Abstract
Calcium signaling is essential for coordinating plant responses to diverse stimuli and regulating growth and development. Among calcium sensors, calmodulin (CaM) and CaM-like proteins (CMLs) represent a class that, despite increasing research, remains incompletely characterized in wheat, with many interacting partners and biological functions remaining largely elusive. This study conducted bioinformatics analyses of subgroup II CaM/CMLs, characterizing their phylogenetic relationships, conserved motifs, sequence features, and cis-elements. Expression analysis revealed that TaCML49-B was significantly upregulated in roots under salt stress. Moreover, TaCML49-B was localized to nucleus, cytoplasm, and membrane. Function characterization demonstrated that overexpression of TaCML49-B in Arabidopsis enhanced salt tolerance, whereas the BSMV-VIGS silencing of TaCML49-B reduced salt resistance in wheat. Furthermore, STRING database prediction analysis and bimolecular fluorescence complementation (BiFC) assay confirmed that TaCML49-B can physically interact with TaIQD23, which encodes an IQ67 domain protein, suggesting its potential involvement in the salt stress signaling pathway. Collectively, our findings indicate that TaCML49-B functions as a positive role in wheat salt stress response, thereby providing novel insights into the functions of TaCML genes and calcium signaling in wheat.
1. Introduction
As a universal second messenger, calcium (Ca2+) plays pivotal roles in plant growth, development, and stress signal transduction [1]. Calmodulin (CaM) and CaM-like proteins (CMLs) are the primary calcium sensors in eukaryotes [2]. By binding Ca2+ through characteristic EF-hand motifs, these proteins undergo conformational changes and regulate the activity of multiple downstream effectors in response to Ca2+ signaling [3]. Most CMLs contain two, four, or six EF-hand domains but lack other recognizable functional motifs, and they typically share at least 16% amino acid sequence identity with canonical CaMs [4].
CaM/CMLs interpret intracellular Ca2+ signatures and modulate diverse physiological and stress-related processes through interactions with specific target proteins [5]. Functional studies across different plant species have highlighted the critical roles of CaM/CMLs in development and stress adaptation [6]. For example, in Arabidopsis, CML16 and CML15 display distinct promoter activities during development [7], while CML41 contributes to bacterial defense by mediating Ca2+-dependent signaling specificity [8]. In tobacco, the CaM isoform rgsCAM (regulator of gene silencing, CaM) enhances viral resistance by inhibiting the activity of the helper component protease HC-Pro [9]. Overexpression of ShCML44 promotes seed germination and seedling vigor [10], and CML10 regulates ascorbic acid synthesis to enhance tolerance to drought, ozone, and UV stress [11]. Similarly, CML14 undergoes structural changes upon Ca2+ binding, which stabilizes the protein and modulates its functional specificity [12]. In rice, OsCML16 is transcriptionally regulated by OsERF48, thereby promoting root growth and drought tolerance [13]. In contrast, heterologous expression of GsCML27 reduces salt tolerance by disrupting ion homeostasis and osmotic regulation [14]. In wheat, several TaCML genes, including TaCML17, TaCML21, TaCML30, TaCML50, TaCML59, and TaCML75, are implicated in responses to abiotic stresses [15]. Moreover, overexpression of TaCML20 enhances the accumulation of water-soluble carbohydrates and improve yield-related traits under stress conditions [16]. TaCML36 has been reported to positively contribute to the wheat immune response against Rhizoctonia cerealis by modulating the expression of defense-associated genes [17]. In addition, Arabidopsis overexpressing TaCAM2-D exhibited enhanced tolerance to both drought and salt stress. TaCAM2-D was further verified to interact with TaMPK8, supporting the functional involvement of TaCAMs in wheat stress responses [18]. These findings collectively highlight the functional diversity of CaM/CMLs and their importance in mediating plant adaptation to environmental stress.
IQ67-domain (IQD) proteins have been implicated in both plant defense and organ development [19]. GhIQD10 interacts with GhCaM7 to control cotton fiber elongation via calcium signaling, and the interaction was inhibited by Ca2+ [20]. In soybean, GmIQD63 interacts with GmCDPK38 to mediate defense against the common cutworm [21]. Moreover, the consistent upregulation of PvIQD4, PvIQD10, PvIQD14, and PvIQD32 suggests that IQD genes may also play an important role in the response to salt stress [22]. However, the potential role of TaIQD in wheat salt stress signaling remains largely unexplored.
Wheat (Triticum aestivum L.) is one of the most important staple crops worldwide, providing essential calories and nutrients for the global population. However, wheat production is severely threatened by soil salinity, which has become one of the major abiotic stresses limiting crop productivity [23]. Excessive salinity impairs seedling establishment, vegetative growth, reproductive development, and grain yield, ultimately resulting in substantial agricultural losses [24]. According to FAO 2024, more than 1.38 billion hectares of land worldwide are currently affected by salinity [25]. By 2050, global warming and freshwater scarcity will result in over 50% of arable land being affected by salt, saline conditions drastically reduce wheat yields, with losses exceeding 60% compared to non-saline soils [26]. Salt tolerance is a genetically and physiologically complex trait, largely regulated by multiple genes and intricate signaling networks. Unfortunately, most modern wheat cultivars exhibit only limited tolerance to salinity, making them highly vulnerable to yield reductions in salt-affected areas [27]. Therefore, the identification and functional characterization of novel salt-responsive genes is a crucial step toward developing salt-tolerant wheat varieties, which is of the great significance for global food security.
Genome-wide transcriptome profiling of CaM/CMLs provides an effective strategy to identify novel regulators of wheat abiotic stress responses. However, the specific roles of CaM/CMLs in wheat stress adaptation remain poorly understood. This study performed the comprehensive analysis of subgroup II CaM/CMLs, including the phylogenetic relationships, conserved motifs, sequence features, and cis-elements analysis. Transcriptome analysis and quantitative real-time PCR (qRT-PCR) analysis revealed that TaCML49-B was strongly induced by salt stress in wheat roots. We performed the functional identification and mechanism analysis of TaCML49-B. TaCML49-B was localized to nucleus and plasma membrane. Overexpression of TaCML49-B in Arabidopsis increased the root growth under NaCl treatment; transgenic plants showed less wilting rate and cell membrane damage than WT under salt treatment in soil. To further study the function of TaCML49-B, BSMV-VIGS-mediated silencing was performed in wheat, and the silenced plants exhibited reduced salt tolerance and increased membrane damage. The prediction analysis and bimolecular fluorescence complementation assay (BiFC) indicated that TaCML49-B interacted with TaIQD23. Collectively, our results demonstrate that TaCML49-B functions as a positive regulator in wheat salt stress response. Our findings advance the understanding of wheat CaM/CMLs function, thereby providing foundational genetic resources for developing salt-tolerant wheat varieties.
2. Results
2.1. Subgroup II CaM/CMLs Are Involved in Responses to Multiple Abiotic Stresses
In a previous study, 128 CaM/CMLs were identified in wheat and designated according to their chromosomal locations. Phylogenetic analysis classified these genes into nine subgroups (group I-IX) [18]. Within subgroup II, 21 proteins from wheat, rice, and Arabidopsis were identified (Figure 1A, Table S1). Multiple subgroup II genes have been reported to participate in abiotic stress responses in Arabidopsis [7,28] and rice [29,30]. In wheat, subgroup II contains six homologous members corresponding to two genes, TaCML41 and TaCML49 (Figure 1A).
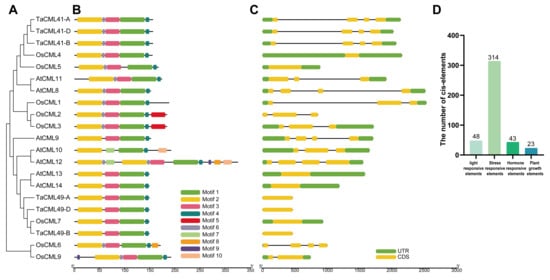
Figure 1.
Phylogenetic relationships, conserved motifs, gene structures, and cis-elements of subgroup II CaM/CMLs in wheat, rice, and Arabidopsis. (A) A maximum-likelihood (ML) phylogenetic tree of CaM/CML proteins from wheat (Ta), rice (Os), and Arabidopsis (At) constructed using MEGA X with 1000 bootstrap replicates. (B) Conserved motif distributions of 21 CaM/CMLs. (C) Gene structures of 21 CaM/CMLs. (D) Predicted cis-elements in promoter regions analyzed using the PlantCARE database.
Gene structure analysis is essential for understanding gene expression and function. To characterize subgroup II members, the 10 most statistically significant conserved motifs were identified, designated as motifs 1–10 (Figure 1B, Table S2). The number of motifs per protein ranged from 4 to 10, and most subgroup II CaM/CMLs exhibited relatively conserved motif compositions. Structural analysis further revealed that the majority of subgroup II CaM/CMLs contained two untranslated regions (UTRs), a single exon, and no introns (Figure 1C, Table S3).
Since gene expression is often regulated by cis-elements in promoter regions, we examined the upstream sequences of subgroup II CaM/CMLs and identified a total of 428 potential cis-elements (Figure 1D, Table S4). These elements were grouped into four major categories: light-responsive elements (11.21%), hormone-responsive elements (10.04%), environmental stress-responsive elements (73.36%), and plant growth-related elements (5.37%) (Figure 1D). The predominance of stress-related cis-elements suggests that subgroup II CaM/CMLs may play key roles in mediating plant responses to abiotic stresses.
To investigate the expression profiles of TaCML41 and TaCML49, wheat salt-related transcriptome data (PRJNA293629, PRJNA355905 and PRJNA374931) were analyzed. The expression of TaCML41 showed no significant variation across tissues or treatment conditions. In contrast, TaCML49 was downregulated in leaves and shoots (Figure 2A,B), but markedly upregulated in roots (Figure 2C). Based on this root-specific induction, subsequent analyses were focused on TaCML49-B.
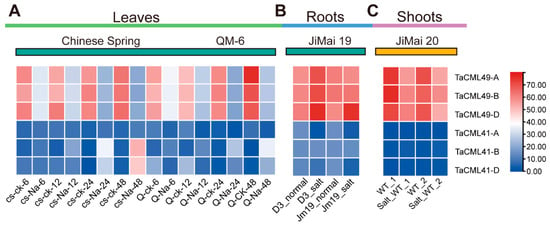
Figure 2.
Expression profiles of TaCML41 and TaCML49 in different wheat tissues. Transcriptome data from the Expression Atlas were used to analyze transcript accumulation in (A) leaves, (B) roots, and (C) shoots. Heatmaps were generated using EvolView, with transcript levels indicated by color gradients. CS, Chinese Spring; CK, control; QM, Qingmai; Na, salt stress; D3, overexpression of DREB in wheat (Jimai19); Jm, Jimai; WT, wild type.
2.2. Expression Pattern Analysis of TaCML49-B Under Salt Stress
The expression patterns of TaCML49-B under salt stress were examined using qRT-PCR. Samples from leaves and roots were collected at 0 h, 2 h, 4 h, 8 h, 12 h, 24 h, and 36 h after salt treatment. In leaves, the transcript levels of TaCML49-B decreased significantly under salt stress (Figure 3A), whereas the levels were markedly upregulated in roots (Figure 3B). These quantitative results were consistent with the transcriptome data, confirming that TaCML49-B is involved in salt stress response.
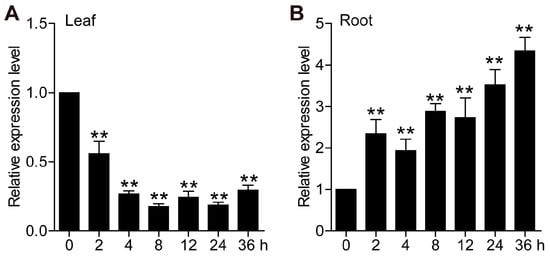
Figure 3.
Expression patterns of TaCML49-B under salt stress. qRT-PCR analysis of TaCML49-B expression in (A) leaves and (B) roots of seven-day-old wheat seedlings subjected to 200 mM NaCl treatment. Wheat TaActin was used as an internal control. Data represent mean ± SD from three biological replicates. Significant differences were determined by Student’s t-test (** p < 0.01).
2.3. TaCML49-B Was Located to Nucleus, Cytoplasm and Membrane
The subcellular localization of TaCML49-B was examined in wheat protoplasts leaves using a green fluorescent protein (GFP) fusion construct. Confocal microscopy revealed that TaCML49-B predominant localized to the nucleus, cytoplasm and membrane (Figure 4).
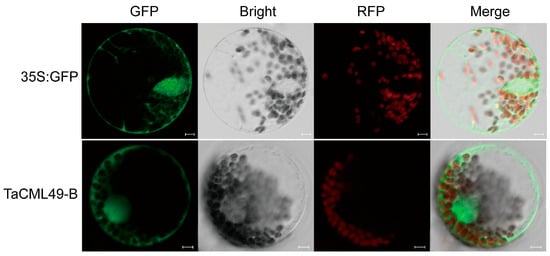
Figure 4.
Subcellular localization of TaCML49-B. The p16318hGFP (control) and TaCML49-B-GFP recombinant were transiently expressed in wheat protoplasts The green indicates GFP signals, and the red indicates chloroplast autofluorescence. Results were observed after transformation for 18 h with confocal microscopy. Scale bars = 5 mm.
2.4. Overexpression of TaCML49-B Enhances Salt Tolerance in Arabidopsis
To assess the role of TaCML49-B in salt-stress tolerance, transgenic lines overexpressing TaCML49-B were generated in Arabidopsis, and three overexpression (OE) lines with higher levels (OE1, OE2, and OE3) were selected by qRT-PCR. Five-day-old seedlings of wild type (WT) and OE lines were grown on 1/2 murashige and skoog (MS) medium supplemented with 0 (control), 75, or 100 mM NaCl (Figure 5A). After 7 days, primary root length, lateral root number, shoot fresh weight, and root fresh weight were measured (Figure 5B). Under normal conditions, no significant differences were observed between WT and OE lines. In contrast, under NaCl treatment, overexpressing TaCML49-B seedlings exhibited longer primary roots, increased lateral root numbers, and higher shoot and root fresh weights compared with WT plants. These results indicate that overexpression of TaCML49-B enhances salt-stress tolerance in Arabidopsis.
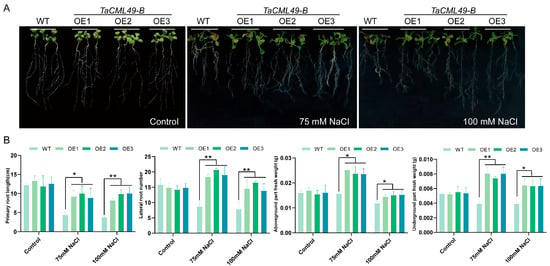
Figure 5.
Phenotypic analysis of TaCML49-B transgenic Arabidopsis under salt stress. (A) Root length assays of WT and TaCML49-B OE lines on MS medium and MS medium supplemented with 75 mM or 100 mM NaCl. (B) The primary root length, lateral root number, shoot fresh weight, and root fresh weight of WT and OE lines. Five-day-old seedlings were transferred to the indicated media and grown for 7 days. Data represent mean ± SD from three biological replicates. Significant differences were determined by Student’s t-test (* p < 0.05, ** p < 0.01).
To assess salt tolerance in soil, 21-day-old WT and TaCML49-B OE lines were irrigated with 0, 125, 150, or 200 mM NaCl solutions (Figure 6A). The wilting rate and shoot fresh weight were measured under different salt treatments (Figure 6B,C), and the physiological parameters including malondialdehyde (MDA), catalase (CAT), peroxidase (POD), and proline (Pro) content were analyzed (Figure 6D–G). Under normal growth conditions, no significant differences were observed between WT and OE lines in wilting rate, shoot fresh weight, or physiological parameters. Under different salt treatments, the aboveground fresh weight of OE lines was significantly higher than WT, while the wilting rate of OE lines was lower than WT. Under 125 mM and 150 mM NaCl, OE lines exhibited higher CAT, POD, and Pro contents, whereas MDA levels were lower compared with WT, which revealed that TaCML49-B OE lines had less cell membrane damage. These results indicate that overexpression of TaCML49-B enhances salt tolerance in Arabidopsis.
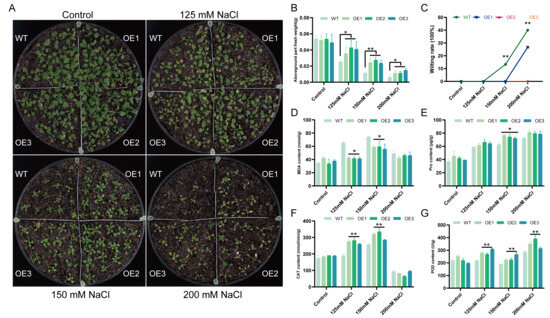
Figure 6.
Overexpression of TaCML49-B enhances salt tolerance in soil-grown Arabidopsis. (A) Phenotypes of 21-day-old WT and TaCML49-B overexpression plants under 0, 125, 150, or 200 mM NaCl treatments. (B) Aboveground fresh weight of WT and OE lines under different salt treatments. (C) Wilting rate of WT and OE lines under different salt treatments. (D–G) Physiological parameters of WT and overexpression plants under normal and salt stress conditions, including MDA (D), Pro (E), CAT (F), and POD (G) contents. Data represent mean ± SD from three biological replicates. Significant differences were determined by Student’s t-test (* p < 0.05, ** p < 0.01).
2.5. Silencing of TaCML49-B Alters Salt Response in Wheat
To further investigate the function of TaCML49-B in wheat, the BSMV-VIGS (barley stripe mosaic virus–virus-induced gene silencing) technique was employed. A conserved 250-bp cDNA fragment from all three TaCML49-B homologs was selected from the coding sequence (CDS) to generate the recombinant virus BSMV: TaCML49-B. Ten days after virus inoculation, virus-infected plants exhibited speckled stripes (Figure 7A). qRT-PCR analysis confirmed that TaCML49-B expression was significantly reduced in the leaves of BSMV: TaCML49-B-infected plants compared with control BSMV:00 plants (Figure 7B). Subsequently, virus-infected wheat plants were subjected to NaCl stress. After seven days, BSMV: TaCML49-B plants displayed higher sensitivity to salt stress than BSMV:00 plants (Figure 7C). Phenotype analysis revealed that MDA content was elevated, whereas Pro content was decreased in BSMV: TaCML49-B plants compared with controls (Figure 7D,E). These results indicate that silencing of TaCML49-B reduces salt resistance in wheat, demonstrating that TaCML49-B acts as a positive regulator in salt stress response.
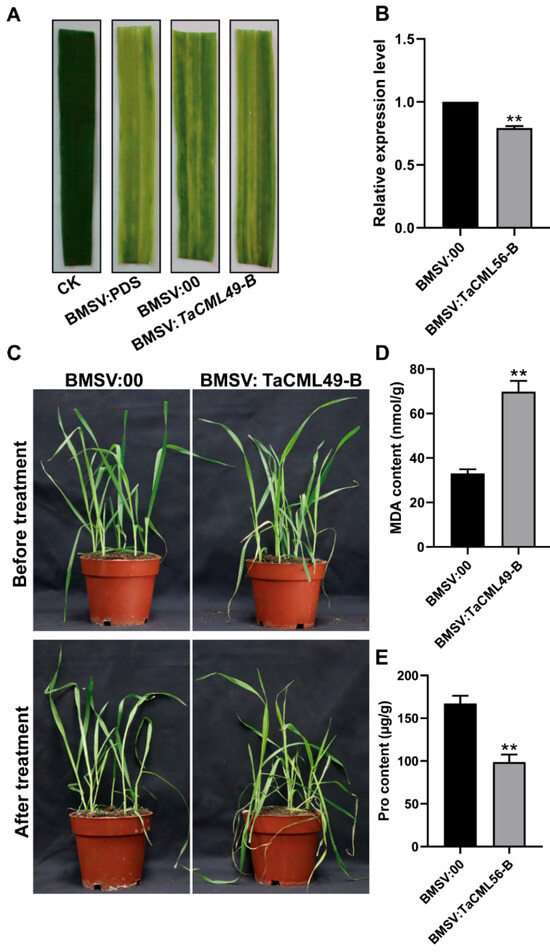
Figure 7.
Functional analysis of wheat TaCML49-B using BSMV-VIGS. (A) Phenotypes of wheat plants following BSMV-VIGS infection. (B) qRT-PCR analysis of TaCML49-B expression in plants infected with BSMV: TaCML49-B or BSMV:00 (control). (C) Phenotypic response of BSMV: TaCML49-B and control plants under salt stress. (D,E) Physiological parameters including MDA and Pro contents in BSMV: TaCML49-B and control plants under salt stress. Data represent mean ± SD from three biological replicates. Significant differences were determined by Student’s t-test (** p < 0.01).
2.6. Protein Interaction Network of TaCML49-B
To predict potential protein interactions, the STRING web server was used. The analysis suggested a possible interaction between TaCML49-B and TaIQD23 (Figure 8A). To further validate this interaction, BiFC assay was performed. TaCML49-B fused to the N-terminal half of YFP (nYFP) and TaIQD23 fused to the C-terminal half of YFP (cYFP) were transiently co-expressed in wheat protoplasts. Yellow fluorescence signals were detected, confirming TaCML49-B interacted with TaIQD23 (Figure 8B).
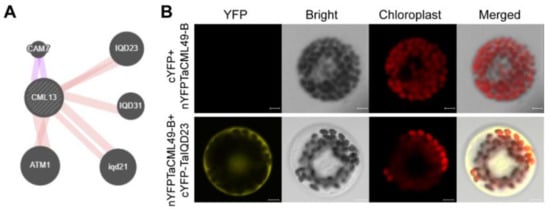
Figure 8.
TaCML49-B interact with TaIQD23. (A) Predicted protein–protein interaction network of TaCML49-B generated using the STRING database. (B) BiFC assay in wheat protoplasts. TaCML49-B coding sequence was fused to the N-terminal half of YFP (pSPYNE, nYFP), and TaIQD23 coding sequence was fused to the C-terminal half of YFP (pSPYCE, cYFP). cYFP and nYFP-TaCML49-B were served as control. Fluorescence signals indicate the interaction between TaCML49-B and TaIQD23. Scale bars = 5 mm.
3. Discussion
Plant Ca2+ sensor proteins play essential roles in Ca2+ signaling networks, maintaining Ca2+ homeostasis during diverse cellular processes [31,32]. CaM/CMLs constitute a major group of EF-hand-containing Ca2+ sensors in plants, which bind Ca2+ and regulate downstream targets in response to stimulus-induced Ca2+ fluctuations and signal transduction [33]. Although 128 TaCaM/CMLs have been classified into nine subgroups in wheat [18], functional data on these genes remain limited. In the present study, a comprehensive analysis of Subgroup II CaM/CMLs from wheat, rice, and Arabidopsis was performed, including phylogenetic relationships, conserved motifs, and gene structures. Furthermore, TaCML49-B was significantly upregulated in roots under salt stresses and was, thus, selected for functional identification and mechanism analysis.
A phylogenetic analysis was performed to compare CaM/CMLs from wheat, Arabidopsis, and rice (Figure 1). The results indicated that both sequence and function of CaM/CMLs are largely conserved among these species. However, wheat CaM/CMLs exhibited a closer phylogenetic relationship with rice than with Arabidopsis (Figure 1). Moreover, although differences in intron-exon structures were observed among the 21 CaM/CMLs, members within the same phylogenetic branch shared common structural characteristics (Figure 1). These findings suggest that the combination of conservation and diversity in motifs and gene structures contributes to the evolution and functional diversification of this gene family.
Abiotic stresses activate genes critical for stress resistance. Numerous stress-responsive cis-elements (CREs) were identified in the promoter regions of the 21 CaM/CMLs analyzed, indicating potential roles in plant stress responses. For instance, AtCML9 expression in seedlings is rapidly induced by abiotic stress and abscisic acid (ABA) [34]. RNA-seq analysis highlighted regulatory relationships between CML8 and genes involved in growth and brassinosteroid (BR) signaling, and co-immunoprecipitation experiments demonstrated that CML8 interacts with the BR receptor BRI1 in a ligand-dependent manner [35]. Additionally, RNA interference of AtCML13 and AtCML14 in mature plants resulted in shortened siliques, reduced root systems, and accelerated leaf senescence [28].
Subgroup II of CaM/CMLs includes six homologous genes corresponding to two wheat genes (TaCML41 and TaCML49), the functions of which in wheat have not been fully characterized. In the present study, transcript levels of these six homologs were analyzed in three wheat tissues at different developmental stages using previously reported transcriptome data. Transcript accumulation of TaCML41 and TaCML49 (Figure 2), together with qRT-PCR results, indicated that TaCML41-B is responsive to salt stress and may regulate plant responses to salinity. Interestingly, TaCML49-B was downregulated in leaves but upregulated in roots under salt stress, indicating tissue-specific regulation (Figure 3). Such contrasting expression patterns suggest that TaCML49-B may function not only in salt-stress signaling but also in processes related to stress-induced aging or senescence. Consistently, in Oryza sativa, OsCML4 is highly expressed under 150 mM NaCl stress [30]; as TaCML41 is homologous to OsCML4, it is likely involved in salt and drought stress responses in wheat.
To further investigate TaCML41-B function under salt stress, transgenic Arabidopsis overexpressing TaCML41-B was generated. TaCML41-B remained relatively insensitive to 100 mM NaCl, whereas WT plants showed a hypersensitive phenotype (Figure 5), suggesting that TaCML41-B is crucial for salt tolerance. Physiological analyses showed that, under salinity, MDA content was significantly reduced, whereas Pro content was significantly elevated in TaCML41-B overexpressing plants (Figure 6). These results indicate that overexpression of TaCML41-B enhances salt tolerance in Arabidopsis. Conversely, silencing of TaCML41-B in wheat using BSMV- VIGS led to decreased salt tolerance, accompanied by increased MDA content and decreased Pro levels (Figure 7). As MDA reflects lipid peroxidation and membrane injury and Pro functions as both an osmoprotectant and ROS scavenger, these changes suggest that TaCML41-B enhances salt tolerance by alleviating oxidative damage and maintaining cellular homeostasis.
By predicting the interaction network of TaCML41-B, it was found that TaCML41-B interacts with TaIQD23 (Figure 8). Previous studies have reported that Arabidopsis IQD1 can interact with CaM/CMLs in vitro in a Ca2+-dependent manner [36]. Moreover, two IQD genes in cotton, Gh_S06G0014 and Gh_S09G1608, were shown to enhance tolerance to drought and salt stress [37]. These findings suggest that the interaction between TaCML41-B and TaIQD23 may contribute to improved salt-stress tolerance in wheat (Figure 9). Collectively, our results indicate that TaCML41-B functions as a positive regulator of salt-stress tolerance, underscoring its important role in wheat responses to abiotic stress.
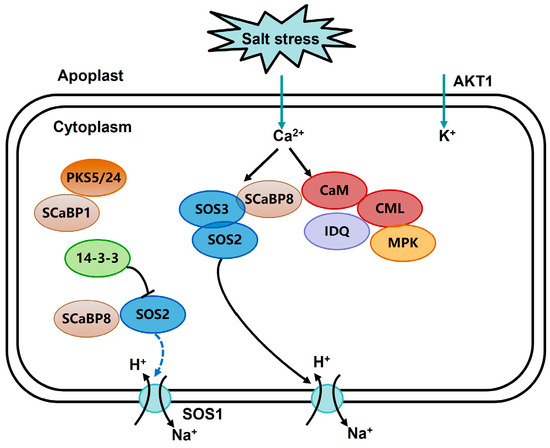
Figure 9.
Schematic diagram of salt-stress response, including CML regulation.
4. Materials and Methods
4.1. Sequence Characteristics and Phylogenetic Analysis of 21 CaM/CMLs
The nucleic acid and protein databases of 21 CaM/CMLs of Arabidopsis, rice, and wheat were downloaded from the Ensemble Plants database (http://plants.ensembl.org/index.html, accessed on 2 June 2025). The CaM/CMLs protein sequences were aligned using ClustalX 2.0 with the default parameters [38]. A phylogenetic tree was constructed using the maximum likelihood method with 1000 bootstrap replicates as implemented in MEGA X [39].
4.2. Exon-Intron Structure, Motif Analysis and Cis-Elements Analysis of 21 CaM/CMLs
The 21 CaM/CMLs gene information was extracted from the GFF3 file and the information was visualized using the gene structure plate in TBtools software V2.326 [40]. The wheat gene protein sequences were integrated, the conserved motifs were analyzed by the online tool MEME (http://alternate.meme-suite.org/tools/meme, accessed on 1 August 2025) [41], and the predicted results were put into TBtools software for visualization.
To analysis the possible biological functions and transcriptional regulation of 21 CaM/CMLs. The 2.0 kb region sequences upstream from start codons were downloaded from Ensemble Plants database and then submitted to PlantCARE database for cis-elements analysis (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 2 August 2025)).
4.3. Expression Patterns Analysis of TaCML41 and TaCML49
The expression data of TaCML41 and TaCML49 gene in leaves and roots after salt stress were downloaded from the abiotic stress transcriptome. The abiotic stress transcriptome data can be searched from the NCBI website (https://www.ncbi.nml.nih.gov, accessed on 3 August 2025). TBtools software is then used to visualize the data obtained above [42].
4.4. Plant Material and Stress Treatments
We examined the expression patterns of candidate genes in wheat variety ‘Fielder’. The seeds were treated with H2O2 and transferred for 4 °C to break dormancy. After germination, the seeds were transferred to a hydroponic box for 25 °C cultivate with photoperiod of 16/8 h. The root and leaf tissues of wheat plants were collected at 0 h, 2 h, 4 h, 8 h, 12 d, 24 d, and 36 h after 200 mM NaCl treatment. The collected samples were immediately frozen in liquid nitrogen and stored in a −80 °C refrigerator for RNA extraction.
4.5. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
RNA was extracted from wheat using the Novozan kit, and the first strand cDNA was synthesized using the full-type Gold Company kit during reverse transcription. ABI Prism 7500 real-time PCR system (Applied Bio systems, Foster City, CA, USA) was used for qRT-PCR and the reagents of Vazyme Taq Pro Universal SYBR qPCR Master Mix. Gene cluster-specific and internal reference was conducted using gene TaActin (accession no. AB181991). The qRT-PCR program was carried out as follows: pre-denaturation at 95 °C for 30 s; denaturation at 94 °C for 10 s, annealing at 60 °C for 30 s, extension at 72 °C for 34 s, 40 cycles. At least three biological replicates were used for qRT-PCR analysis, and the 2−ΔΔCt method was used to analyze the data [43]. The amplification efficiency of primers was determined by melting curve analysis. Primers were listed in Table S5.
4.6. Subcellular Localization of TaCML49-B
The full-length coding sequence (CDS) of TaCML49-B with the stop codon removed was cloned into p16318hGFP vector under control of the CaMV35S promoter. For transient expression assays, 7-day-old wheat seedlings were used for the isolation of wheat protoplasts. The p16318hGFP-TaCML49-B and control plasmids were transformed into wheat protoplasts mediated by PEG4000, as described by [44]. The transfected protoplasts were incubated at 22 °C for 18 h in darkness, after which GFP signals were observed with 488 nm and 543 nm illumination by a confocal laser scanning microscopy (LSM700, CarlZeiss, Oberkochen, Germany). Primers were listed in Table S5.
4.7. Transformation of Arabidopsis and Propagation of Positive Seedlings
The CDS of TaCML49-B was cloned into the vector pCAMBIA2300 and the recombinant vector 2300-TaCML49-B was obtained. The correct recombinant vector was transformed into Agrobacterium tumefaciens strain GV3101. Transgenic Arabidopsis thaliana was obtained by Agrobacterium-mediated inflorescence method [45]. Columbia-0 (WT) was used as control. T0 generation transgenic seeds were placed in 1/2 MS medium supplemented with 50 mg/L kanamycin to screen positive seedlings until to T3 generation.
4.8. Salt-Stress Assays of Transgenic Arabidopsis Plants
Three homozygous T3 lines were selected for phenotypic analysis. For the root assay, WT and transgenic seeds were disinfected and vernalized for 3 days to break dormancy. After 5 days of growth, WT and transgenic Arabidopsis plants were transplanted to 1/2 MS solid medium with different concentrations of NaCl (75, and 100 mM) for salt-stress treatment. After 7 days of treatment, Arabidopsis seedlings were scanned using the Epson Expression 11000XL flatbed scanner (Epson, Suwa, Japan) to determine the length of taproot and number of lateral roots. Then samples were taken to determine the fresh weight of aboveground and underground Arabidopsis seedlings after treatment.
In the phenotypic observation experiment, seeds of WT and transgenic Arabidopsis plants were seeded into flowerpots and salt-stress tests were carried out when they were 3 weeks old. The flowerpots were watered with different concentrations of NaCl solution (125 mM, 150 mM, and 200 mM NaCl). After 7 days of salt treatment, the phenotypes were observed, and the wilting rate and fresh weight of above-ground parts were calculated. The contents of MDA, PRO, CAT, and POD in transgenic Arabidopsis seedlings treated with salt solution at different concentrations were detected with the physiological index assay kit (Suzhou Comin Biotechnology, Suzhou, China). The test was carried out according to the determination method in the kit instructions, and three biological replicates were performed for each experiment.
4.9. BSMV-VIGS-Induced TaCML49-B Silencing Assays
A conserved 200–300 bp cDNA fragment was isolated from TaCML49-B using the online tool SGN VIGS (https://vigs.solgenomics.net/, accessed on 3 July 2025). The γ-TaCML49-B was sequenced by inserting it into a γ plasmid with the restriction site Nhe I. The α, β, and γ plasmids (γ-TaCML49-B and γ-GFP) were linearized, respectively. The restriction sites of α and γ plasmids were Mul I, and the restriction sites of β plasmids were Spe I. RNA was synthesized according to the instructions of the Ribo MAX™Large Scale RNA Production System-T7 kit (Promega, Fitchburg, WI, USA). The synthesized RNA-α, RNA-β, RNA-γ- TaCML49-B (or control RNA-γ-GFP) were mixed and friction-inoculated into two-leaf wheat leaves. Ten days after virus transfection, the leaves were collected to monitor the virus infection [46,47]. The expression level of TaCML49-B in the leaves after inoculation was detected using qRT-PCR. Then, the silenced plants were treated with salt stress to observe the changes of phenotype and relative expression.
4.10. Prediction and Validation of TaCML49-B Interaction Networks
Protein interaction networks of the TaCML49-B were predicted using the STRING database (https://cn.string-db.org/ (accessed on 10 August 2025)).
4.11. Bimolecular Fluorescence Complementation (BiFC) Assay
The vectors used in the BiFC experiment are pSPYNE (nYFP) and pSPYCE (cYFP). The BamHI restriction enzyme from the NEB company was used to cleave nYFP and cYFP vectors. The design adapter was primed, the stop codons were removed, and the target gene with an adapter from the constructed zero background vector was amplified. Construct nYFP-TaCML49-B and nYFP-TaIQD23 vectors. Extract plasmids from the correctly sequenced bacterial solution, extract wheat protoplasts, and add two types of plasmids (10 μg each) to verify their interaction, co-transformed cYFP and nYFP-TaCML49-B constructs as control. Observe the yellow fluorescence signal under a confocal laser microscope. The sequences for the primers used are listed in Table S5.
5. Conclusions
In this study, a comprehensive analysis of Subgroup II of the CaM/CMLs was conducted, including characterization of sequence features, phylogenetic relationships, conserved motifs, and cis-elements. To investigate the molecular mechanisms underlying abiotic stress tolerance, the function of a key CML protein, TaCML49-B, was characterized. Gene expression analyses indicated that TaCML49-B may play important roles in both development and stress responses. Overexpression of TaCML49-B in Arabidopsis enhanced tolerance to salt stress, whereas silencing of TaCML49-B in wheat reduced salt tolerance, demonstrating that TaCML49-B functions as a positive regulator of salt-stress responses. Furthermore, the interaction between TaCML49-B and TaIQD23 was confirmed, supporting its involvement in wheat stress-response pathways. Collectively, these findings provide comprehensive insights into the TaCML gene family in wheat and offer a foundation for further functional characterization aimed at developing stress-resistant wheat varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14203163/s1, Table S1: List of sequence IDs used in the phylogenetic analysis; Table S2: List of conserved motifs of 21 CaM/CMLs proteins; Table S3: Exon and intron numbers of 21 CaM/CMLs; Table S4: Cis-elements in the promoter region of 21 CaM/CMLs; Table S5: The primers used in this study.
Author Contributions
Conceptualization, J.R., J.H. and H.S.; methodology, J.R. and J.H.; software, J.H. and X.J.; validation, B.H. and J.Y.; formal analysis, H.W., J.H. and J.R.; investigation, B.Q.; resources, P.G. and J.Z.; data curation, J.R., Z.X. and H.S.; writing—original draft preparation, B.H., J.R. and Z.X.; writing—review and editing, J.R., Z.X. and H.S.; funding acquisition, J.R. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project of Shanxi Province Key Lab Construction (Z135050009017-2-9), the Shanxi Province Science Foundation for Youths (202303021222044), the Scientific and Technological Innovation Programs of Shanxi Province (2023L045), the Talent Introduction and Research Launch Project of Shanxi Agricultural University (2023BQ83 and 2024BQ18), and the Shanxi Provincial Doctoral Graduates and Postdoctoral Researchers Working in Shanxi Reward Fund Research Project (SXBYKY2023025 and SXBYKY2024102).
Data Availability Statement
Data Availability Statement: Data are contained within this article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, Y.; Du, Y.; Du, J.; Han, Y. Genome-wide analysis of the CML gene family and its response to melatonin in common bean (Phaseolus vulgaris L.). Sci. Rep. 2023, 13, 1196. [Google Scholar] [CrossRef]
- Gifford, J.L.; Walsh, M.P.; Vogel, H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007, 405, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Bergey, D.R.; Kandel, R.; Tyree, B.K.; Dutt, M.; Dhekney, S.A. The Role of Calmodulin and Related Proteins in Plant Cell Function: An Ever-Thickening Plot. Springer Sci. Rev. 2014, 2, 145–159. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, L.; Singh, A.; Wang, H.; Du, L.; Poovaiah, B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 600. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling—ScienceDirect. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef]
- Adenike, O.; Kim, M.; Alexandra, D.; Midhat, U.; Snedden, W.A. Arabidopsis calmodulin-like proteins, cml15 and cml16 possess biochemical properties distinct from calmodulin and show non-overlapping tissue expression patterns. Front. Plant Sci. 2017, 8, 2175. [Google Scholar] [CrossRef]
- Xu, B.; Cheval, C.; Laohavisit, A.; Hocking, B.; Chiasson, D.; Olsson, T.S.G.; Shirasu, K.; Faulkner, C.; Gilliham, M. A calmodulin-like protein regulates plasmodesmal closure during bacterial immune responses. New Phytol. 2017, 215, 77–84. [Google Scholar] [CrossRef]
- Nakahara, K.S.; Masuta, C.; Yamada, S.; Shimura, H.; Kashihara, Y.; Wada, T.S.; Meguro, A.; Goto, K.; Tadamura, K.; Sueda, K.; et al. Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc. Natl. Acad. Sci. USA 2012, 109, 10113–10118. [Google Scholar] [CrossRef]
- Munir, S.; Liu, H.; Xing, Y.; Hussain, S.; Ouyang, B.; Zhang, Y.; Li, H.; Ye, Z. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016, 6, 31772. [Google Scholar] [CrossRef]
- Cho, K.M.; Nguyen, H.T.; Kim, S.Y.; Shin, J.S.; Cho, D.H.; Hong, S.B.; Shin, J.S.; Ok, S.H. CML10, a variant of calmodulin, modulates ascorbic acid synthesis. New Phytol. 2016, 209, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Vallone, R.; Verde, V.L.; D’Onofrio, M.; Giorgetti, A.; Astegno, A. Metal binding affinity and structural properties of calmodulin-like protein 14 from Arabidopsis thaliana. Protein Sci. 2016, 25, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Chung, P.J.; Park, S.H.; Redillas, M.C.F.R.; Kim, Y.S.; Suh, J.W.; Kim, J.K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant Biotechnol. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, X.; Duanmu, H.; Zhu, D.; Yu, Y.; Cao, L.; Liu, A.; Jia, B.; Xiao, J.; Zhu, Y. GsCML27, a Gene Encoding a Calcium-Binding Ef-Hand Protein from Glycine soja, Plays Differential Roles in Plant Responses to Bicarbonate, Salt and Osmotic Stresses. PLoS ONE 2015, 10, e0141888. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Liu, L.; Su, Y.; Li, Y.; Jia, W.; Jiao, B.; Wang, J.; Yang, F.; Dong, F.; et al. Genome-wide identification and expression analysis of calmodulin and calmodulin-like genes in wheat (Triticum aestivum L.). Plant Signal. Behavior 2022, 17, 2013646. [Google Scholar] [CrossRef]
- Kalaipandian, S.; Xue, G.P.; Rae, A.L.; Glassop, D.; Bonnett, G.D.; Mcintyre, L.C. Overexpression of TaCML20, a calmodulin-like gene, enhances water soluble carbohydrate accumulation and yield in wheat. Physiol. Plant. 2019, 165, 790–799. [Google Scholar] [CrossRef]
- Lu, L.; Rong, W.; Zhou, R.; Huo, N.; Zhang, Z.J. TaCML36, a wheat calmodulin-like protein, positively participates in an immune response to Rhizoctonia cerealis. Crop J. 2019, 7, 608–618. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Dong, F.; Zou, J.; Gao, C.; Zhu, Z.; Liu, Y. Multiple roles of wheat calmodulin genes during stress treatment and TaCAM2-D as a positive regulator in response to drought and salt tolerance. Int. J. Biol. Macromol. 2022, 220, 985–997. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Li, D. New Insights Into Functions of IQ67-Domain Proteins. Front. Plant Sci. 2020, 11, 614851. [Google Scholar] [CrossRef]
- Xu, F.; Wang, L.; Xu, J.; Chen, Q.; Ma, C.; Huang, L.; Li, G.; Luo, M. GhIQD10 interacts with GhCaM7 to control cotton fiber elongation via calcium signaling. Crop J. 2023, 11, 447–456. [Google Scholar] [CrossRef]
- Li, X.; Su, F.; Xiang, J.; Zhang, M.; Chen, X.; Hu, D.; Yu, D.; Wang, H. GmIQD63 functions as a novel GmCDPK38-interacting protein in soybean defense against the common cutworm. Planta 2025, 262, 103. [Google Scholar] [CrossRef] [PubMed]
- Kirlioğlu, T.; Okay, A.; Aras, E.S.; Büyük, I. Investigation and computational analysis of the IQD gene family in common bean (Phaseolus vulgaris L.): Expression profiling under salt stress. Turk. J. Bot. 2024, 48, 532–550. [Google Scholar] [CrossRef]
- Farooq, M.; Zahra, N.; Ullah, A.; Nadeem, F.; Rehman, A.; Kapoor, R.; Al-Hinani, M.S.; Siddique, K.H. Salt Stress in Wheat: Effects, Tolerance Mechanisms, and Management. J. Soil Sci. Plant Nutr. 2025, 24, 8151–8173. [Google Scholar] [CrossRef]
- Guo, X.; Wu, C.; Wang, D.; Wang, G.; Jin, K.; Zhao, Y.; Tian, J.; Deng, Z. Conditional QTL mapping for seed germination and seedling traits under salt stress and candidate gene prediction in wheat. Sci. Rep. 2022, 12, 21010. [Google Scholar] [CrossRef]
- Abdellaoui, R.; Elkelish, A.; El-Keblawy, A.; Mighri, H.; Boughalleb, F.; Bakhshandeh, E. Editorial: Halophytes: Salt stress tolerance mechanisms and potential use. Front. Plant Sci. 2023, 14, 1218184. [Google Scholar] [CrossRef]
- Khan, M.S.; Rizvi, A.; Saif, S.; Zaidi, A. Phosphate-Solubilizing Microorganisms in Sustainable Production of Wheat: Current Perspective. Probiotics Agroecosyst. 2017, 3, 51–81. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Anwar, S.; Perveen, S.; Iqbal, N.; Noman, A.; Ali, M. Influence of melatonin on antioxidant defense system and yield of wheat (Triticum aestivum L.) genotypes under saline condition. Pak. J. Bot. 2019, 51, 1987–1994. [Google Scholar] [CrossRef]
- Symonds, K.; Teresinski, H.; Hau, B.; Chiasson, D.; Benidickson, K.; Plaxton, W.; Snedden, W.A. Arabidopsis CML13 and CML14 Have Essential and Overlapping Roles inPlant Development. Plant Cell Physiol. 2024, 65, 228–242. [Google Scholar] [CrossRef]
- Yan, Z.; Deng, R.; Zhang, H.; Li, J.; Zhu, S. Transcriptome analysis of floret opening and closure both Indica and Japonica rice. 3 Biotech 2022, 12, 188. [Google Scholar] [CrossRef]
- Chinpongpanich, A.; Limruengroj, K.; Phean, O.P.S.; Limpaseni, T.; Buaboocha, T. Expression analysis of calmodulin and calmodulin-like genes from rice, Oryza sativa L. BMC Res. Notes 2012, 5, 625. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Brownlee, C. The generation of Ca(2+) signals in plants. Annu. Rev. Plant Biol. 2004, 55, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Pandey, G.K. Expressional analysis and role of calcium regulated kinases in abiotic stress signaling. Curr. Genom. 2010, 11, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.P.; Aldon, D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008, 56, 575–589. [Google Scholar] [CrossRef]
- Lucchin, A.; Fouassier, H.; Robe, E.; Mbengue, M.; Aguilar, M.; San Clemente, H.; Vert, G.; Galaud, J.P.; Aldon, D. The calcium sensor AtCML8 contributes to Arabidopsis plant cell growth by modulating the brassinosteroid signaling pathway. Plant J. 2025, 121, e17179. [Google Scholar] [CrossRef]
- Bürstenbinder, K.; Savchenko, T.; Müller, J.; Adamson, A.W.; Stamm, G.; Kwong, R.; Zipp, B.J.; Dinesh, D.C.; Abel, S. Arabidopsis calmodulin-binding protein IQ67-domain 1 localizes to microtubules and interacts with kinesin light chain-related protein-1. J. Biol. Chem. 2013, 288, 1871–1882. [Google Scholar] [CrossRef]
- Yang, X.; Kirungu, J.N.; Magwanga, R.O.; Xu, Y.; Pu, L.; Zhou, Z.; Hou, Y.; Cai, X.; Wang, K.; Liu, F. Knockdown of GhIQD31 and GhIQD32 increases drought and salt stress sensitivity in Gossypium hirsutum. Plant Physiol. Biochem. 2019, 144, 166–177. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; Mcwilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2. 0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Shi, W.-Y.; Du, Y.-T.; Ma, J.; Min, D.-H.; Jin, L.-G.; Chen, J.; Chen, M.; Zhou, Y.-B.; Ma, Y.-Z.; Xu, Z.-S.; et al. The WRKY Transcription Factor GmWRKY12 Confers Drought and Salt Tolerance in Soybean. Int. J. Mol. Sci. 2018, 19, 4087. [Google Scholar] [CrossRef]
- Udvardi, M.K.; Czechowski, T.; Scheible, W.R. Eleven golden rules of quantitative RT-PCR. Plant Cell 2008, 20, 1736–1737. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Gu, B.; Huang, G.; Tian, Y.; Quan, J.; Lindqvist-Kreuze, H.; Shan, W. Conserved RXLR Effector Genes of Phytophthora infestans Expressed at the Early Stage of Potato Infection Are Suppressive to Host Defense. Front. Plant Sci. 2017, 8, 2155. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-F.; Zhao, W.-Y.; Fu, J.-D.; Liu, Y.-W.; Chen, M.; Zhou, Y.-B.; Ma, Y.-Z.; Xu, Z.-S.; Xi, Y.-J. Genome-Wide Analysis of CDPK Family in Foxtail Millet and Determination of SiCDPK24 Functions in Drought Stress. Front. Plant Sci. 2018, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, G.; Li, G.; Yuan, S.; Wang, C.; Xie, Y.; Guo, T.; Kang, G.; Wang, D. TaPHT1;9-4B and its transcriptional regulator TaMYB4-7D contribute to phosphate uptake and plant growth in bread wheat. New phytol. 2021, 231, 1968–1983. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Ren, Q.; Yan, B.; Zhao, Y.; Yu, P.; Gao, G.; Shi, H.; Chang, S.; Chang, Y.Z. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021, 12, 447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).