Chilean Aloysia Essential Oils: A Medicinal Plant Resource for Postharvest Disease Control
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Essential Oils
2.2. Antifungal Activity of Essential Oils and Compounds
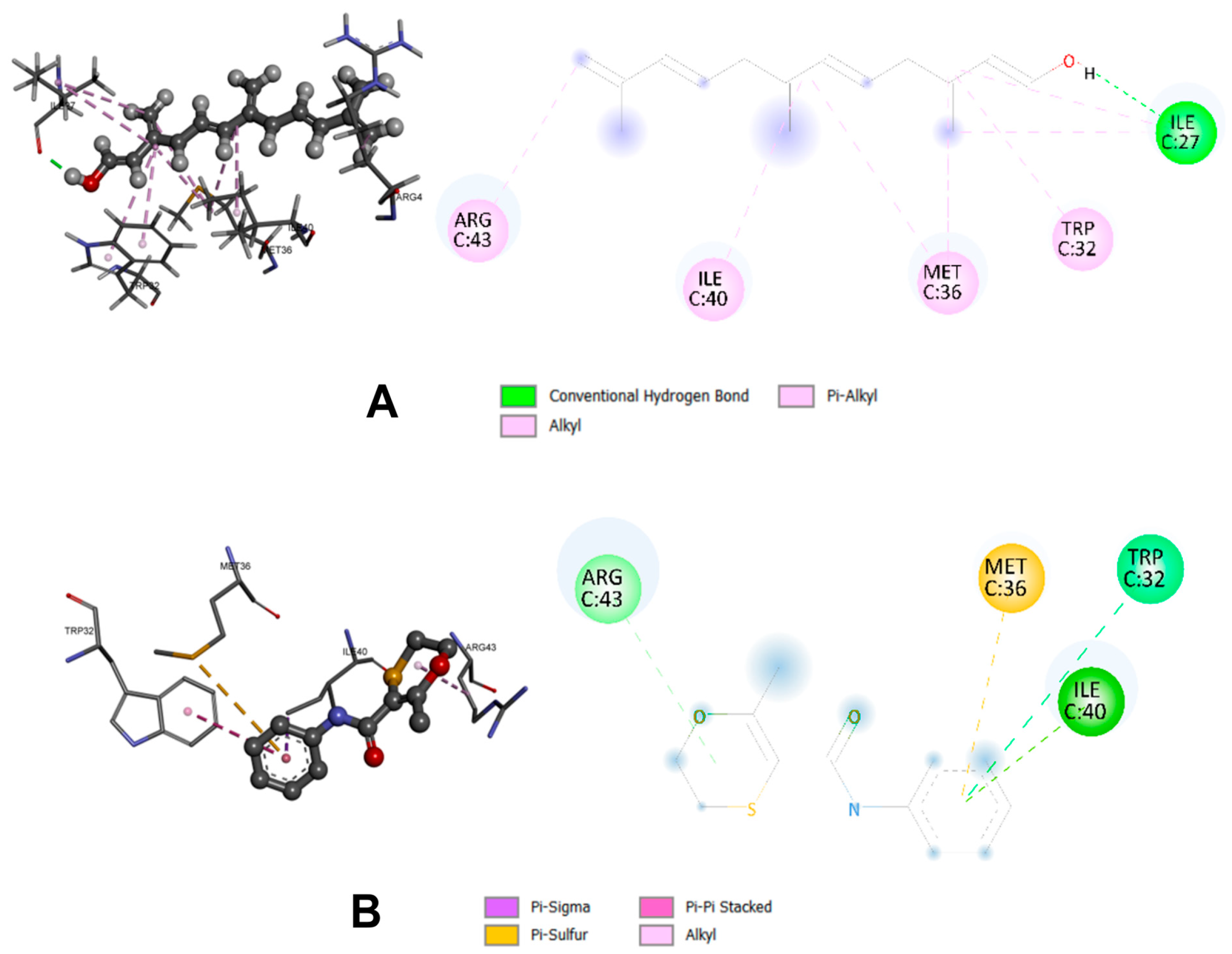
2.3. Molecular Docking
3. Materials and Methods
3.1. General Data
3.2. Plant Material
3.3. Essential Oil Extraction
3.4. Gas Chromatography Analyses
3.5. Antifungal Activity
Fungal Growth Conditions
3.6. Antifungal Assay In Vitro
3.7. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martini, C.; Mari, M. Monilinia fructicola, Monilinia laxa (Monilinia Rot, Brown Rot). In Postharvest Decay; Elsevier: Amsterdam, The Netherlands, 2014; pp. 233–265. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Jeffries, P.; Xu, X.-M. Epidemiology and Management of Brown Rot on Stone Fruit Caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Kou, J.; Wei, Y.; He, X.; Xu, J.; Xu, F.; Shao, X. Infection of Post-Harvest Peaches by Monilinia fructicola Accelerates Sucrose Decomposition and Stimulates the Embden–Meyerhof–Parnas Pathway. Hortic. Res. 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.U.; Zhu, Q.; Wu, Z.; Li, X.; Chen, W.; Xiong, T.; Zhu, X. Current Insights into the Biocontrol and Biotechnological Approaches for Postharvest Disease Management of Botrytis cinerea. Postharvest Biol. Technol. 2024, 216, 113055. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Landi, L.; Raguseo, C.; Pollastro, S.; Faretra, F.; Romanazzi, G. Tracking of Diversity and Evolution in the Brown Rot Fungi Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Microbiol. 2022, 13, 854852. [Google Scholar] [CrossRef]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing Softly: A Roadmap of Botrytis cinerea Pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I.; Cotes Prado, A.M.; Stewart, A. Fillinger, S., Elad, Y., Eds.; Plant Hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Champaign, IL, USA, 2016. [Google Scholar] [CrossRef]
- Mustafa, M.H.; Bassi, D.; Corre, M.-N.; Lino, L.O.; Signoret, V.; Quilot-Turion, B.; Cirilli, M. Phenotyping Brown Rot Susceptibility in Stone Fruit: A Literature Review with Emphasis on Peach. Horticulturae 2021, 7, 115. [Google Scholar] [CrossRef]
- Fidanoğlu, B.T.; Mestav, B.; Özkılınç, H. A Case Study of the Effect of Temperature on Aggressiveness in the Monilinia-Peach Pathosystem. Eur. J. Plant. Pathol. 2023, 167, 1–10. [Google Scholar] [CrossRef]
- Rupp, S.; Weber, R.W.S.; Rieger, D.; Detzel, P.; Hahn, M. Spread of Botrytis cinerea Strains with Multiple Fungicide Resistance in German Horticulture. Front. Microbiol. 2017, 7, 2075. [Google Scholar] [CrossRef]
- Bai, Y.; Hou, Y.; Wang, Q.; Lu, C.; Ma, X.; Wang, Z.; Xu, H. Analysis of the Binding Modes and Resistance Mechanism of Four Methyl Benzimidazole Carbamates Inhibitors Fungicides with Monilinia fructicola β2-Tubulin Protein. J. Mol. Struct. 2023, 1291, 136057. [Google Scholar] [CrossRef]
- Holb, I.J.; Schnabel, G. Effect of Fungicide Treatments and Sanitation Practices on Brown Rot Blossom Blight Incidence, Phytotoxicity, and Yield for Organic Sour Cherry Production. Plant Dis. 2005, 89, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Keiblinger, K.M.; Schneider, M.; Gorfer, M.; Paumann, M.; Deltedesco, E.; Berger, H.; Jöchlinger, L.; Mentler, A.; Zechmeister-Boltenstern, S.; Soja, G.; et al. Assessment of Cu Applications in Two Contrasting Soils—Effects on Soil Microbial Activity and the Fungal Community Structure. Ecotoxicology 2018, 27, 217–233. [Google Scholar] [CrossRef]
- Sreedevi, M.A.; Harikumar, P.S. Occurrence, Distribution, and Ecological Risk of Heavy Metals and Persistent Organic Pollutants (OCPs, PCBs, and PAHs) in Surface Sediments of the Ashtamudi Wetland, South-West Coast of India. Reg. Stud. Mar. Sci. 2023, 64, 103044. [Google Scholar] [CrossRef]
- Ouédraogo, D.-Y.; Mell, H.; Perceval, O.; Burga, K.; Domart-Coulon, I.; Hédouin, L.; Delaunay, M.; Guillaume, M.M.M.; Castelin, M.; Calvayrac, C.; et al. What Are the Toxicity Thresholds of Chemical Pollutants for Tropical Reef-Building Corals? A Systematic Review. Environ. Evid. 2023, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S.; Zhang, J.G.; Wei, Z.J. Comparison of Antifungal Activity of Essential Oils from Different Plants against Three Fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef] [PubMed]
- Kesraoui, S.; Andrés, M.; Berrocal-Lobo, M.; Soudani, S.; Gonzalez-Coloma, A. Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection. Plants 2022, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon Verbena): A Review of Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P.; Alves, R.C. Contribution of Phenolics and Free Amino Acids on the Antioxidant Profile of Commercial Lemon Verbena Infusions. Antioxidants 2023, 12, 251. [Google Scholar] [CrossRef]
- Oukerrou, M.A.; Tilaoui, M.; Mouse, H.A.; Leouifoudi, I.; Jaafari, A.; Zyad, A. Chemical Composition and Cytotoxic and Antibacterial Activities of the Essential Oil of Aloysia citriodora Palau Grown in Morocco. Adv. Pharmacol. Pharm. Sci. 2017, 2017, 7801924. [Google Scholar] [CrossRef]
- Fontana, D.C.; Neto, D.D.; Pretto, M.M.; Mariotto, A.B.; Caron, B.O.; Kulczynski, S.M.; Schmidt, D. Using Essential Oils to Control Diseases in Strawberries and Peaches. Int. J. Food Microbiol. 2021, 338, 108980. [Google Scholar] [CrossRef]
- Mariotto, A.B.; Schmidt, D.; Fontana, D.C.; Neto, D.D.; Ozi, P.F.; Pascholati, S.F.; Caron, B.O. Aloysia citriodora Essential Oil: Antimicrobial Potential and Induced Resistance in Controlling Tomato Black Spot Fungus. Eur. J. Plant. Pathol. 2023, 167, 395–405. [Google Scholar] [CrossRef]
- Freddo, Á.R.; Lewandowski, A.; Busso, C.; Cechim, F.E.; Zorzzi, I.C.; Rey, M.D.S.; Dalacosta, N.L.; Mazaro, S.M. Óleo Essencial De Aloysia citriodora No Controle De Sclerotinia sclerotiorum Em Pepino E Atividade Antifúngica In Vitro. Ca 2016, 25, 373–386. [Google Scholar] [CrossRef]
- Fontana, D.C.; Schmidt, D.; Kulczynski, S.M.; Caron, B.O.; Pretto, M.M.; Mariotto, A.B.; Santos, J.D.; Holz, E. Fungicidal Potential of Essential Oils in Control of Fusarium spp. and Sclerotinia sclerotiorum. Arq. Inst. Biol. 2020, 87, e0612019. [Google Scholar] [CrossRef]
- Carmona, F.; Pereira, A.M.S. Prescription Patterns of Herbal Medicines at a Brazilian Living Pharmacy: The Farmácia Da Natureza Experience, 2013–2019. J. Herb. Med. 2022, 36, 100597. [Google Scholar] [CrossRef]
- Phootha, N.; Yongparnichkul, N.; Fang, Z.; Gan, R.-Y.; Zhang, P. Plants and Phytochemicals Potentials in Tackling Anxiety: A Systematic Review. Phytomed. Plus 2022, 2, 100375. [Google Scholar] [CrossRef]
- Moreno, K.G.T.; De Almeida, T.L.; Marques, A.A.M.; Donadel, G.; Lourenço, E.L.B.; De Almeida, D.A.T.; Gasparotto, A. Evidence of the Cardioprotective Effects of Aloysia polystachya in Isoproterenol-Induced Myocardial Infarction in Rats. J. Med. Food 2023, 26, 36–39. [Google Scholar] [CrossRef]
- Marques, A.A.M.; Lorençone, B.R.; Romão, P.V.M.; Guarnier, L.P.; Palozi, R.A.C.; Moreno, K.G.T.; Tirloni, C.A.S.; Dos Santos, A.C.; Souza, R.I.C.; Klider, L.M.; et al. Ethnopharmacological Investigation of the Cardiovascular Effects of the Ethanol-Soluble Fraction of Aloysia polystachya (Griseb.) Moldenke Leaves in Spontaneously Hypertensive Rats. J. Ethnopharmacol. 2021, 274, 114077. [Google Scholar] [CrossRef] [PubMed]
- Campos-Navarro, R.; Scarpa, G.F. The Cultural-Bound Disease “Empacho” in Argentina. A Comprehensive Botanico-Historical and Ethnopharmacological Review. J. Ethnopharmacol. 2013, 148, 349–360. [Google Scholar] [CrossRef]
- Consolini, A.E.; Berardi, A.; Rosella, M.A.; Volonté, M. Antispasmodic Effects of Aloysia polystachya and A. Gratissima Tinctures and Extracts Are Due to Non-Competitive Inhibition of Intestinal Contractility Induced by Acethylcholine and Calcium. Rev. Bras. Farmacogn. 2011, 21, 889–900. [Google Scholar] [CrossRef]
- Arena, J.S.; Merlo, C.; Defagó, M.T.; Zygadlo, J.A. Insecticidal and Antibacterial Effects of Some Essential Oils against the Poultry Pest Alphitobius diaperinus and Its Associated Microorganisms. J. Pest. Sci. 2020, 93, 403–414. [Google Scholar] [CrossRef]
- López, A.G.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Aromatic Plants Essential Oils Activity on Fusarium verticillioides Fumonisin B1 Production in Corn Grain. Mycopathologia 2004, 158, 343–349. [Google Scholar] [CrossRef]
- Oliva, M.; Gallucci, N.; Zygadlo, J.A.; Demo, M.S. Cytotoxic Activity of Argentinean Essential Oils on Artemia salina. Pharm. Biol. 2007, 45, 259–262. [Google Scholar] [CrossRef]
- Hudaib, M.; Tawaha, K.; Bustanji, Y. Chemical Profile of the Volatile Oil of Lemon Verbena (Aloysia citriodora Paláu) Growing in Jordan. J. Essent. Oil-Bear. Plants 2013, 16, 568–574. [Google Scholar] [CrossRef]
- Jaradat, N.; Hawash, M.; Abualhasan, M.N.; Qadi, M.; Ghanim, M.; Massarwy, E.; Ammar, S.A.; Zmero, N.; Arar, M.; Hussein, F. Spectral Characterization, Antioxidant, Antimicrobial, Cytotoxic, and Cyclooxygenase Inhibitory Activities of Aloysia citriodora Essential Oils Collected from Two Palestinian Regions. BMC Complement. Med. Ther. 2021, 21, 143. [Google Scholar] [CrossRef]
- Sprea, R.M.; Fernandes, L.H.M.; Pires, T.C.S.P.; Calhelha, R.C.; Rodrigues, P.J.; Amaral, J.S. Volatile Compounds and Biological Activity of the Essential Oil of Aloysia citrodora Paláu: Comparison of Hydrodistillation and Microwave-Assisted Hydrodistillation. Molecules 2023, 28, 4528. [Google Scholar] [CrossRef] [PubMed]
- Elechosa, M.A.; Di Leo Lira, P.; Juárez, M.A.; Viturro, C.I.; Heit, C.I.; Molina, A.C.; Martínez, A.J.; López, S.; Molina, A.M.; Van Baren, C.M. Essential Oil Chemotypes of Aloysia citrodora (Verbenaceae) in Northwestern Argentina. Biochem. Syst. Ecol. 2017, 74, 19–29. [Google Scholar] [CrossRef]
- Olmedo, R.; Ribotta, P.; Grosso, N.R. Antioxidant Activity of Essential Oils Extracted from Aloysia triphylla and Minthostachys mollis That Improve the Oxidative Stability of Sunflower Oil under Accelerated Storage Conditions. Euro. J. Lipid. Sci. Tech. 2018, 120, 1700374. [Google Scholar] [CrossRef]
- Tammar, S.; Salem, N.; Aidi Wannes, W.; Limam, H.; Bourgou, S.; Fares, N.; Dakhlaoui, S.; Hammami, M.; Saber, K.; Del Re, G. Chemical Profiling and Bioactivity of Aloysia citriodora Essential Oils from Four Localities in Tunisia. J. Essent. Oil Res. 2024, 36, 200–213. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Salama, Y.; Al-Hajj, N.; Jaradat, N.; Jobran, N.T.; Warad, I.; Hamdan, L.; Alrob, M.A.; Sawafta, A.; Hidmi, A. Chemical Composition, Anticancer, Antimicrobial Activity of Aloysia Citriodora Palau Essential Oils from Four Different Locations in Palestine. BMC Complement. Med. Ther. 2024, 24, 94. [Google Scholar] [CrossRef]
- Moller, A.C.; Parra, C.; Said, B.; Werner, E.; Flores, S.; Villena, J.; Russo, A.; Caro, N.; Montenegro, I.; Madrid, A. Antioxidant and Anti-Proliferative Activity of Essential Oil and Main Components from Leaves of Aloysia polystachya Harvested in Central Chile. Molecules 2020, 26, 131. [Google Scholar] [CrossRef]
- Pina, E.S.; Coppede, J.D.S.; Sartoratto, A.; Fachin, A.L.; Bertoni, B.W.; França, S.D.C.; Pereira, A.M. Antimicrobial Activity and Chemical Composition of Essential Oils from Aloysia polystachya (Griseb.) Moldenke Grown in Brazil. J. Med. Plants Res. 2012, 6, 5412–5416. [Google Scholar] [CrossRef]
- Pérez, C.M.; Torres, C.A.; Aguado, M.I.; Bela, A.J.; Nuñez, M.B.; Bregni, C. Antibacterial activity of essential oils of Aloysia polystachya and Lippia turbinata (Verbenaceae). Bol. Latinoam. Caribe Plantas Med. Aromat. 2016, 15, 199–205. [Google Scholar]
- Chebli, B.; Hmamouchi, M.; Achouri, M.; Hassani, L.I. Composition and in vitro fungitoxic activity of 19 essential oils against two post-harvest pathogens. J. Essent. Oil Res. 2024, 16, 507–511. [Google Scholar] [CrossRef]
- Balsells-Llauradó, M.; Vall-Llaura, N.; Usall, J.; Silva, C.J.; Blanco-Ulate, B.; Teixidó, N.; Caballol, M.; Torres, R. Transcriptional profiling of the terpenoid biosynthesis pathway and in vitro tests reveal putative roles of linalool and farnesal in nectarine resistance against brown rot. Plant Sci. 2023, 327, 111558. [Google Scholar] [CrossRef]
- Cotoras, M.; Castro, P.; Vivanco, H.; Melo, R.; Mendoza, L. Farnesol induces apoptosis-like phenotype in the phytopathogenic fungus Botrytis cinerea. Mycologia 2013, 105, 28–33. [Google Scholar] [CrossRef]
- Kamou, N.N.; Kalogiouri, N.P.; Tryfon, P.; Papadopoulou, A.; Karamanoli, K.; Dendrinou-Samara, C.; Menkissoglu-Spiroudi, U. Impact of Geraniol and Geraniol Nanoemulsions on Botrytis cinerea and Effect of Geraniol on Cucumber Plants’ Metabolic Profile Analyzed by LC-QTOF-MS. Plants 2022, 11, 2513. [Google Scholar] [CrossRef]
- Tsao, R.; Zhou, T. Antifungal activity of monoterpenoids against postharvest pathogens Botrytis cinerea and Monilinia fructicola. J. Essent. Oil Res. 2000, 12, 113–121. [Google Scholar] [CrossRef]
- Corrêa, A.N.R.; Clerici, N.J.; de Paula, N.O.; Brandelli, A. Inhibition of Food Spoilage Fungi, Botrytis cinerea and Rhizopus sp., by Nanoparticles Loaded with Baccharis dracunculifolia Essential Oil and Nerolidol. Foods 2024, 13, 3403. [Google Scholar] [CrossRef]
- Ogundajo, A.L.; Ewekeye, T.; Sharaibi, O.J.; Owolabi, M.S.; Dosoky, N.S.; Setzer, W.N. Antimicrobial Activities of Sesquitepene-Rich Essential Oils of Two Medicinal Plants, Lannea egregia and Emilia sonchifolia, from Nigeria. Plants 2021, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.C.; Alves, C.C.F.; Forim, M.R.; Matos, A.P.; Cunha, G.O.S.; Cazal, C.D.M. Chemical Composition and Antifungal Activity of the Essential Oil from the Hymenaea stigonocarpa Mart. Ex Hayne (Jatobá-Do-Cerrado) Fruit Peel. Nat. Prod. Res. 2024, 38, 1945–1949. [Google Scholar] [CrossRef] [PubMed]
- Semighini, C.P.; Hornby, J.M.; Dumitru, R.; Nickerson, K.W.; Harris, S.D. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 2006, 59, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Diao, Y.D.; Yang, Q.L.; Zhao, F.Y.; Ju, J. Geraniol causes apoptosis in Colletotrichum gloeosporioides by inducing a burst of ROS. Food Health 2025, 7, 2. [Google Scholar] [CrossRef]
- Molińska, E.; Klimczak, U.; Komaszyło, J.; Derewiaka, D.; Obiedziński, M.; Kania, M.; Danikiewicz, W.; Swiezewska, E. Double Bond Stereochemistry Influences the Susceptibility of Short-Chain Isoprenoids and Polyprenols to Decomposition by Thermo-Oxidation. Lipids 2015, 50, 359–370. [Google Scholar] [CrossRef]
- Semighini, C.P.; Murray, N.; Harris, S.D. Inhibition of Fusarium graminearum Growth and Development by Farnesol. FEMS Microbiol. Lett. 2008, 279, 259–264. [Google Scholar] [CrossRef]
- Liu, P.; Deng, B.; Long, C.A.; Min, X. Effect of farnesol on morphogenesis in the fungal pathogen Penicillium expansum. Ann. Microbiol. 2009, 59, 33–38. [Google Scholar] [CrossRef]
- Nassimi, Z.; Taheri, P.; Tarighi, S. Farnesol Altered Morphogenesis and Induced Oxidative Burst–Related Responses in Rhizoctonia solani AG1-IA. Mycologia 2019, 111, 359–370. [Google Scholar] [CrossRef]
- Yu, M.; Yu, J.; Pan, X.; Cao, H.; Song, T.; Liu, Y. Farnesol Inhibits Growth and Development of Ustilaginoidea virens. New Plant Prot. 2024, 1, e17. [Google Scholar] [CrossRef]
- Areco, V.A.; Achimón, F.; Almirón, C.; Nally, M.C.; Zunino, M.P.; Yaryura, P. Antifungal Activity of Essential Oils Rich in Ketones against Botrytis cinerea: New Strategy for Biocontrol. Biocatal. Agric. Biotechnol. 2024, 59, 103233. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, T.; Chen, Y.; Li, B.; Tian, S.; Chen, T. Carvone Fumigation Suppresses Postharvest Fruit Diseases by Disturbing Mitochondrial Functions and Impairing Membrane Integrity of Fungal Pathogens. Postharvest Biol. Technol. 2025, 230, 113810. [Google Scholar] [CrossRef]
- Madrid, A.; Silva, V.; Reyes, C.; Werner, E.; Besoain, X.; Montenegro, I.; Muñoz, E.; Díaz, K. Control of Peach Brown Rot Disease Produced by Monilinia fructicola and Monilinia laxa Using Benzylidene-Cycloalkanones. J. Fungi 2024, 10, 609. [Google Scholar] [CrossRef]
- Huang, S.; Millar, A.H. Succinato deshidrogenasa: Las funciones complejas de una enzima simple. Curr. Opin. Plant Biol. 2013, 16, 344–349. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, A.; Wang, X.; Tao, K.; Jin, H.; Hou, T. Novel Pyrazole Carboxamide Containing a Diarylamine Scaffold Potentially Targeting Fungal Succinate Dehydrogenase: Antifungal Activity and Mechanism of Action. J. Agric. Food Chem. 2022, 70, 13464–13472. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Y.; Wang, Y.; Xing, Z.; Peng, J.; Chen, J. Design, Synthesis and Antifungal Activity of Novel Amide Derivatives Containing a Pyrrolidine Moiety as Potential Succinate Dehydrogenase Inhibitors. Mol. Divers. 2024, 28, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Yang, S.-S.; Zhang, Q.; Zhang, T.-T.; Zhang, T.-Y.; Zhou, B.-H.; Zhou, L. Discovery of N -Phenylpropiolamide as a Novel Succinate Dehydrogenase Inhibitor Scaffold with Broad-Spectrum Antifungal Activity on Phytopathogenic Fungi. J. Agric. Food Chem. 2023, 71, 3681–3693. [Google Scholar] [CrossRef]
- Soto, M.; Estevez-Braun, A.; Amesty, Á.; Kluepfel, J.; Restrepo, S.; Diaz, K.; Espinoza, L.; Olea, A.F.; Taborga, L. Synthesis and Fungicidal Activity of Hydrated Geranylated Phenols against Botrytis cinerea. Molecules 2021, 26, 6815. [Google Scholar] [CrossRef] [PubMed]
- Sachivkina, N.; Senyagin, A.; Podoprigora, I.; Vasilieva, E.; Kuznetsova, O.; Karamyan, A.; Ibragimova, A.; Zhabo, N.; Molchanova, M. Enhancement of the Antifungal Activity of Some Antimycotics by Farnesol and Reduction of Candida albicans Pathogenicity in a Quail Model Experiment. Vet. World 2022, 15, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.M.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-Induced Apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 2392–2401. [Google Scholar] [CrossRef]
- Liu, P.; Luo, L.; Guo, J.; Liu, H.; Wang, B.; Deng, B.; Long, C.; Cheng, Y. Farnesol Induces Apoptosis and Oxidative Stress in the Fungal Pathogen Penicillium expansum. Mycologia 2010, 102, 311–318. [Google Scholar] [CrossRef]
- Montenegro, I.; Villarroel, C.; Muñoz, E.; Mena-Ulecia, K.; Silva, V.; Madrid, A. Anticandidal Activity of Clinopodium chilense Essential Oil. Front. Pharmacol. 2025, 16, 1634250. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Silva, V.; Muñoz, E.; Valle, G.; Martínez-Lobos, M.; Valdés, F.; Díaz, K.; Montenegro, I.; Godoy, P.; Caro, N.; et al. The Effect of 3′,4′-Methylenedioxychalcone Derivatives on Mycelial Growth and Conidial Germination of Monilinia fructicola: An In Silico and In Vitro Study. Agriculture 2025, 15, 983. [Google Scholar] [CrossRef]
- Madrid, A.; Díaz, K.; González, C.; Catalán, K.; Espinoza, L. Antiphytopathogenic Activity of Psoralea glandulosa (Fabaceae) against Botrytis cinerea and Phytophthora cinnamomi. Nat. Prod. Res. 2015, 29, 586–588. [Google Scholar] [CrossRef] [PubMed]

| No | Rt (min) | Compound | RI a | RL b | A. citriodora | A.polystachya | Identification |
|---|---|---|---|---|---|---|---|
| % Area | % Area | ||||||
| 1 | 11.10 | R-limonene | 1031 | 1031 | 3.90 | RL, MS, Co | |
| 2 | 13.16 | Linalool | 1107 | 1107 | 0.78 | RL, MS, Co | |
| 3 | 13.75 | trans-p-mentha-2,8-dienol | 1113 | 1113 | 0.48 | RL, MS | |
| 4 | 14.73 | 8,9-dehydrothymol | 1120 | 1121 | 1.72 | RL, MS | |
| 5 | 15.11 | Nerol | 1205 | 1205 | 1.48 | RL, MS, Co | |
| 6 | 15.55 | Neral | 1209 | 1209 | 1.64 | RL, MS, Co | |
| 7 | 15.82 | α-terpineol | 1160 | 1160 | 0.48 | RL, MS, Co | |
| 8 | 15.97 | Geraniol | 1232 | 1232 | 2.36 | RL, MS, Co | |
| 9 | 15.98 | dihydrocarvone | 1200 | 1200 | 4.57 | RL, MS, Co | |
| 10 | 16.53 | Citral | 1241 | 1241 | 3.16 | RL, MS, Co | |
| 11 | 17.31 | R-carvone | 1242 | 1242 | 88.41 | RL, MS, Co | |
| 12 | 18.91 | Piperitenone | 124 | 1243 | 7.22 | RL, MS | |
| 13 | 22.04 | caryophyllene | 1418 | 1418 | 0.62 | RL, MS | |
| 14 | 23.29 | α-curcumene | 1482 | 1483 | 8.57 | 0.21 | RL, MS |
| 15 | 23.81 | bicyclogermacrene | 1474 | 1475 | 2.25 | RL, MS | |
| 16 | 24.35 | Cubebol | 1510 | 1511 | 2.07 | RL, MS | |
| 17 | 25.65 | Nerolidol | 1548 | 1548 | 2.11 | RL, MS, Co | |
| 18 | 26.21 | Spathulenol | 1572 | 1572 | 38.84 | RL, MS | |
| 19 | 26.39 | caryophyllene oxide | 1578 | 1578 | 17.80 | RL, MS | |
| 20 | 26.54 | t-cadinol | 1636 | 1638 | 1.90 | RL, MS | |
| 21 | 27.98 | Z-nuciferol | 1699 | 1700 | 1.07 | RL, MS | |
| 22 | 28.40 | E-nuciferol | 1725 | 1726 | 2.61 | RL, MS | |
| 23 | 28.45 | Farnesol | 1730 | 1730 | 5.13 | RL, MS, Co | |
| Monoterpene hydrocarbons | 3.9 | ||||||
| Oxygenated monoterpenes Sesquiterpene hydrocarbons | 17.58 | 94.72 | |||||
| 13.82 | 0.83 | ||||||
| Oxygenated sesquiterpenes | 68.53 | ||||||
| Total | 99.93 | 99.45 | |||||
| Sample | B. cinerea a | M. fructicola a | M. laxa a |
|---|---|---|---|
| A. citriodora essential oil | 183.26 ± 1.7 b | 61.89 ± 1.16 b | 73.05 ± 3.1 c |
| Citral | >250 | >250 | >250 |
| Nerol | >250 | >250 | >250 |
| Nerolidol | >250 | >250 | 173.5 ± 2.0 d |
| Gerianol | >250 | 163.4 ± 0.79 d | >250 |
| Farnesol | 230.0 ± 3.1 c | 72.18 ± 0.07 c | 45.32 ± 3.3 b |
| A. polystachya essential oil | >250 | >250 | >250 |
| R-limonene | >250 | >250 | >250 |
| Linalool | >250 | >250 | >250 |
| α-terpineol | >250 | >250 | >250 |
| Dihydrocarvone | >250 | >250 | >250 |
| R-carvone | >250 | >250 | >250 |
| BC-1000® | 45.97 ± 2.7 a | 10.55 ± 1.74 a | 13.64 ± 1.3 a |
| Ligand | Binding Energy [kcal/mol] | Interactions |
|---|---|---|
| Farnesol | −7.5 | Ile27; Trp32; Met36; Ile40; Arg43 |
| CBE | −7.2 | Arg43; Met36; Trp32; Ile40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Ferreira, C.; Flores, S.; Muñoz, E.; Reyes, C.; Trujillo, C.; Gálvez, E.; Díaz, K.; Madrid, A. Chilean Aloysia Essential Oils: A Medicinal Plant Resource for Postharvest Disease Control. Plants 2025, 14, 3121. https://doi.org/10.3390/plants14203121
Silva V, Ferreira C, Flores S, Muñoz E, Reyes C, Trujillo C, Gálvez E, Díaz K, Madrid A. Chilean Aloysia Essential Oils: A Medicinal Plant Resource for Postharvest Disease Control. Plants. 2025; 14(20):3121. https://doi.org/10.3390/plants14203121
Chicago/Turabian StyleSilva, Valentina, Catalina Ferreira, Susana Flores, Evelyn Muñoz, Constanza Reyes, Carmen Trujillo, Esperanza Gálvez, Katy Díaz, and Alejandro Madrid. 2025. "Chilean Aloysia Essential Oils: A Medicinal Plant Resource for Postharvest Disease Control" Plants 14, no. 20: 3121. https://doi.org/10.3390/plants14203121
APA StyleSilva, V., Ferreira, C., Flores, S., Muñoz, E., Reyes, C., Trujillo, C., Gálvez, E., Díaz, K., & Madrid, A. (2025). Chilean Aloysia Essential Oils: A Medicinal Plant Resource for Postharvest Disease Control. Plants, 14(20), 3121. https://doi.org/10.3390/plants14203121






