The Ionome–Hormone–Flavonoid Network Shapes Genotype-Dependent Yield Adaptation in Sugarcane
Abstract
1. Introduction
2. Results
2.1. Genotype-Dependent Variation in Sucrose Content and Yield Among Different Varieties
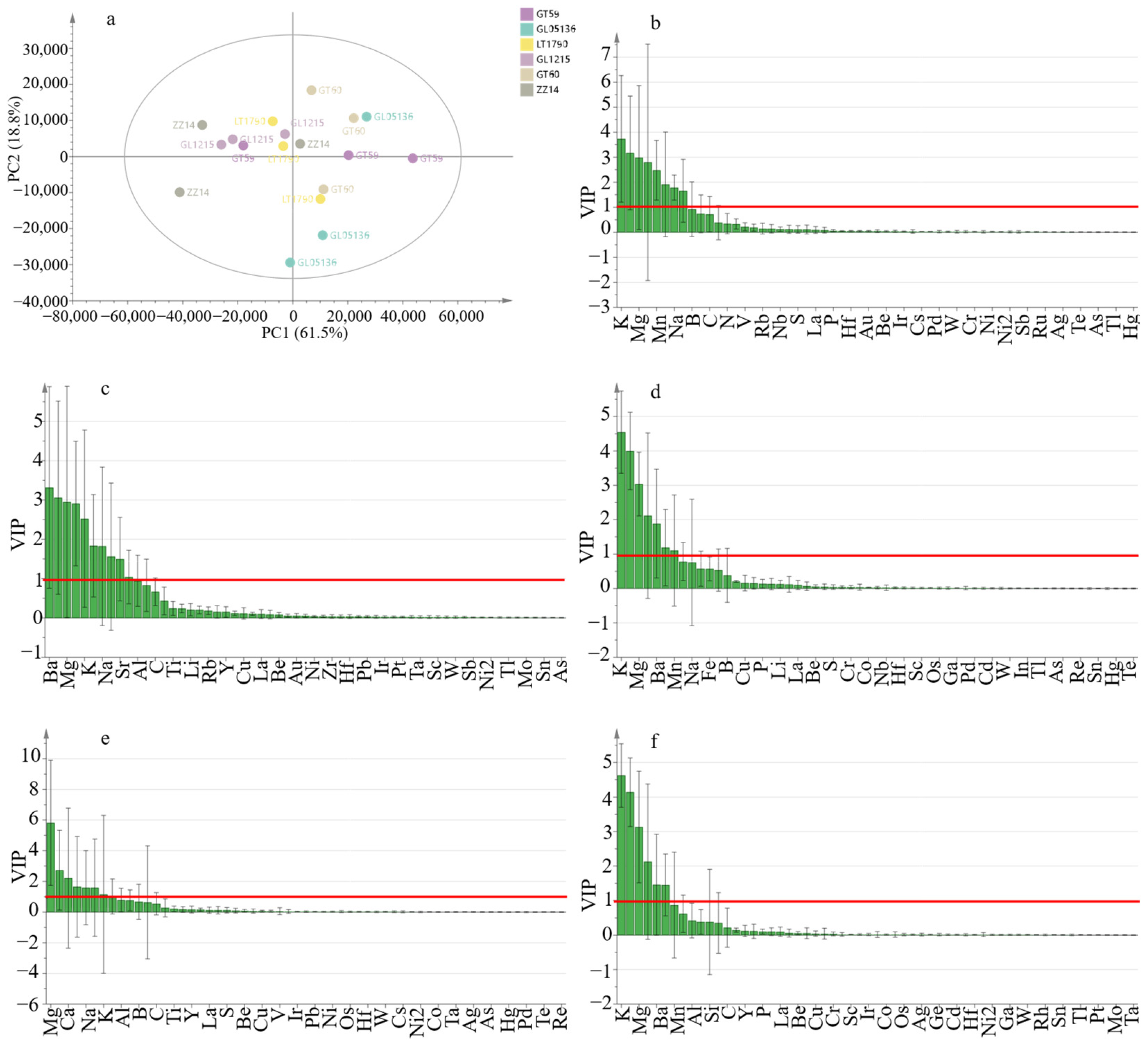
2.2. Ionomic Profiling and Multivariate Analysis of Sugarcane Genotypes
2.3. Phytohormone Profiling of Different Genotypes Reveals Genotype-Dependent Variations
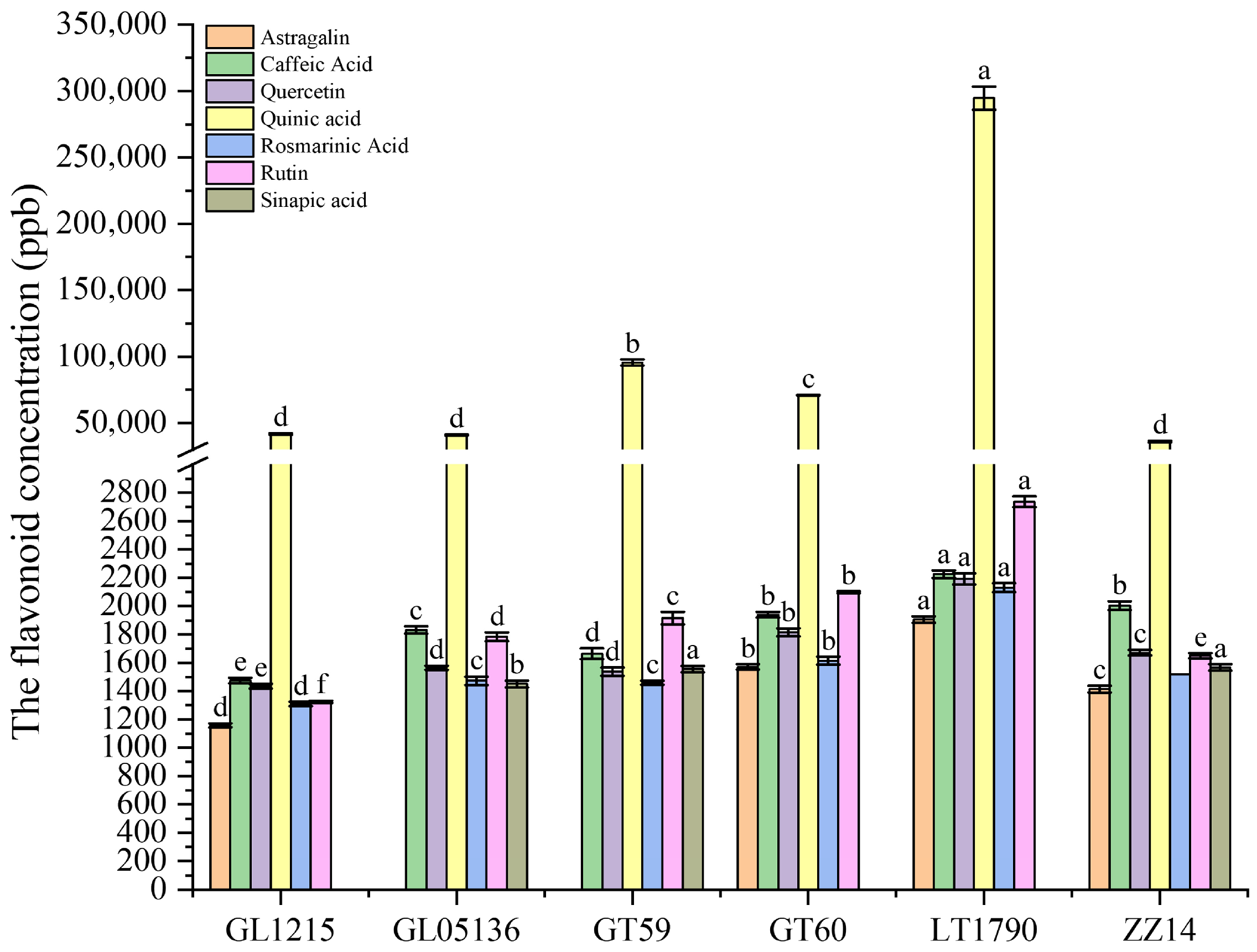
2.4. Genotype-Dependent Variation in Flavonoid Profiles of Different Sugarcane Varieties
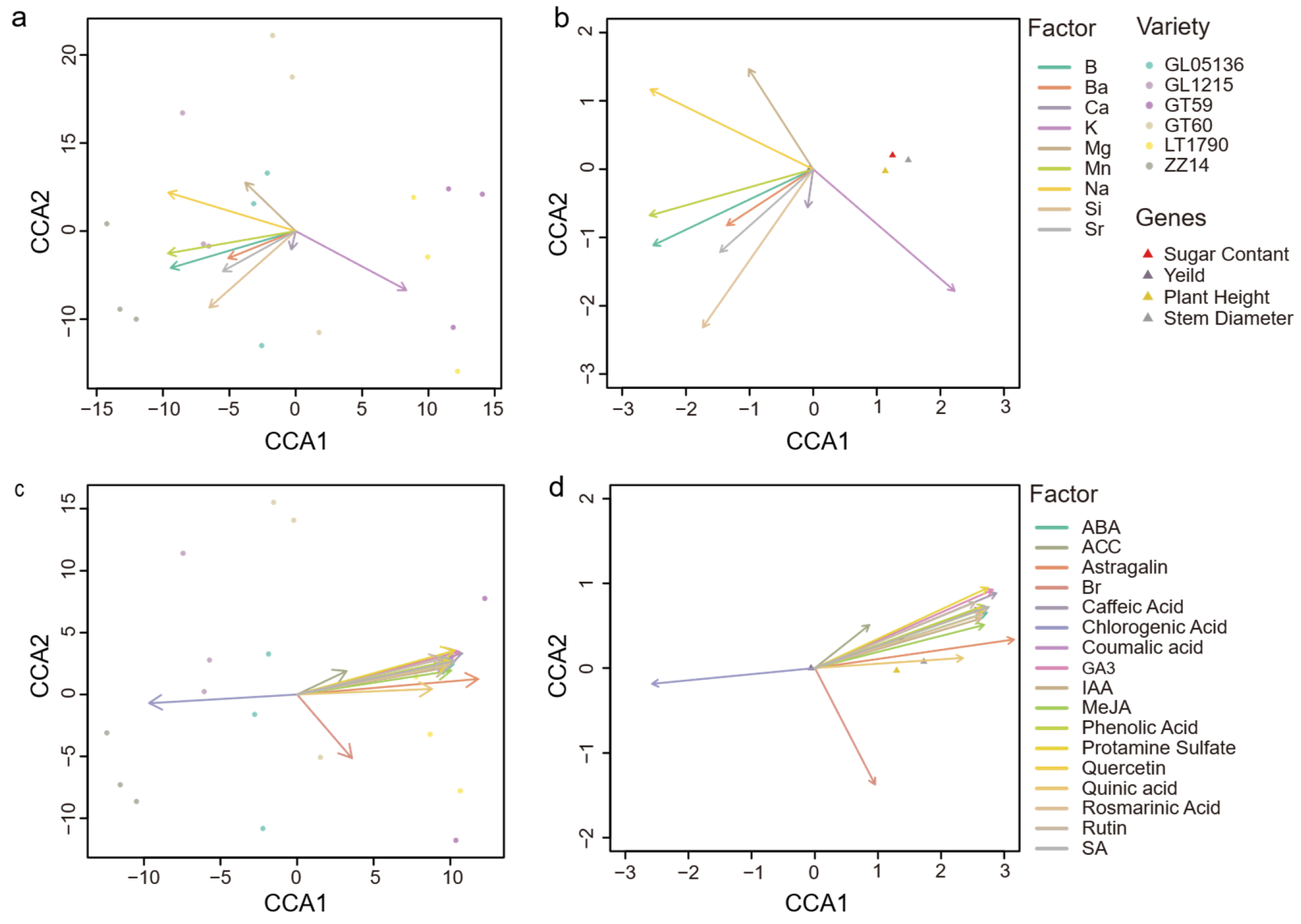
2.5. CCA of Yield Components with Ionomic and Metabolite Data in Different Sugarcane
3. Discussion
3.1. Ionomic Composition and Nutrient-Use Efficiency
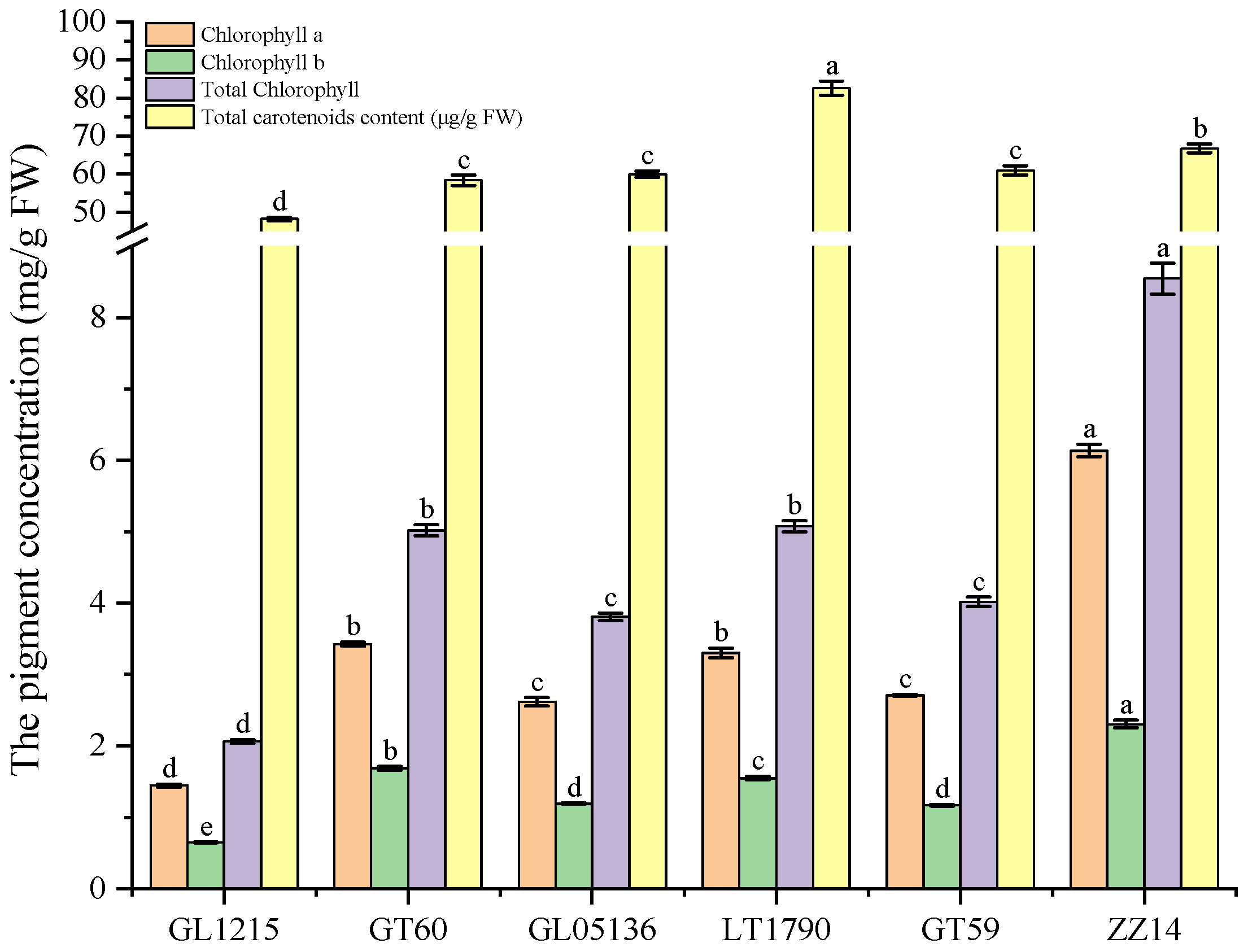
3.2. Photosynthetic Pigments and Productivity
3.3. Endogenous Hormone Profiles and Growth Regulation
3.4. Flavonoid Accumulation and Stress Tolerance
3.5. Implications for Breeding High-Yield, High-Resilience Cultivars
4. Materials and Methods
4.1. Plant Materials
4.2. Sample Collection and Preparation
4.3. Agronomic Parameter Quantification
4.4. Ionomics Analysis
4.5. Plant Hormone Analysis
4.6. Flavonoid Analysis
4.7. Photosynthetic Pigments Analysis
4.8. Statistics and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De França E Silva, N.R.; Chaves, M.E.D.; Luciano, A.C.D.S.; Sanches, I.D.; De Almeida, C.M.; Adami, M. Sugarcane Yield Estimation Using Satellite Remote Sensing Data in Empirical or Mechanistic Modeling: A Systematic Review. Remote Sens. 2024, 16, 863. [Google Scholar] [CrossRef]
- Garcia, V.F.; Ensinas, A.V. Simultaneous Optimization and Integration of Multiple Process Heat Cascade and Site Utility Selection for the Design of a New Generation of Sugarcane Biorefinery. Entropy 2024, 26, 501. [Google Scholar] [CrossRef]
- Harrison, M.D. Sugarcane-Derived Animal Feed; O’Hara, I.M., Mundree, S.G., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 281–310. [Google Scholar]
- K, M.P.; Patil, S.B.; G, H.N.; Patil, C.R.; Patil, P.V. Identification of Promising Sugarcane Clones Based on Cane Yield and Quality Traits in First Clonal Selection Stage. Int. J. Multidiscip. Res. 2024, 6, 12902. [Google Scholar]
- Rakesh, G.; Reddy, G.E.; Dinesh, A.; Swapna, N.; Saicharan, M.; Naik, B.B.; Kumar, M.V. Genetic Studies in Advanced Sugarcane Mid-Late Clones Through Yield and Quality Traits. Int. J. Environ. Clim. Chang. 2023, 13, 4535–4542. [Google Scholar] [CrossRef]
- Hoarau, J.; Grivet, L.; Offmann, B.; Payet, J.; Raboin, L.; Hellmann, M.; Roques, D.; D’Hont, A.; Glaszmann, J.; Hogarth, D. Quantitative Trait Allele (QTA) for Yield Components in a Modern Sugarcane Cultivar. In Proceedings of the International Society of Sugar Cane Technologists (ISSCT) Congress, Brisbane, Australia, 24–28 August 2001; Available online: https://www.semanticscholar.org/paper/Quantitative-Trait-Allele-(QTA)-for-yield-in-a-Hoarau-Grivet/e3d304016e0ae875e9ca9d2f258c4d0351737077 (accessed on 15 October 2025).
- Neisi, A.; Bhatt, R. Investigating the Effect of Applying Liquid Organic Fertilizer and Different Amounts of Nitrogen on Sugarcane Yield. J. Nat. Resour. Conserv. Manag. 2024, 5, 58–65. [Google Scholar] [CrossRef]
- Bamrungrai, J.; Tubana, B.S.; Tre-loges, V.; Promkhambut, A.; Polthanee, A. Effects of Water Stress and Auxin Application on Growth and Yield of Two Sugarcane Cultivars Under Greenhouse Conditions. Agriculture 2021, 11, 613. [Google Scholar] [CrossRef]
- Zhu, S.; Xiao, J.; Han, S.; Li, X.; Li, Z.; Wei, B.; Zhang, D.; Wang, R.; Li, R.; Yang, L.; et al. Transcriptomics Combined with Photosynthetic Physiology and Leaf Structure Analysis Revealed Increased Sugarcane Yield by Fenlong-Ridging. Agronomy 2023, 13, 1196. [Google Scholar] [CrossRef]
- Ruhela, S.K.; Ruhela, M. The Control of the Sugarcane Pest Top Borer (Scirpophaga Nivella FAB*) Through Many Approaches as Integrated Pest Management. J. Adv. Biol. Biotechnol. 2024, 27, 512–520. [Google Scholar] [CrossRef]
- Dlamini, N.E.; Franke, A.C.; Zhou, M. Impact of Soil Type and Harvest Season on the Ratooning Ability of Sugarcane Varieties. Exp. Agric. 2024, 60, e15. [Google Scholar] [CrossRef]
- Mulla, S.; Singh, S.K. Decoding Climate Change : Modeling Resilience of Sugarcane Co86032 in Padegaon (Maharashtra), India with DSSAT and Random Forest. MAUSAM 2025, 76, 431–448. [Google Scholar] [CrossRef]
- de Jager, N.; Shukla, V.; Koprivova, A.; Lyčka, M.; Bilalli, L.; You, Y.; Zeier, J.; Kopriva, S.; Ristova, D. Traits Linked to Natural Variation of Sulfur Content in Arabidopsis Thaliana. J. Exp. Bot. 2023, 75, 1036–1050. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumar, A.; Benazir, I.; Kumar, G. Reassessing the Role of Ion Homeostasis for Improving Salinity Tolerance in Crop Plants. Physiol. Plant. 2021, 171, 502–519. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Synergistic Effects of Salicylic Acid and Melatonin on Modulating Ion Homeostasis in Salt-Stressed Wheat (Triticum Aestivum L.) Plants by Enhancing Root H+-Pump Activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef]
- Vasilachi-Mitoseru, I.-C.; Stoleru, V.; Gavrilescu, M. Integrated Assessment of Pb(II) and Cu(II) Metal Ion Phytotoxicity on Medicago Sativa L., Triticum Aestivum L., and Zea Mays L. Plants: Insights into Germination Inhibition, Seedling Development, and Ecosystem Health. Plants 2023, 12, 3754. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants Under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Nam, Y.-J.; Tran, L.-S.P.; Kojima, M.; Sakakibara, H.; Nishiyama, R.; Shin, R. Regulatory Roles of Cytokinins and Cytokinin Signaling in Response to Potassium Deficiency in Arabidopsis. PLoS ONE 2012, 7, e47797. [Google Scholar] [CrossRef]
- García, M.J.; Lucena, C.; Romera, F.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene and Nitric Oxide Involvement in the Up-Regulation of Key Genes Related to Iron Acquisition and Homeostasis in Arabidopsis. J. Exp. Bot. 2010, 61, 3885–3899. [Google Scholar] [CrossRef]
- Ain, N.; Habiba; Ming, R. Allele-Specific Hormone Dynamics in Highly Transgressive F2 Biomass Segregants in Sugarcane (Saccharum Spp.). Plants 2024, 13, 2247. [Google Scholar] [CrossRef] [PubMed]
- Zamarreño, A.M.; Valduga, G.; Garcia-Mina, J.M. Cane Vinasses Contain Bioactive Concentrations of Auxin and Abscisic Acid in Their Composition. Int. J. Mol. Sci. 2022, 23, 9976. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Deng, Y.; Lv, R.; Akhtar, K.; Muhammad, I.; Farooq, M.; Wen, R. Foliar Application of Plant Growth Regulators Enhances Drought Tolerance by Modulating Growth and Biochemical Responses in Sugarcane Varieties. J. Agron. Crop Sci. 2024, 210, e12784. [Google Scholar] [CrossRef]
- Nong, Q.; Malviya, M.K.; Lin, L.; Xie, J.; Mo, Z.; Solanki, M.K.; Solanki, A.C.; Wang, Z.; Song, X.; Li, Y.; et al. Enhancing Sugarcane Seedling Resilience to Water Stress Through Exogenous Abscisic Acid: A Study on Antioxidant Enzymes and Phytohormone Dynamics. ACS Omega 2024, 9, 31684–31693. [Google Scholar] [CrossRef] [PubMed]
- Chavan, C.S.; Chavan, S.S.; Velhal, R.S.; Patil, A.G.; Deshmukh, L.P.; Bapat, V.A. Effect of Brassinolide on Invitro Growth of Sugarcane Co 86032 (Saccharum Spp.). AIP Conf. Proc. 2018, 1989, 030004. [Google Scholar] [CrossRef]
- Zhou, G.; Shabbir, R.; Sun, Z.; Chang, Y.; Liu, X.; Chen, P. Transcriptomic Analysis Reveals Candidate Genes in Response to Sorghum Mosaic Virus and Salicylic Acid in Sugarcane. Plants 2024, 13, 234. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, R.; Fan, Y.; Huang, X.; Luo, H.; Xiong, F.; Liu, J.; Zhang, R.; Lei, J.; Zhou, H.; et al. Integrated mRNA and Small RNA Sequencing Reveals microRNA Regulatory Network Associated with Internode Elongation in Sugarcane (Saccharum Officinarum L.). BMC Genom. 2019, 20, 817. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Yang, M.; Li, M.; Wang, L. Sugarcane Rind Secondary Metabolites and Their Antioxidant Activities in Eleven Cultivated Sugarcane Varieties. Sugar Tech 2022, 24, 1570–1582. [Google Scholar] [CrossRef]
- Fallah, N.; Pang, Z.; Zhang, C.; Tayyab, M.; Yang, Z.; Lin, Z.; Lin, W.; Ishimwe, C.; Ntambo, M.S.; Zhang, H. Complementary Effects of Biochar, Secondary Metabolites, and Bacteria Biocontrol Agents Rejuvenate Ratoon Sugarcane Traits and Stimulate Soil Fertility. Ind. Crops Prod. 2023, 202, 117081. [Google Scholar] [CrossRef]
- Jan, R.; Aaqil Khan, M.; Asaf, S.; Lubna; Park, J.-R.; Lee, I.-J.; Kim, K.-M. Flavonone 3-Hydroxylase Relieves Bacterial Leaf Blight Stress in Rice via Overaccumulation of Antioxidant Flavonoids and Induction of Defense Genes and Hormones. Int. J. Mol. Sci. 2021, 22, 6152. [Google Scholar] [CrossRef] [PubMed]
- Musilova, L.; Ridl, J.; Polivkova, M.; Macek, T.; Uhlik, O. Effects of Secondary Plant Metabolites on Microbial Populations: Changes in Community Structure and Metabolic Activity in Contaminated Environments. Int. J. Mol. Sci. 2016, 17, 1205. [Google Scholar] [CrossRef]
- Shi, Y.; Xia, H.; Cheng, X.; Zhang, L. Genome-Wide miRNA Analysis and Integrated Network for Flavonoid Biosynthesis in Osmanthus Fragrans. BMC Genom. 2021, 22, 141. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Jia, X.; Wang, W.K.; Liu, T.; Huang, S.P.; Yang, M.Y. Growth Under Elevated Air Temperature Alters Secondary Metabolites in Robinia Pseudoacacia L. Seedlings in Cd- and Pb-Contaminated Soils. Sci. Total Environ. 2016, 565, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Zhang, X.; Mu, Q.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.; Yu, X. Differences in Total Phenolics, Antioxidant Activity and Metabolic Characteristics in Peach Fruits at Different Stages of Ripening. LWT 2023, 178, 114586. [Google Scholar] [CrossRef]
- Kuhn, B.M.; Errafi, S.; Bucher, R.; Dobrev, P.; Geisler, M.; Bigler, L.; Zažímalová, E.; Ringli, C. 7-Rhamnosylated Flavonols Modulate Homeostasis of the Plant Hormone Auxin and Affect Plant Development. J. Biol. Chem. 2016, 291, 5385–5395. [Google Scholar] [CrossRef]
- Luo, M.; Wan, S.; Sun, X.; Ma, T.; Huang, W.; Zhan, J. Interactions Between Auxin and Quercetin During Grape Berry Development. Sci. Hortic. 2016, 205, 45–51. [Google Scholar] [CrossRef]
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, C.; Yu, C.; Dong, J.; Hu, J. Integration of Multi-Omics Technologies for Crop Improvement: Status and Prospects. Front. Bioinform. 2022, 2, 1027457. [Google Scholar] [CrossRef]
- Mehdi, F.; Cao, Z.; Zhang, S.; Gan, Y.; Cai, W.; Peng, L.; Wu, Y.; Wang, W.; Yang, B. Factors Affecting the Production of Sugarcane Yield and Sucrose Accumulation: Suggested Potential Biological Solutions. Front. Plant Sci. 2024, 15, 1374228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, Y.-X.; Liu, K.-X. Influence of Magnesium on Photosynthetic Characteristics and Growth Effects of Sugarcane at Seedling Stage. Am. J. Biochem. Biotechnol. 2019, 15, 33–38. [Google Scholar] [CrossRef]
- Dias, M.S.; Silva, F.A.; Fernandes, P.D.; Farias, C.H.A.; Silva, I.J.; Silva, M.F.C.; Lima, R.F.; Lacerda, C.N.; Lima, A.M.; Lima, V.R.N.; et al. Calcium Pyruvate in the Attenuation of the Water Deficit on the Agro-Industrial Quality of Ratoon Sugarcane. Braz. J. Biol. 2023, 83, e275046. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.R.; Karpe, A.H.; Patil, V.S.; Solanke, A.V.; Surve, U.S. Effect of Polyhalite as Potassium Source on Growth, Yield and Quality of Sugarcane Ratoons Under Different Irrigation Regimes. Int. J. Res. Agron. 2024, 7, 422–428. [Google Scholar] [CrossRef]
- Radasai, N.; Ketrot, D.; Tawornpruek, S.; Inboonchuay, T.; Wongsuksri, A. Effects of Foliar Potassium Supplementation on Yield and Nutrient Uptake of Plant Sugarcane. Sugar Tech 2024, 26, 1665–1675. [Google Scholar] [CrossRef]
- Islam, M.; Begum, M. Comparative Studies of Chlorophyll Content, Yield and Juice Quality of Eight Sugarcane Varieties. J. Agrofor. Environ. 2012, 6, 121–124. [Google Scholar]
- Wirojsirasak, W.; Songsri, P.; Jongrungklang, N.; Tangphatsornruang, S.; Klomsa-ard, P.; Ukoskit, K. Determination of Morpho-Physiological Traits for Assessing Drought Tolerance in Sugarcane. Plants 2024, 13, 1072. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, S.; Jiang, Y.; Lin, Z.; Luo, J.; Li, M.; Guo, J.; Su, Y.; Xu, L.; Que, Y. The Physiological and Agronomic Responses to Nitrogen Dosage in Different Sugarcane Varieties. Front. Plant Sci. 2019, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Pan, Y.-B.; Wang, Z.; Xu, F.; Cheng, W.; Huang, X.; Ren, H.; Pang, C.; Que, Y.; Xu, L. Utilization of a Sugarcane100K Single Nucleotide Polymorphisms Microarray-Derived High-Density Genetic Map in Quantitative Trait Loci Mapping and Function Role Prediction of Genes Related to Chlorophyll Content in Sugarcane. Front. Plant Sci. 2021, 12, 817875. [Google Scholar] [CrossRef]
- de Oliveira, R.I.; de Medeiros, M.R.F.A.; Freire, C.S.; Freire, F.J.; Neto, D.E.S.; de Oliveira, E.C.A. Nutrient Partitioning and Nutritional Requirement in Sugarcane. Aust. J. Crop Sci. 2016, 10, 69–75. [Google Scholar]
- Wickramasinghe, K.P.; Mao, J.; Xu, C.; Lin, X.; Li, X.; Qin, W.; Liu, H.; Liu, X.; Mehdi, F.; Zhao, P.; et al. Comparative Analysis of Seven Endogenous Hormones in a Novel Dual-Axillary Bud Mutant Genotype of Sugarcane. Sugar Tech 2024, 26, 1590–1600. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef]
- Han, W.-H.; Zhang, F.-B.; Ji, S.-X.; Liang, K.-L.; Wang, J.-X.; Fan, X.-P.; Liu, S.-S.; Wang, X.-W. Auxin-Salicylic Acid Seesaw Regulates the Age-Dependent Balance Between Plant Growth and Herbivore Defense. Sci. Adv. 2025, 11, eadu5141. [Google Scholar] [CrossRef]
- Desalegn, B.; Kebede, E.; Legesse, H.; Fite, T. Sugarcane Productivity and Sugar Yield Improvement: Selecting Variety, Nitrogen Fertilizer Rate, and Bioregulator as a First-Line Treatment. Heliyon 2023, 9, e15520. [Google Scholar] [CrossRef]
- Hewawansa, U.H.A.J.; Houghton, M.J.; Barber, E.; Costa, R.J.S.; Kitchen, B.; Williamson, G. Flavonoids and Phenolic Acids from Sugarcane: Distribution in the Plant, Changes During Processing, and Potential Benefits to Industry and Health. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13307. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Li, Y.-R.; Liao, J.-X. Involvement of Anthocyanins in the Resistance to Chilling-Induced Oxidative Stress in Saccharum Officinarum L. Leaves. Plant Physiol. Biochem. 2013, 73, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araújo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane Water Stress Tolerance Mechanisms and Its Implications on Developing Biotechnology Solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Abbas, S.R.; Sabir, S.M.; Ahmad, S.D.; Boligon, A.A.; Athayde, M.L. Phenolic Profile, Antioxidant Potential and DNA Damage Protecting Activity of Sugarcane (Saccharum Officinarum). Food Chem. 2014, 147, 10–16. [Google Scholar] [CrossRef]
- Kölln, O.T.; Boschiero, B.N.; Franco, H.C.J.; Soldi, M.C.M.; Sanches, G.M.; Castro, S.G.d.Q.; Trivelin, P.C.O. Preferential Mineral N Form Uptake by Sugarcane Genotypes Contrasting in Nitrogen Use Efficiency. Exp. Agric. 2022, 58, e32. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Ilieva, D.; Angelova, L.; Drochioiu, G.; Murariu, M.; Surleva, A. Estimation of Soil and Tailing Dump Toxicity: Development and Validation of a Protocol Based on Bioindicators and ICP-OES. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012110. [Google Scholar] [CrossRef]
- Kojima, M.; Kamada-Nobusada, T.; Komatsu, H.; Takei, K.; Kuroha, T.; Mizutani, M.; Ashikari, M.; Ueguchi-Tanaka, M.; Matsuoka, M.; Suzuki, K.; et al. Highly Sensitive and High-Throughput Analysis of Plant Hormones Using MS-Probe Modification and Liquid Chromatography–Tandem Mass Spectrometry: An Application for Hormone Profiling in Oryza Sativa. Plant Cell Physiol. 2009, 50, 1201–1214. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, J.; Jiang, W.; Chen, S. Metabolomics Study of Flavonoids in Coreopsis Tinctoria of Different Origins by UPLC–MS/MS. PeerJ 2022, 10, e14580. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. A Simple, Sensitive HPLC-DAD Method for Simultaneous Determination of Carotenoids, Chlorophylls and α-Tocopherol in Leafy Vegetables. J. Food Meas. Charact. 2017, 11, 979–986. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Chen, S.; Shan, B.; Wei, A.; Luo, Y.; Wu, L.; Jiang, Q.; Chen, Z. The Ionome–Hormone–Flavonoid Network Shapes Genotype-Dependent Yield Adaptation in Sugarcane. Plants 2025, 14, 3181. https://doi.org/10.3390/plants14203181
Lu Q, Chen S, Shan B, Wei A, Luo Y, Wu L, Jiang Q, Chen Z. The Ionome–Hormone–Flavonoid Network Shapes Genotype-Dependent Yield Adaptation in Sugarcane. Plants. 2025; 14(20):3181. https://doi.org/10.3390/plants14203181
Chicago/Turabian StyleLu, Qinyu, Shimiao Chen, Bin Shan, Ailin Wei, Yuhuan Luo, Lanfang Wu, Qiang Jiang, and Zhendong Chen. 2025. "The Ionome–Hormone–Flavonoid Network Shapes Genotype-Dependent Yield Adaptation in Sugarcane" Plants 14, no. 20: 3181. https://doi.org/10.3390/plants14203181
APA StyleLu, Q., Chen, S., Shan, B., Wei, A., Luo, Y., Wu, L., Jiang, Q., & Chen, Z. (2025). The Ionome–Hormone–Flavonoid Network Shapes Genotype-Dependent Yield Adaptation in Sugarcane. Plants, 14(20), 3181. https://doi.org/10.3390/plants14203181





