Abstract
Sitophilus oryzae, Tribolium castaneum, Tribolium confusum, Oryzaephilus surinamensis, Rhyzopertha dominica, Tenebrio molitor, Trogoderma granarium, Acarus siro, and Alphitobius diaperinus represent significant arthropod stored-product pests worldwide. To combat these noxious arthropods, the current study examines the pesticidal effect of essential oils (EOs) derived from four aromatic plants, i.e., Illicium verum Hook. F., Citrus reticulata Blanco, Monodora myristica (Gaertn.) Dunal, and Xylopia aethiopica (Dunal) A. Rich. Considering the challenge of pesticide resistance, the current study focuses on assessing the efficacy of these EOs as an eco-friendly alternative to traditional synthetic insecticides. Two EO concentrations (500 and 1000 µL/kg wheat) were applied to different life stages of these pests in the bioassays. Mortality rates were monitored over several days under controlled environmental conditions. The findings demonstrated that C. reticulata and I. verum EOs had elevated insecticidal effects, especially against larval stages, resulting in 100% mortality in several species. On the contrary, M. myristica and X. aethiopica EOs showed less overall efficacy despite their potency against some pests. Both I. verum and C. reticulata EOs outperformed the positive control, pirimiphos-methyl, in several assays. The results of the current study highlight the potential of several EOs as effective alternatives in reducing synthetic pesticide use for integrated pest control management.
1. Introduction
The bostrychid Rhyzopertha dominica (F.), the tenbrionids Alphitobius diaperinus (Panzer), Tribolium castaneum (Herbst), Tribolium confusum Jacquelin du Val, and Tenebrio molitor L., the curculionid Sitophilus oryzae (L.), the dermestid Trogoderma granarium Everts, the sylvanid Oryzaephilus surinamensis (L.), and the acarid Acarus siro L., are major pests of stored commodities that are spread globally [1,2]. These species cause serious infestations and degradation in storage units containing cereal, grain by-products, pulses, fruits, nuts, vegetables, and even animal-derived products [3]. In addition to the qualitative and quantitative damages caused by these arthropod pests, their activity in different agricultural settings may lead to the accumulation of contaminants, like feces, body fragments, and secretions [4]. Several of these pests are linked with human, pet, and farm animal health hazards such as allergies and diseases [5,6,7]. The constant global distribution of stored-product arthropod populations through international commerce, along with occurring and documented pesticide resistance [8,9,10], necessitates further research and development of novel pest control measures [11,12,13].
Illicium verum Hook. f. (Austrobaileyales: Schisandraceae) is an aromatic evergreen tree known for its reddish, star-shaped fruits and blossoms. It is predominantly cultivated in Southern Asia, particularly in China and Vietnam. The fruit of the tree, commonly known as star anise, is widely used as a cooking spice and has a significant role in traditional Chinese medicine for its therapeutic properties [14]. Illicium verum is widely utilized in herbal medicine, due to the effectiveness of its bioactive constituents, particularly (E)-anethole [15]. Recent research highlights that the fruit of I. verum possesses important antioxidant, antimicrobial, and anti-inflammatory activities [16,17].
Xylopia aethiopica (Dunal) A. Rich. (Magnoliales: Annonaceae) is extensively distributed throughout Africa, particularly in the Western and central regions [18]. It is an evergreen tree, often reaching heights of over 20 m, producing fruits that are slightly hooked cylindrical pods, measuring 2 to 3 mm in width [18]. The plant holds cultural significance in various regions of West Africa due to its medicinal properties and wide usage as a spice. Indeed, many studies have demonstrated its anti-inflammatory, anti-anaphylactic, and antipyretic effects [19,20], including its application for the management of postpartum hemorrhage and contractions [21]. The fruit extracts and stem bark decoctions are traditionally used to cure conditions such as biliousness, bronchitis, and dysentery [22,23]. In addition, it has been proven to be a potent antimicrobial agent against serious infectious microorganisms, like Bacillus subtilis, Staphylococcus aureus, and Candida albicans [19,24].
Monodora myristica (Gaertn.) Dunal (Magnoliales: Annonaceae), commonly known as calabash nutmeg, is a tropical perennial species indigenous to West Africa [25]. This tree typically reaches heights of 15 to 20 m and bears large, glossy leaves [26]. Its distinctive fruit bears a hard, woody shell resembling a calabash, housing seeds that emit a rich, nutmeg-like fragrance [27]. For a long time, these aromatic seeds have been used as a culinary spice and in traditional folk medicine, while recent research focused on their pharmacological potential [28]. The bioactive compounds found in the seeds and stem bark exhibit strong antioxidant and anti-inflammatory properties [29,30]. The stem bark is often utilized as a remedy for ailments such as stomach aches, hemorrhoids, fever, and eye ailments [31]. Additionally, many studies demonstrated its cytotoxicity against cancer cells [32] as well as its antibacterial [33] and antiparasitic effects [34].
Citrus reticulata Blanco (Sapindales: Rutaceae), commonly known as mandarin, represents a prominent and economically significant plant worldwide [35]. Originating in Southeast Asia, this fruit-bearing evergreen tree has become widely cultivated, due to its flavorful and aromatic fruits [36,37]. Unlike common oranges, mandarins can range from oblate to spherical, depending on the variety [38]. Moreover, Citrus fruit peels are rich in phenolics and flavonoids, responsible for many of their bioactivities, generating scientific interest [39]. Previous studies have focused on their antioxidant [40], anti-inflammatory [41], potential anti-cancer [36], antimicrobial [37], and anti-diabetic effects [42].
Given the limited knowledge on the EOs from I. verum, X. aethiopica, M. myristica, and C. reticulata as grain protectants [43,44], the current study aimed at investigating their potential as bio-insecticides against key primary and secondary pests. Given the widespread problem of pesticide resistance and the growing need for sustainable pest management strategies, this research effort aimed to assess the pesticidal potential of these EOs at varying concentrations against eight economically important arthropod pest species. In addition, the present study evaluated the comparative effectiveness of the tested EOs in relation to the conventional pesticide pirimiphos-methyl in reducing pest populations.
2. Results
2.1. EO Chemical Compositions
The GC-MS analysis of M. myristica, X. aethiopica, I. verum, and C. reticulata EOs led to the identification of the 92.9, 99.6, 99.7, and 99.3% of the total composition, respectively (Table 1). As previously reported in the work of Wandjou et al. [45], M. myristica EO was mainly characterized by monoterpene hydrocarbons, i.e., p-cymene (32.8%), α-phellandrene (32.3%), α-pinene (7.6%), limonene (4.4%), and myrcene (4.3%), while X. aethiopica EO was dominated by monoterpene hydrocarbons, oxygenated monoterpenes, and sesquiterpene hydrocarbons with sabinene (26.1%), β-pinene (17.4%), germacrene D (9.7%), α-pinene (9.6%), β-phellandrene (6.2%), and terpinen-4-ol (6.1%) as main compounds (Table 1). Concerning I. verum, its EO was dominated by phenylpropanoids, being (E)-anethole the predominant compound (92.6%). On the other hand, C. reticulata EO was dominated by monoterpene hydrocarbons, with limonene (97.1%) as the main representative of this chemical class (Table 1).

Table 1.
Chemical composition of the essential oils (EOs) determined by GC-MS analysis.
2.2. Effectiveness Against A. diaperinus Adults and Larvae
All main effects and their interactions were significant between and within exposures for both adult and larval stages of A. diaperinus, except the main effect concentration and the three-way interaction between exposure, concentration, and EO for adults (Table 2). Citrus reticulata and I. verum at the highest concentration demonstrated significant effectiveness against A. diaperinus larvae, generally after the fifth day post-exposure, whereas their impact on adults was considerably less pronounced. At 500 ppm, all four EOs exhibited relatively low initial larval mortality (<16%) over a two-day period, with C. reticulata and M. myristica surpassing 50% mortality on day 5, ultimately reaching 66.7 and 56.7% at 7 days post-exposure. Meanwhile, the positive control, pirimiphos-methyl (P-m), demonstrated a consistent increase with time but peaked at 36.7% on day 7. At the higher concentration (1000 ppm), C. reticulata and I. verum achieved complete mortality (100%) on day 6 and day 7, respectively. Monodora myristica followed with 91.1% mortality by day 7. Conversely, X. aethiopica and P-m continued to exhibit elevated efficacy, with X. aethiopica reaching 53.3% and P-m exhibiting a similar mortality trend (37.8%). For adult mortality, recorded rates were generally low across all treatments, with C. reticulata showing the maximum mortality of 11.1% by day 7 at 1000 ppm (Figure 1, Table S1).

Table 2.
MANOVA parameters depicting the main effects and their interactions leading to the observed mortalities of A. diaperinus, T. castaneum, T. confusum, T. molitor, T. granarium, O. surinamensis larvae and adults, R. dominica and S. oryzae adults, and A. siro nymphs and adults, between and within exposure intervals (error df = 80 for all pest species and developmental stages).
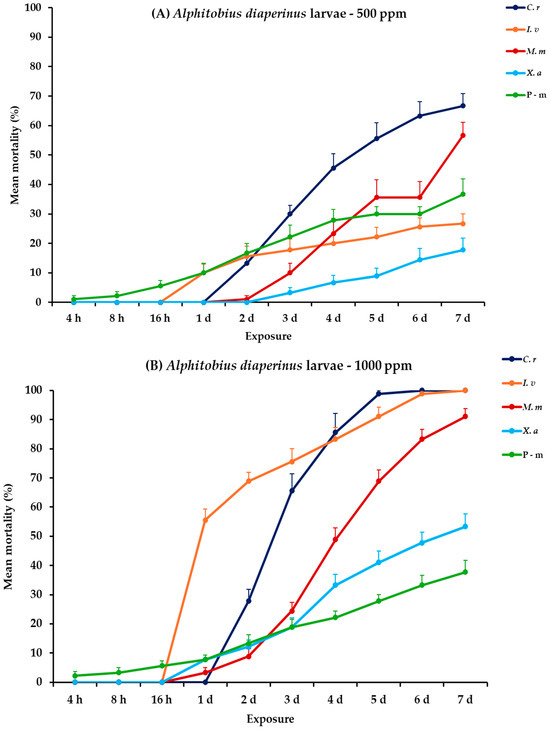
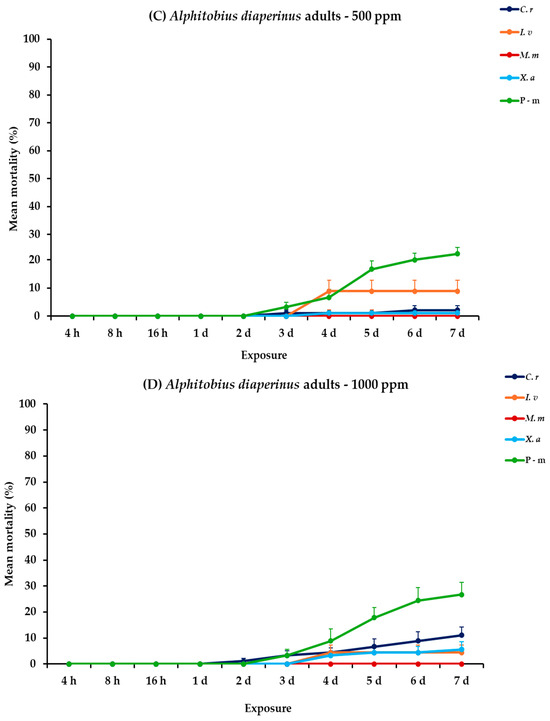
Figure 1.
Mean (%) mortality + standard error (SE) of Alphitobius diaperinus larvae (A,B) and adults (C,D) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.3. Effectiveness Against T. castaneum Adults and Larvae
The analysis of mortality rates for T. castaneum larvae and adults revealed that all the main effects and their interactions between and within exposures were significant. For adults, only concentration had no significant effect (Table 2). Illicium verum, X. aethiopica, and C. reticulata EOs were the most effective in controlling T. castaneum larvae, particularly at the high concentration. At 500 ppm, I. verum showed the highest mortality rate for larvae, reaching 86.7% on day 7, followed closely by X. aethiopica with 85.6%, while C. reticulata and M. myristica exhibited moderate effectiveness, peaking at 56.7 and 24.4%, respectively. The positive control, P-m, achieved 58.9% mortality on day 7. At 1000 ppm, complete mortality (100%) was displayed by I. verum on day 5, and by C. reticulata and X. aethiopica on day 7. Monodora myristica demonstrated a substantial increase in effectiveness at this concentration, attaining 52.2% mortality on day 7. In contrast, the adult T. castaneum showed zero to minimal mortality rates to all treatments at both concentrations, with X. aethiopica at 1000 ppm resulting only at 4.4% by the last day of the bioassay (Figure 2, Table S2).
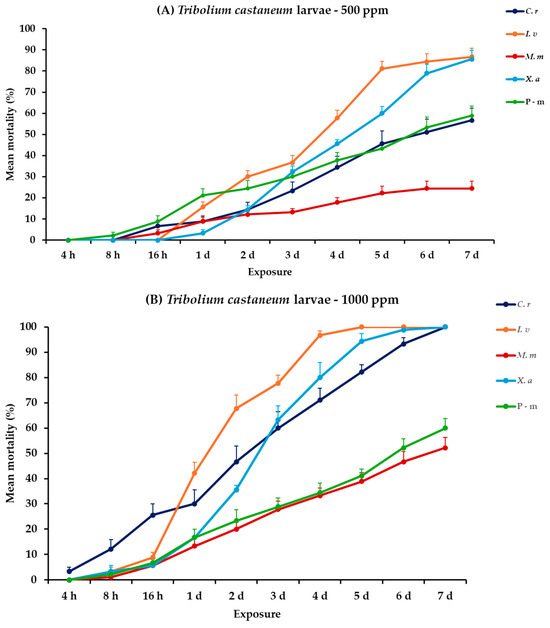
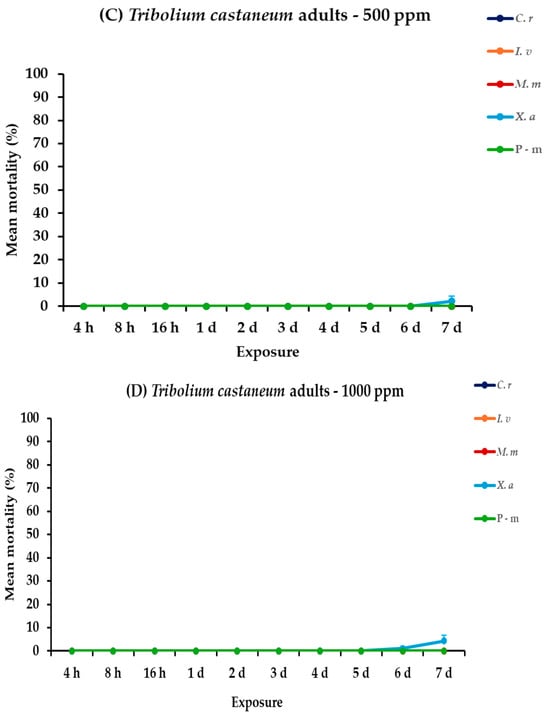
Figure 2.
Mean (%) mortality + standard error (SE) of Tribolium castaneum larvae (A,B) and adults (C,D) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.4. Effectiveness Against T. confusum Adults and Larvae
For T. confusum larvae, all the main effects and respective interactions between and within exposures were significant. For adults, all main effects were significant between exposures, except concentration × EO type. Within exposures the main effects and their interactions were significant (Table 2). Citrus reticulata caused an initial larval mortality of 4.4% at 16 h, eventually reaching 44.4% on day 7 at 500 ppm. Nevertheless, I. verum demonstrated a rapid and significant increase in mortality, achieving 91.1% on day 7 post-exposure. Monodora myristica and X. aethiopica were less effective, with 10 and 42.2% mortality on the last day, respectively. At the highest concentration, the effectiveness of the EOs significantly improved. Specifically, on day 6, I. verum caused complete mortality (100%). On day 7, C. reticulata caused a near-total mortality of 97.8%, while M. myristica and X. aethiopica showed elevated efficacy with rates of 62.2 and 76.7% mortality, respectively. The positive control caused the same level of mortality (66.7%) either compared to EOs tested at 500 ppm or 1000 ppm. For adults of T. confusum, at 500 ppm, only X. aethiopica exhibited slight activity, reaching 5.6%, whereas the other EOs, including the positive control, P-m, showed no significant mortality. At 1000 ppm, again X. aethiopica displayed a visible increase and peaked at 18.9%; meanwhile, the rest of the EOs showed minimal lethal effects, with recorded mortality remaining below 6% (Figure 3, Table S3).
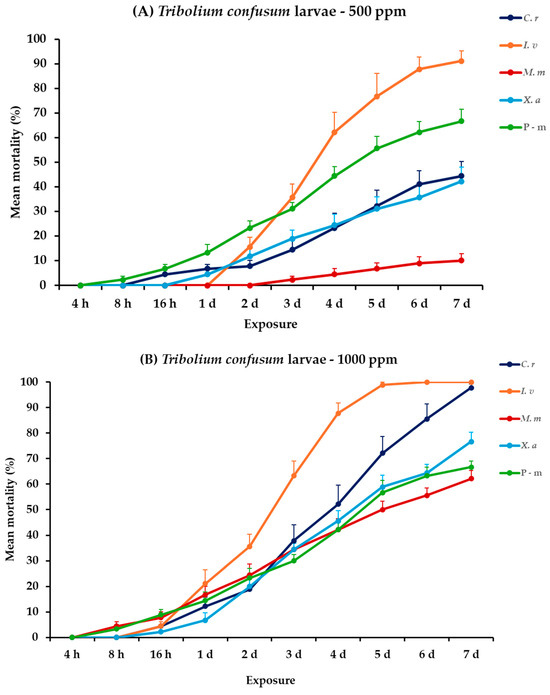
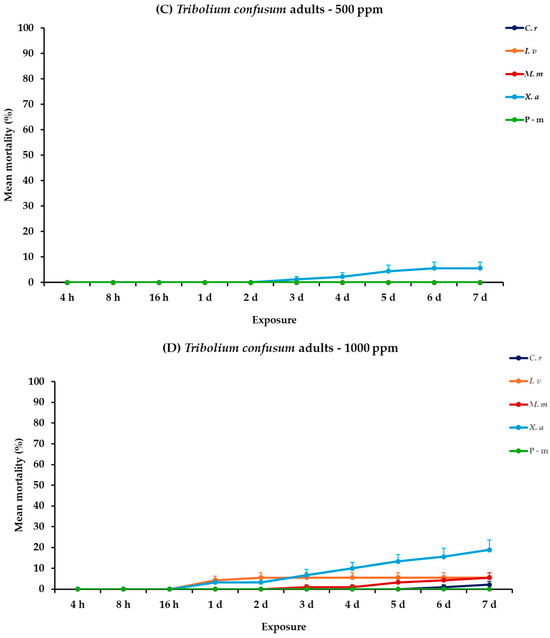
Figure 3.
Mean (%) mortality + standard error (SE) of Tribolium confusum larvae (A,B) and adults (C,D) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.5. Effectiveness Against T. molitor Adults and Larvae
For the analysis of the adults and larvae of T. molitor, all main effects and respective interactions were significant both between and within exposures for adults. For larvae, the main effect exposure × concentration and the interaction exposure × concentration × EO type had no significant effect (Table 2). At 500 ppm, larvae exhibited minimal mortality rates across all EO treatments (<6.7%), with the only significant increase being observed with P-m on the last day (31.1%). In contrast, adult mortality was significantly more pronounced, particularly with C. reticulata and X. aethiopica displaying the highest mortality rate at 47.8 and 37.8%, respectively, on day 7, without surpassing the P-m rate of 66.7%. A similar trend was detected at 1000 ppm, both larvae and adults displayed higher but still moderate mortality values, notably with C. reticulata larvae reaching 10.0% and adults peaking at 78.9% on day 7. Similarly, X. aethiopica displayed a distinct difference between larvae and adults, with 14.4 and 83.3% on day 7, respectively (Figure 4, Table S4).
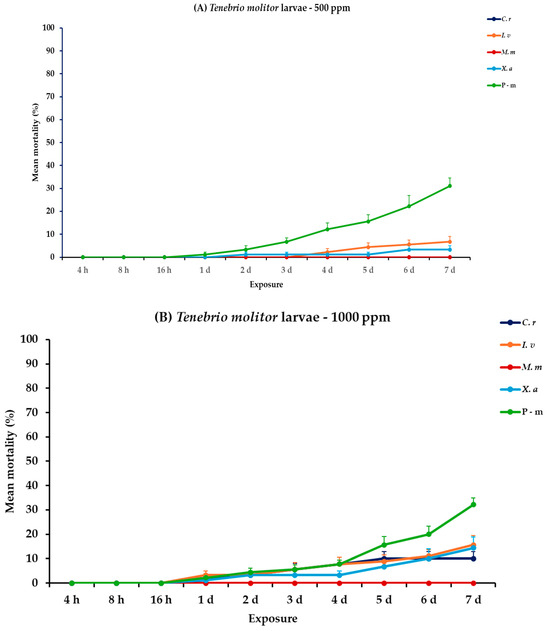
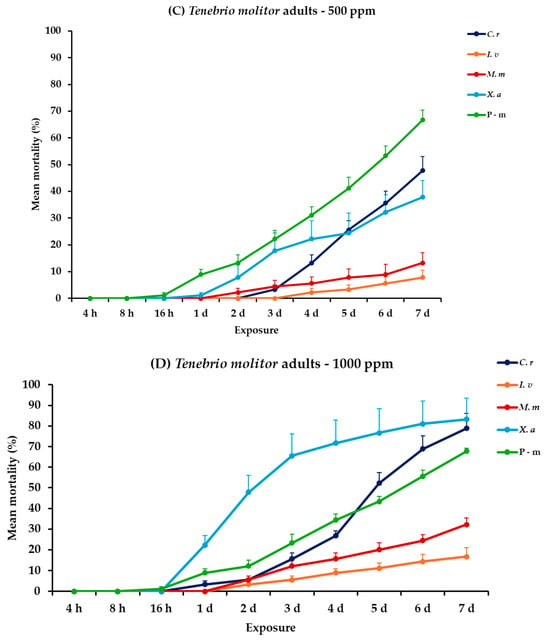
Figure 4.
Mean (%) mortality + standard error (SE) of Tenebrio molitor larvae (A,B) and adults (C,D) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.6. Effectiveness Against T. granarium Adults and Larvae
For T. granarium, all main effects and corresponding interactions were significant for adults and larvae, both within and between exposures (Table 2). At the lower concentration, larval mortality remained low at 7 days post-exposure. Citrus reticulata and X. aethiopica showed no significant larval mortality at this concentration. Recorded data for I. verum and M. myristica were initially minimal and increased consistently to 30.0 and 23.3%, respectively. For adults of T. granarium, mortality increased substantially with time. Monodora myristica achieved the highest adult mortality of 80.0%, followed by X. aethiopica (67.8%) at 7 days post-exposure. At 1000 ppm, a slight increase but no significant difference was observed in larval mortality for both I. verum and M. myristica (47.8 and 31.1%, respectively), while X. aethiopica exhibited minimal efficacy (5.6%). Recorded larval mortality of positive control, when compared to EOs tested at 500 or 1000 ppm, also remained stable at approximately 25.0%. Adult mortality reached 100% for M. myristica after 6 days, followed by X. aethiopica (93.3%), I. verum (90.0%), and C. reticulata (84.4%) at the end of the exposure period. The positive control in both exposure trials also yielded relatively lower results but remained high, with approximately 70% mortality (Figure 5, Table S5).
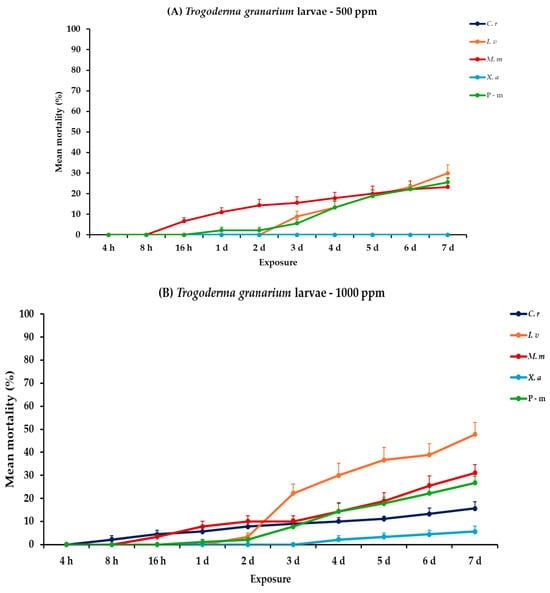
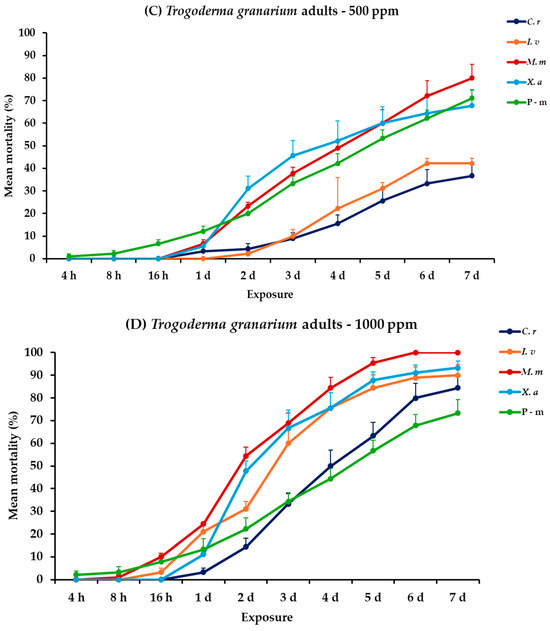
Figure 5.
Mean (%) mortality + standard error (SE) of Trogoderma granarium larvae (A,B) and adults (C,D) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.7. Effectiveness Against O. surinamensis Adults and Larvae
For O. surinamensis, significance was demonstrated for all the main effects and their respective interactions for both life stages, within and between exposures, except the exposure × concentration × EO type interaction for adults (Table 2). At 500 ppm, larval mortality increased gradually over 7 days for all EOs, with C. reticulata and I. verum demonstrating relatively moderate mortality rates at the end of the experiment (35.6 and 28.9%, respectively), though none approached the 63.3% mortality observed with P-m. Adult mortality trends at 500 ppm suggested that I. verum and X. aethiopica were the most lethal, achieving 56.7 and 45.6% mortality, respectively, at the last day, whereas P-m remained at 10%. At 1000 ppm, larval mortality was significantly higher. Specifically, I. verum caused 92.2%, followed by X. aethiopica and C. reticulata, which both peaked at 64.4% after a seven-day period. Accordingly, I. verum also exhibited the highest mortality for adults of this species, reaching a high percentage (90%), while C. reticulata and X. aethiopica also showed a significant increase in effectiveness (71.1 and 80%, respectively) (Figure 6, Table S6).
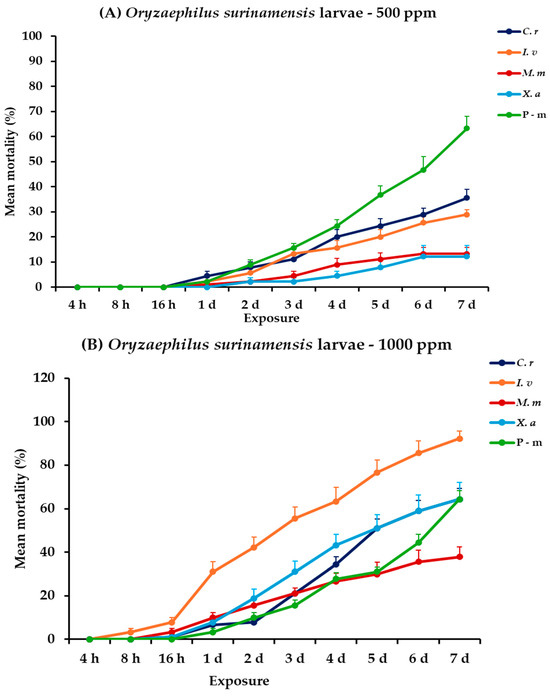
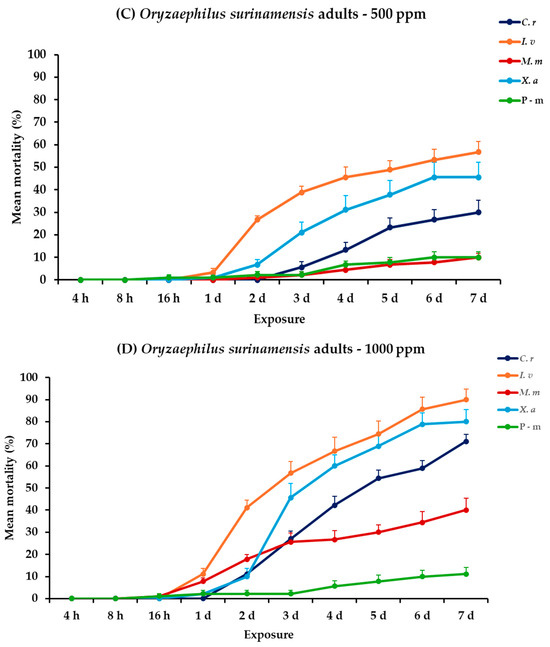
Figure 6.
Mean (%) mortality + standard error (SE) of Oryzaephilus surinamensis larvae (A,B) and adults (C,D) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.8. Effectiveness Against R. dominica Adults
For R. dominica adults, all main effects were significant, along with their associated interactions, between and within exposures (Table 2). At 500 ppm of tested EOs, C. reticulata and X. aethiopica exhibited minimal efficacy, with mortality rates remaining below 15% even after the 7-day period. Additionally, I. verum and M. myristica showed slightly higher mortality rates, both reaching up to 15.6% on the seventh day. At 1000 ppm, all EOs procured elevated mortality percentages in correlation with exposure time. Citrus reticulata and X. aethiopica exhibited substantial increases in mortality, up to 46.7 and 63.3%, respectively, on day 7, while I. verum and M. myristica showed low improvements in mortality, reaching 21.1 and 20.0%, respectively, without significant differences. Interestingly, P-m remained the most effective treatment against this species, achieving 81.1% mortality rate either compared to EOs tested at 500 or 1000 ppm (Figure 7, Table S7).
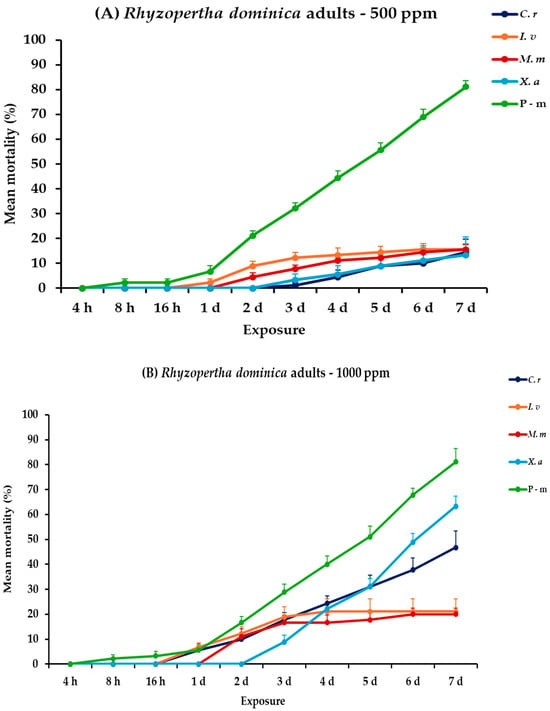
Figure 7.
Mean (%) mortality + standard error (SE) of Rhyzopertha dominica adults (A,B) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.9. Effectiveness Against S. oryzae Adults
For S. oryzae adults, all main effects and their interactions across different exposure times were significant (Table 2). At 500 ppm, C. reticulata showed a marked increase in mortality, reaching 100% at the end of the trial. Contrarily, I. verum, M. myristica, and X. aethiopica exhibited significantly lower effectiveness, with mortalities peaking at 7.8, 3.3, and 4.4%, respectively. At the higher concentration, the efficacy of C. reticulata remained high, achieving 98.9% mortality. Following C. reticulata, I. verum showed improved results at this concentration, with mortality rates rising to 44.4% on day 7, whereas M. myristica and X. aethiopica continued to show limited effectiveness, with recorded mortalities of 11.1 and 8.9%, respectively, on day 7. Positive control also remained highly effective at both dose scenarios against S oryzae, achieving mortality rates of approximately 90% (Figure 8, Table S8).
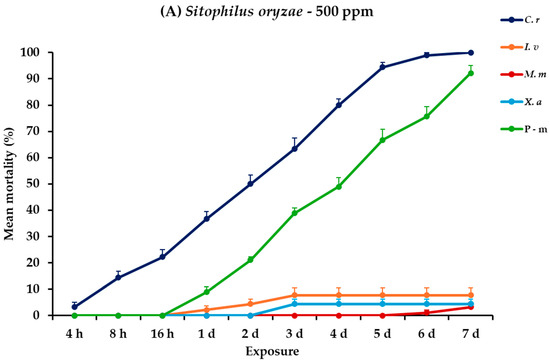
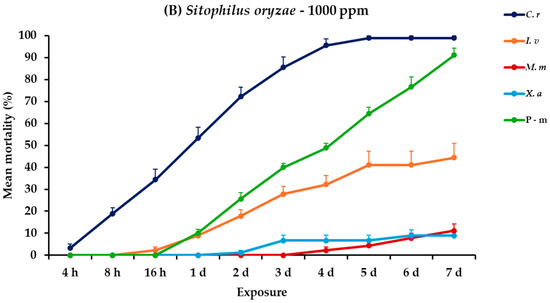
Figure 8.
Mean (%) mortality + standard error (SE) of Sitophilus oryzae adults (A,B) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
2.10. Effectiveness Against A. siro Adults and Nymphs
For A. siro nymphs and adults, all main effects exhibited significance between exposures, apart from the concentration × EO type interaction. Within exposures, significance was displayed for only the exposure and exposure × EO type interaction (Table 2). For nymphs at 500 ppm, C. reticulata and I. verum demonstrated increasing mortality from initially 1.1% on day 1 to 48.9 and 58.9% on day 7, respectively. On the contrary, M. monodora and X. aethiopica showed significantly lower mortality rates, reaching 7.8 and 28.9%, respectively. Furthermore, at 1000 ppm, C. reticulata and I. verum maintained similar efficacy with the previous concentration with moderate results of 58.9 and 65.6%, respectively. Moreover, M. monodora and X. aethiopica demonstrated a slight elevation in efficacy at the end of the experiment (14.4 and 40.0%, respectively). Concerning A. siro adults, C. reticulata, I. verum and X. aethiopica caused significantly higher mortalities than M. monodora, reaching 68.9% after 7 d at 500 ppm. At the highest concentration (1000 ppm), mortality rates increased for all tested EOs, with I. verum killing 75.6%, followed by C. reticulata (66.7%), X. aethiopica (55.6%), and M. monodora (21.1%), at 7 d. Lastly, P-m caused approximately 50.0 and 70.0% nymphal and adult mortality, respectively, on day 7 either compared to EOs tested at 500 or 1000 ppm (Figure 9, Table S9).
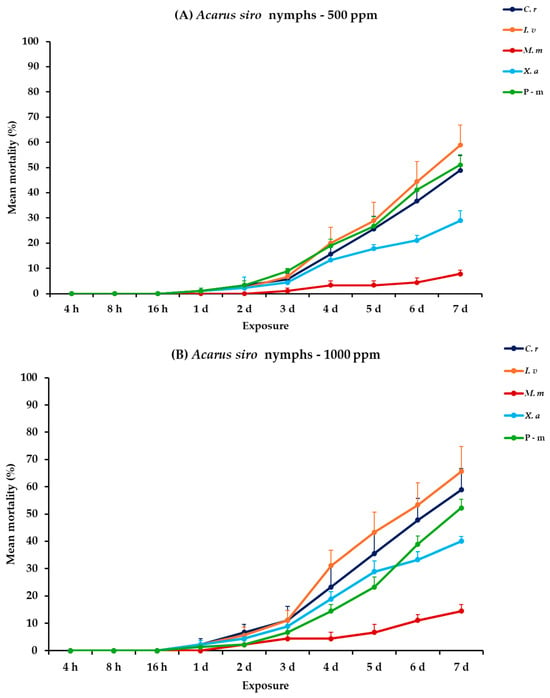
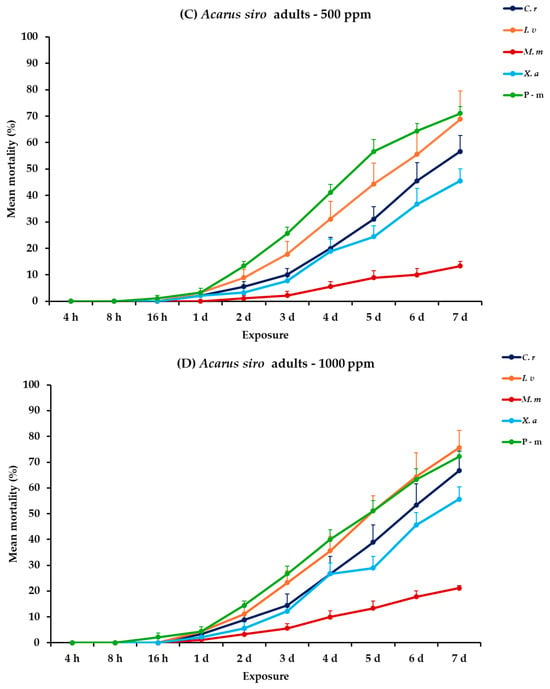
Figure 9.
Mean (%) mortality + standard error (SE) of Acarus siro nymphs (A,B) and adults (C,D) after 4–16 h and 1–7 days in wheat treated with C. reticulata, I. verum, M. myristica, and X. aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations and pirimiphos-methyl (under the abbreviation P-m) as positive control.
3. Discussion
The EOs employed in this study were chemically characterized by GC-MS. More details on the chemical composition of M. myristica and X. aethiopica can be found in the work of Wandjou et al. [45]. Regarding I. verum, the chemical composition of its EO was quite linear with those previously published. Indeed, this EO is commonly characterized by phenylpropanoids, mainly represented by (E)-anethole, with average percentages ranging from 79.9 to 92.4% [49,50,51,52,53]. On the other hand, the chemical composition of C. reticulata EO, which was dominated by limonene, is consistent with those already published. Indeed, it is commonly characterized by high percentages of this compound, ranging from 76 to 85% [54,55]. However, the chemical composition of the EO varies according to factors such as plant part used, extraction technique, developmental stage, and environmental conditions [56].
The present study highlights the pesticidal effects of EOs derived from I. verum, C. reticulata, M. myristica, and X. aethiopica against several key arthropod pests of stored products. Among the four botanical EOs tested, those from I. verum and C. reticulata demonstrated the highest toxic activity, particularly against larval stages, where complete mortality was often achieved. Notably, I. verum EO was perticularly effective at the highest concentration, showing pronounced mortality across most pest species, although T. molitor larvae exhibited a negligible response. Similarly, C. reticulata EO displayed substantial efficacy, achieving total mortality against A. diaperinus, T. castaneum larvae, and S. oryzae adults. These findings align with previous reports of contact, fumigation, and repellency bioassays, where I. verum demonstrated strong activity against important insect species such as Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae), Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae), and A. diaperinus [57,58,59,60]. A clear trend emerged from our study, indicating that higher concentrations and longer exposure times were directly correlated with increased mortality. This time- and concentration-dependent effect is consistent with other studies, such as that of Matos et al. [61], who reported that I. verum exhibited strong fumigant efficacy against Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae) at a relatively low lethal concentration (LC50 = 22.36 μL/L). Furthermore, in contact and repellency assays in the same study, I. verum was shown to reduce oviposition and inhibit insect emergence, highlighting its multifunctional potential. Time-dependent effects were also demonstrated from Ho et al. [43], reporting a notably higher mortality rate of T castaneum at 21 days exposure (69.5%) to I. verum-treated rice vs. 7 days (0.5%).
Citrus-derived EOs have also been studied for their insecticidal activity against significant pest species. Fouad and da Camara [62] documented that C. reticulata peel EO exhibited significantly higher insecticidal activity against S. zeamais adults across multiple bioassays, including contact, ingestion, and fumigant trials, when compared to Citrus aurantiifolia (Christm.) Swingle (Sapindales: Rutaceae) EO, with more pronounced effects observed after just one day of exposure. In a fumigant assay conducted by Safavi and Mobki [63], C. reticulata EO, at the highest concentration (63 µL/L air), caused significant insect mortality (79%) after 48 h against T. castaneum. Both studies highlighted the improvement in efficacy with increased exposure time and dose, further supporting the trend observed in our study. The promising pesticidal action of C. reticulata is linked to the presence of limonene. The latter has been reported for its potent anti-acetylcholinesterase (AChE) activity [64] as well as for its contact toxicity on different pests [65,66,67,68,69]. Regarding I. verum EO, its pesticidal action is due to the high content of (E)-anethole. This phenylpropanoid has been reported for the inhibition of AChE activity [59], thus affecting the normal neurological activity of the insect. Additionally, it is well known that phenylpropanoids affect the insect defense system neutralizing enzymes such as P450, esterases, or glutathione-S-transferases) [70].
Despite the overall effectiveness of the tested EOs, M. myristica and X. aethiopica exhibited comparatively lower efficacy, with mortality rates generally below 50%. However, M. myristica achieved complete mortality in T. granarium adults at 1000 ppm on day 6, while X. aethiopica was lethal to the larvae of both Tribolium species and T. molitor adults. Similarly, × Hesperotropsis leylandii (A.B. Jacks. and Dallim.) Garland and G. Moore (Cupressales: Cupressaceae) E.O. was 100% lethal to the larvae of both T. confusum and T. molitor species at 1000 ppm exposure by day 7, while adults remained completely unaffected. Likewise, Juniperus × pfitzeriana (Spath) P.A. Schmidt (Cupressales: Cupressaceae) achieved 100% mortality in T. granarium adults at 1000 ppm by day 7, while less than 6% in larvae [12]. However, after the exposure to treated millet seeds with a notably higher concentration of X. aethiopica (40,000 ppm), T. castaneum adults achieved a greater mortality rate (68.3%) [44], compared to the present study (4.4% at 1000 ppm). These findings provide valuable data that can help develop targeted applications for different pest species and their developmental stages.
The different susceptibility observed between larvae and adults in our study emphasizes the importance of targeting specific life stages in pest management. Although larvae were generally more susceptible to higher EO concentrations, previous research has indicated that T. molitor adults can sometimes be more vulnerable to botanical treatments than larvae [11,71]. This discrepancy may stem from species- or treatment-specific differences, reflecting the complexity of insect physiology in response to different compounds. Physiological factors contributing to this response could include variations in cuticle structure between species [72,73].
EOs have gained recognition as effective botanical active ingredients in pest control across several agricultural and urban settings [74,75,76]. Their bioactivity, low toxicity to non-target organisms, high volatility, and sensitivity to temperature and UV light degradation make them eco-friendly alternatives to synthetic insecticides [77,78]. The active components in EOs are recognized for their pesticidal, antioxidant, and antimicrobial properties, contributing to their effectiveness in biological applications [79]. While synthetic insecticides, such as organophosphates and pyrethroids, have proven effective in managing stored-product pests [80,81], reliance on them raises concerns due to resistance development and environmental risks from chemical residues [82]. Many pests, such as Tribolium spp., S. oryzae, A. diaperinus, and R. dominica, have developed resistance to these chemicals [83,84,85,86,87,88,89,90,91]. Even mite pests like A. siro have shown resistance to organophosphates, such as pirimiphos-methyl, which has been used for decades as a grain protectant [92,93]. Interestingly, natural pesticides like Carlina acaulis L. (Asterales: Asteraceae) EO have demonstrated significant efficacy against both life stages of A. siro in contact bioassays, achieving approximately 90% mortality [94]. In our study, A. siro adults were more susceptible to 1000 ppm of I. verum and X. aethiopica (>70% mortality) EO than to the positive control (~50% mortality), suggesting that they can be effective against both insect and mite species occurring in stored-product facilities.
Research on Tribolium species has focused on overcoming the challenge of controlling these pests due to the increasing incidence of resistant strains worldwide [95,96,97]. One of the notable findings of our study is the susceptibility of larval individuals of both Tribolium species to three EOs, which could prove essential for developing more environmentally sustainable pest management strategies. In this study, pirimiphos-methyl, used as a positive control, peaked at 66.7% mortality, often remaining below this rate. Notably, zero toxicity was recorded against the adult stages of the two Tribolium species, further highlighting the declining efficacy of conventional insecticides.
The outcomes of this research emphasize the significant potential of EOs, particularly those from I. verum and C. reticulata, in surpassing the performance of the conventional synthetic insecticide pirimiphos-methyl. These findings advocate for the inclusion of specific EOs in integrated pest management (IPM) approaches, providing a more sustainable and eco-friendlier alternative with reduced environmental consequences. Future research should focus on the development and optimization of EO-based formulations, ensuring their long-term efficacy under different storage conditions. This type of research should also focus on the commercialization of EOs as effective insecticides for the management of stored-product pests and potential replacement of chemicals, chiefly organophosphates and pyrethroids. Our study clearly showed that in several cases the tested EOs outperformed pirimiphos-methyl, during the followed short exposure scenario, against A. diaperinus larvae, T. castaneum larvae, Tribolium confusum larvae, Tenebrio molitor adults, Trogoderma granarium larvae and adults, O. surinamensis larvae or adults, S. oryzae adults, and A. siro nymphs or adults. This knowledge should be considered when research involves steps for commercial development of EOs as insecticides in storages. Investigating potential synergies with other biological or chemical controls to counteract resistance development will also be crucial. Finally, expanding these studies to cover a broader spectrum of pests and conducting environmental assessments will solidify the role of EOs in managing resistant pest populations in stored-product systems.
4. Materials and Methods
4.1. Essential Oils (EOs) and Their Chemical Analysis in GC-MS
Illicium verum EO was kindly provided by FD Copeland & Sons Limited (batch P61T, 10/10/07) (London, UK), while X. aethiopica and M. myristica EOs were obtained as previously described in the work of Wandjou et al. [45]. As regards C. reticulata EO, it was obtained from C. reticulata peels deriving from commercial fruits. In detail, dry peels (1100 g) were placed in a 20 L round flask with 11 L of distilled water and subjected to hydrodistillation using a Clevenger-type apparatus for 4 h. The system was heated using a mantle system Falc MA (Falc Instruments, Treviglio, Italy). The EO presented a pale-yellow color and was obtained with a yield of 2.9% (w/w). Xylopia aethiopica and M. myristica EOs were obtained as previously described in the work of Wandjou et al. [45].
The EOs chemical composition was determined by gas chromatography-mass spectrometry analysis (GC-MS) employing the analytical method previously reported by Wandjou et al. [45]. Specifically, the instrument was an Agilent 6890N equipped with a 5973N MS system working at 70 eV in the EI mode (Agilent Technologies, Santa Clara, California, USA). The separation of compounds was achieved through an HP-5MS capillary column (5% phenylmethylpolysiloxane, 30 m length, 0.25 mm internal diameter, and 0.1 µm film thickness). The identification of compounds was achieved as previously published [45].
4.2. Commodity
For the bioassays, pesticide-free and uninfested hard wheat, Triticum durum Desf. (Poales: Poales) var. Claudio, was implemented. The grain moisture content of the wheat was measured at 12.2% using a moisture meter (mini-GAC plus, Dickey-John Europe S.A.S., Colombes, France) immediately before the assays.
4.3. Arthropod Species and Rearing Media
The arthropod individuals utilized in this study were collected from colonies maintained at the Laboratory of Agricultural Zoology and Entomology (LAZE), Agricultural University of Athens, Greece. The colonies were reared under controlled conditions in total darkness. Acarus siro was reared at 25 °C with 80% relative humidity (RH), while all other insect species were maintained at 30 °C with 65% RH. The rearing medium for A. siro constituted a blend of wheat, flaked oats, and brewer’s yeast in a 10:10:1 ratio. Rhyzopertha dominica and S. oryzae were reared on whole wheat grains. Tribolium spp. were reared in wheat flour with 5% brewer’s yeast. Tenebrio molitor was kept in a medium of oat bran and potato cubes to maintain moisture, while A. diaperinus was reared on wheat bran with brewer’s yeast and sliced apples for moisture. Finally, O. surinamensis was reared on a mixture of processed wheat, oat flakes, and yeast in a ratio of 5:5:1.
4.4. Experimental Procedure and Arthropod Selection
Adults of T. molitor, both Tribolium species, S. oryzae, O. surinamensis, and R. dominica were all under 14 days old, while T. granarium adults were <24 h old. Alphitobius diaperinus adults were younger than one week. The larvae of O. surinamensis and Tribolium spp. were used during their 3rd to 4th instar stage, with T. molitor larvae measuring 1 to 1.4 cm in length, T. granarium larvae ranging between 2–4 mm, and A. diaperinus larvae less than 0.7 cm in length. Acarus siro individuals, whose sex was not determined, were from colonies between 1 and 21 days old. Nymphs and adults of A. siro were distinguished based on external characteristics, specifically the shorter body setae found in nymphs [12].
The selection of the two concentrations tested (500 and 1000 μL EO/kg wheat) was reached after the conduction of preliminary screening trials. Solutions were prepared, and the wheat grains were treated following the methods described in Kavallieratos et al. [94]. Additional batches of wheat were treated separately with pure ethanol, water, or ethanol mixed with Tween 80 plus water as negative controls. Pirimiphos-methyl (Actellic EC 50%) at the commercially recommended dose served as the positive control (5 μL/kg grain). From each treated lot (controls or EOs), 1 g for mites or 10 g for insects were sampled using different scoops. Each wheat sample was placed individually on a Precisa XB3200D laboratory scale (Alpha Analytical Instruments, Gerakas, Greece) for weighing and transported inside glass vials (6.0 × 2.7 cm for A. siro and 12.5 × 7.5 cm for the insects). Small vials were sealed with perforated cover lids to ensure adequate aeration, while larger vials were equipped with lids equipped with a 1.5 cm circular opening covered with gauze [98].
Ten arthropods per species and life stage were located in separate vials. To prevent escape, a polytetrafluoroethylene coating was set on the inner portion of the vials close to the lids (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany). All vials were incubated at 25 °C/80% RH for A. siro and 30 °C/65% relative humidity for insect species until the tests were completed. Due to the cannibalistic behavior of A. diaperinus [12], 10 vials containing one individual each were used, constituting one sub-replication. Mortality assessments were conducted under a stereoscope, checking for movement at 4, 8, and 16 h, and from 1 to 7 days. Arthropods were gently prodded with a small fine brush to check for any sign of movement, using separate brushes for each type of essential oil and positive or negative controls. Three replications, each with three sub-replications for each arthropod, were performed, with new solutions, wheat samples, and arthropods prepared each time [13].
4.5. Data Analysis
No adjustments were required for the control mortality, as it remained below 5% for all tested species. To normalize variance the dataset was transformed by implementing the formula log (x + 1) beforehand [99]. Data analysis for each pest species or developmental stage was conducted using a repeated measures model [100], incorporating interactions of main effects. Exposure, mortality, and EO type/concentrations were the response variable, the repeated factor, and the main effects, respectively. All analyses were performed using JMP 16.2 [101]. Mean separation was conducted using the Tukey HSD test at a significance level of 0.05 [102].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14020192/s1, Table S1: Mean (%) mortality ± standard error (SE) of Alphitobius diaperinus larvae and adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S2: Mean (%) mortality ± standard error (SE) of Tribolium castaneum larvae and adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S3: Mean (%) mortality ± standard error (SE) of Tribolium confusum larvae and adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S4: Mean (%) mortality ± standard error (SE) of Tenebrio molitor larvae and adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S5: Mean (%) mortality ± standard error (SE) of Trogoderma granarium larvae and adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S6: Mean (%) mortality ± standard error (SE) of Oryzaephilus surinamensis larvae and adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S7: Mean (%) mortality ± standard error (SE) of Rhyzopertha dominica adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S8: Mean (%) mortality ± standard error (SE) of Sitophilus oryzae adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m); Table S9: Mean (%) mortality ± standard error (SE) of Acarus siro nymphs and adults after 4–16 h, and 1–7 days in wheat treated with Citrus reticulata, Illicium verum, Monodora myristica, and Xylopia aethiopica (under the abbreviations C. r, I. v, M. m, and X. a, respectively) EOs at two concentrations, and with positive control, pirimiphos-methyl (under the abbreviation P-m).
Author Contributions
Conceptualization, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., M.F., E.S., R.P. and F.M.; methodology, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., D.N., M.F., E.S., R.P. and F.M.; software, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S. and D.N.; validation, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., D.N., M.F., E.S., R.P. and F.M.; formal analysis, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., D.N., M.F., E.S., R.P. and F.M.; investigation, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., D.N., M.F., E.S., R.P. and F.M.; resources, N.G.K., C.S.F. and F.M.; data curation, C.S.F., M.F., E.S., R.P. and F.M.; writing—original draft preparation, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., D.N., M.F., E.S., R.P. and F.M.; writing—review and editing, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., D.N., M.F., E.S., R.P. and F.M.; visualization, N.G.K., N.E., C.S.F., M.C.B., D.L.S.G., A.S., D.N., M.F., E.S., R.P. and F.M.; supervision, N.G.K. and F.M.; project administration, N.G.K., C.S.F. and F.M.; funding acquisition, C.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hellenic Entomological Society grant number [02-029].
Data Availability Statement
Data are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, R. Insect Pests of Stored Grain: Biology, Behavior, and Management Strategies; Apple Academic Press: Waretown, MJ, USA, 2017. [Google Scholar]
- Nayak, M.K.; Daglish, G.J. Importance of stored product insects. In Recent Advances in Stored Product Protection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–17. [Google Scholar]
- Hagstrum, D.; Subramanyam, B. Stored-Product Insect Resource; AACC International: Saint Paul, MN, USA, 2009. [Google Scholar]
- Stejskal, V.; Hubert, J. Arthropods as sources of contaminants of stored products: An overview. In Proceedings of the 9th International Working Conference on Stored Product Protection, São Paulo, Brazil, 15–18 October 2006; pp. 15–18. [Google Scholar]
- Langer, K.; Breuer, K.; Kapp, A.; Werfel, T. Staphylococcus aureus-derived enterotoxins enhance house dust mite-induced patch test reactions in atopic dermatitis. Exp. Dermatol. 2007, 16, 124–129. [Google Scholar] [CrossRef]
- Zurek, L.; Gorham, J.R. Insects as vectors of foodborne pathogens. In Handbook of Science and Technology for Homeland Security; Voeller, J.G., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–16. [Google Scholar]
- Zheng, L.; Crippen, T.L.; Sheffield, C.L.; Poole, T.L.; Yu, Z.; Tomberlin, J.K. Evaluation of Salmonella movement through the gut of the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Vector Borne Zoonotic Dis. 2012, 12, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lambkin, T.A.; Rice, S.J.; Furlong, M. Responses of susceptible and cyfluthrin-resistant broiler house populations of lesser mealworm (Coleoptera: Tenebrionidae) to γ-cyhalothrin. J. Econ. Entomol. 2010, 103, 2155–2163. [Google Scholar] [CrossRef]
- Riaz, T.; Shakoori, F.R.; Ali, S.S. Toxicity of phosphine against tolerant and susceptible populations of Trogoderma granarium collected from Punjab, Pakistan. Punjab Univ. J. Zool. 2016, 31, 2530. [Google Scholar]
- Drummond, J.B.; Chapman, R.B. A comparison of two methods to determine the susceptibility of sawtoothed grain beetle (Oryzaephilus surinamensis) populations to pirimiphos-methyl from Canterbury, New Zealand. N. Z. Plant Prot. 2019, 72, 245–252. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalaka, C.T.; Skourti, A.; Nika, E.P.; Maggi, F.; Spinozzi, E.; Mazzara, E.; Petrelli, R.; Lupidi, G.; et al. Efficacy of 12 commercial essential oils as wheat protectants against stored-product beetles, and their acetylcholinesterase inhibitory activity. Entomol. Gen. 2021, 41, 385–414. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Skourti, A.; Filintas, C.S.; Eleftheriadou, N.; Gidari, D.L.S.; Spinozzi, E.; Ferrati, M.; Petrelli, R.; Cianfaglione, K.; et al. Essential oils from three Cupressaceae species as stored wheat protectants: Will they kill different developmental stages of nine noxious arthropods? J. Stored Prod. Res. 2024, 105, 102232. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Eleftheriadou, N.; Boukouvala, M.C.; Skourti, A.; Filintas, C.S.; Gidari, D.L.S.; Maggi, F.; Rossi, P.; Drenaggi, E.; Morshedloo, M.R.; et al. Exploring the efficacy of four Apiaceae essential oils against nine stored-product pests in wheat protection. Plants 2024, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Hu, W.T.; Huang, B.K.; Qin, L.P. Illicium verum: A review on its botany, traditional use, chemistry and pharmacology. J. Ethnopharmacol. 2011, 136, 10–20. [Google Scholar] [CrossRef]
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (star anise) and trans-anethole as valuable raw materials for medicinal and cosmetic applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Bose, S.; Banerjee, S.; Vishnuprasad, C.N.; del Pilar Rodriguez-Torres, M.; Shin, H.S. Star anise (Illicium verum): Chemical compounds, antiviral properties, and clinical relevance. Phytother. Res. 2020, 34, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Huang, Y.; Zhang, W.; Lu, C.; Yuan, J. A comprehensive review of the pharmacology, chemistry, traditional uses and quality control of star anise (Illicium verum Hook. F.): An aromatic medicinal plant. Molecules 2023, 28, 7378. [Google Scholar] [CrossRef]
- Fetse, J.; Kofie, W.; Adosraku, R. Ethnopharmacological importance of Xylopia aethiopica (Dunal) A. Rich (Annonaceae)—A review. J. Pharm. Res. Int. 2016, 11, 1–21. [Google Scholar] [CrossRef]
- Fleischer, T. Xylopia aethiopica A Rich: A chemical and biological perspective. J. Univ. Sci. Technol. 2003, 23, 24–31. [Google Scholar]
- Obiri, D.D.; Osafo, N. Aqueous ethanol extract of the fruit of Xylopia aethiopica (Annonaceae) exhibits anti-anaphylactic and anti-inflammatory actions in mice. J. Ethnopharmacol. 2013, 148, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Alolga, R.N.; Chávez León, M.A.; Osei-Adjei, G.; Onoja, V. GC-MS-based metabolomics, antibacterial and anti-inflammatory investigations to characterize the quality of essential oil obtained from dried Xylopia aethiopica fruits from Ghana and Nigeria. J. Pharm. Pharmacol. 2019, 71, 1544–1552. [Google Scholar] [CrossRef]
- Asekun, O.T.; Adeniyi, B.A. Antimicrobial and cytotoxic activities of the fruit essential oil of Xylopia aethiopica from Nigeria. Fitoterapia 2004, 75, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Anyamele, T.; Ugbogu, E.A.; Nwankwo, V.C.; Ibe, C. A review of the traditional uses, phytochemistry and toxicological profile of Xylopia aethiopica A. Rich. Pharmacol. Res.-Nat. Prod. 2023, 1, 100001. [Google Scholar]
- Owokotomo, I.A.; Jabar, J.M.; Alabi, O.O. Composition and radical scavenging activity of essential oil from Xylopia aethiopica: A new chemotype grown in Nigeria. Eur. J. Adv. Chem. Res. 2021, 2, 25–30. [Google Scholar] [CrossRef]
- Burubai, W.; Akor, A.J.; Igoni, A.H.; Puyate, Y.T. Fracture resistance of African nutmeg (Monodora myristica) to compressive loading. Am. Eurasian J. Agric. Environ. Sci. 2008, 3, 15–18. [Google Scholar]
- Couvreur, T.L.P.; Dagallier, L.P.M.J.; Crozier, F.; Ghogue, J.P.; Hoekstra, P.H.; Kamdem, N.G.; Johnson, D.M.; Murray, N.A.; Sonke, B. Flora of Cameroon—Annonaceae. PhytoKeys 2022, 207, 1–532. [Google Scholar] [CrossRef] [PubMed]
- Omobuwajo, T.O.; Omobuwajo, O.R.; Sanni, L.A. Physical properties of calabash nutmeg (Monodora myristica) seeds. J. Food Eng. 2003, 57, 375–381. [Google Scholar] [CrossRef]
- Ezeuko, A.S.; Bamgboye, O.A.; Jonathan, H.; Uchenna, D. Extraction, physicochemical, phytochemical analysis and identification of some important compounds of Monodora myristica (African nutmeg) seed oil. Int. J. Innov. Res. Sci. Stud. 2017, 4, 406–410. [Google Scholar]
- Isiogugu, O.N.; Ezike, A.C.; Peter, I.E.; Onwuka, A.M.; Obi, B.C.; Abonyi, U.C. Effects of Monodora myristica (Gaertn, Dunal.) (Annonaceae) root bark on acute and chronic inflammation. In Proceedings of the 9th Sub-Regional Scientific Meeting of WANNPRES, Nsukka, Enugu State, Nigeria, 30 May–2 June 2018. [Google Scholar]
- Okechukwu, Q.N.; Ugwuona, F.U.; Ofoedu, C.E.; Juchniewicz, S.; Okpala, C.O.R. Chemical composition, antibacterial efficacy, and antioxidant capacity of essential oil and oleoresin from Monodora myristica and Tetrapleura tetraptera in Southeast Nigeria. Sci. Rep. 2022, 12, 19861. [Google Scholar] [CrossRef]
- Nwaoguikpe, R.N.; Uwakwe, A.A. In vitro antisickling effects of Xylopia aethiopica and Monodora myristica. J. Med. Plant Res. 2005, 2, 119–125. [Google Scholar]
- Bakarnga-Via, I.; Hzounda, J.B.; Fokou, P.V.T.; Tchokouaha, L.R.Y.; Gary-Bobo, M.; Gallud, A.; Garcia, M.; Walbadet, L.; Secka, Y.; Dongmo, P.M.J.; et al. Composition and cytotoxic activity of essential oils from Xylopia aethiopica (Dunal) A. Rich, Xylopia parviflora (A. Rich) Benth.) and Monodora myristica (Gaertn) growing in Chad and Cameroon. BMC Complement. Altern. Med. 2014, 14, 125. [Google Scholar] [CrossRef]
- Awojide, S.H.; Akinlade, B.; Oyewole, K.A.; Adeyemo, A.G.; Adeniyi, E.O.; Fadunmade, O.E.; Anifowose, A.J. Synergistic and antagonistic medicinal activities of essential oil of Monodora myristica. CTU J. Innov. Sustain. Dev. 2023, 15, 1–11. [Google Scholar] [CrossRef]
- Héritier, V.N.V.; Arthur, D.N.; Augustin, M.M.; Nadège, N.K.; Roger, K.V.; Jude-Thaddée, M.N. Antibacterial and antiplasmodial potentials of essential oils from two plants of Tangawisi products: Zingiber officinalis Roscoe and Monodora myristica (Gaertn) Dunal. J. Pharmacogn. Phytochem. 2018, 7, 643–648. [Google Scholar]
- Li, S.; Lo, C.Y.; Ho, C.T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, J.; Cao, J.; Wang, D.; Liu, C.; Yang, R.; Li, X.; Sun, C. Antioxidant capacity, anticancer ability and flavonoids composition of 35 citrus (Citrus reticulata Blanco) varieties. Molecules 2017, 22, 1114. [Google Scholar] [CrossRef]
- Shorbagi, M.; Fayek, N.M.; Shao, P.; Farag, M.A. Citrus reticulata Blanco (the common mandarin) fruit: An updated review of its bioactive, extraction types, food quality, therapeutic merits, and bio-waste valorization practices to maximize its economic value. Food Biosci. 2022, 47, 101699. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Chen, H.; Chen, S.; Liu, Y. Analysis of flavonoid metabolites in citrus peels (Citrus reticulata “Dahongpao”) using UPLC-ESI-MS/MS. Molecules 2019, 24, 2680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Xi, W.; Shen, Y.; Qiao, L.; Zhong, L.; Ye, X.; Zhou, Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014, 145, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Lin, C.C. Investigation of heat treating conditions for enhancing the anti-inflammatory activity of citrus fruit (Citrus reticulata) peels. J. Agric. Food Chem. 2008, 56, 7976–7982. [Google Scholar] [CrossRef]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic potency, antioxidant effects, and mode of actions of Citrus reticulata fruit peel hydroethanolic extract, hesperidin, and quercetin in nicotinamide/streptozotocin-induced wistar diabetic rats. Oxid. Med. Cell. Longev. 2020, 2020, 730492. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Ma, Y.; Goh, P.M.; Sim, K.Y. Star anise, Illicium verum Hook f. as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol. Technol. 1995, 6, 341–347. [Google Scholar] [CrossRef]
- Babarinde, S.A.; Adeyemo, Y.A. Toxic and repellent properties of Xylopia aethiopica (Dunal) A. Richard on Tribolium castaneum Herbst infesting stored millets, Pennisetum glaucum (L.) R. Br. Arch. Phytopathol. Plant Prot. 2010, 43, 810–816. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Baldassarri, C.; Ferrati, M.; Maggi, F.; Pavela, R.; Tsabang, N.; Petrelli, R.; Ricciardi, R.; Desneux, N.; Benelli, G. Essential oils from Cameroonian aromatic plants as effective insecticides against mosquitoes, houseflies, and moths. Plants 2022, 11, 2353. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mass Spectral Library (NIST/EPA/NIH MS/MS); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Mondello, L. Flavour and Fragrance Natural and Synthetic Compounds; Shimadzu Corps: Kyoto, Japan, 2012. [Google Scholar]
- Hanif, M.A.; Al-Maskari, M.Y.; Al-Maskari, A.; Al-Shukaili, A.; Al-Maskari, A.Y.; Al-Sabahi, J.N. Essential oil composition, antimicrobial and antioxidant activities of unexplored Omani basil. J. Med. Plant Res. 2011, 5, 751–757. [Google Scholar]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Noumi, E.; Ahmad, I.; Adnan, M.; Patel, H.; Merghni, A.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Kadri, A.; Caputo, L.; et al. Illicium verum L. (Star anise) essential oil: GC/MS profile, molecular docking study, in silico ADME profiling, quorum sensing, and biofilm-inhibiting effect on foodborne bacteria. Molecules 2023, 28, 7691. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.U.; Bitencourt, R.D.O.B.; de Moraes Freitas, A.; Moreira, H.V.S.; de Amorim Magalhães, K.L.; de Souza, B.A.; Golo, P.S.; de Almeida Chaves, D.S.; Bittencourt, V.R.E.P.; da Costa Angelo, I. Exploiting the combination of entomopathogenic fungi and Illicium verum essential oil against Aedes aegypti larvae. Biol. Control 2024, 193, 105526. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Cui, H.; Chen, X.; Wang, L.; An, P.; Zhou, H.; Dong, Y. Essential oils from Citrus reticulata cv. shatangju peel: Optimization of hydrodistillation extraction by response surface methodology and evaluation of their specific adhesive effect to polystyrene. Am. Chem. Soc. Omega 2021, 6, 13695–13703. [Google Scholar] [CrossRef] [PubMed]
- Job, J.T.; Visakh, N.U.; Pathrose, B.; Alfarhan, A.; Rajagopal, R.; Thayyullathil, J.; Thejass, P.; Ramesh, V.; Narayanankutty, A. Chemical composition and biological activities of the essential oil from Citrus reticulata Blanco peels collected from agrowastes. Chem. Biodivers. 2024, 21, e202301223. [Google Scholar] [CrossRef]
- Chaubey, M.K. Insecticidal activities of Cinnamomum tamala (Lauraceae) essential oil against Sitophilus oryzae L. (Coleoptera: Curculionidae). Int. J. Entomol. Res. 2016, 4, 91–98. [Google Scholar]
- Li, S.G.; Li, M.Y.; Huang, Y.Z.; Hua, R.M.; Lin, H.F.; He, Y.J.; Wei, L.L.; Liu, Z.Q. Fumigant activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione S-transferase activities in adult Sitophilus zeamais. J. Pest Sci. 2013, 86, 677–683. [Google Scholar] [CrossRef]
- Wei, L.; Hua, R.; Li, M.; Huang, Y.; Li, S.; He, Y.; Shen, Z. Chemical composition and biological activity of star anise Illicium verum extracts against maize weevil, Sitophilus zeamais adults. J. Insect Sci. 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, Y.; Sabier, M.; Zhang, T.; Deng, J.; Song, X.; Liao, Z.; Li, Q.; Yang, S.; Cao, Y.; et al. Trans-anethole is a potent toxic fumigant that partially inhibits rusty grain beetle (Cryptolestes ferrugineus) acetylcholinesterase activity. Ind. Crop. Prod. 2021, 161, 113207. [Google Scholar] [CrossRef]
- Peter, R.; Josende, M.E.; da Silva Barreto, J.; da Costa Silva, D.G.; da Rosa, C.E.; Maciel, F.E. Effect of Illicium verum (Hook) essential oil on cholinesterase and locomotor activity of Alphitobius diaperinus (Panzer). Pestic. Biochem. Physiol. 2022, 181, 105027. [Google Scholar] [CrossRef]
- Matos, L.F.; da Cruz Lima, E.; de Andrade Dutra, K.; Navarro, D.M.D.A.F.; Alves, J.L.R.; Silva, G.N. Chemical composition and insecticidal effect of essential oils from Illicium verum and Eugenia caryophyllus on Callosobruchus maculatus in cowpea. Ind. Crop. Prod. 2020, 145, 112088. [Google Scholar] [CrossRef]
- Fouad, H.A.; da Camara, C.A. Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 30–36. [Google Scholar] [CrossRef]
- Safavi, S.A.; Mobki, M. Fumigant toxicity of essential oils from Citrus reticulata Blanco fruit peels against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). J. Crop Prot. 2012, 1, 115–120. [Google Scholar]
- Abdelgaleil, S.A.; Mohamed, M.I.; Badawy, M.E.; El-Arami, S.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, D.W. Toxicity of basil and orange essential oils and their components against two coleopteran stored products insect pests. J. Asia. Pac. Entomol. 2014, 17, 13–17. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, S.E.; Kang, M.S.; Park, B.; Lee, S.G.; Lee, H.S. Acaricidal and insecticidal properties of Coriandrum sativum oils and their major constituents extracted by three different methods against stored product pests. Appl. Biol. Chem. 2018, 1, 481–488. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Okunowo, W.O.; Osuntoki, A.A.; Olabode, T.B.; Ayo-Folorunso, F. Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 2020, 168, 104643. [Google Scholar] [CrossRef] [PubMed]
- Prates, H.T.; Santos, J.P.; Waquil, J.M.; Fabris, J.D.; Oliveira, A.B.; Foster, J.E. Insecticidal activity of monoterpenes against Rhyzopertha dominica (F.) and Tribolium castaneum (Herbst). J. Stored Prod. Res. 1998, 34, 243–249. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Prajapati, V.; Khanuja, S.P.S.; Kumar, S. Effect of d-limonene on three stored-product beetles. J. Econ. Entomol. 2003, 96, 990–995. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig. (Apiaceae). Fitoterapia 2009, 80, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.; Skourti, A.; Nika, E.P.; Boukouvala, M.C.; Kavallieratos, N.G. Five natural compounds of botanical origin as wheat protectants against adults and larvae of Tenebrio molitor L. and Trogoderma granarium Everts. Environ. Sci. Pollut. Res. Int. 2021, 28, 42763–42775. [Google Scholar] [CrossRef]
- Doucet, D.; Retnakaran, A. Targeting cuticular components for pest management. In Extracellular Composite Matrices in Arthropods; Cohen, E., Mousisian, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 369–407. [Google Scholar]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect cuticle and insecticide development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef] [PubMed]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Suwannayod, S.; Sukontason, K.L.; Pitasawat, B.; Junkum, A.; Limsopatham, K.; Jones, M.K.; Somboon, P.; Leksomboon, R.; Chareonviriyaphap, T.; Tawatsin, A.; et al. Synergistic toxicity of plant essential oils combined with pyrethroid insecticides against blow flies and the house fly. Insects 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Demeter, S.; Lebbe, O.; Hecq, F.; Nicolis, S.C.; Kenne Kemene, T.; Martin, H.; Fauconnier, M.L.; Hance, T. Insecticidal activity of 25 essential oils on the stored product pest, Sitophilus granarius. Foods 2021, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. Int. J. Environ. Sci. Technol. 2016, 9, 354. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Arthur, F.H.; Subramanyam, B. Chemical Control in Stored Products; Kansas State University: Manhattan, KS, USA, 2012; pp. 95–100. [Google Scholar]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Holloway, J.C.; Emery, R.N.; Pavic, H.; Bartlet, J.; Collins, P.J. Strong resistance to phosphine in the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae): Its characterisation, a rapid assay for diagnosis and its distribution in Australia. Pest Manag. Sci. 2013, 69, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Johnson, D. Baseline susceptibility and cross-resistance in adult and larval Alphitobius diaperinus (Coleoptera: Tenebrionidae) collected from poultry farms in Arkansas. J. Econ. Entomol. 2015, 108, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.C.; Falk, M.G.; Emery, R.N.; Collins, P.J.; Nayak, M.K. Resistance to phosphine in Sitophilus oryzae in Australia: A national analysis of trends and frequencies over time and geographical spread. J. Stored Prod. Res. 2016, 69, 129–137. [Google Scholar] [CrossRef]
- Lyons, B.N.; Crippen, T.L.; Zheng, L.; Teel, P.D.; Swiger, S.L.; Tomberlin, J.K. Susceptibility of Alphitobius diaperinus in Texas to permethrin-and β-cyfluthrin-treated surfaces. Pest Manag. Sci. 2017, 73, 562–567. [Google Scholar] [CrossRef]
- Andrić, G.G.; Golić, M.P.; Kljajić, P. Toxicity of several contact insecticides to Tribolium castaneum (Herbst) populations after selection with pirimiphos-methyl and deltamethrin. Pestic. Fitomed. 2015, 30, 209–216. [Google Scholar] [CrossRef]
- Attia, M.A.; Wahba, T.F.; Shaarawy, N.; Moustafa, F.I.; Guedes, R.N.C.; Dewer, Y. Stored grain pest prevalence and insecticide resistance in Egyptian populations of the red flour beetle Tribolium castaneum (Herbst) and the rice weevil Sitophilus oryzae (L.). J. Stored Prod. Res. 2020, 87, 101611. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Usman, M.; Gulzar, S.; El-Shafie, H.A. Detection of phosphine resistance in field populations of four key stored-grain insect pests in Pakistan. Insects 2021, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Khan, H.A.A.; Haider, M.S.; Anwar, W.; Akhter, A. Selection for resistance to pirimiphos-methyl, permethrin and spinosad in a field strain of Sitophilus oryzae: Resistance risk assessment, cross-resistance potential and synergism of insecticides. Environ. Sci. Pollut. Res. Int. 2023, 30, 29921–29928. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.M.; Mustapha, M.A.; Aliyu, M.; Ibrahim, S.S. Multiple pesticide resistance in rust-red flour beetle (Tribolium castaneum, Herbst 1797) from northern Nigeria is probably driven by metabolic mechanisms. Agrochemicals 2023, 2, 170–180. [Google Scholar] [CrossRef]
- Szlendak, E.; Conyers, C.; Muggleton, J.; Thind, B.B. Pirimiphos-methyl resistance in two stored product mites, Acarus siro and Acarus farris, as detected by impregnated paper bioassay and esterase activity assays. Exp. Appl. Acarol. 2000, 24, 45–54. [Google Scholar] [CrossRef]
- Hubert, J.; Navratilova, B.; Sopko, B.; Nesvorna, M.; Phillips, T.W. Pesticide residue exposure provides different responses of the microbiomes of distinct cultures of the stored product pest mite Acarus siro. BMC Microbiol. 2022, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Spinozzi, E.; Ferrati, M.; Petrelli, R.; Maggi, F.; Benelli, G. Carlina acaulis essential oil: A candidate product for agrochemical industry due to its pesticidal capacity. Ind. Crops Prod. 2022, 188, 115572. [Google Scholar] [CrossRef]
- Rossi, E.; Cosimi, S. Insecticide resistance in Italian populations of Tribolium flour beetles. Bull. Insectol. 2010, 63, 251–258. [Google Scholar]
- Dukić, N.; Andrić, G.; Ninkovic, V.; Golić, M.P.; Kljajić, P.; Radonjić, A. Behavioural responses of Tribolium castaneum (Herbst) to different types of uninfested and infested feed. Bull. Entomol. Res. 2020, 110, 550–557. [Google Scholar] [CrossRef]
- Korunić, Z.; Liška, A.; Lucić, P.; Hamel, D.; Rozman, V. Evaluation of diatomaceous earth formulations enhanced with natural products against stored product insects. J. Stored Prod. Res. 2020, 86, 101565. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Perinelli, D.R.; Spinozzi, E.; Bonacucina, G.; Cappellacci, L.; Morshedloo, M.R.; Canale, A.; Benelli, G.; et al. Apiaceae essential oil nanoemulsions as effective wheat protectants against five arthropod pests. Ind. Crops Prod. 2022, 186, 115001. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson: Essex, UK, 2014. [Google Scholar]
- Sall, J.; Lehman, A.; Creighton, L. JMP Start Statistics. A Guide to Statistics and Data Analysis Using JMP and JMP IN Software; Duxbury Press: Belmont, CA, USA, 2001. [Google Scholar]
- SAS Institute Inc. Using JMP 16.2; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Freeman & Company: New York, NY, USA, 1995. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).