Rejuvenation of Mature Ilex paraguariensis Plants Through Serial Rooted Cuttings: Exploring the Roles of miRNAs in Reversing Adult Phase, Promoting Root Formation, and Determining Root Structure
Abstract
1. Introduction
2. Results
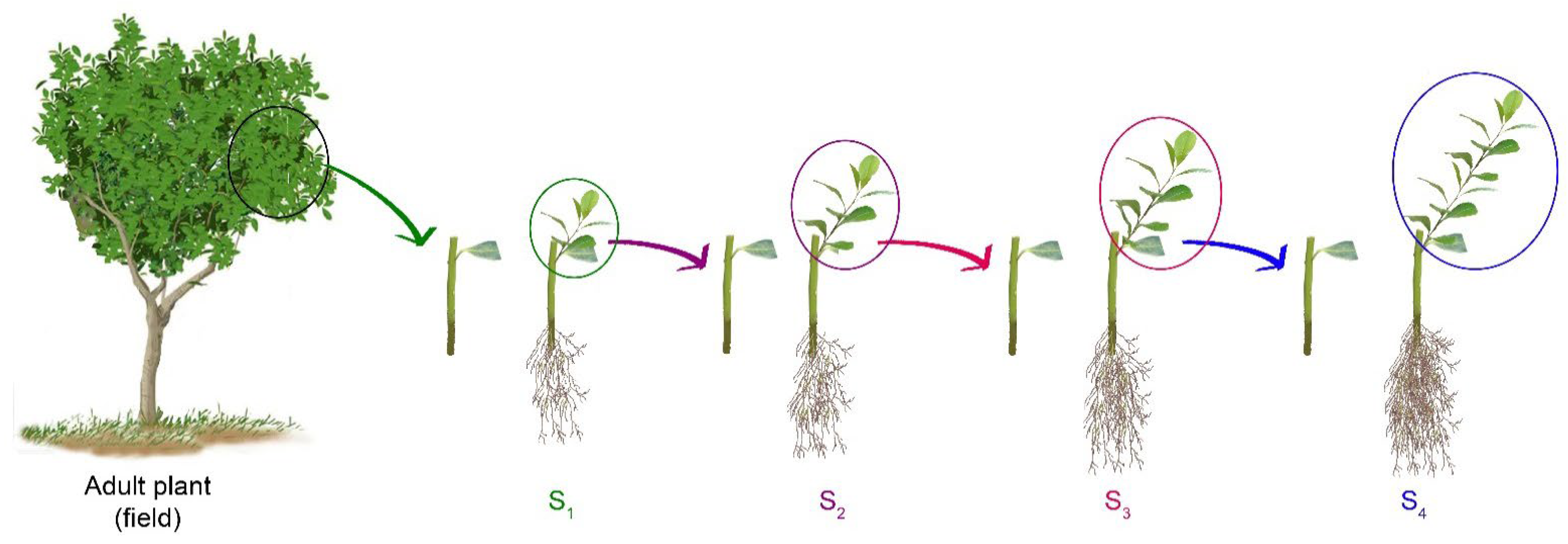
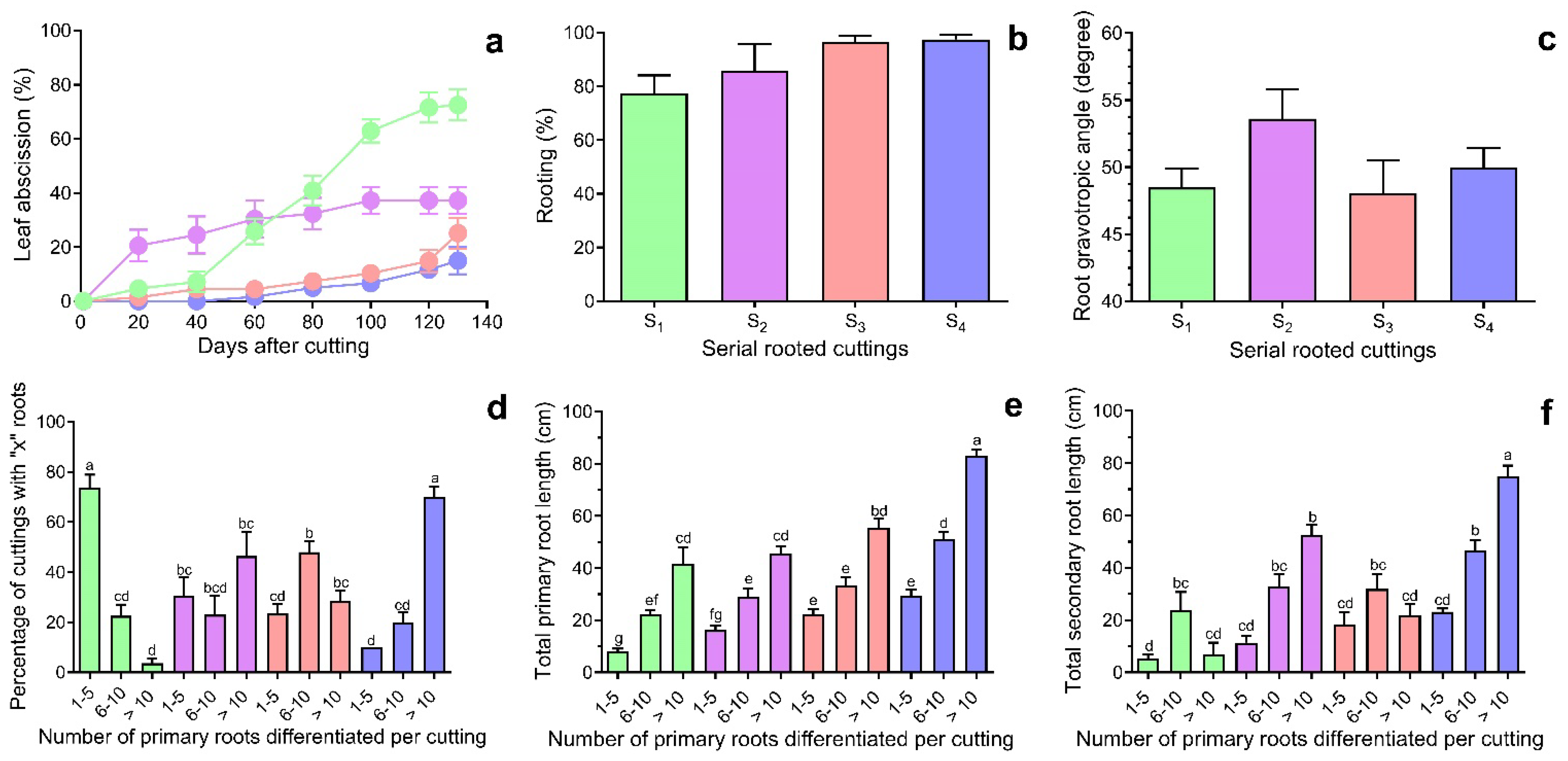
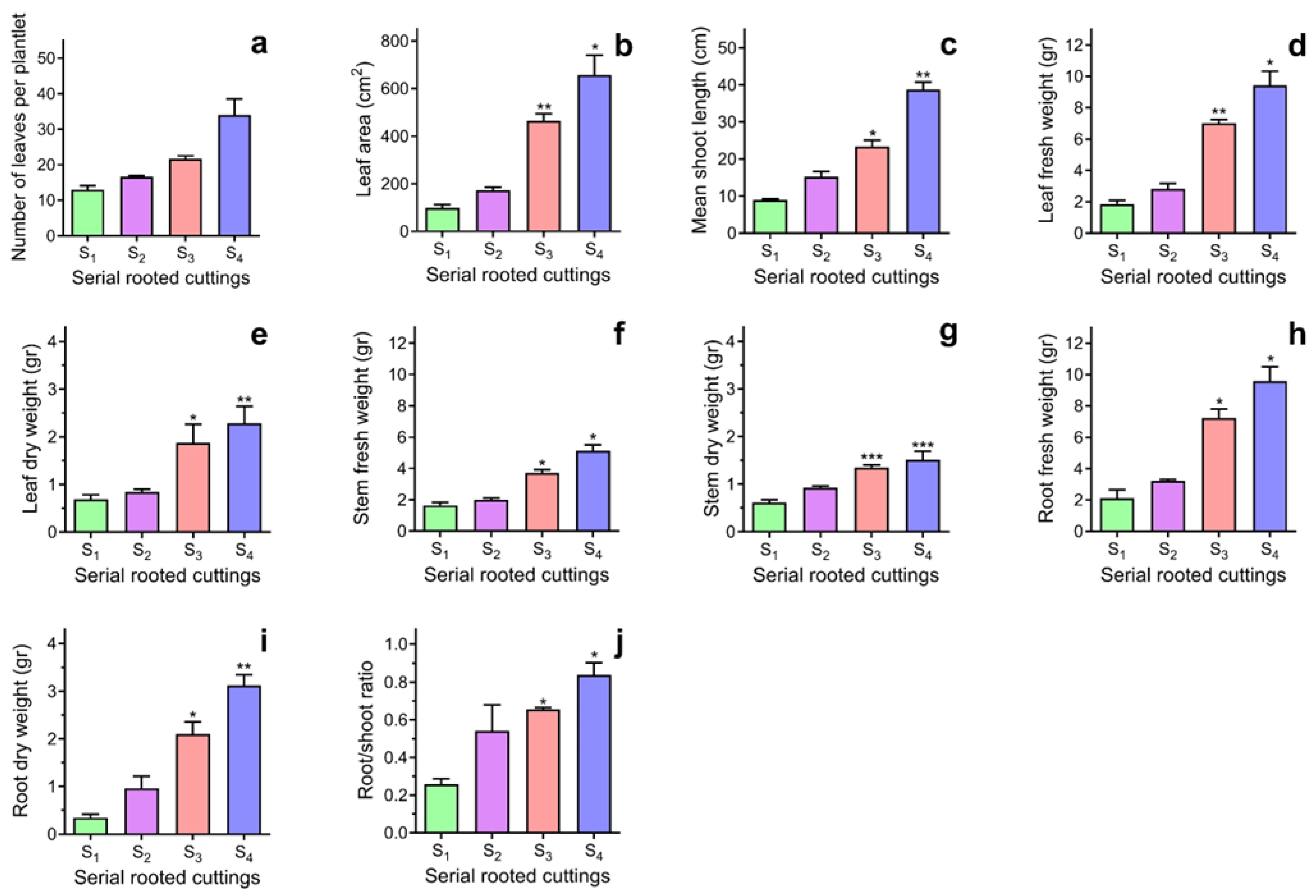
2.1. The Effects of Rejuvenation on Adventitious Root Development and Plant Growth
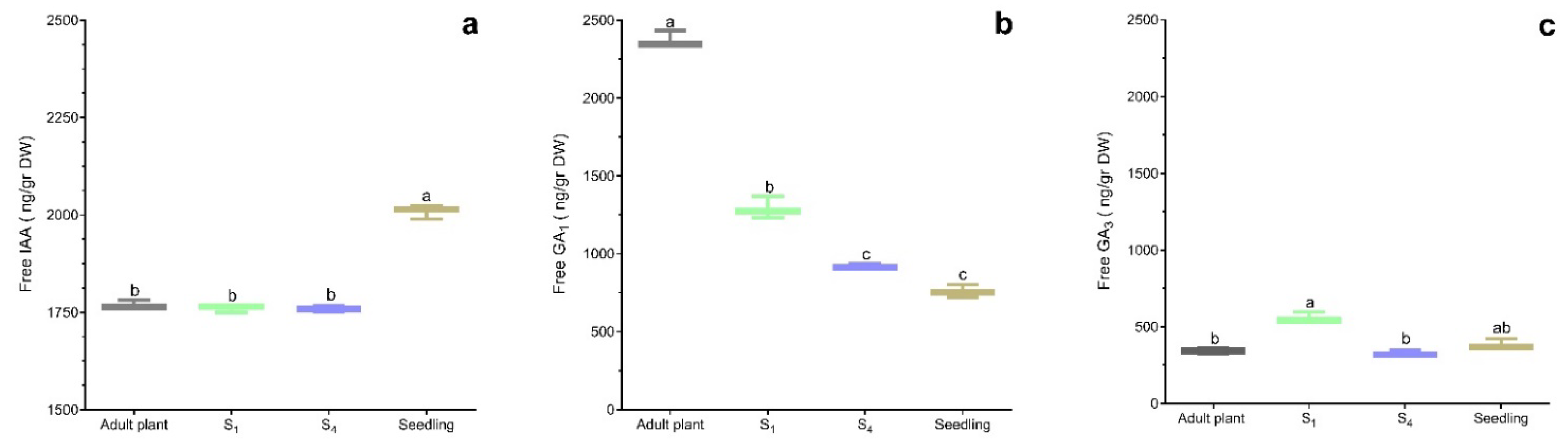
2.2. The Effects of Rejuvenation on Free-Hormones and Trehalose Content
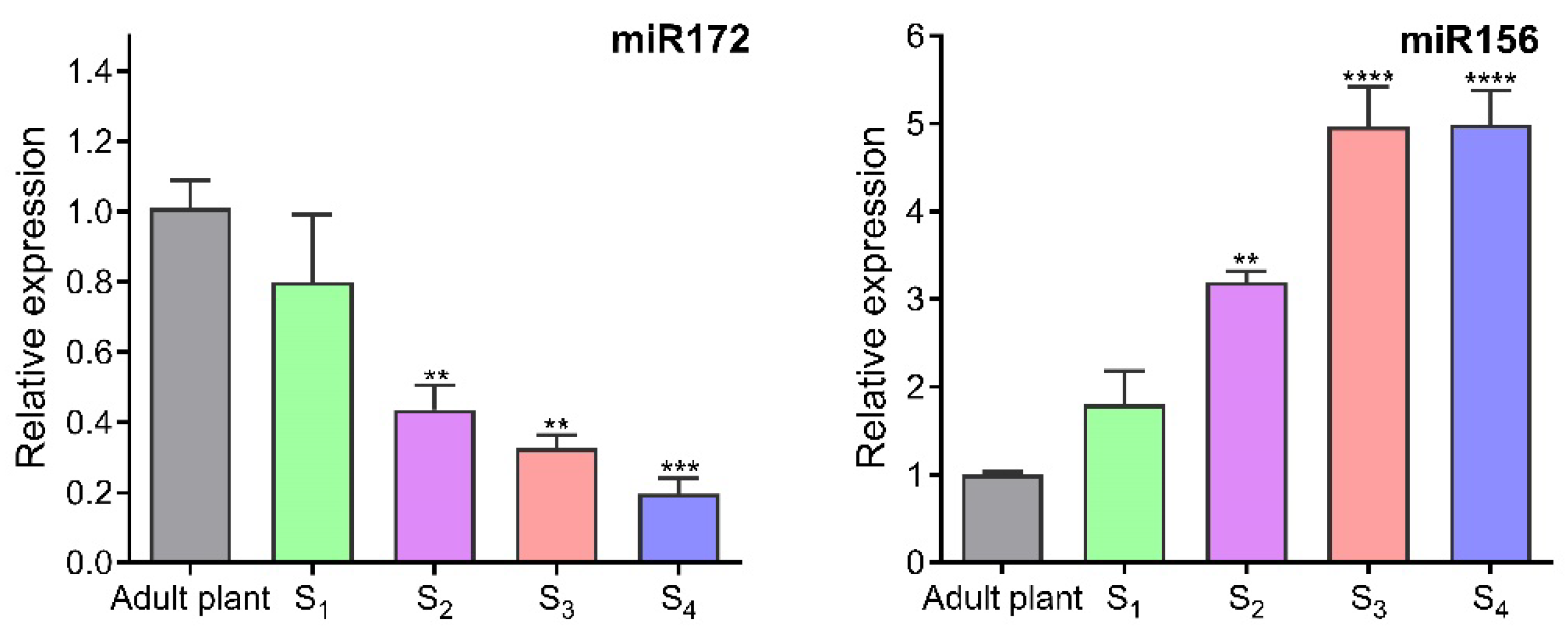
2.3. Plant Reversal Age Estimation by miRNA Expression
2.4. Expression of Genes Involved in Adventitious Root Formation
2.5. Small RNA Sequencing and miRNA Identification
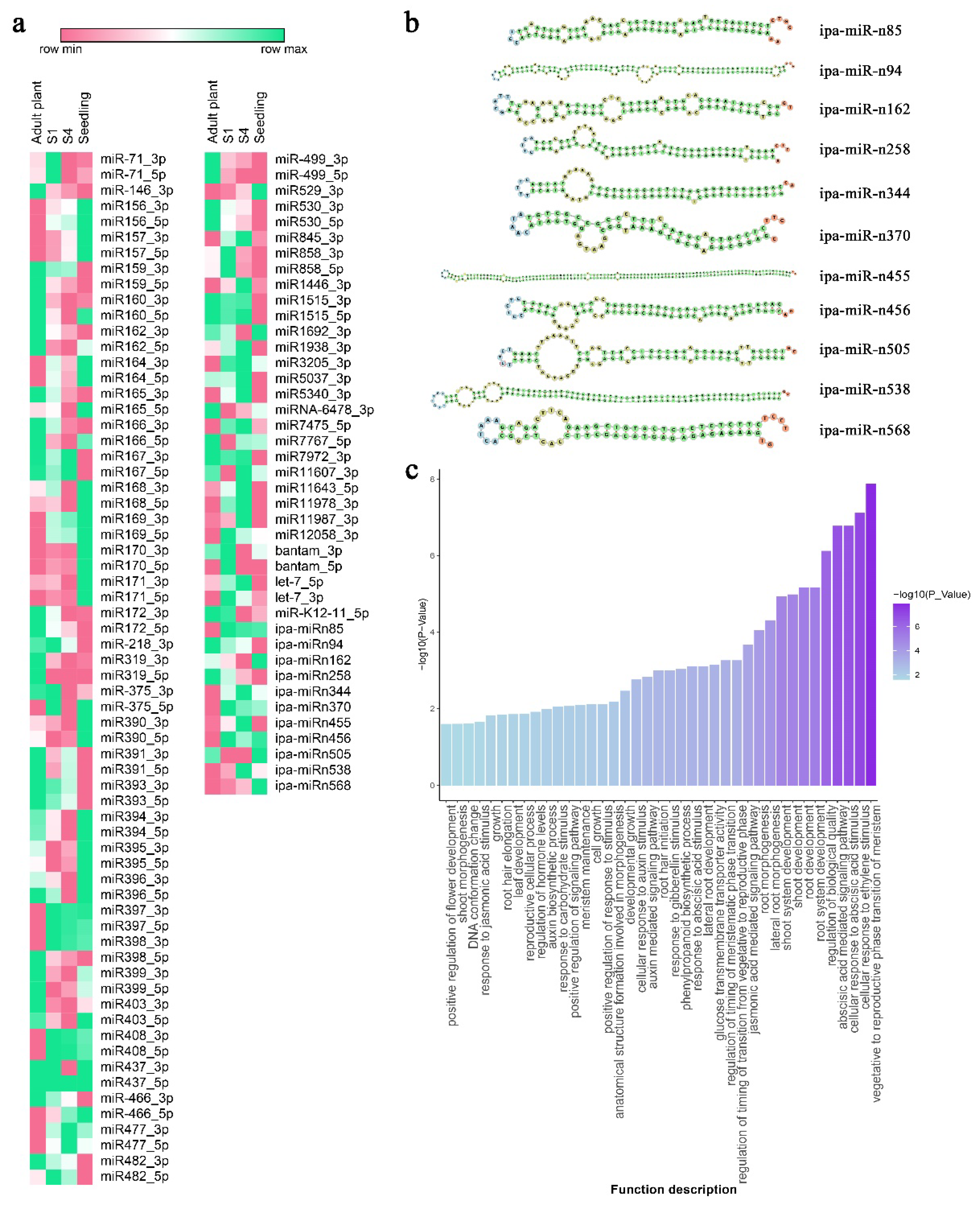
2.6. Changes in miRNAs Expression Among Plants at Different Physiological Ages, Target Prediction and Functional Analysis
3. Discussion
4. Materials and Methods
4.1. Establishing a Plant Population with Different Physiological Ages
4.2. Measurements for Growth Traits
4.3. Plant Reversal Age Estimation by miRNAs RT-qPCR
4.4. RNA Isolation, Library Construction, and Sequencing
4.5. RNA-Seq Data Processing
4.6. Differential Expression Analysis of miRNAs
4.7. Expression of Genes Involved in Adventitious Root Formation
4.8. Hormones and Trehalose Determination
4.9. Statistical
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giberti, G.C. Yerba mate (Ilex paraguariensis, Aquifoliaceae), according to early Río de La Plata Bonpland’s handwritings: Its southern South American geographical distribution. Bonplandia 2011, 20, 203–212. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.; Chanaj-Kaczmarek, J.; Cielecka-Piontek, J. Yerba mate—A long but current history. Nutrients 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.; Nunes, A.; Moreira, B.R.; Maraschin, M. Yerba mate (Ilex paraguariensis A. St.-Hil.) For new therapeutic and nutraceutical interventions: A review of patents issued in the last 20 years (2000–2020). Phytotherapy Res. 2023, 37, 527–548. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Yan, Y.; Zheng, B.; Yan, D. The applications of plant polyphenols: Implications for the development and biotechnological utilisation of Ilex species. Plants 2024, 13, 3271. [Google Scholar] [CrossRef]
- Sansberro, P.; Rey, H.; Bernardis, A.; Luna, C.; Collavino, M.; Mroginski, L. Plant regeneration of Ilex paraguariensis St. Hil. (Aquifoliaceae) by in vitro culture of nodal segments. BioCell 2000, 24, 53–63. [Google Scholar]
- Luna, C.V.; Duarte, M.J.; Brugnoli, E.A.; Ayala, P.G.; Espasandin, F.D.; Bernardis, A.C.; Mroginski, L.A.; Sansberro, P.A. In vitro plantlet production of Ilex paraguariensis adult plants using BIT bioreactors. Plant Cell Tiss. Org. Cult. 2024, 157, 19. [Google Scholar] [CrossRef]
- Tarragó, J.; Sansberro, P.; Filip, R.; López, P.; González, A.; Luna, C.; Mroginski, L. Effect of leaf retention and flavonoids on rooting of Ilex paraguariensis cuttings. Sci. Hort. 2005, 103, 479–488. [Google Scholar] [CrossRef]
- Tarragó, J.; Filip, R.; Mroginski, L.; Sansberro, P. Influence of the irradiance on phenols content and rooting of Ilex paraguariensis cuttings collected from adult plants. Acta Physiol. Plant. 2012, 34, 2419–2424. [Google Scholar] [CrossRef]
- Pimentel, N.; Pedroso, M.F.; Lencina, K.H.; Santos de Oliveira, J.M.; Bisognin, D.A. Anatomical characterisation of the adventitious roots of Mate (Ilex paraguariensis A. St. Hil.) mini-cuttings. Braz. Arch. Biol. Technol. 2020, 63, e20190359. [Google Scholar] [CrossRef]
- Bisognin, D.A.; Lencina, K.H.; da Luz, L.V.; Fleig, F.D.; Gazzana, D. Adventitious rooting competence and rescue of adult mate plants by cuttings. Rev. Árvore 2018, 42, e420312. [Google Scholar] [CrossRef]
- Poethig, R.S. Phase change and the regulation of shoot morphogenesis in plants. Science 1990, 250, 923–930. [Google Scholar] [CrossRef]
- Kiefer, C.; Bergonzi, S.; Brand, L.; Wötzel, S.; Koch, M.A. Contrasting life history traits in monocarpic versus polycarpic plants from a molecular-evolutionary point of view. Ann. Plant Rev. 2019, 2, 1–25. [Google Scholar] [CrossRef]
- Von Aderkas, P.; Bonga, J.M. Influencing micropropagation and somatic embryogenesis in mature trees by manipulation of phase change, stress and culture environment. Tree Physiol. 2000, 20, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, Y.; Li, Y. Plant rejuvenation: From phenotypes to mechanisms. Plant Cell Rep. 2020, 39, 1249–1262. [Google Scholar] [CrossRef]
- Dong, Q.; Hu, B.; Zhang, C. microRNAs and their roles in plant development. Front. Plant Sci. 2022, 13, 824240. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, M.; Willmann, M.R.; McCormick, K.; Hu, T.; Yang, L.; Starker, C.G.; Voytas, D.F.; Meyers, B.C.; Poethig, R.S. Threshold-dependent repression of SPL gene expression by miR156/miR157 controls vegetative phase change in Arabidopsis thaliana. PLoS Genet. 2018, 14, e1007337. [Google Scholar] [CrossRef]
- Wu, G.; Poethig, R.S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 2006, 133, 3539–3547. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Hu, X.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. High miR156 expression is required for auxin-induced adventitious root formation via MxSPL26 independent of PINs and ARFs in Malus xiaojinensis. Front. Plant Sci. 2017, 8, 1059. [Google Scholar] [CrossRef]
- Feng, S.J.; Xu, Y.M.; Guo, C.K.; Zheng, J.R.; Zhou, B.Y.; Zhang, Y.T.; Zhu, Z.; Wang, H.; Wu, G. Modulation of miR156 to identify traits associated with vegetative phase change in tobacco (Nicotiana tabacum). J. Exp. Bot. 2016, 67, 1493–1504. [Google Scholar] [CrossRef]
- Abu-Abied, M.; Szwerdszarf, D.; Mordehaev, I.; Levy, A.; Rogovoy, O.; Belausov, E.; Yaniv, Y.; Uliel, S.; Katzenellenbogen, M.; Riov, J.; et al. Microarray analysis revealed upregulation of nitrate reductase in juvenile cuttings of Eucalyptus grandis, which correlated with increased remodeling during adventitious root formation. BMC Genom. 2012, 15, 826. [Google Scholar] [CrossRef]
- Li, T.; Gonzalez, N.; Inzé, D.; Dubois, M. Emerging connections between small RNAs and phytohormones. Trends Plants Sci. 2020, 25, 912–929. [Google Scholar] [CrossRef]
- Yan, X.-X.; Liu, X.-L.; Cui, H.; Zhao, M.-Q. The roles of microRNAs in regulating root formation and growth in plants. J. Integr. Agric. 2022, 21, 901–916. [Google Scholar] [CrossRef]
- Li, K.; Liu, Z.; Xing, L.; Wei, Y.; Mao, J.; Meng, Y.; Bao, L.; Han, M.; Zhao, C.; Zhang, D. miRNAs associated with auxin signaling, stress response, and cellular activities mediate adventitious root formation in apple rootstocks. Plant Physiol. Biochem. 2019, 139, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Raihan, T.; Geneve, R.L.; Perry, S.E.; Rodriguez Lopez, C.M. The regulation of plant vegetative phase transition and rejuvenation: miRNAs, a key regulator. Epigenomes 2021, 5, 24. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.-W.; Weigel, D.; Poethig, R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Lauter, N.; Kampani, A.; Carlson, S.; Goebel, M.; Moose, S.P. microRNA172 down-regulates glossy15 to promote vegetative phase change in maise. Proc. Natl. Acad. Sci. USA 2005, 102, 9412–9417. [Google Scholar] [CrossRef]
- Spanudakis, E.; Jackson, S. The role of microRNAs in the control of flowering time. J. Exp. Bot. 2014, 65, 365–380. [Google Scholar] [CrossRef]
- Wang, J.-W.; Park, M.Y.; Wang, L.-J.; Koo, Y.; Chen, X.-Y.; Weigel, D.; Poethig, R.S. MiRNA control of vegetative phase change in trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Werner, S.; Bartrina, I.; Schmülling, T. Cytokinin regulates vegetative phase change in Arabidopsis thaliana through the miR172/TOE1-TOE2 module. Nat. Commun. 2021, 12, 5816. [Google Scholar] [CrossRef]
- Krivmane, B.; Runge, K.S.; Samsone, I.; Rungis, D.E. Differentially expressed conserved plant vegetative phase-change-related microRNAs in mature and rejuvenated silver birch in vitro propagated tissues. Plants 2023, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Poethig, R.S.; Fouracre, J. Temporal regulation of vegetative phase change in plants. Dev. Cell 2024, 59, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Sansberro, P.A.; Mroginski, L.A.; Bottini, R.A. In vitro morphogenetic responses of Ilex paraguariensis nodal segments treated with different gibberellins and Prohexadione-Ca. Plant Growth Regul. 2001, 34, 209–214. [Google Scholar] [CrossRef]
- Sansberro, P.A.; Mroginski, L.A.; Bottini, R.A. Shoot growth in Ilex paraguariensis plants grown under varying photosynthetically active radiation is affected through gibberellin levels. Plant Growth Regul. 2002, 38, 231–236. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Koo, Y.; He, J.; Poethig, R.S. Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. eLlife 2013, 2, e00260. [Google Scholar] [CrossRef]
- Proveniers, M. Sugars speed up the circle of life. eLife 2013, 2, e00625. [Google Scholar] [CrossRef]
- Ponnu, J.; Schlereth, A.; Zacharaki, V.; Działo, M.; Abel, C.; Feil, R.; Schmid, M.; Wahl, V. The trehalose 6-phosphate pathway impacts vegetative phase change in Arabidopsis thaliana. Plant J. 2020, 104, 768–780. [Google Scholar] [CrossRef]
- Fichtner, F.; Lunn, J.E. The Role of Trehalose 6-Phosphate (Tre6P) in plant metabolism and development. Annu. Rev. Plant Biol. 2021, 72, 737–760. [Google Scholar] [CrossRef]
- Han, Y.; Liang, A.; Xu, D.; Zhang, Y.; Shi, J.; Li, M.; Liu, T.; Qi, H. Versatile roles of trehalose in plant growth and development and responses to abiotic stress. Veg. Res. 2024, 4, e007. [Google Scholar] [CrossRef]
- Xu, M.Y.; Zhang, L.; Li, W.W.; Hu, X.L.; Wang, M.B.; Fan, Y.L.; Zhang, C.Y.; Wang, L. Stress-induced early flowering is mediated by miR169 in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 89–101. [Google Scholar] [CrossRef]
- Waheed, S.; Zeng, L. The critical role of miRNAs in regulation of flowering time and flower development. Genes 2020, 11, 319. [Google Scholar] [CrossRef]
- Kim, W.; Ahn, H.J.; Chiou, T.J.; Ahn, J.H. The role of the miR399-PHO2 module in the regulation of flowering time in response to different ambient temperatures in Arabidopsis thaliana. Mol. Cells 2011, 32, 83–88. [Google Scholar] [CrossRef]
- Bernardi, Y.; Ponso, M.A.; Belén, F.; Vegetti, A.C.; Dotto, M.C. MicroRNA miR394 regulates flowering time in Arabidopsis thaliana. Plant Cell Rep. 2022, 41, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, M.; Futagami, K.; Shimamura, M.; Inoue, C.; Kunimoto, K.; Oogami, T.; Tomita, Y.; Inoue, K.; Kohchi, T.; Yamaoka, S.; et al. An early arising role of the MicroRNA156/529-SPL module in reproductive development revealed by the liverwort Marchantia polymorpha. Curr. Biol. 2019, 29, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Y.; Qin, T.; Guo, X.; Xu, K.; Xu, C.; Yuan, W. Functional conservation and divergence of miR156 and miR529 during rice development. Crop J. 2023, 11, 692–703. [Google Scholar] [CrossRef]
- Hu, Z.; Nie, Z.; Yan, C.; Huang, H.; Ma, X.; Wang, Y.; Ye, N.; Tuskan, G.A.; Yang, X.; Yin, H. Transcriptome and degradome profiling reveal a role of miR530 in the circadian regulation of gene expression in Kalanchoë marnieriana. Cells 2021, 10, 1526. [Google Scholar] [CrossRef]
- Barakat, A.; Sriram, A.; Park, J.; Zhebentyayeva, T.; Main, D.; Abbott, A. Genome wide identification of chilling responsive microRNAs in Prunus persica. BMC Genom. 2012, 13, 481. [Google Scholar] [CrossRef]
- Li, J.; Song, Q.; Zuo, Z.-F.; Liu, L. MicroRNA398: A master regulator of plant development and stress responses. Int. J. Mol. Sci. 2022, 23, 10803. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, B.; Gao, C.; Zhang, H.; Wen, F.; Tao, L.; Fu, G.; Xiong, J. The evolution and functional roles of mir408 and its targets in plants. Int. J. Mol. Sci. 2022, 23, 530. [Google Scholar] [CrossRef]
- Ayala, P.G.; Acevedo, R.M.; Luna, C.V.; Rivarola, M.; Acuña, C.; Marcucci Poltri, S.; González, A.M.; Sansberro, P.A. Transcriptome dynamics of rooting zone and leaves during in vitro adventitious root formation in Eucalyptus nitens. Plants 2022, 11, 3301. [Google Scholar] [CrossRef]
- Li, S.-W. Molecular bases for the regulation of adventitious root generation in plants. Front. Plant Sci. 2021, 12, 614072. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, R.M.; Avico, E.H.; Ruiz, O.A.; Sansberro, P.A. Assessment of reference genes for quantitative real-time PCR normalisation in Ilex paraguariensis leaves during drought acclimatisation. Biol. Plantarum. 2018, 62, 89–96. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Garg, V.; Varshney, R.K. Analysis of Small RNA Sequencing Data in Plants. Methods Mol Biol. 2022, 2443, 497–509. [Google Scholar] [CrossRef]
- Vignale, F.A.; Garcia, A.H.; Modenutti, C.P.; Sosa, E.J.; Defelipe, L.A.; Oliveira, R.; Nunes, G.L.; Acevedo, R.M.; Burguener, G.F.; Rossi, S.M.; et al. Yerba mate (Ilex paraguariensis) genome provides new insights into convergent evolution of caffeine biosynthesis. eLife 2025, 14, e104759. [Google Scholar] [CrossRef]
- Acevedo, R.M.; Avico, E.H.; González, S.; Rodrigues Salvador, A.; Rivarola, M.; Paniego, N.; Nunes-Nesi, A.; Ruiz, O.A.; Sansberro, P.A. Transcript and metabolic adjustments triggered by drought in Ilex paraguariensis leaves. Planta 2019, 250, 445–462. [Google Scholar] [CrossRef]
- Iparraguirre, J.; ·Masciarelli, O.; Villasuso, A.L.; Piatti, D.; Llanes, A. Macrocystis pyrifera alga extracts combined with Azospirillum argentinense improve growth and hormonal responses in Zea mays plants under drought stress. J. Soil Sci. Plant Nutr. 2024, 24, 3209–3223. [Google Scholar] [CrossRef]
- Kretschmer, P.M.; Bannister, A.M.; O’Brien, M.K.; MacManus-Spencer, L.A.; Paulick, M.G. A liquid chromatography-tandem mass spectrometry assay for the detection and quantification of trehalose in biological samples. J. Chromatogr. 2016, 1033–1034, 9–16. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, M.J.; Acevedo, R.M.; Ortiz, N.L.; Álvarez, M.Y.; Sansberro, P.A. Rejuvenation of Mature Ilex paraguariensis Plants Through Serial Rooted Cuttings: Exploring the Roles of miRNAs in Reversing Adult Phase, Promoting Root Formation, and Determining Root Structure. Plants 2025, 14, 1668. https://doi.org/10.3390/plants14111668
Duarte MJ, Acevedo RM, Ortiz NL, Álvarez MY, Sansberro PA. Rejuvenation of Mature Ilex paraguariensis Plants Through Serial Rooted Cuttings: Exploring the Roles of miRNAs in Reversing Adult Phase, Promoting Root Formation, and Determining Root Structure. Plants. 2025; 14(11):1668. https://doi.org/10.3390/plants14111668
Chicago/Turabian StyleDuarte, María J., Raúl M. Acevedo, Nicolás L. Ortiz, Mayra Y. Álvarez, and Pedro A. Sansberro. 2025. "Rejuvenation of Mature Ilex paraguariensis Plants Through Serial Rooted Cuttings: Exploring the Roles of miRNAs in Reversing Adult Phase, Promoting Root Formation, and Determining Root Structure" Plants 14, no. 11: 1668. https://doi.org/10.3390/plants14111668
APA StyleDuarte, M. J., Acevedo, R. M., Ortiz, N. L., Álvarez, M. Y., & Sansberro, P. A. (2025). Rejuvenation of Mature Ilex paraguariensis Plants Through Serial Rooted Cuttings: Exploring the Roles of miRNAs in Reversing Adult Phase, Promoting Root Formation, and Determining Root Structure. Plants, 14(11), 1668. https://doi.org/10.3390/plants14111668





