Metabolite Profiling and Association Analysis of Leaf Tipburn in Heat-Tolerant Bunching Onion Varieties
Abstract
1. Introduction
2. Results
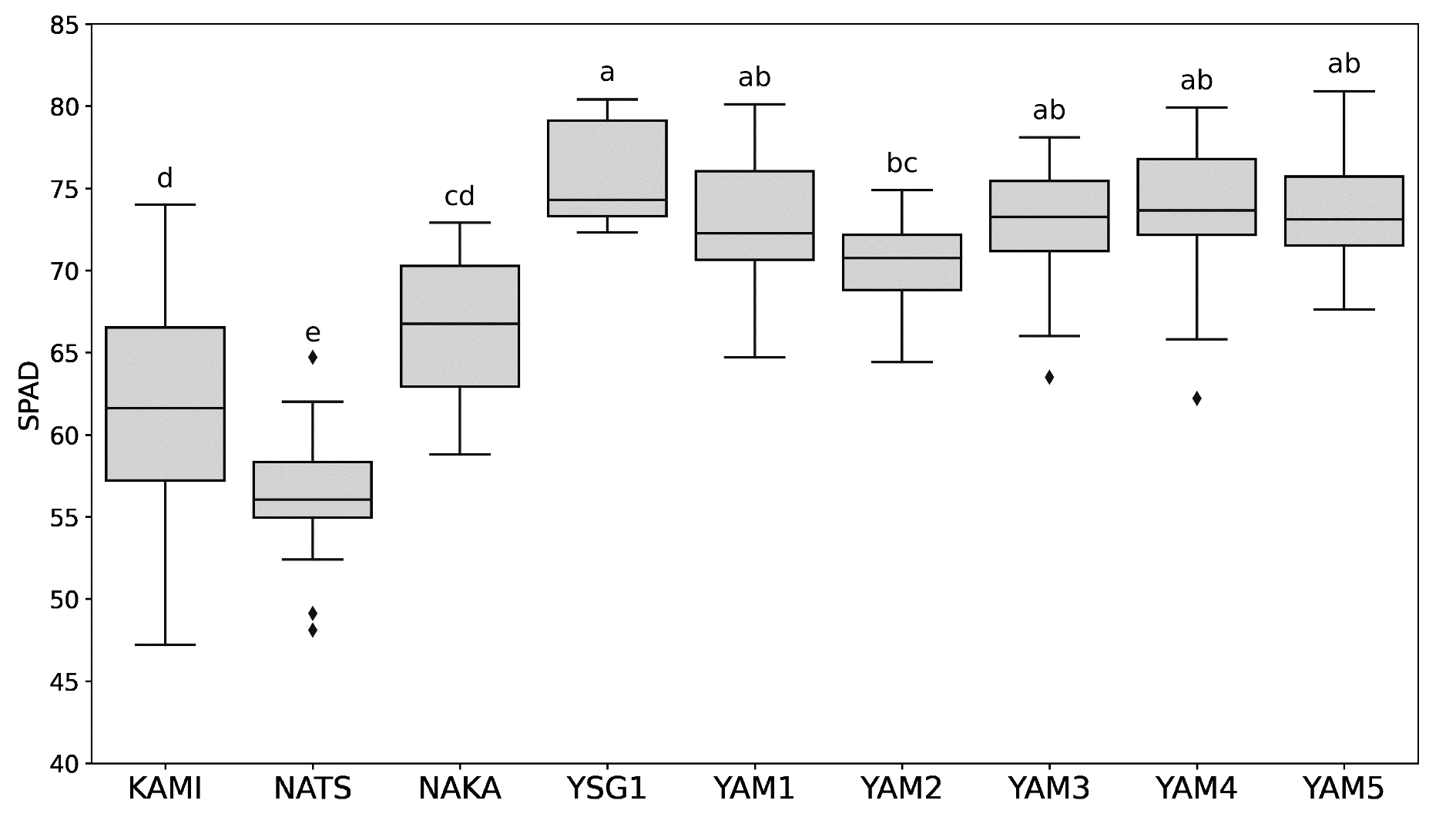
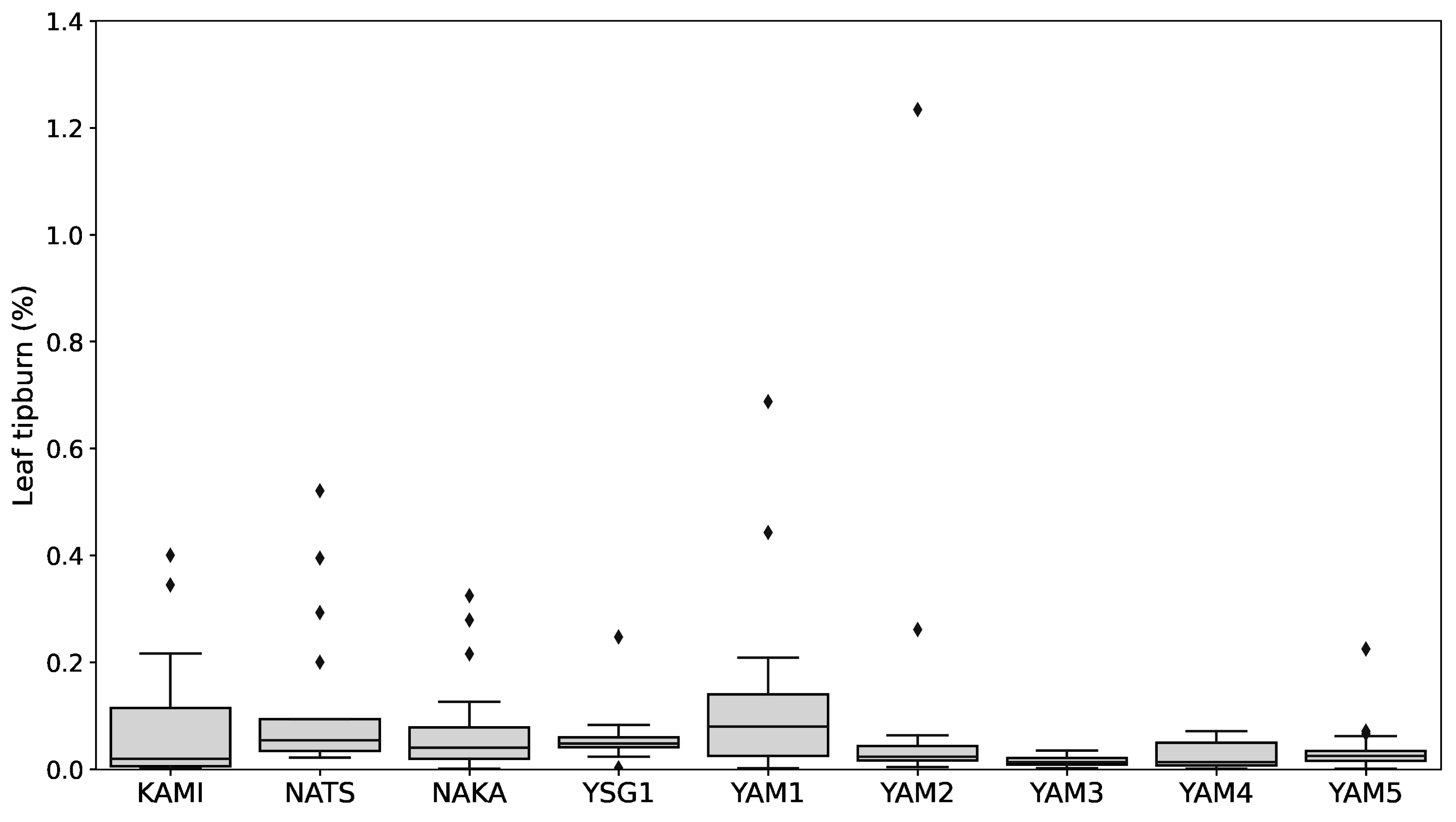
2.1. Variation in Pigment Contents and Functional Components Among Different Bunching Onion Varieties and Breeding Lines Across Different Growing Conditions
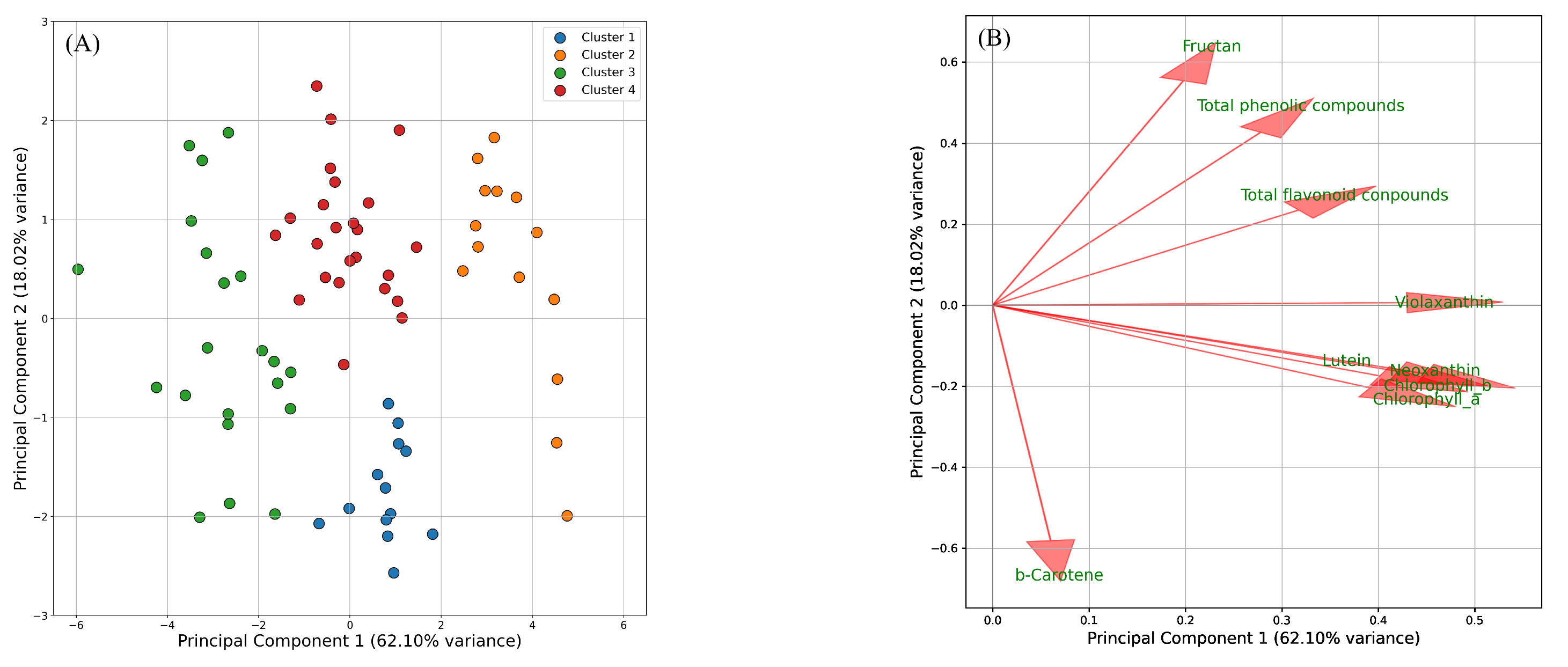
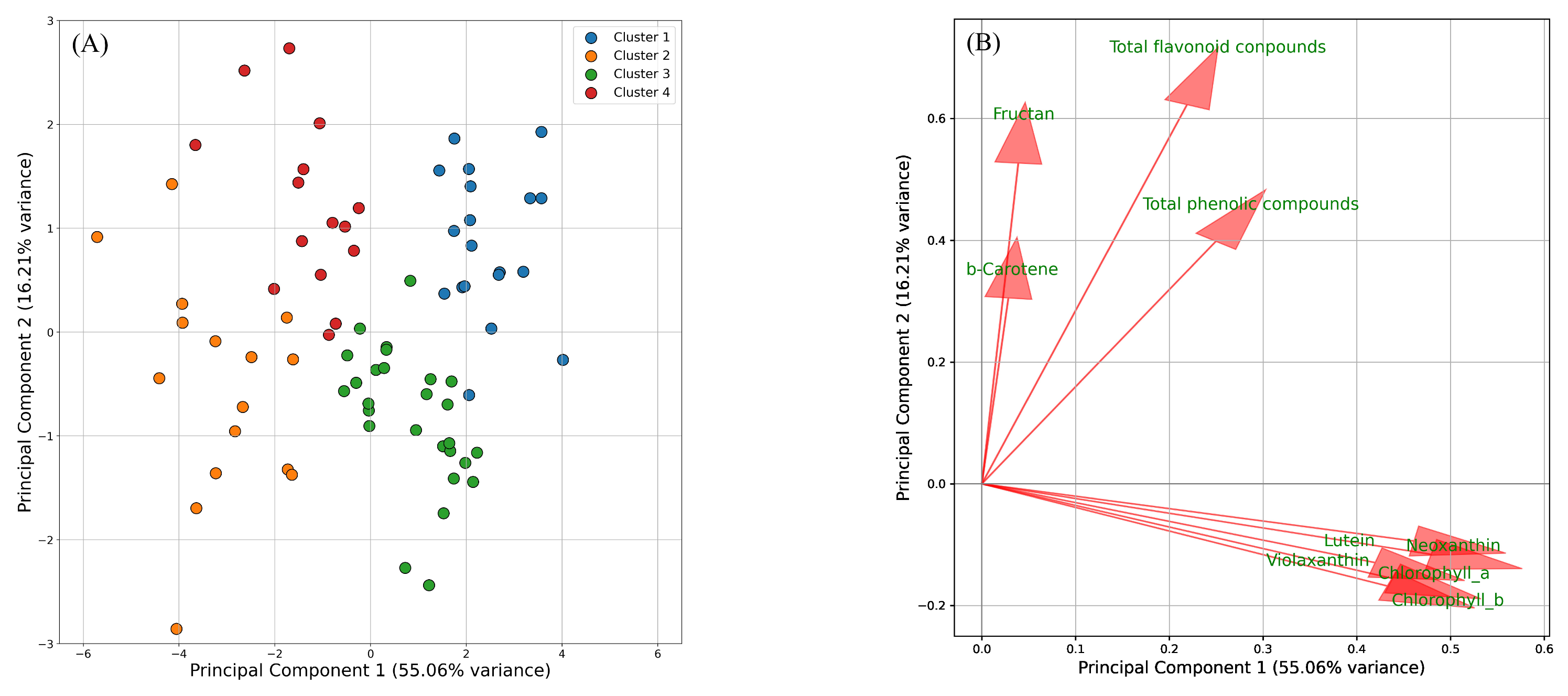
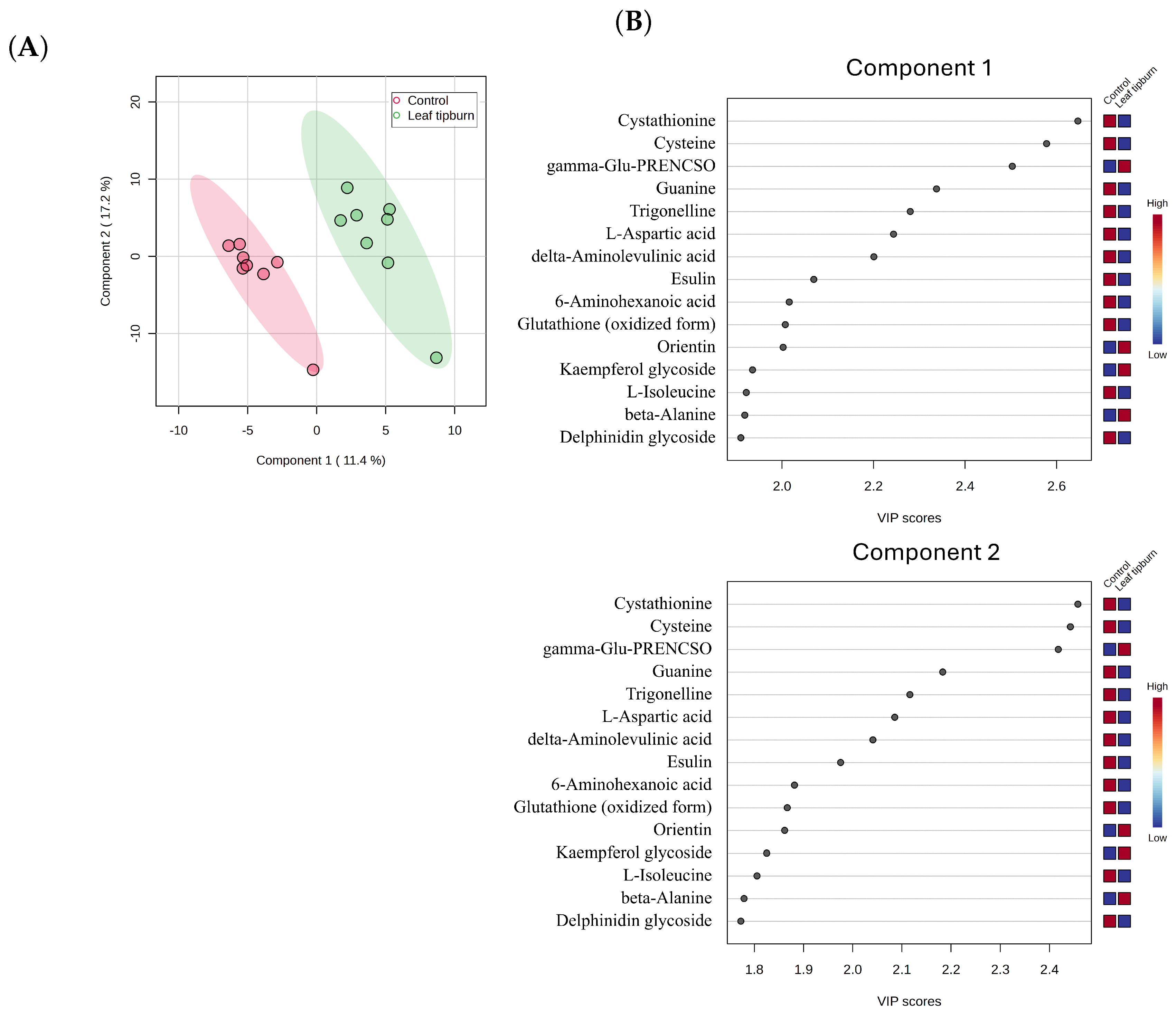
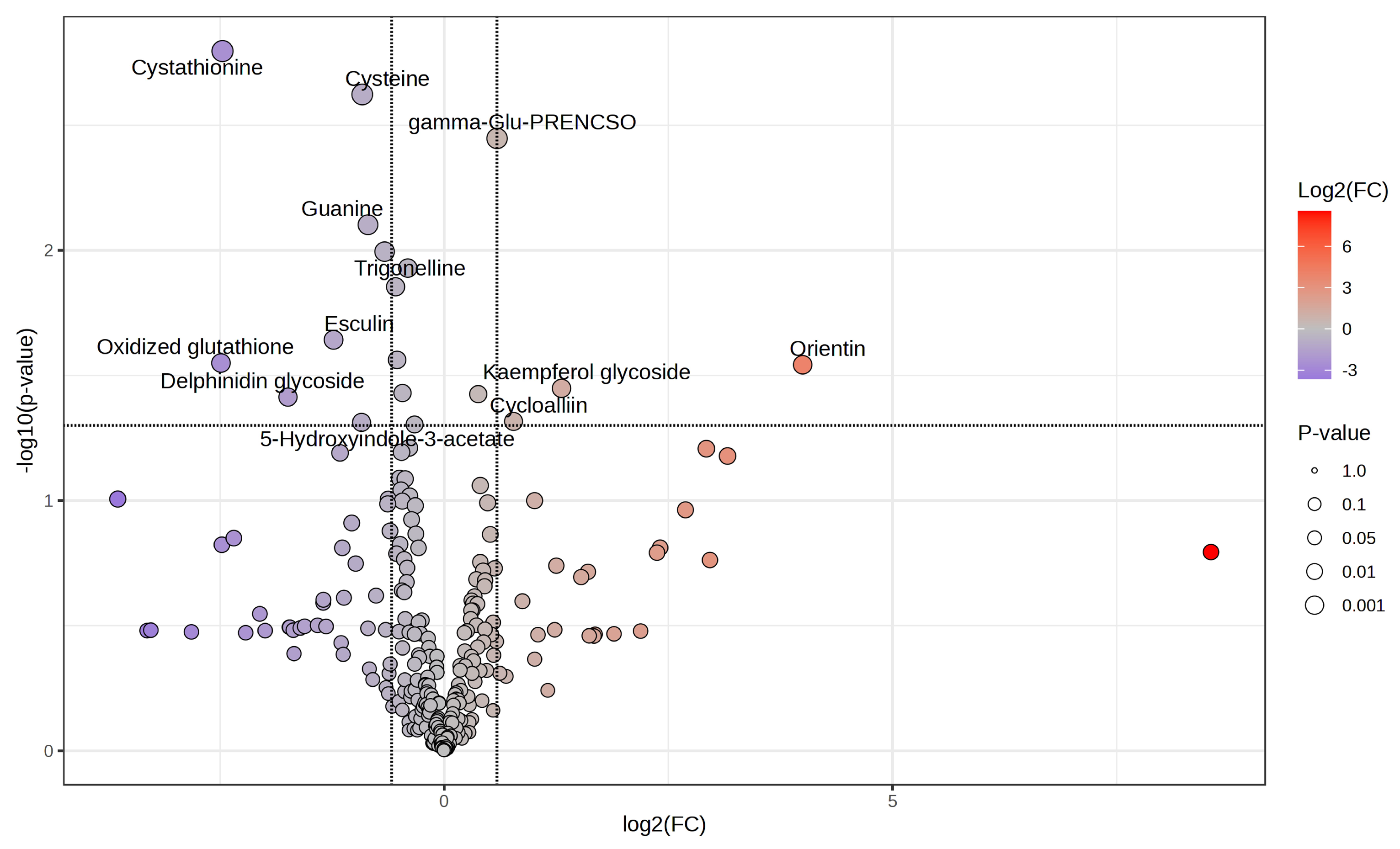
2.2. Estimating the Metabolites Involved in Leaf Tipburn
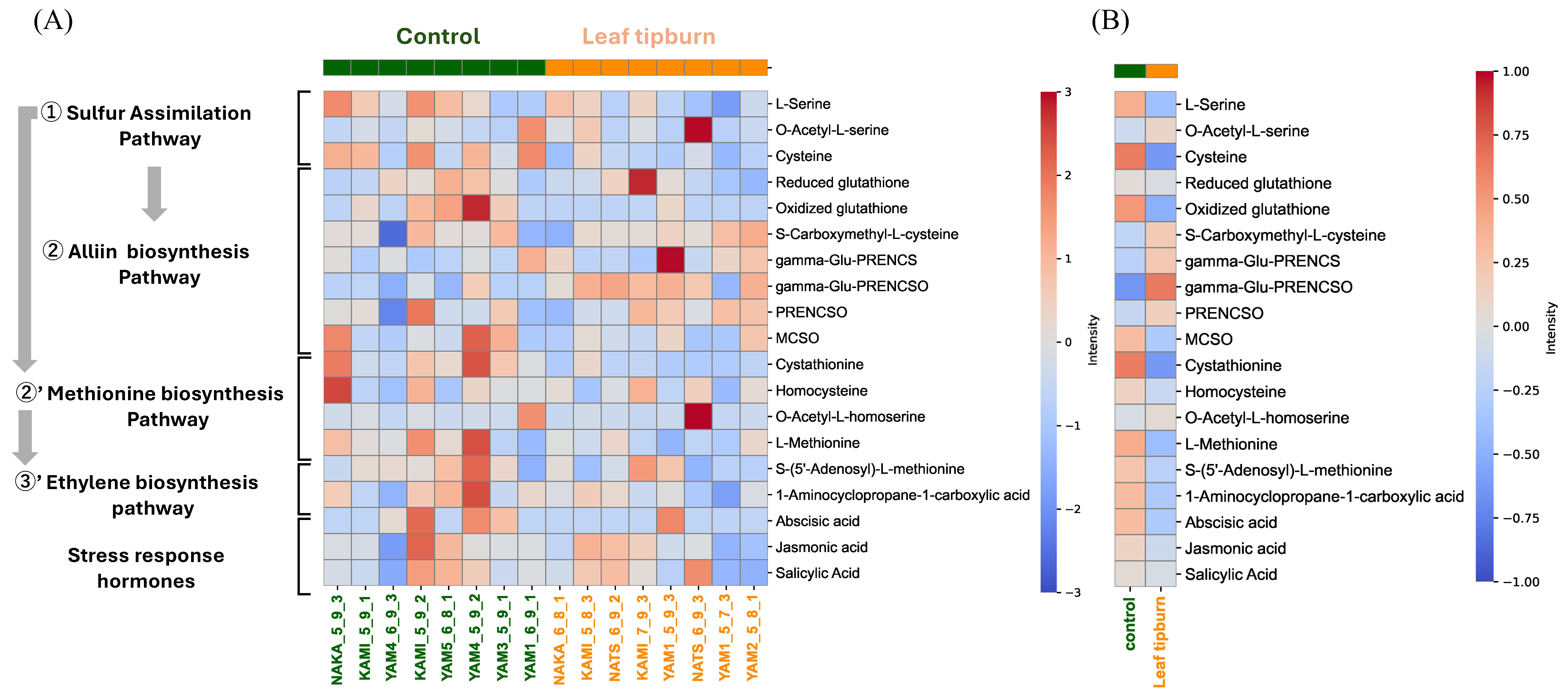
2.3. Variations in Organosulfur Compounds and Plant Hormones
3. Discussion
3.1. Clarification of the Differences in Pigment Compounds and Functional Components Among Varieties and Lines Based on the Growing Conditions
3.2. Estimation of the Metabolites Involved in Leaf Tipburn
4. Materials and Methods
4.1. Materials and Growth Conditions
4.2. Leaf Tipburn Measurement
4.3. SPAD Value Measurement
4.4. Pigment Compound Measurement
4.5. 70% Ethanol Extraction
4.6. Total Phenolic Compounds Measurement
4.7. Total Flavonoid Compound Measurement
4.8. Fructan Measurement
4.9. Metabolome Analysis
4.10. Data Analysis
4.10.1. Statistical Analysis
4.10.2. Estimate Compounds Associated with Leaf Tipburn Using Metabolomics
4.10.3. Machine Learning Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamasaki, A.; Alliums, H.E. Botany, Production and Uses; CABI: Wallingford, UK, 2022; p. 111. [Google Scholar]
- Kim, S.H.; Yoon, J.; Han, J.; Seo, Y.; Kang, B.H.; Lee, J.; Ochar, K. Green Onion (Allium fistulosum): An Aromatic Vegetable Crop Esteemed for Food, Nutritional and Therapeutic Significance. Foods 2023, 12, 4503. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Ramakrishna, Y. Welsh onion (Allium fistulosum L.): A promising spicing-culinary herb of Mizoram. Indian J. Hill Farming 2017, 30, 201–208. [Google Scholar]
- Padula, G.; Xia, X.; Hołubowicz, R. Welsh Onion (Allium fistulosum L.) Seed Physiology, Breeding, Production and Trade. Plants 2022, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Kołota, E.; Adamczewska-Sowińska, K.; Uklańska-Pusz, C. Yield and nutritional value of Japanese bunching onion (Allium fistulosum L.) depending on the growing season and plant maturation stage. J. Elem. 2012, 17, 587–596. [Google Scholar]
- Liu, X.; Gao, S.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Alterations in leaf photosynthetic electron transport in Welsh onion (Allium fistulosum L.) under different light intensity and soil water conditions. Plant Biol. 2021, 23, 83–90. [Google Scholar] [CrossRef]
- Maruo, T.; Johkan, M. Chapter 12—Tipburn. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 173–176. [Google Scholar]
- Hutchings, J. Food Colour and Appearance; Springer Science & Business Media: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Nilsson, T. Postharvest handling and storage of vegetables. In Fruit and Vegetable Quality; CRC Press: Boca Raton, FL, USA, 2000; pp. 112–138. [Google Scholar]
- Kuronuma, T.; Watanabe, Y.; Ando, M.; Watanabe, H. Relevance of tipburn incidence to the competence for Ca acquirement and Ca distributivity in lisianthus [Eustoma grandiflorum (Raf.) Shinn.] cultivars. Sci. Hortic. 2019, 246, 805–811. [Google Scholar] [CrossRef]
- Su, T.; Li, P.; Wang, H.; Wang, W.; Zhao, X.; Yu, Y.; Zhang, D.; Yu, S.; Zhang, F. Natural variation in a calreticulin gene causes reduced resistance to Ca2+ deficiency-induced tipburn in Chinese cabbage (Brassica rapa ssp. pekinensis). Plant Cell Environ. 2019, 42, 3044–3060. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Wei, Q.; Li, B.; Zhong, X.; Hu, T.; Hu, H.; Bao, C. Transcriptome-Wide Identification and Characterization of Circular RNAs in Leaves of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) in Response to Calcium Deficiency-Induced Tip-burn. Sci. Rep. 2019, 9, 14544. [Google Scholar] [CrossRef]
- Inden, H.; Asahira, T.; Okui, H. Analysis of factors influencing leaf tip drying of Japanese bunching onion in summer. In Proceedings of the Abstract Proceedings of the Horticultural Science Spring Meeting, Tokyo, Japan, 2 April 1987; p. 272. (In Japanese). [Google Scholar]
- Abbey, L.; Joyce, D.; Aked, J.; Smith, B. Genotype, sulphur nutrition and soil type effects on growth and dry-matter production of spring onion. J. Hortic. Sci. Biotechnol. 2002, 77, 340–345. [Google Scholar] [CrossRef]
- Jeyakumar, P.; Balamohan, T. Micronutrients for Horticultural Crops; Tamil Nadu Agricultural University: Coimbatore, India, 2007. [Google Scholar]
- Ahmad, M.; Büker, P.; Khalid, S.; Berg, L.V.D.; Shah, H.; Wahid, A.; Emberson, L.; Power, S.; Ashmore, M. Effects of ozone on crops in north-west Pakistan. Environ. Pollut. 2013, 174, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.; Grieve, C. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Riaz, M.; Mahmood, R.; Khan, S.; Haider, M.; Ramzan, S. Onion tip burn: Significance, and response to amount and form of nitrogen. Sci. Hortic. 2020, 261, 108773. [Google Scholar] [CrossRef]
- Saure, M. Causes of the tipburn disorder in leaves of vegetables. Sci. Hortic. 1998, 76, 131–147. [Google Scholar] [CrossRef]
- Uno, Y.; Okubo, H.; Itoh, H.; Koyama, R. Reduction of leaf lettuce tipburn using an indicator cultivar. Sci. Hortic. 2016, 210, 14–18. [Google Scholar] [CrossRef]
- Palencia, P.; Martinez, F.; Ribeiro, E.; Pestana, M.; Gama, F.; Saavedra, T.; de Varennes, A.; Correia, P. Relationship between tipburn and leaf mineral composition in strawberry. Sci. Hortic. 2010, 126, 242–246. [Google Scholar] [CrossRef]
- Hirai, M.; Yano, M.; Goodenowe, D.; Kanaya, S.; Kimura, T.; Awazuhara, M.; Arita, M.; Fujiwara, T.; Saito, K. Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 10205–10210. [Google Scholar] [CrossRef] [PubMed]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Bergo, A.M.; Leiss, K.; Havlik, J. Twenty Years of 1H NMR Plant Metabolomics: A Way Forward toward Assessment of Plant Metabolites for Constitutive and Inducible Defenses to Biotic Stress. J. Agric. Food Chem. 2024, 72, 8332–8346. [Google Scholar] [CrossRef] [PubMed]
- Saviano, G.; Paris, D.; Melck, D.; Fantasma, F.; Motta, A.; Iorizzi, M. Metabolite variation in three edible Italian Allium cepa L. by NMR-based metabolomics: A comparative study in fresh and stored bulbs. Metabolomics 2019, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Hirata, S.; Sawada, Y.; Hirai, M.; Sato, S.; Hirakawa, H.; Mine, Y.; Tanaka, K.; Shigyo, M. Widely targeted metabolome and transcriptome landscapes of Allium fistulosum—A. cepa chromosome addition lines revealed a flavonoid hot spot on chromosome 5A. Sci. Rep. 2019, 9, 3541. [Google Scholar] [CrossRef]
- Medina-Melchor, D.; Zapata-Sarmiento, D.; Becerra-Martínez, E.; Rodríguez-Monroy, M.; Vallejo, L.; Sepúlveda-Jiménez, G. Changes in the metabolomic profiling of Allium cepa L. (onion) plants infected with Stemphylium vesicarium. Eur. J. Plant Pathol. 2022, 162, 557–573. [Google Scholar] [CrossRef]
- Hamidon, M.; Ahamed, T. Detection of Tip-Burn Stress on Lettuce Grown in an Indoor Environment Using Deep Learning Algorithms. Sensors 2022, 22, 7251. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C. Leaf Tipburn in Cauliflower as Affected by Cultivar, Calcium Sprays, and Nitrogen Nutrition. HortScience 1990, 25, 660–663. [Google Scholar] [CrossRef]
- Hermanns, A.; Zhou, X.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid Pigment Accumulation in Horticultural Plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Obel, H.; Cheng, C.; Tian, Z.; Njogu, M.; Li, J.; Du, S.; Lou, Q.; Zhou, J.; Yu, X.; Ogweno, J. Transcriptomic and Physiological Analyses Reveal Potential Genes Involved in Photoperiod-Regulated β-Carotene Accumulation Mechanisms in the Endocarp of Cucumber (Cucumis sativus L.) Fruit. Int. J. Mol. Sci. 2022, 23, 12650. [Google Scholar] [CrossRef]
- Efeoglu, B.; Terzioglu, S. Photosynthetic responses of two wheat varieties to high temperature. Eurasian J. Biosci. 2009, 3, 97–106. [Google Scholar] [CrossRef]
- Sato, R.; Ito, H.; Tanaka, A. Chlorophyll b degradation by chlorophyll b reductase under high-light conditions. Photosynth. Res. 2015, 126, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Ooshima, K.; Yuko, Y.; Yoshinori, S.; Sato, R.; Maruo, T. Elucidation of the cause for Chinese chive leaf tip-burn. Bull. Tochigi Agric. Exp. Stn. 2015, 73, 11–20. [Google Scholar]
- Hernández, I.; Alegre, L.; Breusegem, F.V.; Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Li, J.; Wang, X.; Wang, C. Crosstalk between Hydrogen Sulfide and Other Signal Molecules Regulates Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 4593. [Google Scholar] [CrossRef]
- Jin, Z.; Shen, J.; Qiao, Z.; Yang, G.; Wang, R.; Pei, Y. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2011, 414, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wollers, S.; Heidenreich, T.; Zarepour, M.; Zachmann, D.; Kraft, C.; Zhao, Y.; Mendel, R.; Bittner, F. Binding of Sulfurated Molybdenum Cofactor to the C-terminal Domain of ABA3 from Arabidopsis thaliana Provides Insight into the Mechanism of Molybdenum Cofactor Sulfuration. J. Biol. Chem. 2008, 283, 9642–9650. [Google Scholar] [CrossRef]
- Tolin, S.; Arrigoni, G.; Trentin, A.; Veljovic-Jovanovic, S.; Pivato, M.; Zechman, B.; Masi, A. Biochemical and quantitative proteomics investigations in Arabidopsis ggt1 mutant leaves reveal a role for the gamma-glutamyl cycle in plant’s adaptation to environment. Proteomics 2013, 13, 2031–2045. [Google Scholar] [CrossRef]
- Ohkama-Ohtsu, N.; Radwan, S.; Peterson, A.; Zhao, P.; Badr, A.; Xiang, C.; Oliver, D. Characterization of the extracellular γ-glutamyl transpeptidases, GGT1 and GGT2, in Arabidopsis. Plant J. 2007, 49, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.; Petrov, V. Reactive Oxygen Species and Abiotic Stress in Plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef]
- Chung, K.; Demianski, A.; Harrison, G.; Laurie-Berry, N.; Mitsuda, N.; Kunkel, B. Jasmonate Hypersensitive 3 negatively regulates both jasmonate and ethylene-mediated responses in Arabidopsis. J. Exp. Bot. 2022, 73, 5067–5083. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Yamamoto, K. LIA for Win32 (LIA32) Release 0.377 e (English Version) User’s Manual. Currently Version Ver. 0.378. 2004. Available online: https://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/download.html (accessed on 11 November 2024).
- Markwell, J.; Osterman, J.; Mitchell, J. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Dissanayake, P.; Yamauchi, N.; Shigyo, M. Chlorophyll degradation and resulting catabolite formation in stored Japanese bunching onion (Allium fistulosum L.). J. Sci. Food Agric. 2008, 88, 1981–1986. [Google Scholar] [CrossRef]
- Hang, T.; Shigyo, M.; Yaguchi, S.; Yamauchi, N.; Tashiro, Y. Effect of single alien chromosome from shallot (Allium cepa L. Aggregatum group) on carbohydrate production in leaf blade of bunching onion (A. fistulosum L.). Genes Genet. Syst. 2004, 79, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Denis, W. A colorimetric method for the determination of phenols (and phenol derivatives) in urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Vu, Q.; Hang, T.; Yaguchi, S.; Ono, Y.; Pham, T.; Yamauchi, N.; Shigyo, M. Assessment of biochemical and antioxidant diversities in a shallot germplasm collection from Vietnam and its surrounding countries. Genet. Resour. Crop Evol. 2013, 60, 1297–1312. [Google Scholar] [CrossRef]
- Percheron, F. Dosage colorimetrique du fructose et des fructofuranosides par l’acide thiobarbiturique. Compte Rendu 1962, 255, 2521–2522. [Google Scholar]
- Sawada, Y.; Tsukaya, H.; Li, Y.; Sato, M.; Kawade, K.; Hirai, M. A novel method for single-grain-based metabolic profiling of Arabidopsis seed. Metabolomics 2017, 13, 75. [Google Scholar] [CrossRef]

| Cluster | Features | Growth Conditions | KAMI | NATS | NAKA | YAM1 | YAM2 | YAM3 | YAM4 | YAM5 |
|---|---|---|---|---|---|---|---|---|---|---|
| cluster 1 | -carotene_UP | May_July y | 1 | 3 | 1 | 2 | 1 | 3 | ||
| May_August | 2 | |||||||||
| May_September | ||||||||||
| cluster 2 | Pigment cmpds_UP z | May_July | ||||||||
| May_August | ||||||||||
| May_September | 3 | 3 | 3 | 2 | 3 | |||||
| cluster 3 | Pigment cmpds_DOWN | May_July | 3 | 3 | 2 | 2 | 2 | |||
| May_August | 3 | 3 | 2 | |||||||
| May_September | 1 | |||||||||
| cluster 4 | Functional cmpds_UP | May_July | 1 | |||||||
| May_August | 1 | 1 | 3 | 3 | 3 | 3 | ||||
| May_September | 3 | 2 | 3 | 1 |
| Cluster | Features | Growth Conditions | KAMI | NATS | NAKA | YSG1 | YAM1 | YAM2 | YAM3 | YAM4 | YAM5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cluster 1 | Pigment cmpds_UP y Functional cmpds_UP | May_September z | 3 | 3 | 2 | 2 | 2 | ||||
| June_September | 2 | 1 | |||||||||
| July_September | 3 | 1 | |||||||||
| cluster 2 | Pigment cmpds_DOWN Functional cmpds_DOWN | May_September | 2 | 1 | |||||||
| June_September | 3 | 2 | 1 | ||||||||
| July_September | 3 | 3 | 1 | ||||||||
| cluster 3 | Pigment cmpds_UP Functional cmpds_DOWN | May_September | 1 | 1 | |||||||
| June_September | 1 | 2 | 1 | 1 | 3 | 3 | |||||
| July_September | 1 | 3 | 3 | 3 | 3 | 2 | |||||
| cluster 4 | Pigment cmpds_DOWN Functional cmpds_UP | May_September | 3 | 1 | 2 | 1 | |||||
| June_September | 1 | 2 | 1 | 2 | 1 | ||||||
| July_September | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, T.; Yamamoto, R.; Matsuse, K.; Fuji, M.; Fujii, K.; Hirata, S.; Abdelrahman, M.; Sato, M.; Hirai, M.Y.; Shigyo, M. Metabolite Profiling and Association Analysis of Leaf Tipburn in Heat-Tolerant Bunching Onion Varieties. Plants 2025, 14, 187. https://doi.org/10.3390/plants14020187
Nakajima T, Yamamoto R, Matsuse K, Fuji M, Fujii K, Hirata S, Abdelrahman M, Sato M, Hirai MY, Shigyo M. Metabolite Profiling and Association Analysis of Leaf Tipburn in Heat-Tolerant Bunching Onion Varieties. Plants. 2025; 14(2):187. https://doi.org/10.3390/plants14020187
Chicago/Turabian StyleNakajima, Tetsuya, Reina Yamamoto, Kanako Matsuse, Masato Fuji, Koei Fujii, Sho Hirata, Mostafa Abdelrahman, Muneo Sato, Masami Yokota Hirai, and Masayoshi Shigyo. 2025. "Metabolite Profiling and Association Analysis of Leaf Tipburn in Heat-Tolerant Bunching Onion Varieties" Plants 14, no. 2: 187. https://doi.org/10.3390/plants14020187
APA StyleNakajima, T., Yamamoto, R., Matsuse, K., Fuji, M., Fujii, K., Hirata, S., Abdelrahman, M., Sato, M., Hirai, M. Y., & Shigyo, M. (2025). Metabolite Profiling and Association Analysis of Leaf Tipburn in Heat-Tolerant Bunching Onion Varieties. Plants, 14(2), 187. https://doi.org/10.3390/plants14020187








