Exploring the Potential of Selenium-Containing Amine (Se-AMA) to Enhance Photosynthesis and Leaf Water Content: New Avenues for Carbonic Anhydrase Modulation in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
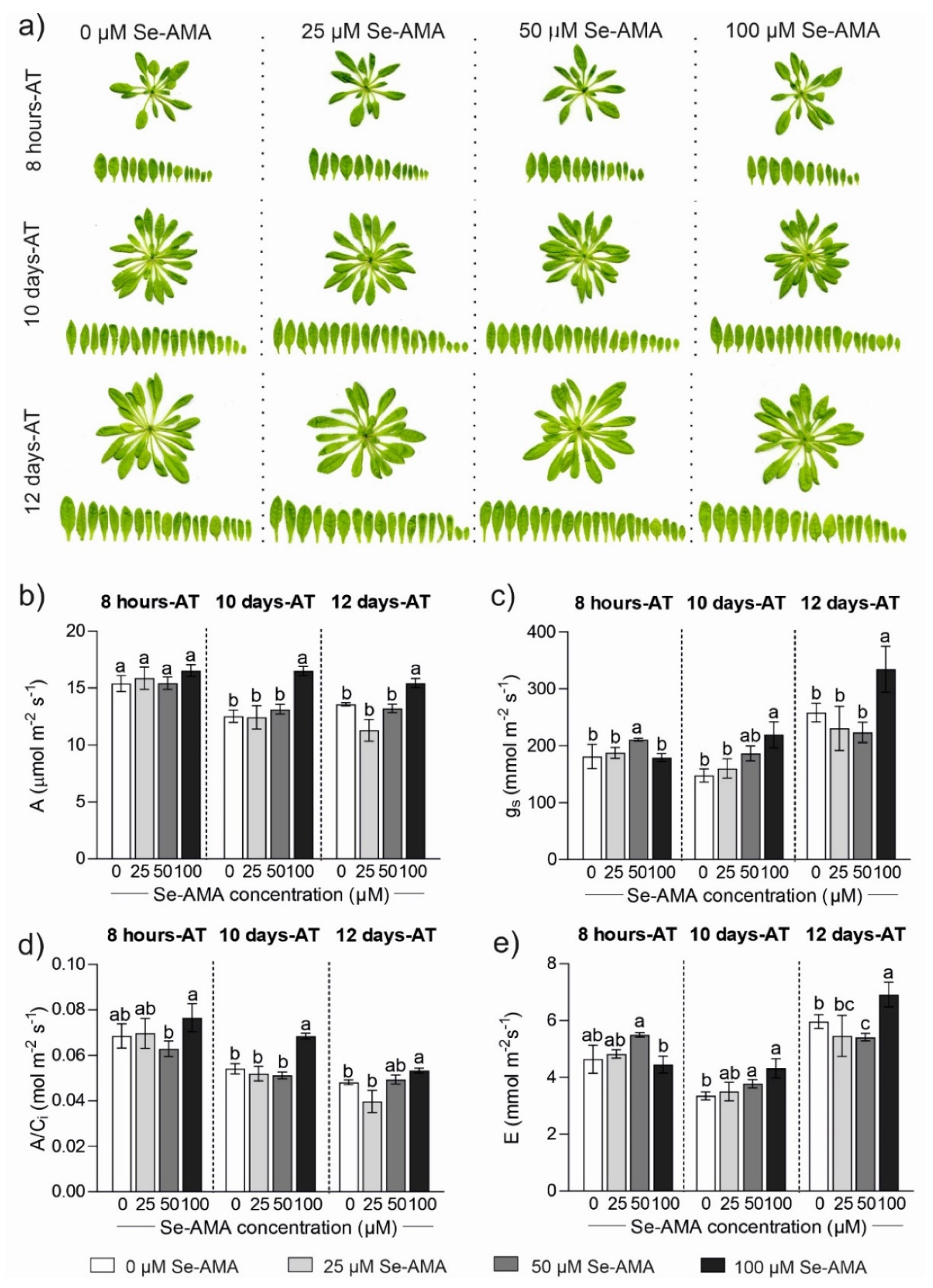
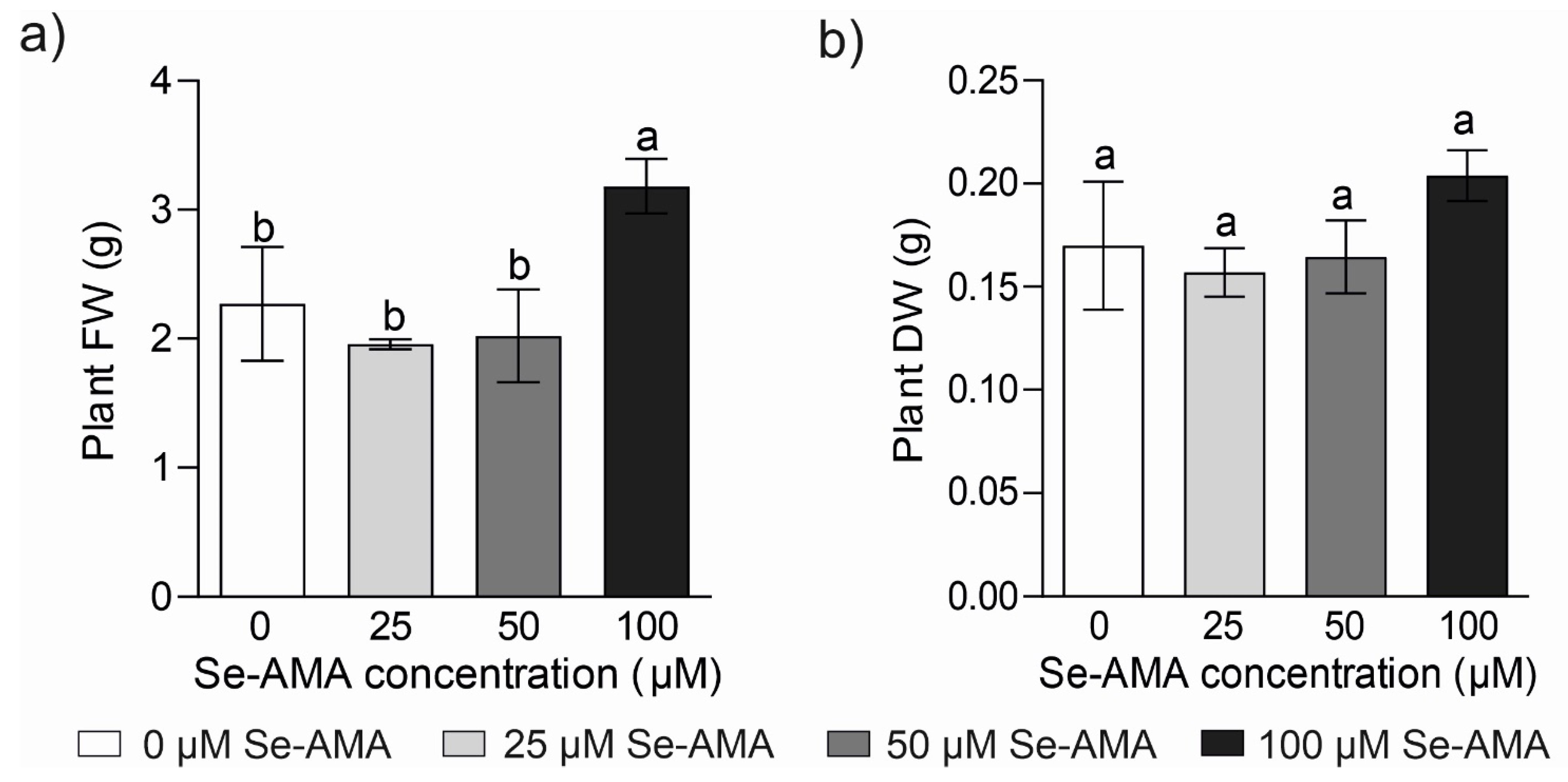
2.1. Plant Physiological Responses to Different Doses of Se-AMA (Experiment 1)
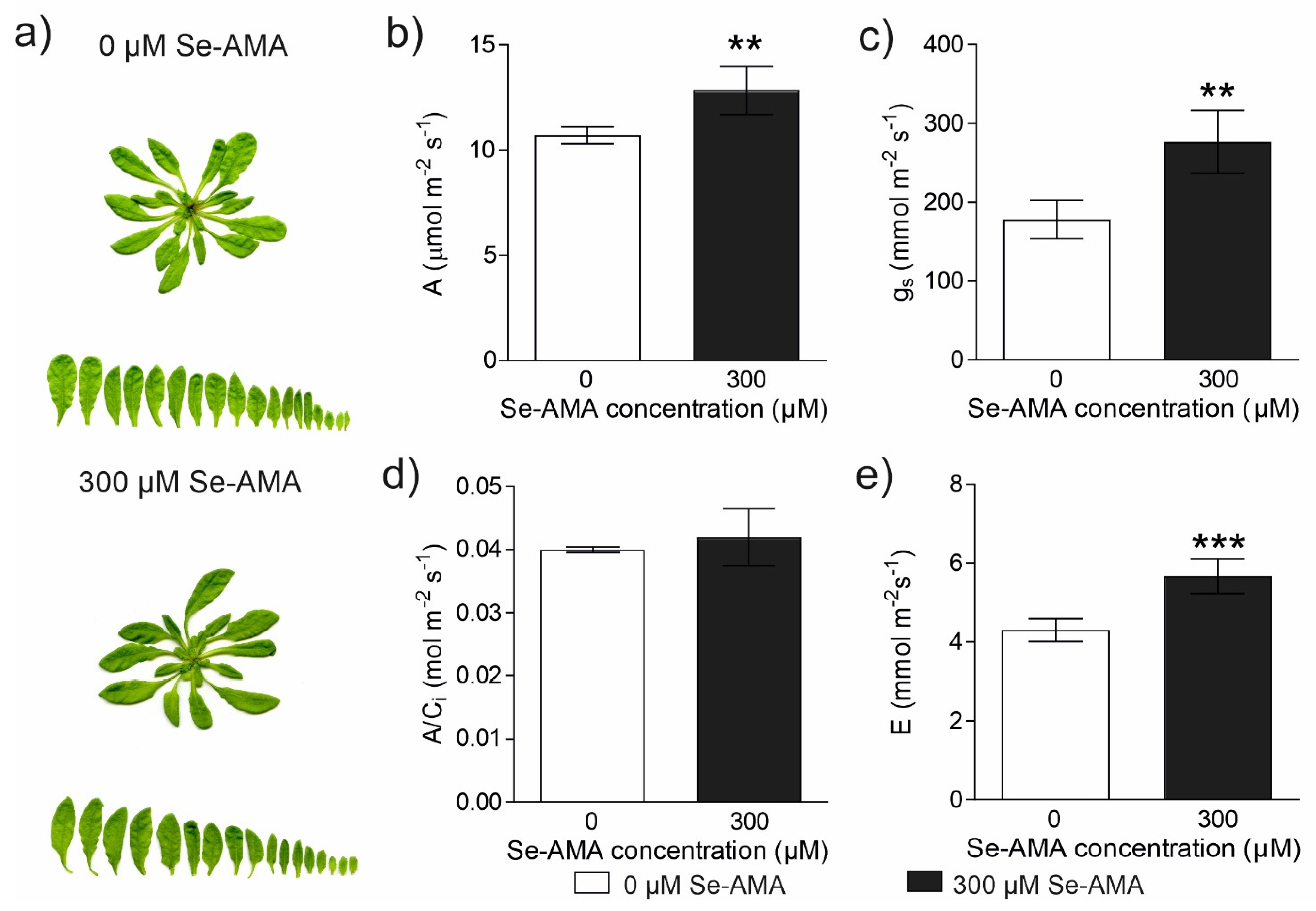
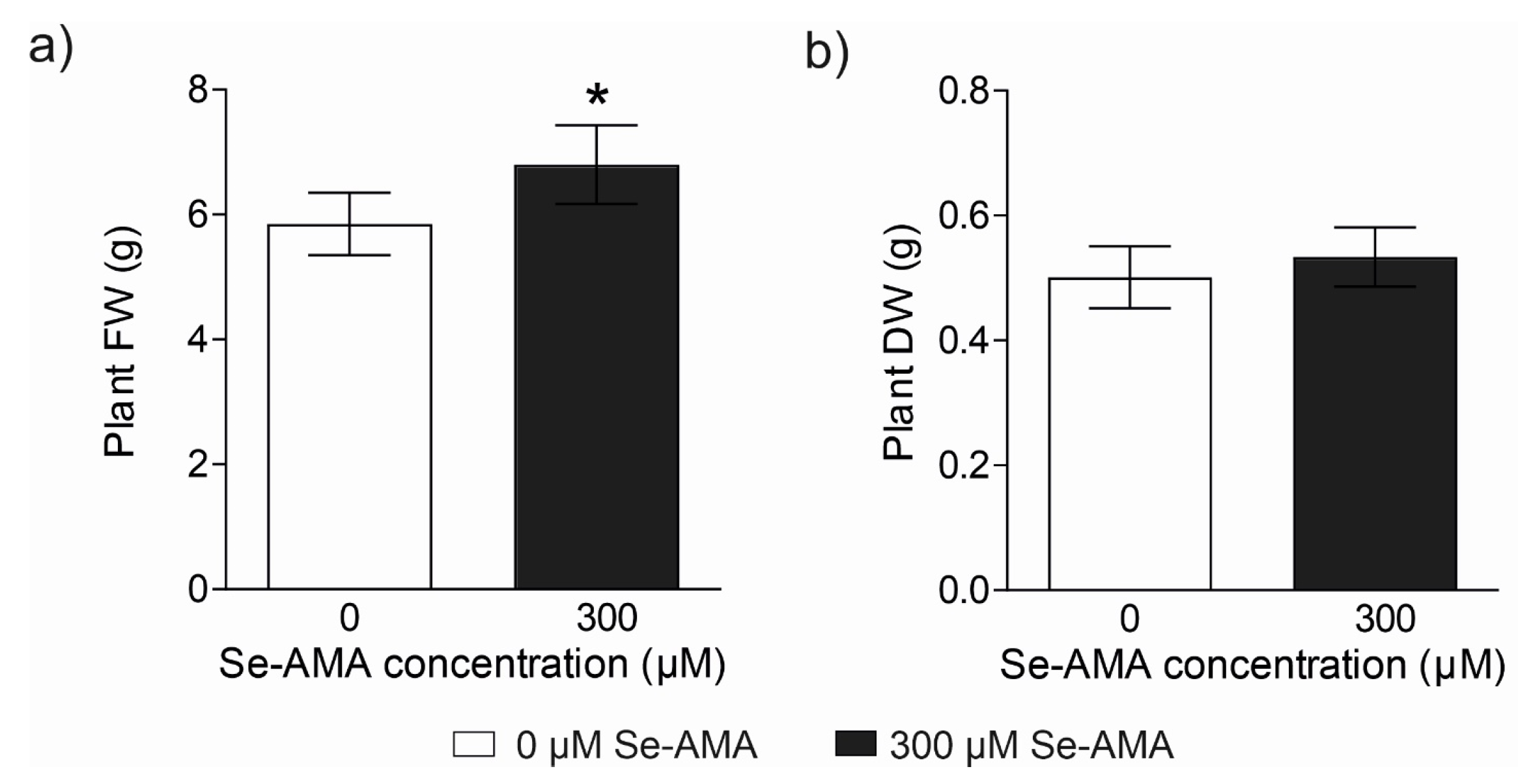
2.2. Effect of High Doses of Se-AMA on Plant Physiological Performances (Experiment 2)
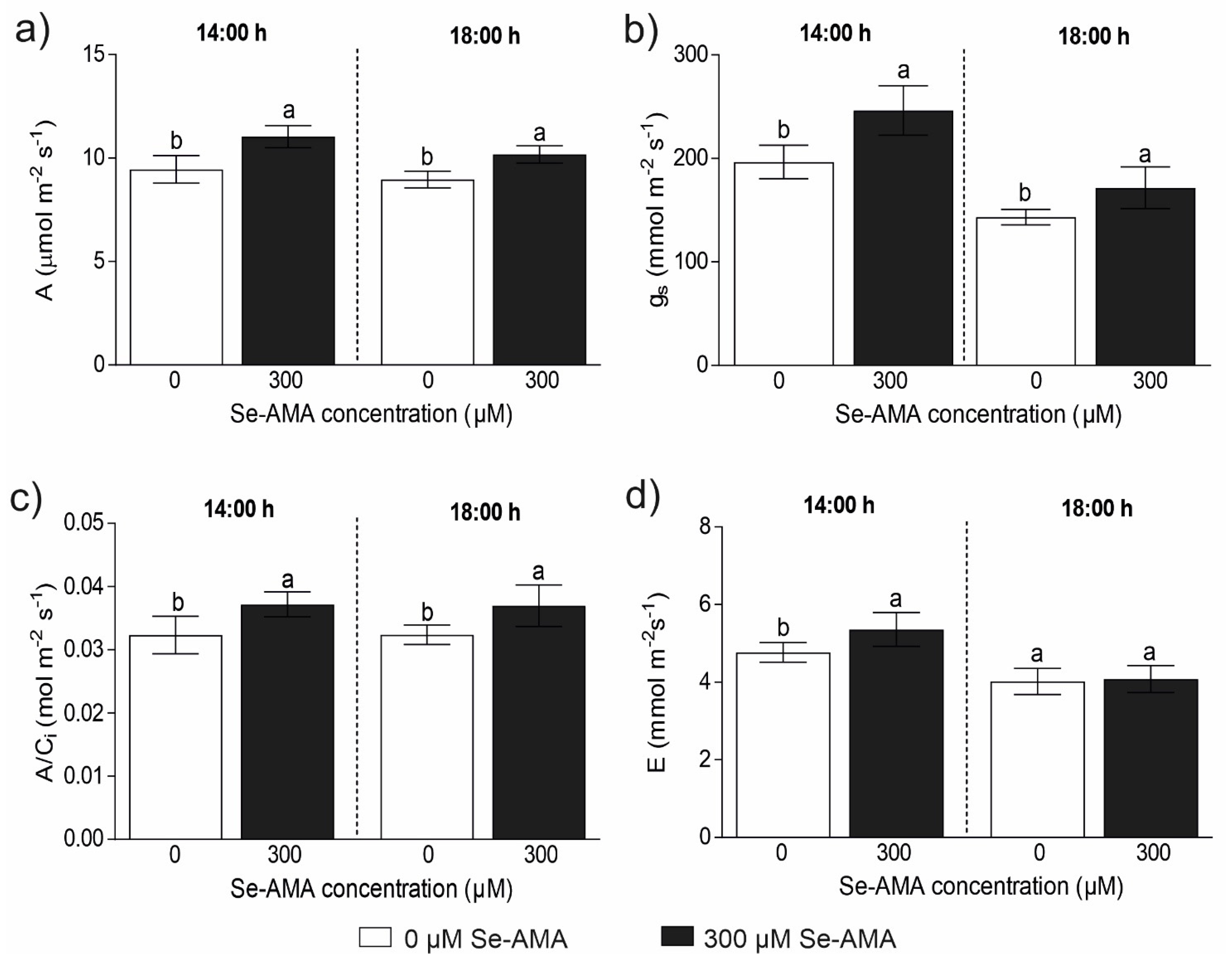
2.3. Daily Effect of Se-AMA on Plant Physiological Performances (Experiment 3)
3. Discussion
3.1. Effect of Se-AMA on Plant Physiological Performances
3.2. Effect of Se-AMA on Plant Water Status
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Growth Conditions
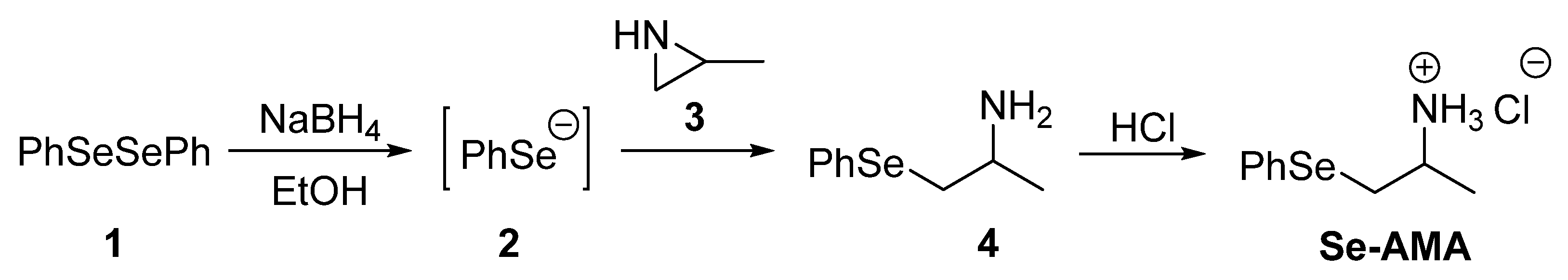
5.2. Synthesis of Aminoselenide 4 [1-(Phenylselanyl)propan-2-amine]
5.3. Synthesis of Aminoselenide Hydrochloride (Se-AMA)
5.4. Se-AMA Analysis
5.5. Se-AMA Treatments
5.6. Evaluation of Plant Photosynthetic Performance
5.7. Plant Growth Parameters
5.8. Leaf Pigments Detection
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying Impacts of Enhancing Photosynthesis on Crop Yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez, C.; Aguiló-Nicolau, P.; Galmés, J. Improving Photosynthesis through the Enhancement of Rubisco Carboxylation Capacity. Biochem. Soc. Trans. 2021, 49, 2007–2019. [Google Scholar] [CrossRef] [PubMed]
- DiMario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant Carbonic Anhydrases: Structures, Locations, Evolution, and Physiological Roles. Mol. Plant 2017, 10, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, N.U.; Roughton, F.J.W. The state of carbon dioxide in blood. J. Physiol. 1933, 80, 143. [Google Scholar] [CrossRef] [PubMed]
- Hewett-Emmett, D.; Tashian, R.E. Functional Diversity, Conservation, and Convergence in the Evolution of the α-, β-, and γ-Carbonic Anhydrase Gene Families. Mol. Phylogenet. Evol. 1996, 5, 50–77. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Ivanov, B.N. Unsolved Problems of Carbonic Anhydrases Functioning in Photosynthetic Cells of Higher C3 Plants. Biochemistry 2021, 86, 1243–1255. [Google Scholar] [CrossRef]
- Tholen, D.; Zhu, X.G. The Mechanistic Basis of Internal Conductance: A Theoretical Analysis of Mesophyll Cell Photosynthesis and CO2 Diffusion. Plant Physiol. 2011, 156, 90–105. [Google Scholar] [CrossRef]
- Ogée, J.; Wingate, L.; Genty, B. Estimating Mesophyll Conductance from Measurements of C18OO Photosynthetic Discrimination and Carbonic Anhydrase Activity. Plant Physiol. 2018, 178, 728–752. [Google Scholar] [CrossRef]
- Momayyezi, M.; McKown, A.D.; Bell, S.C.S.; Guy, R.D. Emerging Roles for Carbonic Anhydrase in Mesophyll Conductance and Photosynthesis. Plant J. 2020, 101, 831–844. [Google Scholar] [CrossRef]
- Wu, Y.; Rao, S. Root-Derived Bicarbonate Assimilation in Plants; Springer: Dordrecht, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Perez-Martin, A.; Michelazzo, C.; Torres-Ruiz, J.M.; Flexas, J.; Fernández, J.E.; Sebastiani, L.; Diaz-Espejo, A. Regulation of Photosynthesis and Stomatal and Mesophyll Conductance under Water Stress and Recovery in Olive Trees: Correlation with Gene Expression of Carbonic Anhydrase and Aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Tang, Y.; Zhu, X.G. Stomata Conductance as a Goalkeeper for Increased Photosynthetic Efficiency. Curr. Opin. Plant Biol. 2022, 70, 102310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiang, X.; Ji, D.; Chen, Q.; Ma, T.; Wang, J.; Liu, C. A Carbonic Anhydrase, ZmCA4, Contributes to Photosynthetic Efficiency and Modulates CO2 Signaling Gene Expression by Interacting with Aquaporin ZmPIP2;6 in Maize. Plant Cell Physiol. 2024, 65, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.N.; Ignatova, L.K.; Zhurikova, E.M.; Yanyushin, M.F.; Ivanov, B.N. The multiplicity of functions of carbonic anhydrases in higher plants. In Carbonic Anhydrases: Biochemistry, Mechanism of Action and Therapeutic Applications; Nova Science Publishers: New York, NY, USA, 2018; pp. 111–137. [Google Scholar]
- Ortega, M.A.; De Leon-Oliva, D.; Gimeno-Longas, M.J.; Boaru, D.L.; Fraile-Martinez, O.; García-Montero, C.; de Castro, A.V.; Barrena-Blázquez, S.; López-González, L.; Amor, S.; et al. Vascular Calcification: Molecular Networking, Pathological Implications and Translational Opportunities. Biomolecules 2024, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Akocak, S.; Supuran, C.T. Activation of α-, β-, γ-δ-, ζ- and η-Class of Carbonic Anhydrases with Amines and Amino Acids: A Review. J. Enzym. Inhib. Med. Chem. 2019, 34, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Mishra, C.B.; Tiwari, M.; Supuran, C.T. Progress in the Development of Human Carbonic Anhydrase Inhibitors and Their Pharmacological Applications: Where Are We Today? Med. Res. Rev. 2020, 40, 2485–2565. [Google Scholar] [CrossRef]
- Barresi, E.; Ravichandran, R.; Germelli, L.; Angeli, A.; Baglini, E.; Salerno, S.; Marini, A.M.; Costa, B.; Da Pozzo, E.; Martini, C.; et al. Carbonic Anhydrase Activation Profile of Indole-Based Derivatives. J. Enzym. Inhib. Med. Chem. 2021, 36, 1783–1797. [Google Scholar] [CrossRef]
- Tanini, D.; Carradori, S.; Capperucci, A.; Lupori, L.; Zara, S.; Ferraroni, M.; Ghelardini, C.; Di Cesare Mannelli, L.; Micheli, L.; Lucarini, E.; et al. Chalcogenides-incorporating carbonic anhydrase inhibitors concomitantly revertedoxaliplatin-induced neuropathy and enhanced antiproliferative action. Eur. J. Med. Chem. 2021, 225, 113793. [Google Scholar] [CrossRef]
- Nocentini, A.; Angeli, A.; Carta, F.; Winum, J.Y.; Zalubovskis, R.; Carradori, S.; Capasso, C.; Donald, W.A.; Supuran, C.T. Reconsidering Anion Inhibitors in the General Context of Drug Design Studies of Modulators of Activity of the Classical Enzyme Carbonic Anhydrase. J. Enzym. Inhib. Med. Chem. 2021, 36, 561–580. [Google Scholar] [CrossRef]
- Zhou, X.-q.; Ma, X.-l.; Ariffin, N.S. The Potential of Carbonic Anhydrase Enzymes as a Novel Target for Anti-Cancer Treatment. Eur. J. Pharmacol. 2024, 976, 176677. [Google Scholar] [CrossRef]
- Temperini, C.; Scozzafava, A.; Vullo, D.; Supuran, C.T. Carbonic Anhydrase Activators. Activation of Isozymes I, II, IV, VA, VII, and XIV with L- and D-Histidine and Crystallographic Analysis of Their Adducts with Isoform II: Engineering Proton-Transfer Processes within the Active Site of an Enzyme. Chem.-A Eur. J. 2006, 12, 7057–7066. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A.; Supuran, C.T.; Angeli, A. Sulfur, Selenium and Tellurium Containing Amines Act as Effective Carbonic Anhydrase Activators. Bioorg. Chem. 2019, 87, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Capperucci, A.; Coronnello, M.; Salvini, F.; Tanini, D.; Dei, S.; Teodori, E.; Giovannelli, L. Synthesis of Functionalised Organochalcogenides and in Vitro Evaluation of Their Antioxidant Activity. Bioorg. Chem. 2021, 110, 104812. [Google Scholar] [CrossRef]
- Kromdijk, J.; Long, S.P. One Crop Breeding Cycle from Starvation? How Engineering Crop Photosynthesis for Rising CO2 and Temperature Could Be One Important Route to Alleviation. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152578. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Simkin, A.J.; McAusland, L.; Lawson, T.; Raines, C.A. Overexpression of the RieskeFeS protein increases electron transport rates and biomass yield. Plant Physiol. 2017, 175, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.A.; Gori, A.; Da-Silva, C.J.; Brunetti, C. Abscisic acid biosynthesis and signaling in plants: Key targets to improve water use efficiency and drought tolerance. Appl. Sci. 2020, 10, 6322. [Google Scholar] [CrossRef]
- Provensi, G.; Costa, A.; Rani, B.; Becagli, M.V.; Vaiano, F.; Passani, M.B.; Tanini, D.; Capperucci, A.; Carradori, S.; Petzer, J.P.; et al. New β-arylchalcogeno amines with procognitive properties targeting Carbonic Anhydrases and Monoamine Oxidases. Eur. J. Med. Chem. 2022, 244, 114828. [Google Scholar] [CrossRef]
- Tanini, D.; Borgogni, C.; Capperucci, A. Mild and selective silicon-mediated access to enantioenriched 1,2-mercaptoamines and β-amino arylchalcogenides. New J. Chem. 2019, 43, 6388–6393. [Google Scholar] [CrossRef]
- Dewar, R.; Mauranen, A.; Mäkelä, A.; Hölttä, T.; Medlyn, B.; Vesala, T. New Insights into the Covariation of Stomatal, Mesophyll and Hydraulic Conductances from Optimization Models Incorporating Nonstomatal Limitations to Photosynthesis. New Phytol. 2018, 217, 571–585. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Groszmann, M.; Evans, J.R. Stomatal, Mesophyll Conductance, and Biochemical Limitations to Photosynthesis during Induction. Plant Physiol. 2021, 185, 146–160. [Google Scholar] [CrossRef]
- Petrík, P.; Petek-petrik, A.; Mukarram, M.; Schuldt, B.; Lamarque, L.J. Emerging Voices in Botany Leaf Physiological and Morphological Constraints of Water- Use Efficiency in C3 Plants. AoB Plants 2023, 15, plad047. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W. Photosynthesis and Respiration; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Simonin, K.A.; Burns, E.; Choat, B.; Barbour, M.M.; Dawson, T.E.; Franks, P.J. Increasing leaf hydraulic conductance with transpiration rate minimizes the water potential drawdown from stem to leaf. J. Exp. Bot. 2015, 66, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Andrews, J.; Mulholland, B.J.; McKee, J.M.; Hilton, H.W.; Horridge, J.S.; Farquhar, G.D.; Smeeton, R.C.; Smillie, I.R.; Black, C.R.; et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol. 2007, 143, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.; Qin, X.; Zeise, B.; Xu, D.; Rappel, W.J.; Boron, W.F.; Schroeder, J.I. Reconstitution of CO2 Regulation of SLAC1 Anion Channel and Function of CO2-Permeable PIP2;1 Aquaporin as CARBONIC ANHYDRASE4 Interactor. Plant Cell 2015, 28, 568–582. [Google Scholar] [CrossRef]
- Del Carmen Martinez-Ballesta, M.; Carvajal, M. New Challenges in Plant Aquaporin Biotechnology. Plant Sci. 2014, 217–218, 71–77. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration Increases under High-Temperature Stress: Potential Mechanisms, Trade-Offs and Prospects for Crop Resilience in a Warming World. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrami, S.; Alderotti, F.; Capperucci, A.; Tanini, D.; Brunetti, C.; Ferrini, F.; Lo Piccolo, E.; Gori, A. Exploring the Potential of Selenium-Containing Amine (Se-AMA) to Enhance Photosynthesis and Leaf Water Content: New Avenues for Carbonic Anhydrase Modulation in Arabidopsis thaliana. Plants 2025, 14, 258. https://doi.org/10.3390/plants14020258
Beltrami S, Alderotti F, Capperucci A, Tanini D, Brunetti C, Ferrini F, Lo Piccolo E, Gori A. Exploring the Potential of Selenium-Containing Amine (Se-AMA) to Enhance Photosynthesis and Leaf Water Content: New Avenues for Carbonic Anhydrase Modulation in Arabidopsis thaliana. Plants. 2025; 14(2):258. https://doi.org/10.3390/plants14020258
Chicago/Turabian StyleBeltrami, Sara, Francesca Alderotti, Antonella Capperucci, Damiano Tanini, Cecilia Brunetti, Francesco Ferrini, Ermes Lo Piccolo, and Antonella Gori. 2025. "Exploring the Potential of Selenium-Containing Amine (Se-AMA) to Enhance Photosynthesis and Leaf Water Content: New Avenues for Carbonic Anhydrase Modulation in Arabidopsis thaliana" Plants 14, no. 2: 258. https://doi.org/10.3390/plants14020258
APA StyleBeltrami, S., Alderotti, F., Capperucci, A., Tanini, D., Brunetti, C., Ferrini, F., Lo Piccolo, E., & Gori, A. (2025). Exploring the Potential of Selenium-Containing Amine (Se-AMA) to Enhance Photosynthesis and Leaf Water Content: New Avenues for Carbonic Anhydrase Modulation in Arabidopsis thaliana. Plants, 14(2), 258. https://doi.org/10.3390/plants14020258











