Production of Malheuran A, a Geranylated Flavonoid with Antimicrobial and Anti-Inflammatory Activities, in Hairy Root Cultures of Dalea purpurea
Abstract
1. Introduction
2. Results and Discussion
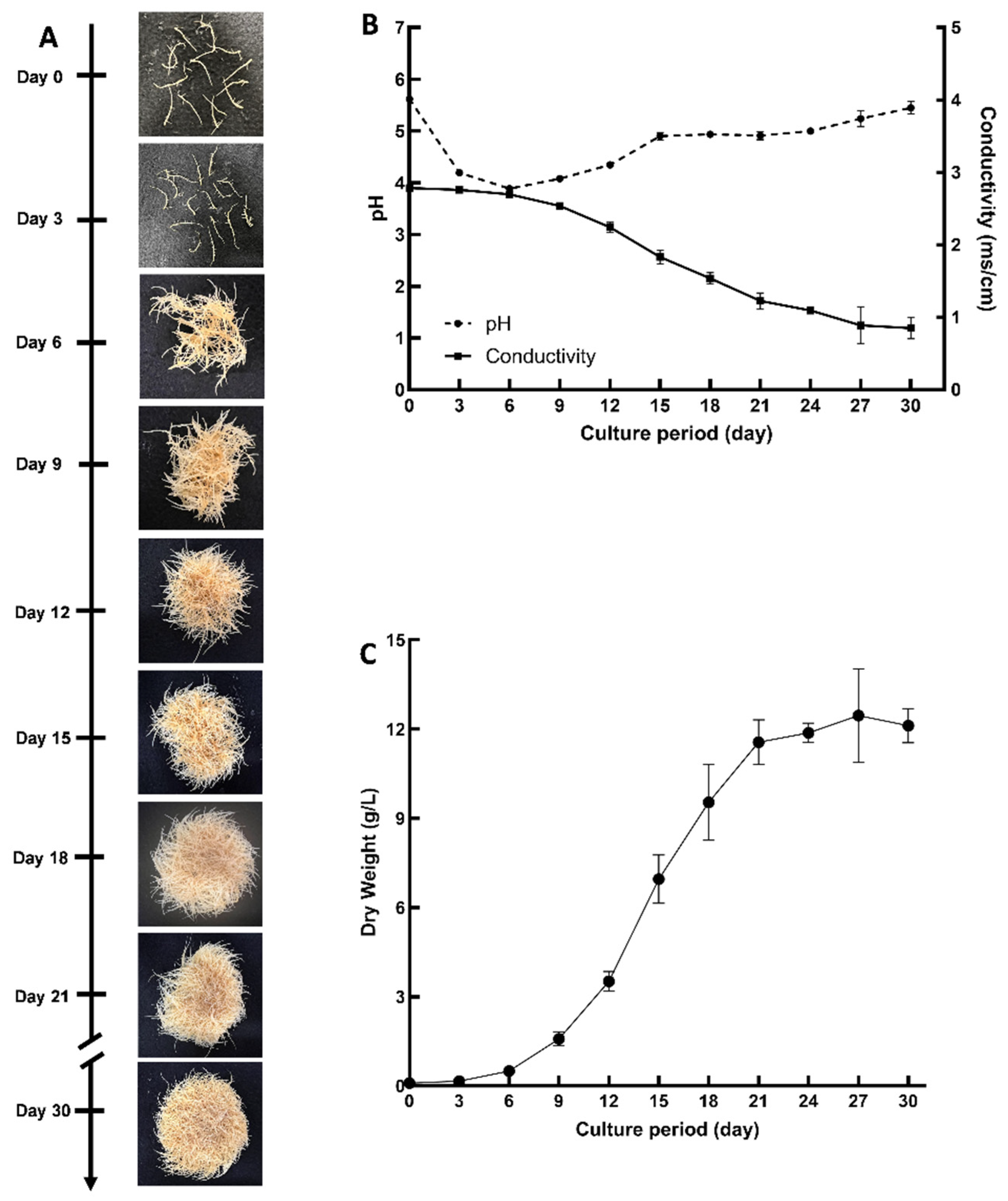
2.1. Establishment and Characterization of D. purpurea Hairy Root Cultures
2.2. Growth Kinetics of D. purpurea Hairy Roots in 500 mL Flasks
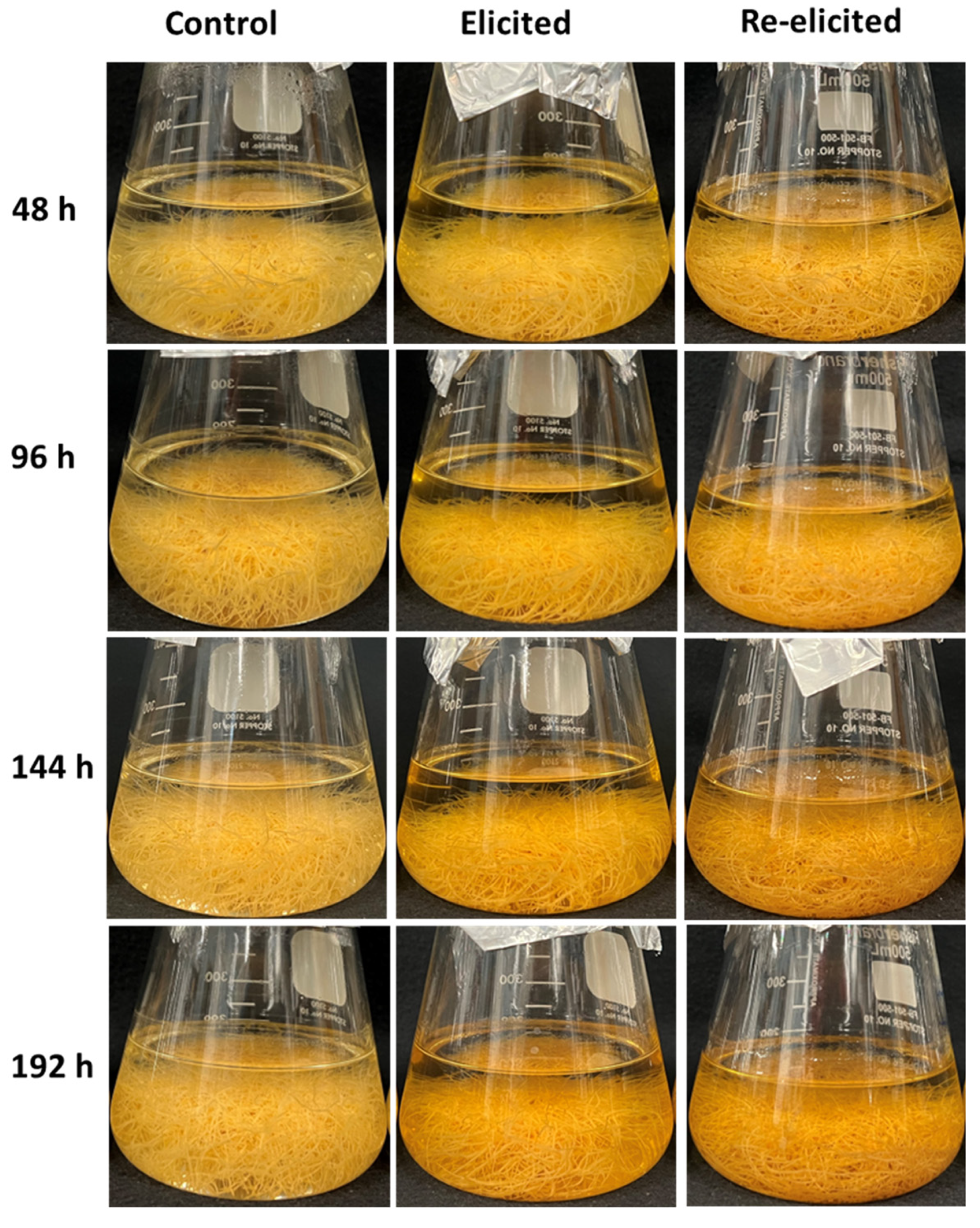
2.3. Phenotype of Hairy Roots upon Elicitor Treatment
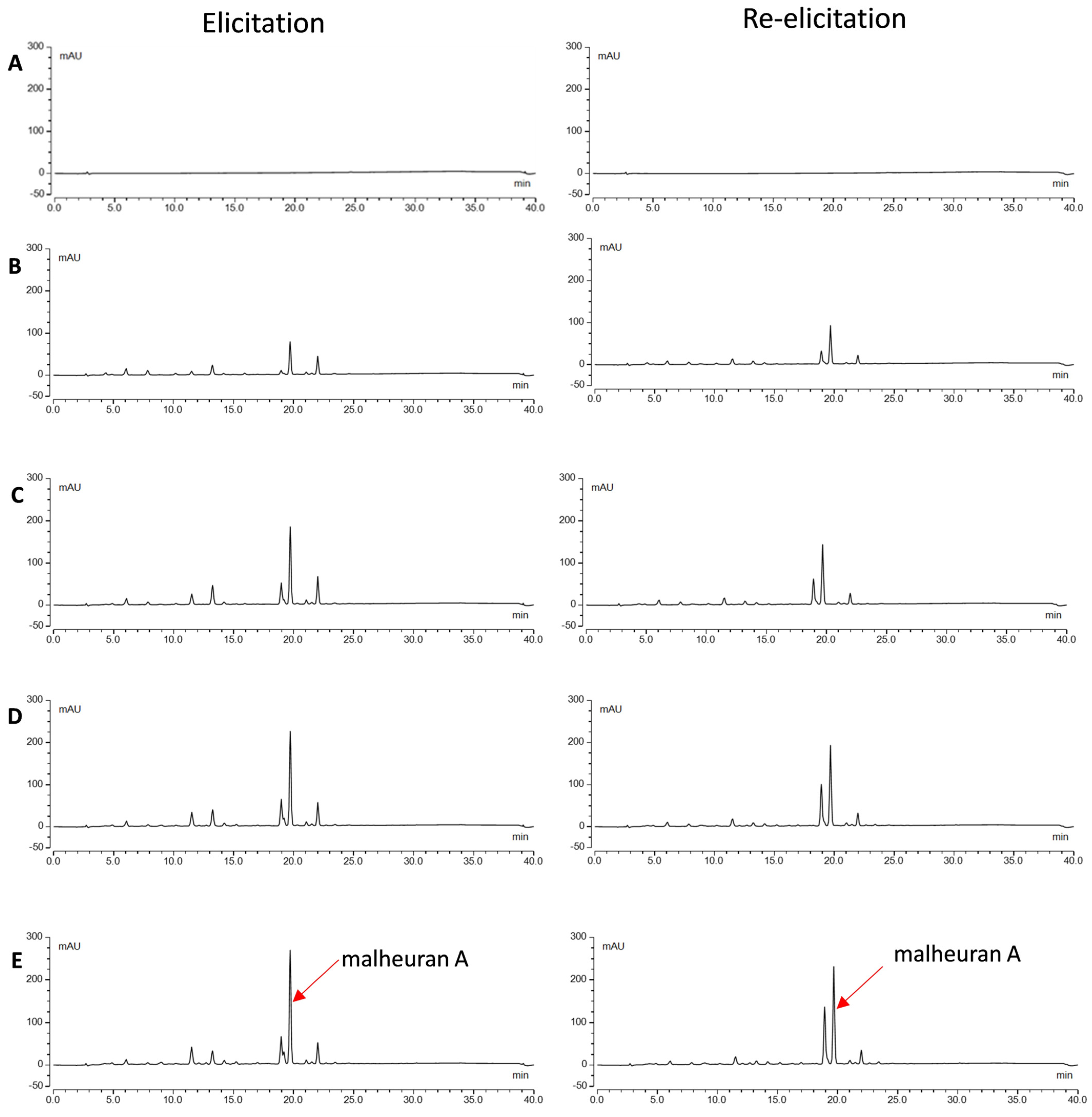
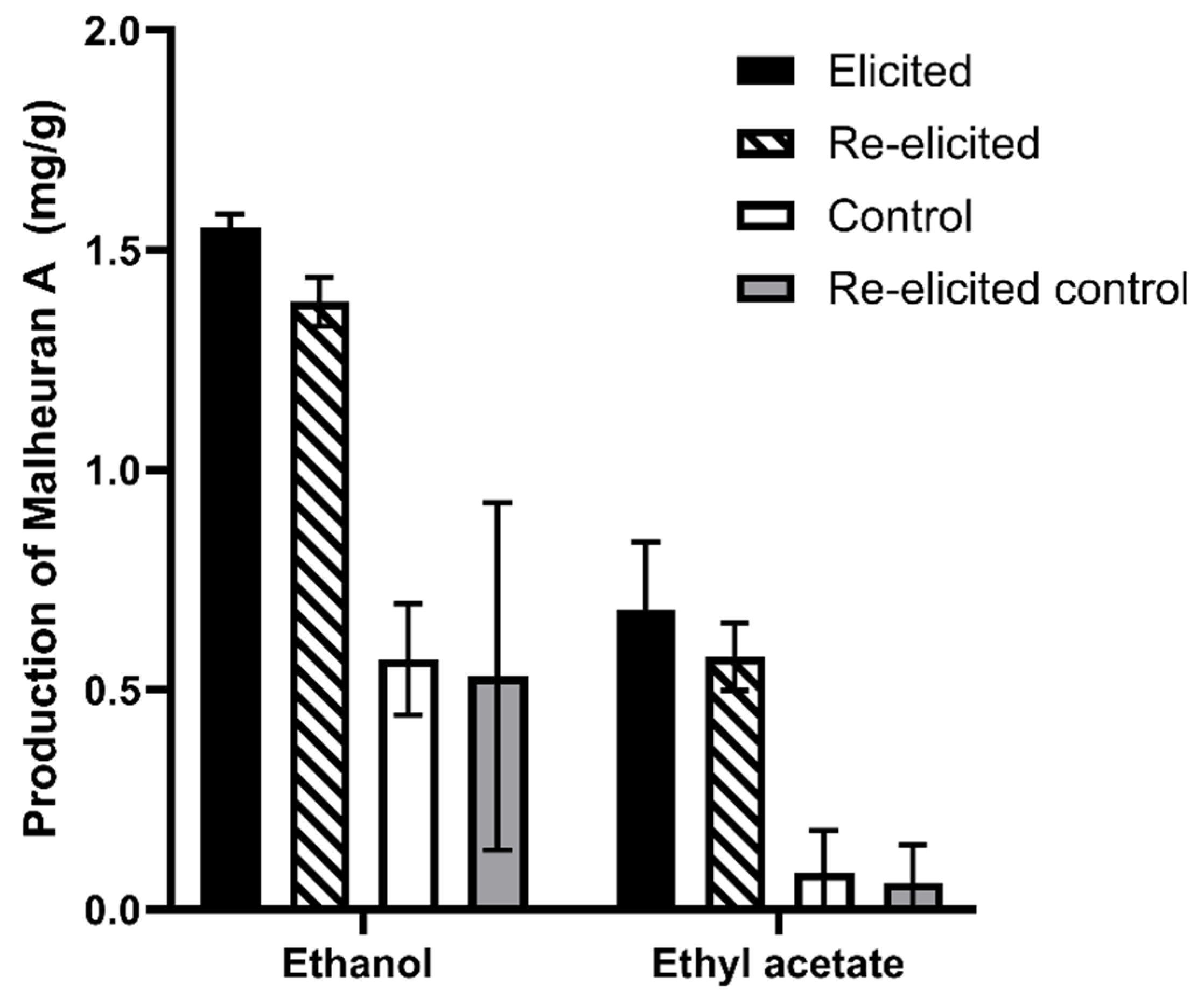
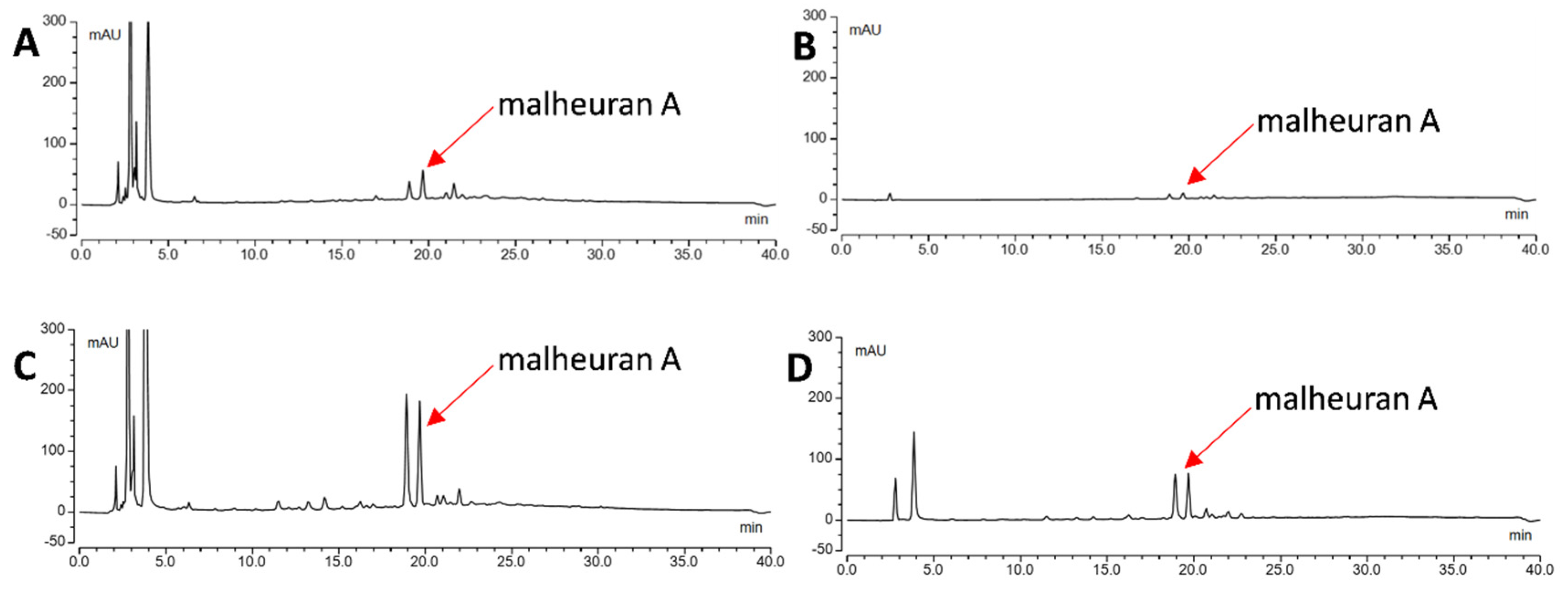
2.4. Effect of Elicitor Treatment on the Yield of Malheuran A in the Culture Medium
2.5. Yield of Malheuran A in the Hairy Root Culture System of D. purpurea
2.6. Re-Elicitation of Elicited Hairy Root Cultures
2.7. Purification of Malheuran A
2.8. Identification of Malheuran A
2.9. Antimicrobial Activity of Malheuran A
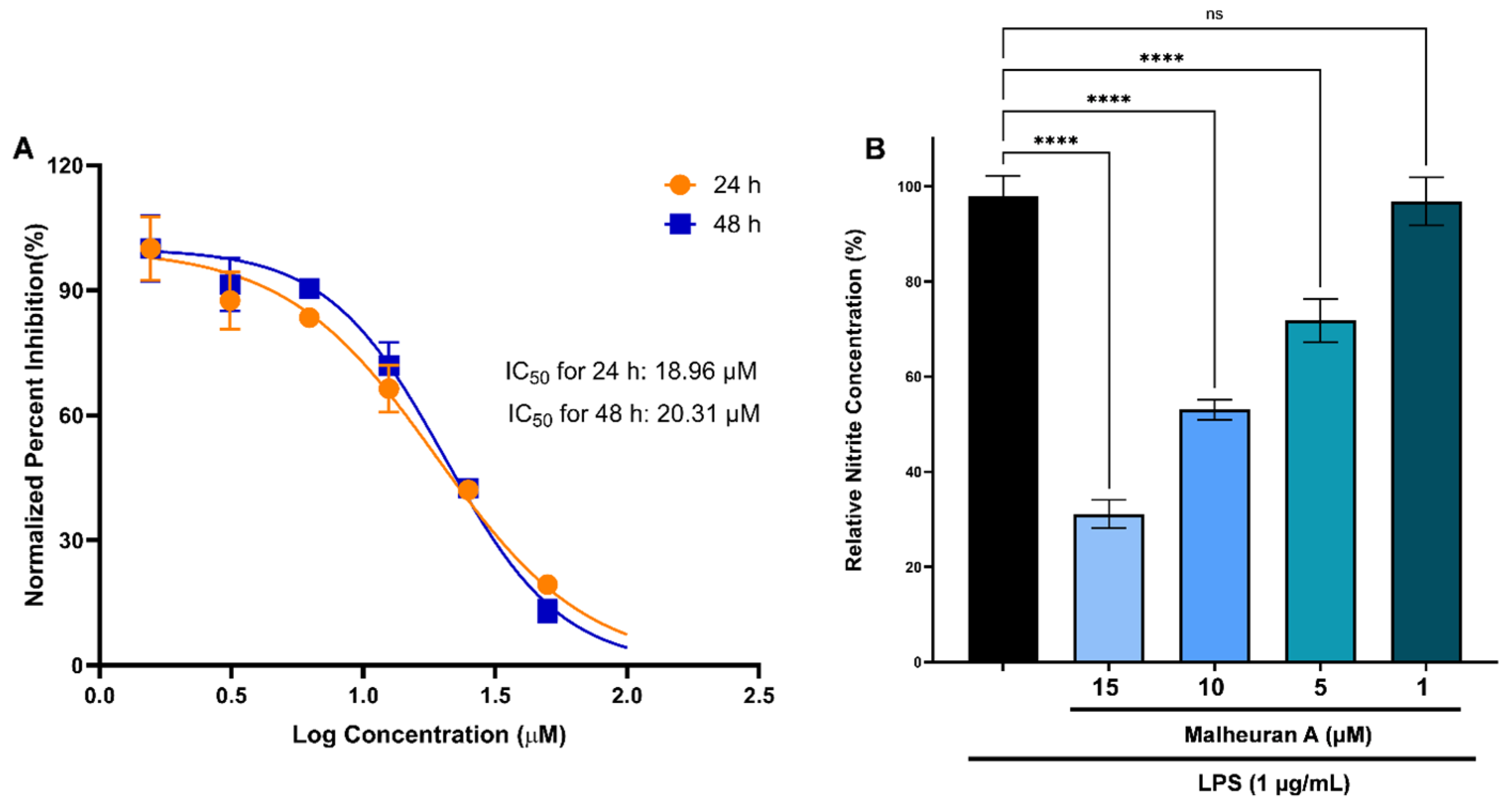
2.10. Anti-Inflammatory Activity of Malheuran A
3. Materials and Methods
3.1. Seed Sterilization and Germination of D. purpurea
3.2. Establishment of Hairy Root Lines of D. purpurea
3.3. Growth Kinetics of D. purpurea Hairy Roots
3.4. Elicitation of D. purpurea Hairy Root Culture and Analytical HPLC Analysis
3.5. Re-Elicitation of D. purpurea Hairy Root Cultures
3.6. Extraction and HPLC Analysis of Polyphenols from D. purpurea Hairy Root Culture Medium and Tissues
3.7. Extraction and Isolation of Malheuran A
3.8. Nuclear Magnetic Resonance Spectrometer (NMR) Analysis
3.9. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis
3.10. Antimicrobial Assay
3.11. Anti-Inflammatory Assay
3.11.1. Cell Culture
3.11.2. Cytotoxicity Assay and Quantification of Nitrite Level
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peralta, M.A.; Santi, M.D.; Cabrera, J.L.; Ortega, M.G. Dalea genus, chemistry, and bioactivity studies. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 307–341. [Google Scholar]
- Dreyer, D.L.; Munderloh, K.P.; Thiessen, W.E. Extractives of Dalea species (Leguminosae). Tetrahedron 1975, 31, 287–293. [Google Scholar] [CrossRef]
- Dictionary of Natural Products, 33.1; CRC Press: Boca Raton, FL, USA; Available online: https://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml (accessed on 7 November 2024).
- Belofsky, G.; French, A.N.; Wallace, D.R.; Dodson, S.L.J. New geranyl stilbenes from Dalea purpurea with in vitro opioid receptor affinity. J. Nat. Prod. 2004, 67, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, X.; Liu, T.; Wang, Y.; Ahmed, N.; Li, Z.; Jiang, H. Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells. Plant Commun. 2021, 2, 100229. [Google Scholar] [CrossRef] [PubMed]
- Vanhakylä, S.; Salminen, J.-P. Seasonal variation in plant polyphenols and related bioactivities across three years in ten tree species as visualized by mass spectrometric fingerprint mapping. Molecules 2023, 28, 6093. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Hairy root culture for mass-production of high-value secondary metabolites. Crit. Rev. Biotechnol. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Ren, F.-C.; Wang, L.-X.; Lv, Y.-F.; Hu, J.-M.; Zhou, J. Structure revision of four classes of prenylated aromatic natural products based on a rule for diagnostic 13C NMR chemical shifts. J. Org. Chem. 2020, 86, 10982–10990. [Google Scholar] [CrossRef]
- Yazaki, K.; Sasaki, K.; Tsurumaru, Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 2009, 70, 1739–1745. [Google Scholar] [CrossRef]
- Belofsky, G.; Engels, L.; McPherson, V.; Nash, K.; Sullivan, K.; Torrey, B.; Ripley, C.; Coria, A.; Bicchieri, T.; Dondji, B. Investigation of Dalea parryi (Fabaceae) metabolites for anthelmintic activity against the human pathogenic hookworm Ancylostoma ceylanicum. Phytochemistry 2020, 177, 112423. [Google Scholar] [CrossRef]
- Deardorff, K.; Ray, W.; Winterstein, E.; Brown, M.; McCornack, J.; Cardenas-Garcia, B.; Jones, K.; McNutt, S.; Fulkerson, S.; Ferreira, D.; et al. Phenolic metabolites of Dalea ornata affect both survival and motility of the human pathogenic hookworm Ancylostoma ceylanicum. J. Nat. Prod. 2016, 79, 2296–2303. [Google Scholar] [CrossRef]
- Navrátilová, A.; Nešuta, O.; Vančatová, I.; Čížek, A.; Varela-M, R.E.; López-Abán, J.; Villa-Pulgarin, J.A.; Mollinedo, F.; Muro, A.; Žemličková, H.; et al. C-Geranylated flavonoids from Paulownia tomentosa fruits with antimicrobial potential and synergistic activity with antibiotics. Pharm. Biol. 2016, 54, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Fang, S.-C.; Wu, C.-H.; Huang, S.-M.; Yen, G.-C. Anti-inflammatory Effect of the 5,7,4′-Trihydroxy-6-geranylflavanone Isolated from the Fruit of Artocarpus communis in S100B-Induced Human Monocytes. J. Agric. Food Chem. 2011, 59, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Šmejkal, K.; Svačinová, J.; Šlapetová, T.; Schneiderová, K.; Dall’Acqua, S.; Innocenti, G.; Závalová, V.; Kollár, P.; Chudík, S.; Marek, R.; et al. Cytotoxic activities of several geranyl-substituted flavanones. J. Nat. Prod. 2010, 73, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Sychrová, A.; Škovranová, G.; Čulenová, M.; Fialová, S.B. Prenylated flavonoids in topical infections and wound healing. Molecules 2022, 27, 4491. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Navrátilová, A.; Schneiderová, K.; Veselá, D.; Hanáková, Z.; Fontana, A.; Dall’Acqua, S.; Cvačka, J.; Innocenti, G.; Novotná, J.; Urbanová, M.; et al. Minor C-geranylated flavanones from Paulownia tomentosa fruits with MRSA antibacterial activity. Phytochemistry 2013, 89, 104–113. [Google Scholar] [CrossRef]
- Šmejkal, K.; Chudík, S.; Klouček, P.; Marek, R.; Cvačka, J.; Urbanová, M.; Julínek, O.; Kokoška, L.; Šlapetová, T.; Holubová, P.; et al. Antibacterial C-geranylflavonoids from Paulownia tomentosa fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef]
- Hanáková, Z.; Hošek, J.; Babula, P.; Dall’Acqua, S.; Václavík, J.; Šmejkal, K. C-Geranylated flavanones from Paulownia tomentosa fruits as potential anti-inflammatory compounds acting via inhibition of TNF-α production. J. Nat. Prod. 2015, 78, 850–863. [Google Scholar] [CrossRef]
- Ryu, H.W.; Park, Y.J.; Lee, S.U.; Lee, S.; Yuk, H.J.; Seo, K.H.; Kim, Y.U.; Hwang, B.Y.; Oh, S.R. Potential anti-inflammatory effects of the fruits of Paulownia tomentosa. J. Nat. Prod. 2017, 80, 2659–2665. [Google Scholar] [CrossRef]
- Hanáková, Z.; Hošek, J.; Kutil, Z.; Temml, V.; Landa, P.; Vaněk, T.; Schuster, D.; Dall’acqua, S.; Cvačka, J.; Polanský, O.; et al. Anti-inflammatory activity of natural geranylated flavonoids: Cyclooxygenase and lipoxygenase inhibitory properties and proteomic analysis. J. Nat. Prod. 2017, 80, 999–1006. [Google Scholar] [CrossRef]
- Elkordy, A.A.; Haj-Ahmad, R.R.; Awaad, A.S.; Zaki, R.M. An overview on natural product drug formulations from conventional medicines to nanomedicines: Past, present and future. J. Drug Deliv. Sci. Technol. 2021, 63, 102459. [Google Scholar] [CrossRef]
- Shenoy, E.S.; Paras, M.L.; Noubary, F.; Walensky, R.P.; Hooper, D.C. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): A systematic review. BMC Infect. Dis. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Elmaidomy, A.H.; Shady, N.H.; Abdeljawad, K.M.; Elzamkan, M.B.; Helmy, H.H.; Tarshan, E.A.; Adly, A.N.; Hussien, Y.H.; Sayed, N.G.; Zayed, A. Antimicrobial potentials of natural products against multidrug resistance pathogens: A comprehensive review. RSC Adv. 2022, 12, 29078–29102. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Zhuravleva, Y. Inflammation: A new look at an old problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Jia, X.H.; Xiao, C.M.; Tang, W.Z. Paulownia C-geranylated flavonoids: Their structural variety, biological activity and application Prospects. Phytochem. Rev. 2019, 18, 549–570. [Google Scholar] [CrossRef]
- Mazzoni-Putman, S.M.; Brumos, J.; Zhao, C.; Alonso, J.M.; Stepanova, A.N. Auxin Interactions with other hormones in plant development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039990. [Google Scholar] [CrossRef]
- Sharma, A.R.; Gajurel, G.; Ahmed, I.; Roedel, K.; Medina-Bolivar, F. Induction of the prenylated stilbenoids arachidin-1 and arachidin-3 and their semi-preparative separation and purification from hairy root cultures of peanut (Arachis hypogaea L.). Molecules 2022, 27, 6118. [Google Scholar] [CrossRef]
- Gajurel, G.; Ñopo-Olazabal, L.; Hendrix, E.; Medina-Bolivar, F. Production and secretion of cajaninstilbene acid in hairy root cultures of pigeon pea (Cajanus cajan) co-treated with multiple elicitors. Planta 2022, 11, 834. [Google Scholar]
- Fang, L.; Sharma, A.R.; Aniemena, C.; Roedel, K.; Henry, F.; Moussou, P.; Samuga, A.; Medina-Bolivar, F. Elicitation of stilbenes and benzofuran derivatives in hairy root cultures of white mulberry (Morus alba). Plants 2023, 12, 175. [Google Scholar] [CrossRef]
- Belofsky, G.; Aronica, M.; Foss, E.; Diamond, J.; Santana, F.; Darley, J.; Dowd, P.F.; Coleman, C.M.; Ferreira, D. Antimicrobial and antiinsectan phenolic metabolites of Dalea searlsiae. J. Nat. Prod. 2014, 77, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.W.; Wang, Q.L.; Luo, M.; Zhu, M.D.; Liang, H.M.; Li, W.J.; Cai, H.; Zhou, Z.B.; Wang, H.; Tong, S.Q.; et al. Phytochemistry and pharmacology of natural prenylated flavonoids. Arch. Pharm. Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Eerdunbayaer; Orabi, M.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of new phenolics isolated from licorice, and the effectiveness of licorice phenolics on vancomycin-resistant Enterococci. Molecules 2014, 19, 13027–13041. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant. Sci. 2022, 13, 881032. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Condori, J.; Sivakumar, G.; Hubstenberger, J.; Dolan, M.C.; Sobolev, V.S.; Medina-Bolivar, F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol. Biochem. 2010, 48, 310–318. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef]
- Oku, N.; Hayashi, S.; Yamaguchi, Y.; Takenaka, H.; Igarashi, Y. Nostochopcerol, a new antibacterial monoacylglycerol from the edible cyanobacterium Nostochopsis lobatus. Beilstein J. Org. Chem. 2023, 19, 133–138. [Google Scholar] [CrossRef]







| Position | δC a | δH, Mult (J in Hz) b | HMBC b,c |
|---|---|---|---|
| 2 | 75.0, CH | 5.66, dd (13.0, 2.7) | 4, 1′, 2′, 6′ |
| 3 | 43.0, CH2 | 2.91, dd (16.7, 13.0) | 2, 1′, 4 |
| 2.68, dd (16.7, 2.7) | 4, 10 | ||
| 4 | 190.7, C | ||
| 5 | 125.5, CH | 7.57, d (8.6) | 4, 7, 9 |
| 6 | 109.5, CH | 6.60, d (8.6) | 8, 10 |
| 7 | 161.6, C | ||
| 8 | 115.5, C | ||
| 9 | 161.3, C | ||
| 10 | 114.5, C | ||
| 1′ | 117.5, C | ||
| 2′ | 155.2, C | ||
| 3′ | 102.5, CH | 6.44, d (2.0) | 1′, 5′, 2′, 4′ |
| 4′ | 158.4, C | ||
| 5′ | 106.9, CH | 6.42, dd (8.3, 2.0) | 3′, 1′, 4′ |
| 6′ | 127.8, CH | 7.35, d (8.3) | 2, 2′, 4′ |
| 1″ | 21.9, CH2 | 3.33, m | 7, 8, 9, 2″, 3″ |
| 2″ | 122.3, CH | 5.25, t (7.1) | 5″, 1″, 4″, 8 |
| 3″ | 134.5, C | ||
| 4″ | 15.4, CH3 | 1.63, s | 5″, 2″, 3″ |
| 5″ | 39.6, CH2 | 1.90, m | 4″, 6″, 2″, 7″, 3″ |
| 6″ | 26.5, CH2 | 2.02 d | 5″, 8″, 7″, 3″ |
| 7″ | 124.3, CH | 5.02, t (6.8) | 6″, 10″, 9” |
| 8″ | 130.7, C | ||
| 9″ | 25.0, CH3 | 1.56, s | 8″, 7″, 10″ |
| 10″ | 16.9, CH3 | 1.50, s | 8″, 7″, 9″ |
| tR (min) | UV Max (nm) | [M + H]+ | MS2 Ions | [M − H]− | MS2 Ions |
|---|---|---|---|---|---|
| 19.4 | 207, 221 (sh), 233 (sh), 287, 322 (sh) | 409 | 273, 391 | 407 | 160, 245, 389 |
| Microorganisms | MIC (µg/mL) | Reference (µg/mL) |
|---|---|---|
| Acinetobacter baumannii ATCC 747 a | >100 | 0.62 c |
| Bacillus subtilis ATCC 6623 b | 3.12 | 0.31 d |
| Enterococcus faecalis ATCC 29212 b | 3.12 | 5 d |
| Enterococcus faecalis ATCC 51299 (VRE) b | 3.12 | 10 e |
| Enterococcus faecium ATCC 700221 (VRE) b | 3.12 | 5 e |
| Escherichia coli ATCC 25922 a | >100 | 0.31 c |
| Klebsiella pneumoniae ATCC 700603 a | >100 | 0.62 c |
| Micrococcus luteus ATCC 10240 b | 1.56 | 0.31 d |
| Staphylococcus aureus ATCC 25923 b | 3.12 | 1.25 d |
| Staphylococcus aureus ATCC 35591 (MRSA) b | 3.12 | 2.5 d |
| Staphylococcus aureus ATCC 00699 (MRSA) b | 3.12 | 5 d |
| Staphylococcus epidermidis ATCC 700296 b | 6.25 | 2.5 d |
| Streptococcus mutans UA159 b | 3.12 | 1.25 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.R.; Gajurel, G.; Abdel-Karim, S.; Alam, M.A.; Shields, R.C.; Medina-Bolivar, F. Production of Malheuran A, a Geranylated Flavonoid with Antimicrobial and Anti-Inflammatory Activities, in Hairy Root Cultures of Dalea purpurea. Plants 2025, 14, 259. https://doi.org/10.3390/plants14020259
Sharma AR, Gajurel G, Abdel-Karim S, Alam MA, Shields RC, Medina-Bolivar F. Production of Malheuran A, a Geranylated Flavonoid with Antimicrobial and Anti-Inflammatory Activities, in Hairy Root Cultures of Dalea purpurea. Plants. 2025; 14(2):259. https://doi.org/10.3390/plants14020259
Chicago/Turabian StyleSharma, Amit Raj, Gaurav Gajurel, Salma Abdel-Karim, Mohammad Abrar Alam, Robert Colquhoun Shields, and Fabricio Medina-Bolivar. 2025. "Production of Malheuran A, a Geranylated Flavonoid with Antimicrobial and Anti-Inflammatory Activities, in Hairy Root Cultures of Dalea purpurea" Plants 14, no. 2: 259. https://doi.org/10.3390/plants14020259
APA StyleSharma, A. R., Gajurel, G., Abdel-Karim, S., Alam, M. A., Shields, R. C., & Medina-Bolivar, F. (2025). Production of Malheuran A, a Geranylated Flavonoid with Antimicrobial and Anti-Inflammatory Activities, in Hairy Root Cultures of Dalea purpurea. Plants, 14(2), 259. https://doi.org/10.3390/plants14020259






