrossRef]
- Li, D.; Miao, L.; Cisse, E.-H.M.; Li, L.; Chen, B.; Yang, F. Dissecting the Below- and Aboveground Specific Responses of Two Waterlogging Tolerant Arbor Species to Nutrient Supply under Waterlogging Conditions. Tree Physiol. 2022, 43, 390–403. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, S.; Zhang, G.; Feng, L.; Zheng, C.; Zhou, Y.; Du, J.; Yuan, M.; Chen, Y.; Wang, C.; et al. Nitric Oxide Induces Monosaccharide Accumulation through Enzyme S-nitrosylation. Plant Cell Environ. 2017, 40, 1834–1848. [Google Scholar] [CrossRef]
- Lucob-Agustin, N.; Sugiura, D.; Kano-Nakata, M.; Hasegawa, T.; Suralta, R.R.; Niones, J.M.; Inari-Ikeda, M.; Yamauchi, A.; Inukai, Y. The Promoted Lateral Root 1 (plr1) Mutation is Involved in Reduced Basal Shoot Starch Accumulation and Increased Root Sugars for Enhanced Lateral Root Growth in Rice. Plant Sci. 2020, 301, 110667. [Google Scholar] [CrossRef]
- Laloum, D.; Magen, S.; Soroka, Y.; Tamar, A.W. Exploring the Contribution of Autophagy to the Excess-Sucrose Response in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 3891. [Google Scholar] [CrossRef]
- Gibson, S.I. Control of Plant Development and Gene Expression by Sugar Signaling. Curr. Opin. Plant Biol. 2004, 8, 93–102. [Google Scholar] [CrossRef]
- Yue, J.; Du, C.; Jing, J.; Xie, T.; Chen, W.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Inhibition of α-ketoglutarate Dehydrogenase Activity Affects Adventitious Root Growth in Poplar via Changes in GABA Shunt. Planta 2018, 248, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Zhao, Z.; Wei, L.; Liu, Z.; Hu, D.; Liao, W. Nitric Oxide is Involved in Hydrogen Sulfide-induced Adventitious Rooting in Tomato (Solanum lycopersicum). Funct. Plant Biol. 2022, 49, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Fishel, D.W.; Zaczek, J.J.; Preece, J.E. Positional Influence on Rooting of Shoots Forced From the Main Bole of Swamp White Oak and Northern Red Oak. Can. J. For. Res. 2003, 33, 705–711. [Google Scholar] [CrossRef]
- Shao, F.; Wang, S.; Huang, W.; Liu, Z. Effects of IBA on the Rooting of Branch Cuttings of Chinese jujube (Zizyphus jujuba Mill.) and Changes to Nutrients and Endogenous Hormones. J. For. Res. 2018, 29, 1557–1567. [Google Scholar]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Haider, M.Z.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef]
- Uğurlar, F.; Kaya, C. Synergistic Mitigation of Nickel Toxicity in Pepper (Capsicum annuum) by Nitric Oxide and Thiourea via Regulation of Nitrogen Metabolism and Subcellular Nickel Distribution. Funct. Plant Biol. 2023, 50, 1099–1116. [Google Scholar] [CrossRef]
- Bano, A.; Noreen, Z.; Tabassum, F.; Zafar, F.; Rashid, M.; Aslam, M.; Shah, A.A.; Shah, A.N.; Jaremko, M.; Alasmael, N.; et al. Induction of Salt Tolerance in Brassica rapa by Nitric Oxide Treatment. Front. Plant Sci. 2022, 13, 995837. [Google Scholar] [CrossRef]
- Bhardwaj, D.R.; Mishra, V.K. Vegetative Propagation of Ulmus villosa: Effects of Plant Growth Regulators, Collection Time, Type of Donor and Position of Shoot on Adventitious Root Formation in Stem Cuttings. New For. 2005, 29, 105–116. [Google Scholar] [CrossRef]
- Rout, G.R. Effect of Auxins on Adventitious Root Development from Single Node Cuttings of Camellia sinensis (L.) kuntze and Associated Biochemical Changes. Plant Growth Regul. 2006, 48, 111–117. [Google Scholar] [CrossRef]
- Macedo, E.; Vieira, C.; Carrizo, D.; Porfirio, S.; Hegewald, H.; Arnholdt-Schmitt, B.; Calado, M.L.; Peixe, A. Adventitious Root Formation in Olive (Olea europaea L.) Microshoots: Anatomical Evaluation and Associated Biochemical Changes in Peroxidase and Polyphenol Oxidase Activities. J. Hortic. Sci. Biotechnol. 2013, 88, 53–59. [Google Scholar] [CrossRef]
- Hao, H.; Xie, B.; Zhao, D.; Kang, J.; Jiang, X.; Gai, Y. Proteomic Insights into Adventitious Root Formation in Larix kaempferi. J. Proteom. 2024, 307, 105288. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, X.; Ding, F.; Zhao, J.; Guo, A.F.; Zhang, L.; Yao, J.; Yang, Y.L. Interaction of Nitric Oxide and Reactive Oxygen Species and Associated Regulation of Root Growth in Wheat Seedlings Under Zinc Stress. Ecotoxicol. Environ. Saf. 2015, 113, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Nitric Oxide Induced Modulations in Adventitious Root Growth, Lignin Content and Lignin Synthesizing Enzymes in the Hypocotyls of Vigna radiata. Plant Physiol. Biochem. 2019, 141, 225–230. [Google Scholar] [CrossRef]
- Staszek, P.; Krasuska, U.; Bederska-Błaszczyk, M.; Gniazdowska, A. Canavanine Increases the Content of Phenolic Compounds in Tomato (Solanum lycopersicum L.) Roots. Plants 2020, 9, 1595. [Google Scholar] [CrossRef]
- Liao, W.; Xiao, H.; Zhang, M. Effect of Nitric Oxide and Hydrogen Peroxide on Adventitious Root Development from Cuttings of Ground-Cover Chrysanthemum and Associated Biochemical Changes. J. Plant Growth Regul. 2010, 29, 338–348. [Google Scholar] [CrossRef]
- Haissig, B.E. Influence of Auxins and Auxin Synergists on Adventitious Root Primordia Initiation and Development. N. Z. J. For. Sci. 1974, 4, 311–323. [Google Scholar]
- Gebhardt, K. Activation of Indole-3-acetic Acid Oxidase from Horseradish and Prunus by Phenols and H2O2. Plant Growth Regul. 1982, 1, 73–84. [Google Scholar] [CrossRef]
- Safronova, V.I.; Stepanok, V.V.; Engqvist, G.L.; Alekseyev, Y.V.; Belimov, A.A. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol. Fertil. Soils 2006, 42, 267–272. [Google Scholar] [CrossRef]
- Ullah, F.; Saqib, S.; Khan, W.; Ayaz, A.; Batool, A.; Wang, W.; Xiong, Y. The multifaceted role of sodium nitroprusside in plants: Crosstalk with phytohormones under normal and stressful conditions. Plant Growth Regul. 2024, 103, 453–470. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, M.; Wei, S.; Zhang, J.; Wang, C.; Liao, W. Roles of nitric oxide in heavy metal stress in plants: Cross-talk with phytohormones and protein S-nitrosylation. Environ. Pollut. 2020, 259, 113943. [Google Scholar] [CrossRef] [PubMed]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Ben-Yaakov, E.; Harpaz-Saad, S.; Galili, D.; Eyal, Y.; Goldschmidt, E. The Relationship between Chlorophyllase Activity and Chlorophyll Degradation During the Course of Leaf Senescence in Various Plant Species. Isr. J. Plant Sci. 2006, 54, 129–135. [Google Scholar] [CrossRef]
- Gupta, P.; Seth, C.S. Nitric Oxide Donor Sodium Nitroprusside Promotes Seed Germination and Ameliorates Adverse Effects of Salinity by Enhancing the Growth Indices and Photosynthetic Traits in Brassica juncea L. cv. Varuna Phytomorphol. Int. J. Plant Sci. 2015, 65, 156–163. [Google Scholar]
- Lu, Y.; Shen, X.; Li, Y.; Xu, Y.; Chen, Y.; Chen, Y.; Hu, X.; Li, X.; Sun, X.; Gong, J. Regulation of Chlorophyll and Carotenoid Metabolism in Citrus Fruit. Hortic. Plant J. 2025, 11, 951–962. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Qiu, X.; Hu, G.; Wang, Y.; Wang, Q. Exogenous Nitric Oxide Alleviates Iron-deficiency Chlorosis in Peanut Growing on Calcareous Soil. Plant Soil. Environ. 2012, 58, 111–120. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Y.; Sheng, H.; Wang, Y.; Kang, H.; Zeng, J. The Salicylic Acid and Nitric Oxide Increase Photosynthesis and Antioxidant Defense in Wheat under UV-B Stress. Biol. Plant. 2016, 60, 686–694. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Effects of Foliar Applications of Nitric Oxide and Spermidine on Chlorophyll Fluorescence, Photosynthesis and Antioxidant Enzyme Activities of Citrus Seedlings under Salinity Stress. Photosynthetica 2018, 56, 1313–1325. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.; Liao, W.; Ma, Z.; Xu, X.; Wang, M.; Ren, P.; Niu, L.; Jin, X.; Zhu, Y. Hydrogen Peroxide is Involved in Abscisic acid-induced Adventitious Rooting in Cucumber (Cucumis sativus L.) under Drought Stress. J. Plant Biol. 2016, 59, 536–548. [Google Scholar] [CrossRef]
- Vanin, A.F.; Svistunenko, D.A.; Mikoyan, V.D.; Serezhenkov, V.A.; Fryer, M.J.; Baker, N.R.; Cooper, C.E. Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. J. Biol. Chem. 2004, 279, 24100–24107. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, W.; Zhang, N.; Wen, S.; Chen, J. Hydrogen sulfide and nitric oxide regulate the adaptation to iron deficiency through affecting Fe homeostasis and thiol redox modification in Glycine max seedlings. Plant Physiol. Biochem. 2023, 194, 1–14. [Google Scholar] [PubMed]
- Song, Y.; Dong, Y.; Kong, J.; Tian, X.; Bai, X.; Xu, L. Effects of root addition and foliar application of nitric oxide and salicylic acid in alleviating iron deficiency induced chlorosis of peanut seedlings. J. Plant Nutr. 2016, 40, 63–81. [Google Scholar] [CrossRef]
- Graziano, M.; Lorenzo, L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J. 2007, 52, 949–960. [Google Scholar] [CrossRef]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric oxide improves internal iron availability in plants. Plant Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Bai, X.; Long, J.; He, X.; Yan, J.; Chen, X.; Tan, Y.; Li, K.; Chen, L.; Xu, H. Overexpression of Spinach Non-Symbiotic Hemoglobin in Arabidopsis Resulted In Decreased NO Content and Lowered Nitrate and Other Abiotic Stresses Tolerance. Sci. Rep. 2016, 6, 26400. [Google Scholar] [CrossRef]
- Wodala, B.; Deák, Z.; Vass, I.; Erdei, L.; Altorjay, I.; Horváth, F. In Vivo Target Sites of Nitric Oxide in Photosynthetic Electron Transport as Studied by Chlorophyll Fluorescence in Pea Leaves. Plant Physiol. 2008, 146, 1920–1927. [Google Scholar] [CrossRef]
- Wu, X.; Ding, H.; Chen, J.; Zhang, H.; Zhu, W. Attenuation of Salt Induced Changes in Photosynthesis by Exogenous Nitric Oxide in Tomato (Lycopersicon esculentum Mill. L.) seedlings. Afr. J. Biotechnol. 2010, 9, 7837–7846. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Wei, S.; Hu, D.; Liu, Y.; Feng, L.; Li, C.; Qi, N.; Wang, C.; Liao, W. Nitric Oxide Enhanced Salt Stress Tolerance in Tomato Seedlings, Involving Phytohormone Equilibrium and Photosynthesis. Int. J. Mol. Sci. 2022, 23, 4539. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhou, Z. Effect of No donor SNP on Photosynthetic Pigments and Chlorophyll Fluorescence under Enhanced UV-B radiation in Larix gmelinii. In Proceedings of the International Conference on Green Energy and Environmental Sustainable Development, Jilin, China, 4–6 November 2011; Volume 2011, pp. 972–976. [Google Scholar]
- Yang, Y.; Li, L.; Zhao, H.; Ma, P.; Zhang, C. Effect of Exogenous Nitric Oxide on Lipid Peroxidation and Chlorophyll Fluorescence in Wheat Leaves under High Temperature and Strong Irradiance Stress. Agric. Sci. Technol. 2008, 9, 25–30. [Google Scholar]
- Ma, L.; Zhang, Z.; Yao, B.; Ma, Z.; Huang, X.; Zhou, B.; Xu, M.; Guo, J.; Zhou, H. Effects of Drought and Heat on the Productivity and Photosynthetic Characteristics of Alpine Meadow Plants on the Qinghai-Tibetan Plateau. J. Mt. Sci. 2021, 18, 2079–2093. [Google Scholar] [CrossRef]
- Verma, N.; Prasad, S.M. Interplay of Hydrogen Peroxide and Nitric Oxide: Systemic Regulation of Photosynthetic Performance and Nitrogen Metabolism in Cadmium Challenged Cyanobacteria. Physiol. Mol. Biol. Plants 2021, 27, 2181–2199. [Google Scholar] [CrossRef]
- Rodrigues, V.; Kumar, A.; Prabhu, K.N.; Pragadheesh, V.S.; Shukla, A.K.; Sundaresan, V. Adventitious root cultures of Decalepis salicifolia for the production of 2-hydroxy-4-methoxybenzaldehyde, a Vanillin isomer flavor metabolite. Appl. Microbiol. Biotechnol. 2021, 105, 3087–3099. [Google Scholar] [CrossRef]
- Handel, E.V. Direct Microdetermination of Sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, D.; Xu, S.; Hu, H.; Pan, J.; Li, P.; Shen, W. Endogenous Hydrogen Sulfide Homeostasis Is Responsible for the Alleviation of Senescence of Postharvest Daylily Flower via Increasing Antioxidant Capacity and Maintained Energy Status. J. Agric. Food. Chem. 2017, 65, 718–726. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Bru, R.; Cabanes, J.; Garcia-Carmona, F. Characterization of Catecholase and Cresolase Activities of Monastrell Grape Polyphenol Oxidase. Phytochemistry 1988, 27, 319–321. [Google Scholar] [CrossRef]
- Beffa, R.; Martin, H.V.; Pilet, P.E. In Vitro Oxidation of Indoleacetic Acid by Soluble Auxin-Oxidases and Peroxidases from Maize Roots. Plant Physiol. 1990, 94, 485–491. [Google Scholar] [CrossRef]
- Wang, C.; Wei, L.; Zhang, J.; Hu, D.; Gao, R.; Liu, Y.; Feng, L.; Gong, W.; Liao, W. Nitric Oxide Enhances Salt Tolerance in Tomato Seedlings by Regulating Endogenous S-nitrosylation Levels. J. Plant Growth Regul. 2022, 42, 275–293. [Google Scholar] [CrossRef]



| Time (Day) | Treatments | Chl a Content (mg·g−1 FW) | Chl b Content (mg·g−1 FW) | Chl (a + b) Content (mg·g−1 FW) |
|---|---|---|---|---|
| 0 | Control | 0.92 ± 0.11 a | 0.32 ± 0.02 a | 1.24 ± 0.13 a |
| SNP | 0.93 ± 0.17 a | 0.30 ± 0.02 a | 1.23 ± 0.19 a | |
| SNP + cPTIO | 0.96 ± 0.11 a | 0.31 ± 0.01 a | 1.27 ± 0.16 a | |
| Control | 1.04 ± 0.12 a | 0.33 ± 0.02 a | 1.37 ± 0.10 ab | |
| 1 | SNP | 1.30 ± 0.16 a | 0.34 ± 0.02 a | 1.64 ± 0.14 a |
| SNP + cPTIO | 0.90 ± 0.04 a | 0.29 ± 0.02 a | 1.19 ± 0.03 b | |
| Control | 1.22 ± 0.13 ab | 0.36 ± 0.02 a | 1.58 ± 0.14 a | |
| 3 | SNP | 1.60 ± 0.10 a | 0.40 ± 0.01 a | 2.00 ± 0.09 a |
| SNP + cPTIO | 0.84 ± 0.05 b | 0.26 ± 0.02 b | 1.10 ± 0.06 b | |
| Control | 1.44 ± 0.09 b | 0.37 ± 0.02 b | 1.81 ± 0.07 b | |
| 5 | SNP | 1.89 ± 0.09 a | 0.45 ± 0.02 a | 2.34 ± 0.10 a |
| SNP + cPTIO | 0.76 ± 0.06 c | 0.22 ± 0.01 c | 0.98 ± 0.05 c | |
| Control | 1.55 ± 0.25 b | 0.41 ± 0.02 b | 1.96 ± 0.27 b | |
| 7 | SNP | 2.57 ± 0.17 a | 0.50 ± 0.02 a | 3.07 ± 0.18 a |
| SNP + cPTIO | 0.53 ± 0.02 c | 0.12 ± 0.02 c | 0.65 ± 0.04 c |
| Time (Day) | Treatments | Fo | Fm | Fv/Fm |
|---|---|---|---|---|
| 0 | Control | 9842.67 ± 373.51 a | 30,173.67 ± 954.54 a | 0.67 ± 0.01 a |
| SNP | 10,718.00 ± 872.97 a | 33,011.33 ± 1539.85 a | 0.68 ± 0.01 a | |
| SNP + cPTIO | 10,678.33 ± 1823.33 a | 28,300.67 ± 1456.82 a | 0.62 ± 0.06 a | |
| Control | 10,982.67 ± 57.78 a | 39,947.33 ± 454.67 b | 0.73 ± 0.003 a | |
| 1 | SNP | 10,942.67 ± 290.80 a | 58,755.33 ± 2325.39 a | 0.81 ± 0.003 ab |
| SNP + cPTIO | 13,084.67 ± 1062.17 a | 32,498.00 ± 3182.41 b | 0.58 ± 0.08 b | |
| Control | 11,807.67 ± 1064.87 a | 45,558.33 ± 1447.70 b | 0.74 ± 0.02 a | |
| 3 | SNP | 11,443.33 ± 538.37 a | 67,368.33 ± 1337.61 a | 0.83 ± 0.01 b |
| SNP + cPTIO | 13,193.67 ± 198.59 a | 40,107.67 ± 2498.11 c | 0.67 ± 0.02 b | |
| Control | 12,340.00 ± 192.16 a | 43,876.33 ± 731.07 a | 0.72 ± 0.01 a | |
| 5 | SNP | 12,053.33 ± 847.66 a | 57,320.67 ± 1509.85 b | 0.79 ± 0.03 a |
| SNP + cPTIO | 14,000.00 ± 977.05 a | 31,314.67 ± 2506.67 c | 0.55 ± 0.06 b | |
| Control | 13,702.67 ± 1691.16 ab | 41,514.00 ± 2295.77 a | 0.67 ± 0.04 a | |
| 7 | SNP | 10,433.33 ± 981.46 b | 54,955.67 ± 2234.24 b | 0.81 ± 0.02 a |
| SNP + cPTIO | 19,526.70 ± 1612.81 a | 23,603.88 ± 1326.81 c | 0.17 ± 0.07 b |
| Time (Day) | Treatments | ABS/RC | TRo/RC | ETo/RC |
|---|---|---|---|---|
| 0 | Control | 2.90 ± 0.07 a | 1.95 ± 0.03 a | 0.35 ± 0.01 a |
| SNP | 2.59 ± 0.28 a | 1.88 ± 0.05 a | 0.36 ± 0.04 a | |
| SNP + cPTIO | 3.01 ± 0.46 a | 1.82 ± 0.11 a | 0.35 ± 0.03 a | |
| Control | 3.57 ± 0.05 b | 2.36 ± 0.29 b | 0.46 ± 0.02 b | |
| 1 | SNP | 4.56 ± 0.21 a | 3.73 ± 0.11 a | 0.64 ± 0.03 a |
| SNP + cPTIO | 3.07 ± 0.10 b | 2.03 ± 0.19 b | 0.36 ± 0.02 c | |
| Control | 3.58 ± 0.16 b | 2.42 ± 0.34 b | 0.55 ± 0.02 b | |
| 3 | SNP | 4.63 ± 0.26 a | 4.27 ± 0.38 a | 0.78 ± 0.02 a |
| SNP + cPTIO | 3.16 ± 0.17 b | 2.29 ± 0.32 b | 0.45 ± 0.01 c | |
| Control | 3.01 ± 0.14 b | 2.08 ± 0.16 b | 0.42 ± 0.001 ab | |
| 5 | SNP | 4.29 ± 0.17 a | 3.66 ± 0.12 a | 0.54 ± 0.08 a |
| SNP + cPTIO | 2.52 ± 0.07 b | 1.78 ± 0.05 b | 0.27 ± 0.02 b | |
| Control | 2.65 ± 0.17 b | 1.58 ± 0.13 b | 0.35 ± 0.02 b | |
| 7 | SNP | 3.92 ± 0.20 a | 3.13 ± 0.23 a | 0.45 ± 0.02 a |
| SNP + cPTIO | 1.72 ± 0.07 c | 1.22 ± 0.10 b | 0.19 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, J.; Wu, Y.; Zhang, H.; Wang, H.; Wang, L. Effect of Nitric Oxide on Adventitious Root Development from Cuttings of Sweetpotato and Associated Biochemical Changes. Plants 2025, 14, 3183. https://doi.org/10.3390/plants14203183
Wang M, Li J, Wu Y, Zhang H, Wang H, Wang L. Effect of Nitric Oxide on Adventitious Root Development from Cuttings of Sweetpotato and Associated Biochemical Changes. Plants. 2025; 14(20):3183. https://doi.org/10.3390/plants14203183
Chicago/Turabian StyleWang, Meng, Jianghui Li, Yuhao Wu, Hongxing Zhang, Hui Wang, and Lingyun Wang. 2025. "Effect of Nitric Oxide on Adventitious Root Development from Cuttings of Sweetpotato and Associated Biochemical Changes" Plants 14, no. 20: 3183. https://doi.org/10.3390/plants14203183
APA StyleWang, M., Li, J., Wu, Y., Zhang, H., Wang, H., & Wang, L. (2025). Effect of Nitric Oxide on Adventitious Root Development from Cuttings of Sweetpotato and Associated Biochemical Changes. Plants, 14(20), 3183. https://doi.org/10.3390/plants14203183






