A Bioeconomically Valuable Essential Oil from Baccharis sinuata Kunth in Southern Ecuador: Chemical Composition and Enantiomeric Profile
Abstract
1. Introduction
2. Results
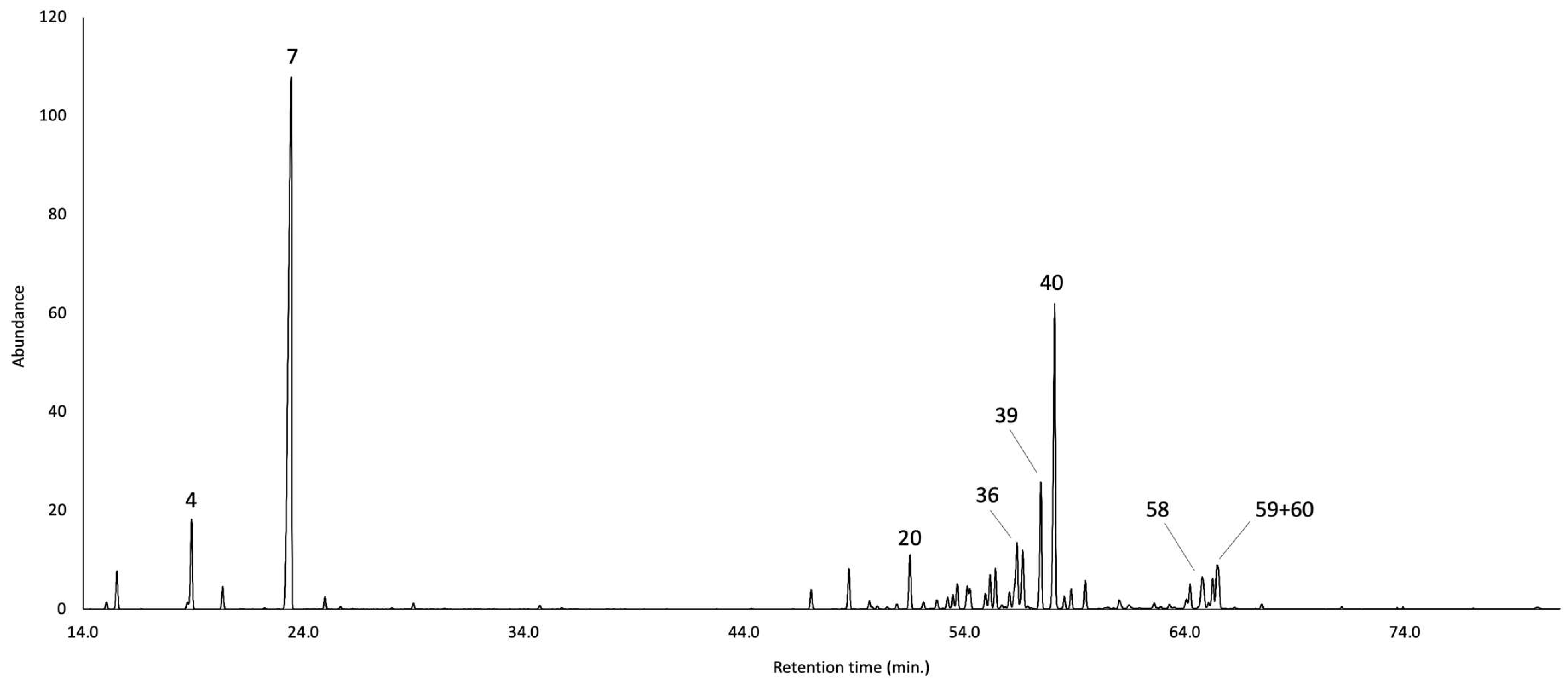
2.1. Qualitative and Quantitative Chemical Analyses
2.2. Enantioselective Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Distillation and Sample Preparation
4.3. Qualitative Chemical Analysis (GC-MS)
4.4. Quantitative Chemical Analysis (GC-FID)
4.5. Enantioselective Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Megadiverse Countries, UNEP-WCMC. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries (accessed on 3 July 2025).
- Malagón, O.; Ramírez, J.; Andrade, J.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.M.; Vinueza, D.R.; Gilardoni, G.; Mota, A.J.; Malagón, O. The Essential Oil from the Roots of Valeriana rigida Ruiz & Pav. Growing in the Paramos of Chimborazo (Ecuador): Chemical Analysis, Enantioselective Profile, and Preliminary Biological Activity. Plants 2025, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, Y.E.; Rodríguez, M.d.C.; Bustamante, M.E.; Cuenca, S.; Malagón, O.; Cumbicus, N.; Gilardoni, G. Gynoxys hallii Hieron., Gynoxys calyculisolvens Hieron., and Gynoxys azuayensis Cuatrec. Essential Oils—Chemical and Enantioselective Analyses of Three Unprecedented Volatile Fractions from the Ecuadorian Biodiversity. Plants 2025, 14, 659. [Google Scholar] [CrossRef]
- Gilardoni, G.; Sgorbini, B.; Pavarino, M.; Cumbicus, N.; Romero, F.; Malagón, O. The leaf essential oil of Ecuadorian Ophryosporus peruvianus (J.F. Gmel.) R.M. King & H. Rob: Chemical composition, enantioselective analysis, and in vitro enzymatic inhibitory activity. J. Essent. Oil Res. 2024, 36, 588–596. [Google Scholar]
- Ramírez, J.; Gilardoni, G.; Jácome, M.; Montesinos, J.; Rodolfi, M.; Guglielminetti, M.L.; Cagliero, C.; Bicchi, C.; Vidari, G. Chemical composition, enantiomeric analysis, AEDA sensorial evaluation and antifungal activity of the essential oil from the Ecuadorian plant Lepechinia mutica Benth (Lamiaceae). Chem. Biodivers. 2017, 14, e1700292. [Google Scholar] [CrossRef]
- WFO Plant List. World Flora Online. Available online: https://wfoplantlist.org/ (accessed on 3 July 2025).
- Tropicos.org. Missouri Botanical Garden. Available online: https://www.tropicos.org (accessed on 3 July 2025).
- INABIO. Instituto Nacional de Biodiversidad. Available online: https://bndb.sisbioecuador.bio/bndb/collections/list.php?usethes=1&taxa=40487 (accessed on 3 July 2025).
- iNaturalist Ecuador. Contribuyendo a La Biodiversidad Del País. Available online: https://ecuador.inaturalist.org (accessed on 3 July 2025).
- Campos, F.R.; Bressan, J.; Jasinski, V.C.G.; Zuccolotto, T.; da Silva, L.E.; Cerqueira, L.B. Baccharis (Asteraceae): Chemical Constituents and Biological Activities. Chem. Biodivers. 2016, 13, 1–17. [Google Scholar] [CrossRef]
- Valarezo, E.; Rosales, J.; Morocho, V.; Cartuche, L.; Guaya, D.; Ojeda-Riascos, S.; Armijos, C.; Gonzalez, S. Chemical composition and biological activity of the essential oil of Baccharis obtusifolia Kunth from Loja, Ecuador. J. Essent. Oil Res. 2015, 27, 212–216. [Google Scholar] [CrossRef]
- Valarezo, E.; Rosillo, M.; Cartuche, L.; Malagon, O.; Meneses, M.; Morocho, V. Chemical composition, antifungal and antibacterial activity of the essential oil from Baccharis latifolia (Ruiz & Pav.) Pers. (Asteraceae) from Loja, Ecuador. J. Essent. Oil Res. 2013, 25, 233–238. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Salehi, P.; Sonboli, A.; Eftekhar, F.; Nejad-Ebrahimi, S.; Yousefzadi, M. Essential Oil Composition, Antibacterial and Antioxidant Activity of the Oil and Various Extracts of Ziziphora clinopodioides subsp. rigida (Boiss.) Rech. f. from Iran. Biol. Pharm. Bull. 2005, 28, 1892–1896. [Google Scholar] [CrossRef]
- Bassole, I.H.N.; Ouattara, A.S.; Nebie, R.; Ouattara, C.A.T.; Kabore, Z.I.; Traore, S.A. Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry 2003, 62, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.-L.; Ollé, D.; Ollitrault, P.; Luro, F.; Brillouet, J.-M. Leaf and peel volatile compounds of an interspecific citrus somatic hybrid [Citrus aurantifolia (Christm.) Swing. + Citrus paradisi Macfayden]. Flavour Fragr. J. 2002, 17, 416–424. [Google Scholar] [CrossRef]
- Rega, B.; Fournier, N.; Guichard, E. Solid phase microextraction (SPME) of orange juice flavor: Odor representativeness by direct gas chromatography olfactometry (D-GC-O). J. Agric. Food Chem. 2003, 51, 7092–7099. [Google Scholar] [CrossRef] [PubMed]
- Shellie, R.; Marriott, P.; Zappia, G.; Mondello, L.; Dugo, G. Interactive use of linear retention indices on polar and apolar columns with an MS-Library for reliable characterization of Australian tea tree and other Melaleuca sp. oils. J. Essent. Oil Res. 2003, 15, 305–312. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Zappia, G.; Bonaccorsi, I.; Cotroneo, A.; Russo, M.T. The composition of the volatile fraction and the enantiomeric distribution of five volatile components of faustrime oil (Monocitrus australatica × Fortunella sp. × Citrus aurantifolia). J. Essent. Oil Res. 2004, 16, 328–333. [Google Scholar] [CrossRef]
- Cozzani, S.; Muselli, A.; Desjobert, J.-M.; Bernardini, A.-F.; Tomi, F.; Casanova, J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005, 20, 436–441. [Google Scholar] [CrossRef]
- Zheng, C.H.; Kim, T.H.; Kim, K.H.; Leem, Y.H.; Lee, H.J. Characterization of potent aroma compounds in Chrysanthemum coronarium L. (Garland) using aroma extract dilution analysis. Flavour Fragr. J. 2004, 19, 401–405. [Google Scholar] [CrossRef]
- Lee, S.-J.; Umano, K.; Shibamoto, T.; Lee, K.-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Högnadóttir, Á.; Rouseff, R.L. Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 998, 201–211. [Google Scholar] [CrossRef]
- Tzakou, O.; Couladis, M.; Slavkovska, V.; Mimica-Dukic, N.; Jancic, R. The essential oil composition of Salvia brachyodon Vandas. Flavour Fragr. J. 2003, 18, 2–4. [Google Scholar] [CrossRef]
- Museli, A.; Pau, M.; Desjobert, J.-M.; Foddai, M.; Usai, M.; Costa, J. Volatile constituents of Achillea ligustica All. by HS-SPME/GC/GC-MS. Comparison with essential oils obtained by hydrodistillation from Corsica and Sardinia. Chromatographia 2009, 69, 575–585. [Google Scholar] [CrossRef]
- Buttery, R.G.; Xu, C.; Ling, L.C. Volatile components of wheat leaves (and stems): Possible insect attractants. J. Agric. Food Chem. 1985, 33, 115–117. [Google Scholar] [CrossRef]
- Yu, E.J.; Kim, T.H.; Kim, K.H.; Lee, H.J. Characterization of aroma-active compounds of Abies nephrolepis (Khingan fir) needles using aroma extract dilution analysis. Flavour Fragr. J. 2004, 19, 74–79. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Lopez-Tamames, E.; Buxaderas, S. Assessment of the Volatile Composition of Juices of Apricot, Peach, and Pear According to Two Pectolytic Treatments. J. Agric. Food Chem. 2005, 53, 7837–7843. [Google Scholar] [CrossRef]
- Lamarque, A.L.; Maestri, D.M.; Zygadlo, J.A.; Grosso, N.R. Volatile constituents from flowers of Acacia caven (Mol.) Mol. var. caven, Acacia aroma Gill. ex Hook., Erythrina crista-galli L. and Calliandra tweedii Benth. Flavour Fragr. J. 1998, 13, 266–268. [Google Scholar] [CrossRef]
- Berlinet, C.; Ducruet, V.; Brillouet, J.-M.; Reynes, M.; Brat, P. Evolution of aroma compounds from orange juice stored in polyethylene terephthalate (PET). Food Addit. Contam. 2005, 22, 185–195. [Google Scholar] [CrossRef]
- Tu, N.T.M.; Onishi, Y.; Son, U.S.; Ogawa, E.; Ukeda, H.; Sawamura, M. Characteristic odour components of Citrus inflata Hort. Ex Tanaka (Mochiyu) cold-pressed peel oil. Flavour Fragr. J. 2003, 18, 454–459. [Google Scholar]
- Beck, J.J.; Higbee, B.S.; Marrill, G.B.; Roitman, J.N. Comparison of volatile emissions from undamaged and mechanically damaged almonds. J. Sci. Food Argic. 2008, 88, 1363–1368. [Google Scholar] [CrossRef]
- Muselli, A.; Rossi, P.-G.; Desjobert, J.-M.; Bernardini, A.-F.; Berti, L.; Costa, J. Chemical composition and antibacterial activity of Otanthus maritimus (L.) Hoffmanns. Link essential oils from Corsica. Flavour Fragr. J. 2007, 22, 217–223. [Google Scholar] [CrossRef]
- Paniandy, J.-C.; Chane-Ming, J.; Pierbattesti, J.-C. Chemical Composition of the Essential Oil and Headspace Solid-Phase Microextraction of the Guava Fruit (Psidium guajava L.). J. Essent. Oil Res. 2000, 12, 153–158. [Google Scholar] [CrossRef]
- Lago, J.H.G.; de Ávila, P., Jr.; de Aquino, E.M.; Moreno, P.R.H.; Ohara, M.T.; Limberger, R.P.; Apel, M.A.; Henriques, A.T. Volatile oils from leaves and stem barks of Cedrela fissilis (Meliaceae): Chemical composition and antibacterial activities. Flavour Fragr. J. 2004, 19, 448–451. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Cervantes, M.; Combariza, Y.; Martínez, J.R. HRGC-FID-MSD Analysis of the Secondary Metabolites Obtained by Different Extraction Methods from Lepechinia schiedeana, and Evaluation of its Antioxidant Activity in vitro. In Proceedings of the 20th International Symposium on Capillary Chromatography, Riva del Garda, Italy, 26–29 May 1998; pp. 1–11. [Google Scholar]
- González, S.; Guerra, P.E.; Bottaro, H.; Molares, S.; Demo, M.S.; Oliva, M.M.; Zunino, M.P.; Zygadlo, J.A. Aromatic plants from Patagonia. Part I. Antimicrobial activity and chemical composition of Schinus polygamus (Cav.) Cabrera essential oil. Flavour Fragr. J. 2004, 19, 36–39. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, H.; Cadwallader, K.R. Aroma-active compounds in Kimchi during fermentation. J. Agric. Food Chem. 1998, 46, 1944–1953. [Google Scholar] [CrossRef]
- Niponsak, A.; Laohakunjit, N.; Kerdchoechuen, O. Changes of volatile compounds and physicochemical qualities of fresh cut Pomelo during storage. Agric. Sci. 2011, 42, 109–112. [Google Scholar]
- Castioni, P.; Kapetanidis, I. Volatile constituents from Brunfelsia grandiflora ssp. grandiflora: Qualitative analysis by GC-MS. Sci. Pharm. 1996, 64, 83–91. [Google Scholar]
- Orav, A.; Kann, J. Determination of peppermint and orange aroma compounds in food and beverages. Proc. Est. Acad. Sci. Chem. 2001, 50, 217–225. [Google Scholar]
- Cai, J.; Lin, P.; Zhu, X.; Su, Q. Comparative analysis of clary sage (S. sclarea L.) oil volatiles by GC-FTIR and GC-MS. Food Chem. 2006, 99, 401–407. [Google Scholar] [CrossRef]
- Choi, H.-S. Character impact odorants of Citrus Hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003, 51, 2687–2692. [Google Scholar] [CrossRef]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Ghrabi, Z.G.; Zarrouk, H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Tigrine-Kordiani, N.; Meklati, B.Y.; Chemat, F. Analysis by gas chromatography—Mass spectrometry of the essential oil of Zygophyllum album L., an aromatic and medicinal plant growing in Algeria. Int. J. Aromather. 2006, 16, 187–191. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.R.; Miguel, M.G.; da Cunha, A.P. Analysis by gas chromatography-mass spectrometry of the volatile components of Teucrium lusitanicum and Teucrium algarbiensis. J. Chromatogr. A 2004, 1033, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Noorizadeh, H.; Farmany, A.; Noorizadeh, M. Quantitative structure-retention relationships analysis of retention index of essential oils. Quim. Nova 2011, 34, 242–249. [Google Scholar] [CrossRef]
- Paolini, J.; Tomi, P.; Bernardini, A.-F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed analysis of the essential oil from Cistus albidus L. by combination of GC/RI, GC/MS and 13C NMR spectroscopy. N. Z. J. Agric. Res. 2008, 22, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Molleken, U.; Sinnwell, V.; Kubeczka, K.H. The essential oil composition of fruits from Smyrnium perfoliatum. Phytochemistry 1998, 47, 1079–1083. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Tomi, F.; Bernardini, A.-F.; Casanova, J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Saroglou, V.; Marin, P.D.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146–152. [Google Scholar] [CrossRef]
- Dugo, G.; Bonaccorsi, I.; Sciarrone, D.; Costa, R.; Dugo, P.; Mondello, L.; Santi, L.; Fakhry, H.A. Characterization of Oils from the Fruits, Leaves and Flowers of the Bitter Orange Tree. J. Essent. Oil Res. 2011, 23, 45–59. [Google Scholar] [CrossRef]
- Budel, J.M.; Matzenbacher, N.I.; Duarte, M.R. Genus Baccharis (Asteraceae): A review of chemical and pharmacological studies. Recent Prog. Med. Plants 2008, 21, 1–18. [Google Scholar]
- Lopez Arze, J.B.; Garneau, F.-X.; Collin, G.; Jean, F.-I.; Gagnon, H. Essential Oils from Bolivia. I. Asteraceae: Baccharis tricuneata (L.f.) Pers. var. ruiziana Cuatrecassas. J. Essent. Oil Res. 2004, 16, 429–431. [Google Scholar] [CrossRef]
- Moreno-Pizani, M.A.; Paredes-Trejo, F.J.; Farias-Ramirez, A.J.; dos Santos, H.T.; Prado Massarioli, A.; Marin, F.R.; Takeyoshi, B.Y.; Alves Marques, P.A.; De Stefano Piedade, S.M.; de Alencar, S.M. Essential Oil Content of Baccharis crispa Spreng. Regulated by Water Stress and Seasonal Variation. AgriEngineering 2020, 2, 458–470. [Google Scholar] [CrossRef]
- de Souza, M.T.; de Souza, M.T.; Bernardi, D.; de Melo, D.J.; Gorgatti Zarbin, P.H.; Cassilha Zawadneak, M.A. Insecticidal and oviposition deterrent effects of essential oils of Baccharis spp. and histological assessment against Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2021, 11, 3944. [Google Scholar] [CrossRef]
- Simões-Pires, C.A.; Debenedetti, S.; Spegazzini, E.; Mentz, L.A.; Matzenbacher, N.I.; Limberger, R.P.; Henriques, A.T. Investigation of the essential oil from eight species of Baccharis belonging to sect. Caulopterae (Asteraceae, Astereae): A taxonomic approach. Plant Syst. Evol. 2005, 253, 23–32. [Google Scholar] [CrossRef]
- Chialva, F.; Doglia, G. Essential Oil from Carqueja (Baccharis genistelloides Pers.). J. Essent. Oil Res. 1990, 2, 173–177. [Google Scholar] [CrossRef]
- Ascari, J.; de Oliveira, M.S.; Nunes, D.S.; Granato, D.; Scharf, D.R.; Simionatto, E.; Otuki, M.; Soley, B.; Heiden, G. Chemical composition, antioxidant and anti-inflammatory activities of the essential oils from male and female specimens of Baccharis punctulata (Asteraceae). J. Ethnopharmacol. 2019, 234, 1–7. [Google Scholar] [CrossRef] [PubMed]
- González, M.D. Chemical Composition of the Leaf Oil from Baccharis punctulata DC. at Two Phenological Stages. J. Essent. Oil Res. 2019, 31, 573–581. [Google Scholar] [CrossRef]
- Bailac, P.N.; Dellacasa, A.D.; Bernasconi, H.O.; Ponzi, M.I.; Firpo, N.H. Essential Oil of Female Plants of Baccharis coridifolia De Candole. J. Essent. Oil Res 2001, 13, 23–24. [Google Scholar] [CrossRef]
- Joung, D.; Song, C.; Ikei, H.; Okuda, T.; Igarashi, M.; Koizumi, H.; Park, B.J.; Yamaguchi, T.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of olfactory stimulation with D-limonene. Adv. Hortic. Sci. 2014, 28, 90–94. [Google Scholar]
- Alkanat, M.; Alkanat, H.Ö. D-Limonene reduces depression-like behaviour and enhances learning and memory through an anti-neuroinflammatory mechanism in male rats subjected to chronic restraint stress. Eur. J. Neurosci. 2024, 60, 4491–4502. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Berliocchi, L.; Chiappini, C.; Adornetto, A.; Gentile, D.; Cerri, S.; Russo, R.; Bagetta, G.; Corasaniti, M.T. Early LC3 lipidation induced by D-limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine 2018, 40, 98–105. [Google Scholar] [CrossRef]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Lee, N.H.; Hyun, C.-G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Catanzaro, I.; Naselli, F.; Sciandrello, G.; Caradonna, F. Abnormal mitotic spindle assembly and cytokinesis induced by d-Limonene in cultured mammalian cells. Mutagenesis 2013, 28, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.N.H.W.; Ahmad, F.; Khong, H.Y.; Zulkifli, R.M. Comparative study of the essential oils of three Beilschmiedia species and their biological activities. Int. J. Food Sci. Technol. 2016, 51, 240–249. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H. Chemical compositions and biological activities of the essential oils of Beilschmiedia madang Blume (Lauraceae). Arch. Pharmacal Res. 2015, 38, 485–493. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef]
- Costa, E.V.; Da Silva, T.B.; Menezes, L.R.A.; Ribeiro, L.H.G.; Gadelha, F.R.; De Carvalho, J.E.; De Souza, L.M.B.; Da Silva, M.A.N.; Siqueira, C.A.T.; Salvador, M.J. Biological activities of the essential oil from the leaves of Xylopia laevigata (Annonaceae). J. Essent. Oil Res. 2013, 25, 179–185. [Google Scholar] [CrossRef]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef]
- Kumar, A. Chemical composition and biological biological activities activities of key constituents of essential oil from the rhizomes of Kaempferia galanga L. Int. J. Pharma. Bio. Sci. 2014, 5, 225–231. [Google Scholar]
- Simionatto, E.; Chagas, M.O.; Peres, M.T.L.P.; Hess, S.C.; da Silva, C.B.; Ré-Poppi, N.; Gebara, S.S.; Corsino, J.; Morel, A.F.; Stuker, C.Z.; et al. Chemical Composition and Biological Activities of Leaves Essential Oil from Schinus molle (Anacardiaceae). J. Essent. Oil Bear. Plants 2011, 14, 590–599. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Sanna, C.; Ballero, M.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Bianco, A. Chemical composition and biological activity of the essential oil from Helichrysum microphyllum Cambess. ssp. tyrrhenicum Bacch., Brullo e Giusso growing in La Maddalena Archipelago, Sardinia. J. Oleo Sci. 2015, 64, 19–26. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Dugo, G.; Mondello, L.; Cotroneo, A.; Bonaccorsi, I.; Lamonica, G. Enantiomeric Distribution of Volatile Components of Citrus Oils by MDGC. Perfum. Flavorist 2001, 26, 20–26. [Google Scholar]
- Brokl, M.; Flores, G.; Blanch, G.P.; Del Castillo, M.L.R. Changes in the enantiomeric distribution of selected volatile constituents of Mentha pulegium L. powders caused by hot water treatment. J. Agric. Food Chem. 2006, 54, 8836–8841. [Google Scholar] [CrossRef] [PubMed]
- Casabianca, H.; Graff, J.B.; Faugier, V.; Fleig, F.; Grenier, C. Enantiomeric Distribution Studies of Linalool and Linalyl Acetate. A Powerful Tool for Authenticity Control of Essential Oils. J. High Resolut. Chromatogr. 1998, 21, 107–112. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of Gynoxys rugulosa Muschl. (Asteraceae) Growing in Southern Ecuador: Chemical and Enantioselective Analyses. Plants 2023, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 3 July 2025).
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Gilardoni, G.; Matute, Y.; Ramírez, J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants 2020, 9, 791. [Google Scholar] [CrossRef]
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]

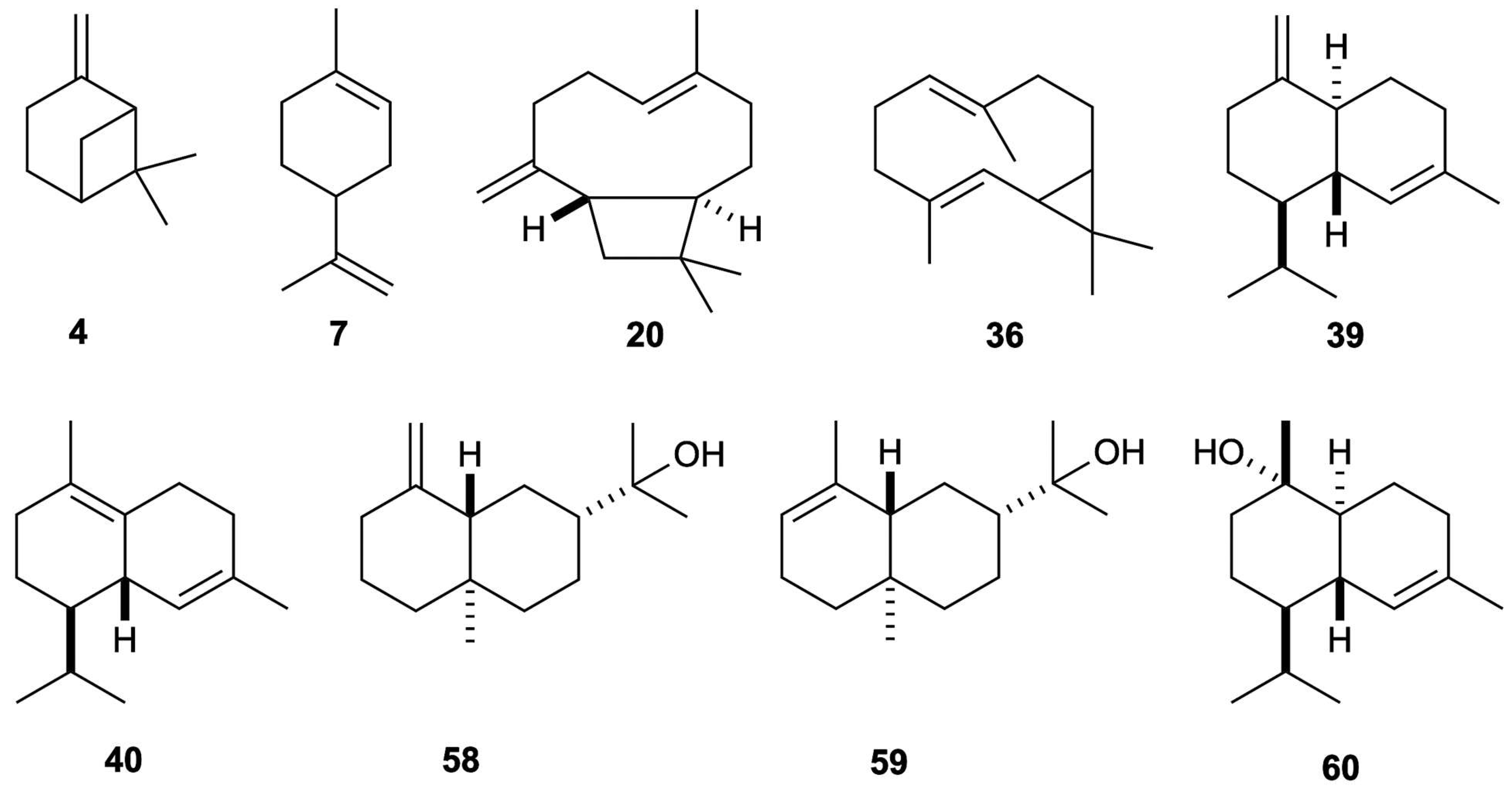

| N. | Compounds | 5% Phenyl Methyl Polysiloxane | Polyethylene Glycol | Average (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRI | % | σ | Lit. | LRI | % | σ | Lit. | |||||
| Calc. | Ref. | Calc. | Ref. | |||||||||
| 1 | α-thujene | 927 | 924 | 0.3 | 0.09 | [15] | 1018 | 1018 | 0.4 | 0.12 | [16] | 0.4 |
| 2 | α-pinene | 933 | 932 | 1.9 | 0.47 | [15] | 1014 | 1014 | 1.9 | 0.40 | [17] | 1.9 |
| 3 | sabinene | 972 | 969 | 0.4 | 0.09 | [15] | 1112 | 1112 | 0.4 | 0.08 | [18] | 0.4 |
| 4 | β-pinene | 974 | 974 | 4.9 | 0.86 | [15] | 1101 | 1100 | 4.9 | 0.62 | [19] | 4.9 |
| 5 | myrcene | 992 | 988 | 1.3 | 0.25 | [15] | 1154 | 1154 | 1.3 | 0.19 | [20] | 1.3 |
| 6 | α-terpinene | 1016 | 1014 | 0.1 | 0.01 | [15] | 1164 | 1164 | 0.1 | 0.02 | [21] | 0.1 |
| 7 | limonene | 1030 | 1024 | 39.0 | 6.99 | [15] | 1190 | 1190 | 39.0 | 4.96 | [22] | 39.0 |
| 8 | (E)-β-ocimene | 1049 | 1044 | 0.5 | 0.10 | [15] | 1245 | 1245 | 0.5 | 0.08 | [23] | 0.5 |
| 9 | γ-terpinene | 1058 | 1054 | 0.1 | 0.02 | [15] | 1233 | 1233 | 0.1 | 0.01 | [24] | 0.1 |
| 10 | linalool | 1099 | 1095 | 0.3 | 0.02 | [15] | 1551 | 1551 | 0.3 | 0.03 | [25] | 0.3 |
| 11 | terpinen-4-ol | 1174 | 1174 | 0.2 | 0.04 | [15] | 1592 | 1592 | 0.1 | 0.04 | [26] | 0.2 |
| 12 | α-terpineol | 1187 | 1186 | 0.1 | 0.02 | [15] | - | - | - | - | - | 0.1 |
| 13 | p-vinyl-guaiacol | 1309 | 1309 | 0.1 | 0.01 | [15] | - | - | - | - | - | 0.1 |
| 14 | α-cubebene | 1350 | 1348 | 0.6 | 0.12 | [15] | 1433 | 1435 | 0.6 | 0.14 | [27] | 0.6 |
| 15 | α-copaene | 1376 | 1374 | 1.3 | 0.16 | [15] | 1460 | 1460 | 1.3 | 0.21 | [28] | 1.3 |
| 16 | β-cubebene | 1390 | 1387 | 0.4 | 0.01 | [15] | 1509 | 1509 | 0.4 | 0.04 | [29] | 0.4 |
| 17 | sibirene | 1395 | 1400 | 0.1 | 0.02 | [15] | 1508 | - | 0.5 | 0.17 | § | 0.3 |
| 18 | methyl eugenol | 1402 | 1403 | 0.1 | 0.05 | [15] | 2016 | 2016 | trace | - | [30] | 0.1 |
| 19 | α-gurjunene | 1409 | 1409 | 0.2 | 0.02 | [15] | 1494 | 1501 | 0.2 | 0.03 | [31] | 0.2 |
| 20 | (E)-β-caryophyllene | 1419 | 1417 | 1.9 | 0.16 | [15] | 1562 | 1562 | 2.0 | 0.29 | [32] | 2.0 |
| 21 | β-copaene | 1428 | 1430 | 0.3 | 0.03 | [15] | 1554 | 1552 | 0.3 | 0.04 | [33] | 0.3 |
| 22 | β-gurjunene | 1434 | 1431 | 0.1 | 0.01 | [15] | - | - | - | - | - | 0.1 |
| 23 | aromadendrene | 1438 | 1439 | 0.3 | 0.08 | [15] | 1607 | 1606 | 0.7 | 0.10 | [34] | 0.5 |
| 24 | 6,9-guaiadiene | 1443 | 1442 | 0.4 | 0.03 | [15] | 1651 | - | - | - | § | 0.4 |
| 25 | cis-muurola-3,5-diene | 1446 | 1448 | [15] | - | - | - | - | - | |||
| 26 | trans-muurola-3,5-diene | 1450 | 1451 | 0.5 | 0.05 | [15] | 1595 | - | 0.3 | 0.10 | § | 0.4 |
| 27 | α-humulene | 1453 | 1452 | 0.9 | 0.05 | [15] | 1633 | 1637 | 1.6 | 0.19 | [35] | 0.9 |
| 28 | 9-epi-(E)-caryophylene | 1460 | 1464 | 1.6 | 0.07 | [15] | 1607 | 1604 | 0.3 | 0.10 | [36] | 0.3 |
| 29 | cis-muurola-4(14),5-diene | 1462 | 1465 | [15] | 1635 | - | o.w. peak 27 | - | § | 1.3 | ||
| 30 | trans-cadina-1(6),4-diene | 1473 | 1475 | 0.5 | 0.05 | [15] | 1625 | - | 0.3 | 0.10 | § | 0.4 |
| 31 | γ-muurolene | 1477 | 1478 | 1.1 | 0.11 | [15] | 1655 | 1655 | 1.1 | 0.19 | [28] | 1.1 |
| 32 | germacrene D | 1481 | 1480 | 1.5 | 0.08 | [15] | 1674 | 1676 | 2.2 | 0.14 | [35] | 1.9 |
| 33 | β-selinene | 1485 | 1489 | 0.1 | 0.02 | [15] | 1679 | 1678 | 0.2 | 0.04 | [37] | 0.2 |
| 34 | β-cis-guaiene | 1488 | 1492 | [15] | 1659 | 1651 | 0.2 | 0.06 | [38] | 0.2 | ||
| 35 | trans-muurola-4(14),5-diene | 1491 | 1493 | 1.1 | 0.10 | [15] | 1687 | - | 0.5 | 0.09 | § | 0.8 |
| 36 | bicyclogermacrene | 1496 | 1500 | 3.0 | 0.13 | [15] | 1698 | 1699 | 2.3 | 0.12 | [35] | 2.7 |
| 37 | α-muurolene | 1500 | 1500 | 1.7 | 0.29 | [15] | 1693 | 1695 | 1.7 | 0.21 | [39] | 1.7 |
| 38 | germacrene A | 1504 | 1508 | 0.1 | 0.00 | [15] | - | - | - | - | - | 0.1 |
| 39 | γ-cadinene | 1514 | 1513 | 4.0 | 0.10 | [15] | 1725 | 1722 | 11.0 | 1.11 | [40] | 4.0 |
| 40 | δ-cadinene | 1525 | 1522 | 7.3 | 0.41 | [15] | 1728 | 1729 | [27] | 7.3 | ||
| 41 | trans-cadina-1,4-diene | 1532 | 1533 | 0.4 | 0.01 | [15] | 1747 | - | 0.4 | 0.04 | § | 0.4 |
| 42 | α-cadinene | 1538 | 1537 | 0.6 | 0.04 | [15] | 1758 | 1751 | 0.6 | 0.06 | [41] | 0.6 |
| 43 | elemol | 1548 | 1548 | 1.5 | 0.69 | [15] | 2075 | 2074 | 1.5 | 0.57 | [42] | 1.5 |
| 44 | palustrol | 1566 | 1567 | 0.1 | 0.06 | [15] | 1899 | 1903 | trace | - | [27] | 0.1 |
| 45 | germacrene D-4-ol | 1575 | 1574 | 1.1 | 0.78 | [15] | 2034 | 2038 | 0.3 | 0.08 | [43] | 0.7 |
| 46 | spathulenol | 1577 | 1577 | [15] | 2111 | 2110 | 0.4 | 0.26 | [44] | 0.4 | ||
| 47 | globulol | 1582 | 1590 | 0.5 | 0.35 | [15] | 2060 | 2061 | 0.1 | 0.04 | [45] | 0.3 |
| 48 | unidentified (MW = 222) | 1590 | 1590 | 0.2 | 0.20 | [15] | - | - | - | - | - | 0.2 |
| 49 | ledol | 1602 | 1602 | 0.5 | 0.29 | [15] | 2006 | 2007 | 0.1 | 0.02 | [46] | 0.3 |
| 50 | β-oplopenone | 1607 | 1607 | 0.2 | 0.22 | [15] | - | - | - | - | - | 0.2 |
| 51 | 1,10-di-epi-cubenol | 1614 | 1618 | 0.3 | 0.22 | [15] | 2038 | 2037 | 0.2 | 0.05 | [47] | 0.3 |
| 52 | 10-epi-γ-eudesmol | 1618 | 1622 | 0.2 | 0.15 | [15] | 2080 | 2084 | 0.2 | 0.12 | [36] | 0.2 |
| 53 | 1-epi-cubenol | 1628 | 1627 | 0.6 | 0.38 | [15] | 2044 | 2047 | 0.3 | 0.25 | [46] | 0.5 |
| 54 | γ-eudesmol | 1631 | 1630 | 2.0 | 1.04 | [15] | 2156 | 2158 | 3.0 | 1.42 | [48] | 2.0 |
| 55 | epi-α-cadinol | 1641 | 1638 | 3.0 | 1.44 | [15] | 2158 | 2158 | [49] | 1.2 | ||
| 56 | epi-α-muurolol | 1642 | 1640 | [15] | 2174 | 2173 | 1.3 | 0.62 | [50] | 1.3 | ||
| 57 | α-muurolol | 1646 | 1644 | 0.4 | 0.24 | [15] | 2190 | 2191 | 0.5 | 0.27 | [51] | 0.5 |
| 58 | β-eudesmol | 1649 | 1649 | 2.0 | 1.30 | [15] | 2217 | 2216 | 2.0 | 1.36 | [52] | 2.0 |
| 59 | α-eudesmol | 1653 | 1652 | 5.0 | 2.80 | [15] | 2207 | 2208 | 3.0 | 1.33 | [48] | 3.0 |
| 60 | α-cadinol | 1654 | 1652 | [15] | 2225 | 2225 | 2.0 | 1.12 | [53] | 2.0 | ||
| 61 | shyobunol | 1689 | 1688 | 0.4 | 0.26 | [15] | - | - | - | - | - | 0.4 |
| 62 | benzyl benzoate | 1769 | 1759 | 0.2 | 0.16 | [15] | - | - | - | - | - | 0.2 |
| 63 | n-docosane | 2200 | 2200 | 0.3 | 0.07 | [15] | - | - | - | - | - | 0.3 |
| monoterpenes | 48.5 | 48.6 | 48.6 * | |||||||||
| oxygenated monoterpenoids | 0.6 | 0.4 | 0.6 * | |||||||||
| sesquiterpenes | 30.0 | 28.7 | 30.4 * | |||||||||
| oxygenated sesquiterpenoids | 18.0 | 14.9 | 17.1 * | |||||||||
| others | 0.7 | - | 0.7 * | |||||||||
| total | 97.8 | 92.6 | 97.4 * | |||||||||
| Chiral Selector | Ion Integration (m/z) | LRI | Enantiomer | Distribution (%) | e.e. (%) |
|---|---|---|---|---|---|
| DET | TIC | 913 | (1S,5S)-(+)-α-thujene * | - | 100.0 |
| DET | TIC | 917 | (1R,5R)-(−)-α-thujene * | 100.0 | |
| DAC | TIC | 914 | (1S,5S)-(−)-α-pinene | 100.0 | 100.0 |
| DAC | TIC | 916 | (1R,5R)-(+)-α-pinene | - | |
| DET | TIC | 949 | (1R,5R)-(+)-β-pinene | 12.0 | 76.0 |
| DET | TIC | 958 | (1S,5S)-(−)-β-pinene | 88.0 | |
| DET | TIC | 977 | (1R,5R)-(+)-sabinene | 12.4 | 75.2 |
| DET | TIC | 991 | (1S,5S)-(−)-sabinene | 87.6 | |
| DET | TIC | 1056 | (S)-(−)-limonene | 5.0 | 90.0 |
| DET | TIC | 1067 | (R)-(+)-limonene | 95.0 | |
| DET | TIC | 1181 | (R)-(−)-linalool | 81.7 | 63.4 |
| DET | TIC | 1194 | (S)-(+)-linalool | 18.3 | |
| DAC | TIC | 1293 | (R)-(−)-terpinen-4-ol | 76.2 | 52.4 |
| DAC | TIC | 1298 | (S)-(+)-terpinen-4-ol | 23.8 | |
| DET | 59 | 1301 | (S)-(−)-α-terpineol | 67.1 | 34.2 |
| DET | 59 | 1313 | (R)-(+)-α-terpineol | 32.9 | |
| DET | TIC | 1322 | (1R,2S,6S,7S,8S)-(−)-α-copaene | 100.0 | 100.0 |
| DET | TIC | 1324 | (1S,2R,6R,7R,8R)-(+)-α-copaene | - | |
| DET | 161 | 1461 | (R)-(+)-germacrene D | 7.2 | 85.6 |
| DET | 161 | 1467 | (S)-(−)-germacrene D | 92.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilardoni, G.; Flores, B.; Cumbicus, N.; Malagón, O. A Bioeconomically Valuable Essential Oil from Baccharis sinuata Kunth in Southern Ecuador: Chemical Composition and Enantiomeric Profile. Plants 2025, 14, 3110. https://doi.org/10.3390/plants14193110
Gilardoni G, Flores B, Cumbicus N, Malagón O. A Bioeconomically Valuable Essential Oil from Baccharis sinuata Kunth in Southern Ecuador: Chemical Composition and Enantiomeric Profile. Plants. 2025; 14(19):3110. https://doi.org/10.3390/plants14193110
Chicago/Turabian StyleGilardoni, Gianluca, Bryan Flores, Nixon Cumbicus, and Omar Malagón. 2025. "A Bioeconomically Valuable Essential Oil from Baccharis sinuata Kunth in Southern Ecuador: Chemical Composition and Enantiomeric Profile" Plants 14, no. 19: 3110. https://doi.org/10.3390/plants14193110
APA StyleGilardoni, G., Flores, B., Cumbicus, N., & Malagón, O. (2025). A Bioeconomically Valuable Essential Oil from Baccharis sinuata Kunth in Southern Ecuador: Chemical Composition and Enantiomeric Profile. Plants, 14(19), 3110. https://doi.org/10.3390/plants14193110








