Abstract
Lepechinia mutica, an endemic species of the Ecuadorian Andes, was studied to identify the seasonal variation in volatile organic compounds emitted from leaves and flowers in winter and summer using solid-phase microextraction–gas chromatography–mass spectrometry (SPME-GC-MS). A total of 101 and 100 volatile compounds were identified in flowers and leaves, respectively. The main compounds in flowers were β-phellandrene (7.81–17.74%), dictamnol (3.57–31.89%) and 9-epi-(E)-caryophyllene (3.93–14.37%), while in the leaves, they were dictamnol (9.85–34.64%), (Z)-β-ocimene (1.24–29.24%) and δ-3-carene (1.14–11.51%). This is the first report of enantiomeric separation in L. mutica using a capillary column with 2,3-diethyldecyl-6-tert-butyl-dimethylsilyl-β-cyclodextrin, revealing three enantiomerically pure compounds as (S)-(-)-β-pinene, (1S,3R)-(+)-δ-3-carene and (S)-(+)-linalool, while (+) (-) α-pinene, (+) (-) δ-cadinene and (+) (-) α-muurolene were found as racemic mixtures. Principal component analysis confirmed distinct chemical profiles between plant parts and seasons. This result has important implications for the future highlighting its potential as a source of seasonally variables components with applications in fragrance and phytotherapy.
1. Introduction
Climate is a primary driver of plant ecosystem dynamics, directly regulating key physiological processes, including photosynthesis, transpiration, nutrient cycling and the biosynthesis of primary and secondary metabolites [1]. While not essential for basic growth or survival, secondary metabolites contribute to the fundamental metabolic functions necessary for plant survival; they play an essential role in enhancing plant performance under biotic and abiotic stress conditions, such as pathogen attacks or nutrient limitation [2,3]. These compounds have been associated with protective and defensive functions, including antioxidant activity, as well as antibiotic, insecticidal and herbicidal properties against external aggressions [4]. Essential oils are complex mixtures of volatile secondary metabolites; they are derived from plants and are highly responsive to both intrinsic (e.g., phenology) and extrinsic (e.g., temperature, UV, drought) factors, leading to dynamic seasonal shifts in their chemical composition [5,6]. These variations directly affect the biological activity of plant extracts, highlighting the importance of understanding how environmental and physiological cues together influence essential oil profiles for successful phytochemical discovery and pharmacological application [7].
The Lamiaceae family, commonly known as the mint or deadnettle family, comprises approximately 236 genera and over 7000 species of herbs, shrubs and trees [8]. It is widely recognized for its rich diversity of essential oils and volatile compounds which confer distinctive aromatic and biological properties [9]. Within this family, Lepechinia is distinguished by unique fragrance and potential as a source of bioactive compounds exhibiting antimicrobial, antioxidant, anti-inflammatory and neuroprotective activities [10]. Lepechinia mutica is an endemic plant species native to Ecuador, commonly found in cloud forests, páramos, tropical forests and grasslands at elevations between 2200 and 3400 m a.s.l. [11,12]. Traditionally, it is used in folk medicine to treat various ailments, headache and nervous disorders and as antiseptic remedy [13,14,15]. However, the limitation of scientific research on this species makes it a promising candidate for further investigation into its chemical composition, aromatic profile and seasonal variability. The analysis of its volatile organic compounds using solid-phase microextraction coupled with gas chromatography–mass spectrometry (SPME-GC-MS) could provide significant contributions to the discovery of novel chemical entities and the development of new pharmaceuticals [16].
We hypothesized that seasonal changes in abiotic stresses (e.g., temperature, ultraviolet radiation and drought) would lead to distinct volatile profiles in leaves and flowers. Chemical characterization of volatile organic compounds in the leaves and flowers of L. mutica is essential to understand its aromatic profile and seasonal variation. Volatile compounds are small, lipophilic molecules that play critical roles in plant environment interactions, including pollinator attraction, defense against pathogens and herbivores and adaptation to changing environmental conditions [17]. While previous studies on Lepechinia spp. have identified sesquiterpenes, monoterpenes and diterpenes as the main constituents [18], our SPME-GC-MS approach, unlike the hydrodistillation method employed by Guzmán et al. (2022), which identified shyobunol, δ-3-carene and globulol [19], revealed dictamnol to be the dominant sesquiterpene. This highlights the critical role of extraction methodology in shaping observed chemical profiles.
This study employs HS-SPME-GC-MS to characterize seasonal variation in the volatile organic compounds from leaves and flowers of L. mutica. Extractions were performed during winter and summer with chemical profiles analyzed using a non-polar column. Additionally, preliminary enantioselective analyzes of the leaves and flowers are reported. It is expected that valuable data for the conservation and sustainable utilization of this endemic species will be provided by this work as well as a greater understanding of its chemical composition.
2. Results
2.1. Chemical Composition
The chemical composition variability of volatile compounds in flowers of L. mutica is presented in Table 1. A total of 101 compounds were identified in the winter and summer flower samples. The major constituents with the ranges included β-phellandrene (7.81–17.74%), dictamnol (3.57–31.89%), 9-epi-(E)-caryophyllene (3.93–14.37%) and bicyclogermacrene (2.4–9.74%), among others. A predominance of sesquiterpenes hydrocarbons was observed, accounting a range of 41.15–54.9%, followed by hydrocarbon monoterpenes 17.69–37% while the oxygenated sesquiterpenes were present at 3.9–8.64% (Figures S1–S6).

Table 1.
Volatile compounds identified in different stages of L. mutica flowers development using SPME-GC-MS.
Chemical composition of L. mutica leaves revealed the presence of 100 compounds. The major constituents were dictamnol (26.45% ± 14.37%), (Z)-β-ocimene (19.05% ± 8.93%), α-cuprenene (7.60% ± 2.81%), camphene (4.81% ± 0.69%) and linalool isovalerate (4%). (79% ± 1.19%), α-zingiberene (4.30% ± 3.73%), α-pinene (3.45% ± 0.43%), trans-β-guaiene (3.11% ± 0.48%), p-mentha-1(7),8-diene (2.21% ± 0.94%) and myrcene (2.07% ± 1.77%). The main compound groups identified were hydrocarbon monoterpenes (47.41%, 27.85% and 25.30%), hydrocarbon sesquiterpenes (42.69%, 61.26% and 63.12%) and oxygenated sesquiterpenes (13.37%, 8.55% and 13.17%) and oxygenated monoterpenes were present in lower proportions with 1.77%, 1.08% and 1.29%, respectively (Table 2) (Figures S7–S12).

Table 2.
Volatile compounds identified in different stages of L. mutica leaves development using SPME-GC-MS.
These differences in chemical profiles between seasons could be explained by variations in biosynthetic stages and the physiological needs of the plant. Sesquiterpenes are larger, less volatile molecules compared to monoterpenes and are often associated with protective and defensive functions. This pattern suggests a possible functional adaptation of the plant’s volatile metabolism in response to seasonal environmental conditions (Figure S13).
2.2. PCA Analysis
PCoA showed clear segregation by plant part (leaves vs. flowers) and season indicating that chemical composition is primarily structured by season and plants parts (PC1 47.7% and 23.8% variance). Evidence of the results obtained using the corresponding tool was presented in the statistical analysis. It was possible to group all the compounds and highlight those that were predominant in the different seasons of the year. In winter, the predominant compounds were aromadendrene and (Z)-β-ocimene. By contrast, the main compounds identified in summer were dictammol and junenol. The Principal Componet Analysis (PCoA) ordination showed a clear separation between chemical composition of L. mutica and parts of plants and season (Figure 1).
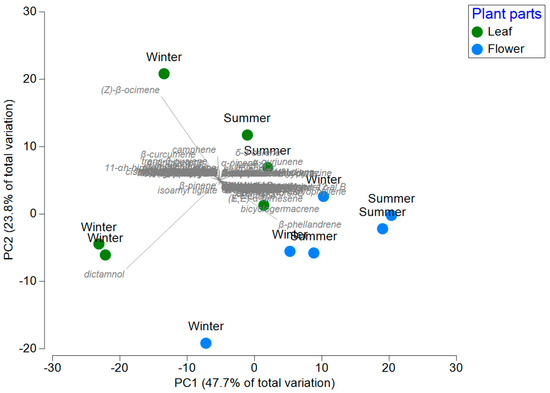
Figure 1.
Principal coordinate analysis showing variation between chemical compounds of L. mutica (Benth.) in different parts of plants and seasons.
PERMANOVA analyses showed that chemical composition of L. mutica was structured according to the parts of plants and season, and a large component of variation was associated with the parts of plants with 22.4% of explained variance in chemical composition of L. mutica. This was followed by the season, accounting for 14.5% of the explained variance (Table 3). Conversely, the interaction between plant parts (leaves and flowers) and season does not have significant effects (p value = 0.074).

Table 3.
Results of two-factor PERMANOVA analysis of chemical composition of L. mutica by parts of plants and season.
Results of pairwise PERMANOVA test between chemical composition of L. mutica according to parts of plants (62.78%, p-value = 0.001) and season (58.73%, p value = 0.002) showed that the highest significant dissimilarity values for chemical composition were associated with parts of plants followed by season.
The SIMPER routine revealed that not all chemical compounds contribute equally to establishing the differences in the parts of plants. We observed that the largest contributions are due to differences in chemical compounds of β-phellandrene (Z)-β-ocimene and bicyclogermacrene (Table 4).

Table 4.
Results of the SIMPER analyses.
2.3. Enantioselective Analysis
Enantioselective analysis using a chiral capillary column (2,3-diethyl-6-tert-butyl dimethylsilyl-β-cyclodextrin as a chiral selector) resolved nine enantiomers in L. mutica flowers and leaves. Three compound as (S)-(-)-β-pinene, (1S,3R)-(+)-δ-3-carene and (S)-(+)-linalool were identified as enantiomerically pure (e.e. 100%). The remaining six as (1R)-(+)-α-pinene, (1S)-(-)-α-pinene (1S,4S,7R)-(+)-δ-cadinene, (1R,4R,7S)-(–)-δ-cadinene, (6R,8S)- (+)-α-muurolene and (6S,8R)-(–)-α-muurolene occurred as racemic mixtures (Table 5 and Table 6).

Table 5.
Enantioselective analysis in flowers of L. mutica using β-cyclodextrin column.

Table 6.
Enantioselective analysis in leaves of L. mutica using β-cyclodextrin column.
3. Discussion
Volatile compounds emitted by flowers and leaves of the endemic species L. mutica using SPME-GC-MS revealed its complex chemical profile, highlighting its potential as a source of bioactive compounds. The results showed significant seasonal variations in chemical composition, with these variations depending on the time at which the samples were collected. These variations are likely influenced by environmental factors such as temperature, humidity, solar radiation, drought stress, and ultraviolet-B radiation affect secondary metabolic pathways in plants [22,23,24].
In flowers, the major compounds identified during winter were (Z)-β-ocimene, aromadendrene and linalool isovalerate, while the summer samples showed higher levels of 9-epi-(E)-caryophyllene and α-gurjunene. These results partially align with those reported by Guzmán et al. [19], who analyzed the essential oil of L. mutica obtained via hydrodistillation and identified shyobunol (10.80%), δ-3-carene (8.69%), δ-cadinene (6.96%), globulol (5.91%) and (E)-caryophyllene (4.55%). The marked compositional differences likely stem from methodological effects, in the hydrodistillation high temperatures can induce thermal degradation or artifact formation [25], whereas HS-SPME-GC-MS provides a non-destructive, in situ snapshot of the plant’s natural volatile profile, better reflecting true biological emissions.
In leaves, the most abundant compounds in winter were (Z)-β-ocimene (18.04 ± 2.43%) and 9-epi-(E)-caryophyllene (14.23 ± 0.12%), while in summer were dictamnol (26.45 ± 14.37%) and (Z)-β-ocimene (19.05 ± 8.93%). Aromadendrene was the most prevalent compound in both seasons, although its concentration varied significantly between them. For instance, Ramírez et al. [25] identified 78 compounds in the essential oil of L. mutica, with hydrocarbon sesquiterpenes and oxygenated monoterpenes being the most abundant groups. However, the results demonstrate considerable variation depending on the collection site and season, suggesting a strong influence of environmental [26,27,28] and phenological factors on secondary metabolite production [29].
Our results indicated that the season is a determining factor in the chemical composition. Similarly, previous studies have pointed out that plants are intimately connected to their environment and are therefore strongly influenced by climatic variables, including seasonal changes [30]. It is consistent with previous studies that have highlighted the influence of environmental and phenological factors on the biosynthesis and accumulation of secondary metabolites in medicinal plants. It is well established that various genetic, ontogenic, morphogenetic, and environmental factors can affect the production of natural products [31,32]. As herbs develop, they progress through predictable stages: seedling, vegetative growth, flowering, fruiting, and senescence, each potentially associated with distinct metabolic profiles [33]. Natural products can be gradually synthesized or upregulated in response to environmental stressors. In this context, it has been demonstrated that temperature, radiation, water availability, and other abiotic factors significantly impact secondary metabolism [34]. Importantly, these variables do not act in isolation but change synergistically throughout the growing season. Therefore, seasonal variation encompasses a set of ecologically relevant and interrelated parameters that collectively shape the chemical phenotype of the plant [35]. Drought and UV radiation, which are key abiotic stressors in the Andean cloud forests where L. mutica thrives, can trigger shifts in secondary metabolism by altering phenology, resource allocation and the synthesis of defensive compounds. These physiological responses can directly affect floral scent profiles, potentially reducing pollinator attraction while increasing herbivore deterrence. These trade-offs highlight how environmental pressures act as selective drivers of chemical variability, influencing not only the quantity, but also the enantiomeric composition, of volatiles that are essential for ecological function. This work contributes novel data on the seasonal variability of volatile compounds in L. mutica, an aspect that has been scarcely explored to date.
Another relevant finding of this study was the identification and separation of enantiomers, a total of nine enantiomers were successfully separated using chiral gas chromatography. Notably, compounds such as (S)-(-)-β-pinene, (1S,3R)-(+)-δ-3-carene and (S)-(+)-linalool were found to be enantiomerically pure. By contrast, racemic mixtures were observed for (+) (-)-α-pinene, (+) (-)-δ-cadinene and (+) (-)-α-muurolene. This is particularly important as enantiomers may exhibit distinct or even opposing, biological activities depending on their spatial configuration [36]. For example, the (+)-α-pinene enantiomer has been shown to have more potent anti-inflammatory effects than its (−) enantiomer [37], highlighting the importance of considering chirality in future pharmacological research.
Furthermore, several of the compounds identified in this study have previously been associated with promising therapeutic properties. In our study, one of the major compounds was Dictamnol; it has been documented to exhibit antimicrobial, anti-inflammatory and cytotoxic activities [38]. Concurrently, (Z)-β-ocimene, a major monoterpene found in the leaves and flowers of the plant, is a well-known generalist pollinator attractant and plays a key role in the plant’s defense against herbivory [39]. Furthermore, molecular docking and simulation studies reveal that (Z)-β-ocimene forms stable interactions with α-glucosidase, suggesting an inhibitory effect that could be relevant to metabolic disorders such as diabetes [40]. (Z)-β-ocimene is more prevalent and emitted in greater abundance in floral aromas than its isomer (E)-β-ocimene [41].
The seasonal variability observed in the chemical composition suggests that we should time biomass collection according to the desired target. This strategic approach could benefit not only the pharmaceutical and cosmetic industries but also contribute to the conservation of the species by promoting sustainable cultivation practices.
This study provides detailed insights into the chemical profile of L. mutica. However, key limitations remain. It would be valuable to investigate the influence of factors such as altitude, soil type, local climatic and land practices (e.g., wild harvesting versus cultivation) on the production of secondary metabolites in this species. There is also an absence of replicated sites across altitudinal gradients and the lack of metabolomic quantification. Another promising area for future research would be to assess the biological activity of individual enantiomers. Given the potential applications of these compounds in health and wellness, it is important to understand how their chiral configuration affects biological activity as this could lead to the development of more selective and effective drugs. Future work will involve multi-site sampling, controlled environmental trials and the targeted quantification of key enantiomers.
4. Materials and Methods
4.1. Plant Material
The leaves and flowers of L. mutica were collected in 2024 during two distinct seasons: winter (February, March and April) and summer (June, July and August). For each sampling period, samples were taken from three independent individuals, with no repeated sampling from the same plants. Each sample (n = 3 per month × part) was collected from a different plant in Loja province, South of Ecuador (coordinates: 3°59′59″ S; 79°12′45″ W). Sampling occurred during mean temperature ranges: winter (12–18 °C, RH 75–85%) and summer (16–22 °C, RH 65–75%) and monthly rainfall <50 mm (winter) vs. >200 mm (summer). The species was authenticated by Dr. Jorge Ramírez from the Universidad Técnica Particular de Loja (UTPL). Collection was carried out under official permission from the Ministry of Environment, Water and Ecological Transition (Authorization No. MAATE-ARSFC-2022-2839). A voucher specimen No. 14778 was deposited at the Herbarium of the Universidad Técnica Particular de Loja (HUTPL) for future reference.
4.2. Headspace Solid-Phase Microextraction
Samples were collected in the early morning hours and analyzed immediately to minimize chemical degradation. Young leaves and flowers were selected for analysis. Following collection, 5 ± 0.1 g of each sample was weighed using an analytical balance (OHAUS Adventurer™ Pro, model 30061977, OHAUS Corporation, Parsippany, NJ, USA). The weighed material was then placed into a transparent 100 mL glass vial (Boeco). For volatile compound extraction, headspace solid-phase microextraction (HS-SPME) was performed using a Carboxen™/DVB/PDMS fiber (50/30 µm, StableFlex 24 Ga, Manual, 3/pk, gray; SUPELCO Inc., Bellefonte, PA, USA). Once inserted into the vial, the fiber remained in the headspace for a defined period to allow adsorption of volatile compounds before thermal desorption and analysis by GC–MS [42]. The fiber was equilibrated at 40 °C for 30 min (previously determined by an adsorption curve) with RSD < 5% (n = 3) for repeatability and r2 > 0.99 for linearity (range 0.1–10 µg/mL).
4.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
For qualitative analysis, volatile compounds were identified using a Thermo Scientific TRACE 1310 gas chromatograph (Waltham, MA, USA) coupled to a Thermo Scientific ISQ 7000 mass spectrometer (Bartlesville, OK, USA). Ultrapure helium (Indura GC grade, Guayaquil, Ecuador) was used as the carrier gas at a constant flow rate of 1 mL/min. The injection mode was set to split (split ratio 10:1), with an injection temperature of 250 °C. A non-polar DB-5ms column (5% phenyl, 95% dimethylpolysiloxane; 30 m × 0.25 mm i.d., film thickness 0.25 µm) was used under the following temperature program: initial temperature of 40 °C held for 5 min, followed by a first ramp of 3 °C/min to 150 °C, then a second ramp of 5 °C/min to 180 °C, and finally a third ramp of 7 °C/min to reach 230 °C, which was held for 5 min. Ion source and quadrupole temperatures were set at 230 °C and 150 °C, respectively. Full-scan mass spectra were acquired in the range of 30–350 m/z at a scan rate of 0.2 scans/s over a total run time of 66 min.
After sample extraction via HS-SPME, the fiber was thermally desorbed directly into the GC injector at 250 °C for 5 min to release the adsorbed volatile compounds prior to chromatographic separation.
4.4. Enantiomeric Analysis
Enantiomeric profiling was carried out using a chiral capillary column MEGA-DEX-DAC Beta (Mega, MI, Italy), composed of 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin as the chiral selector. The column had an internal diameter of 0.25 mm, a phase thickness of 0.25 µm, and a length of 25 m. It was installed on the same GC–MS system used for qualitative analysis. The sample amount, injector temperature, transfer line temperature, and MS parameters remained consistent with those used in the qualitative analysis. The split ratio was adjusted to 20:1. The GC method included an initial oven temperature of 60 °C held for 2 min, followed by a ramp of 2 °C/min until reaching 220 °C, which was maintained for an additional 2 min. To enable accurate compound identification and enantiomeric assignment, a homologous series of n-alkanes (C9–C25) was injected under identical conditions to calculate linear retention indices [43].
4.5. Identification of Volatile Compounds
The volatile compounds isolated from L. mutica were identified using a combination of Linear Retention Index (LRI) and mass spectra matching. LRI were calculated based on a co-injected series of n-alkanes (C9–C24; ChemService, West Chester, PA, USA) following the methodology described by Calva et al. [43]. Mass spectra were compared with NIST [44] and Adams [20] libraries and further confirmed by comparison with LRI values reported from the literature.
4.6. Data Analysis
Principal Coordinate Analysis (PCoA) based on the Bray–Curtis distance index was employed to visualize the chemical compounds of L. mutica (Benth.) in different parts of plants and seasons. In order to show the relationship between chemical compounds of L. mutica (response group) and parts of plants (explanatory group) in different seasons.
To test whether the two parts of plants (leaves and flowers) of L. mutica (Benth.) had significantly different compositions of chemical compound and to detect the effects of parts of plants and season variability, we performed a two-factor permutational multivariate analysis of variance (PERMANOVA) on the chemical composition data [45]. We used the Bray–Curtis distance measure and 999 random permutations. To assess compounds similarity among the different parts of plants and season, we performed additional pairwise PERMANOVA tests [46].
4.7. Statistical Analysis
Statistical analysis was performed to evaluate the consistency of the SPME extractions, with each sample analyzed in triplicate using independent fibers. These replicates were subjected to various statistical procedures as described by Hammer [47].
All statistical analyses were carried out using the software PAST version 5.0 (Paleontological Statistics), which provided visual and numerical outputs for data exploration and pattern recognition [42].
5. Conclusions
Chemical characterization of the L. mutica volatile compounds using HS-SPME/GC-MS technique revelated a complex and seasonally dynamic profile with significant seasonal variations during winter and summer. These findings offer valuable insights for optimizing the use of L. mutica in the pharmaceutical and food industries based on target compounds. PCA confirmed strong separation by plant part and season, underscoring the influence of environmental and phenological factors on secondary metabolism. The identification of enantiomerically pure (S)-(−)-α-pinene, (1S,3R)-(+)-δ-3-carene, and (S)-(+) linalool alongside racemic mixtures of other terpenes, highlights the critical role of chirality in its bioactivity potential. This work emphasizes the importance of considering phenological and climatic factors in phytochemical research. Future work will evaluate the biological activity of isolated enantiomers and assess the impact of altitude and soil on chemotype stability, advancing sustainable conservation and targeted utilization of this endemic Andean species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14193103/s1.
Author Contributions
Conceptualization, J.C. and Á.B.; methodology, D.S., J.R. and Á.B.; investigation, J.C., J.R., C.A. and Á.B.; writing—original draft preparation, J.C. and Á.B.; writing—review and editing, J.R.; supervision, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Universidad Técnica Particular de Loja, grant POA-VIN-056.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the Universidad Técnica Particular de Loja, Ecuador.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumari, A.; Lakshmi, G.A.; Krishna, G.K.; Patni, B.; Prakash, S.; Bhattacharyya, M.; Singh, S.K.; Verma, K.K. Climate Change and Its Impact on Crops: A Comprehensive Investigation for Sustainable Agriculture. Agronomy 2022, 12, 3008. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant Phenolics—Secondary Metabolites with Diverse Functions. Recent Adv. Polyphenol. Res. 2009, 1, 1–35. [Google Scholar] [CrossRef]
- Khan, A.; Kanwal, F.; Ullah, S.; Fahad, M.; Tariq, L.; Altaf, M.T.; Riaz, A.; Zhang, G. Plant Secondary Metabolites—Central Regulators Against Abiotic and Biotic Stresses. Metabolites 2025, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of Seasonal Variation on Secondary Metabolites of Medicinal Plants. Int. J. Pharm. Sci. Res. 2015, 6, 3654–3660. [Google Scholar] [CrossRef]
- Dos Santos, S.M.; Cardoso, C.A.L.; de Oliveira Junior, P.C.; da Silva, M.E.; Pereira, Z.V.; Silva, R.M.M.F.; Formagio, A.S.N. Seasonal and Geographical Variation in the Chemical Composition of Essential Oil from Allophylus edulis Leaves. S. Afr. J. Bot. 2023, 154, 41–45. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Chandra, I. In Vitro Propagation, Phytochemistry and Pharmacology of Basilicum polystachyon (L.) Moench (Lamiaceae): A Short Review. S. Afr. J. Bot. 2023, 155, 178–186. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Socaciu, M.-I.; Socaci, S.A.; Mureșan, V.; Fogarasi, M.; Rotar, A.M. Chemometric Comparison and Classification of Some Essential Oils Extracted from Plants Belonging to Apiaceae and Lamiaceae Families Based on Their Chemical Composition and Biological Activities. Molecules 2018, 23, 2261. [Google Scholar] [CrossRef]
- Ramírez, J.; Gilardoni, G.; Radice, M.; Morocho, V. Phytochemistry, Bioactivity, and Ethnopharmacology of the Genus Lepechinia Willd. (Lamiaceae): A Review. Plants 2024, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Missouri Botanical Garden. Available online: http://www.tropicos.org/Name/17602035?tab=acceptednames (accessed on 7 November 2024).
- Lozano, P. Plant Biodiversity of Ecuador: A Neotropical Megadiverse Country. In Global Biodiversity: Selected Countries in the Americas and Australia; Apple Academic Press: Waretown, NJ, USA, 2018; Volume 4, pp. 185–211. [Google Scholar]
- Naranjo, P.; Escaleras, R. La Medicina Tradicional en el Ecuador: Memorias de las Primeras Jornadas Ecuatorianas de Etnomedicina Andina, 1st ed.; Universidad Andina Simón Bolívar–Corporación Editora Nacional: Quito, Ecuador, 1995. [Google Scholar]
- Drew, B.T.; Sytsma, K.J. The South American Radiation of Lepechinia (Lamiaceae): Phylogenetics, Divergence Times and Evolution of Dioecy. Bot. J. Linn. Soc. 2013, 171, 171–190. [Google Scholar] [CrossRef]
- Esteves, P.F.; Kuster, R.M.; Barbi, N.S.; Menezes, F.S. Chemical Composition and Cytotoxic Activity of Lepechinia speciosa (St. Hil.) Epling. Lat. Am. J. Pharm. 2010, 29, 38–44. [Google Scholar]
- Filipiak, W.; Bojko, B. SPME in Clinical, Pharmaceutical, and Biotechnological Research—How Far Are We from Daily Practice? TrAC Trends Anal. Chem. 2019, 115, 203–213. [Google Scholar] [CrossRef]
- Stashenko, E.; Martínez, J. Ecological Role of Volatile Compounds in Plants. In Bioactive Volatile Compounds from Plants; Stashenko, E., Ed.; Universidad de los Andes: Bogotá, Colombia, 2010; pp. 1–15. [Google Scholar]
- Ramírez, J.; Gilardoni, G.; Ramón, E.; Tosi, S.; Picco, A.M.; Bicchi, C.; Vidari, G. Phytochemical study of the Ecuadorian species Lepechinia mutica (Benth.) Epling and high antifungal activity of carnosol against Pyricularia oryzae. Pharmaceuticals 2018, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, L.; Malla, J.L.; Ramírez, J.; Gilardoni, G.; Calva, J.; Hidalgo, D.; Valarezo, E.; Rey-Valeirón, C. Acaricidal Efficacy of Plants from Ecuador, Ambrosia peruviana (Asteraceae) and Lepechinia mutica (Lamiaceae) against Larvae and Engorged Adult Females of the Common Cattle Tick, Rhipicephalus microplus. Vet. Sci. 2022, 9, 23. [Google Scholar] [CrossRef]
- Adams, D.R.P. Identification of Essential Oil Components by Gas Chromatography; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Ramírez, J.; Gilardoni, G.; Jácome, M.; Montesinos, J.; Rodolfi, M.; Guglielminetti, M.L.; Vidari, G. Chemical Composition, Enantiomeric Analysis, AEDA Sensory Evaluation and Antifungal Activity of the Essential Oil from the Ecuadorian Plant Lepechinia mutica Benth. (Lamiaceae). Chem. Biodiver. 2017, 14, e1700292. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Marčac, N.; Balbino, S.; Tonković, P.; Medved, A.M.; Cegledi, E.; Dragović, S.; Dragović-Uzelac, V.; Repajić, M. Hydrodistillation and Steam Distillation of Fennel Seeds Essential Oil: Parameter Optimization and Application of Cryomilling Pretreatment. Processes 2023, 11, 2354. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum basilicum) Essential Oils Depend on Seasonal Variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Bejenaru, L.E.; Segneanu, A.-E.; Bejenaru, C.; Biţă, A.; Tuţulescu, F.; Radu, A.; Ciocîlteu, M.V.; Mogoşanu, G.D. Seasonal Variations in Chemical Composition and Antibacterial and Antioxidant Activities of Rosmarinus officinalis L. Essential Oil from Southwestern Romania. Appl. Sci. 2025, 15, 681. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Xie, C.; Li, M.; Jim, C.Y.; Liu, D. Environmental Factors Driving the Spatial Distribution Pattern of Venerable Trees in Sichuan Province, China. Plants 2022, 11, 3581. [Google Scholar] [CrossRef]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing Herbivory as You Grow Up: The Ontogeny of Resistance in Plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef]
- Haffner, V.; Enjalric, F.; Lardet, L.; Carron, M.P. Maturation of Woody Plants: A Review of Metabolic and Genomic Aspects. Ann. For. Sci. 1991, 48, 615–630. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C. Seasonal Variation of Natural Products in European Trees. Phytochem. Rev. 2018, 17, 923–935. [Google Scholar] [CrossRef]
- Batista, A.; dos Santos, F.M.; Batista, J.M.; Cass, Q.B. Enantiomeric Mixtures in Natural Product Chemistry: Separation and Absolute Configuration Assignment. Molecules 2018, 23, 492. [Google Scholar] [CrossRef]
- Silva, A.C.R.d.; Lopes, P.M.; Azevedo, M.M.B.d.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Coca-Ruíz, V.; Suárez, I.; Aleu, J.; Collado, I.G. Structures, Occurrences and Biosynthesis of 11,12,13-Tri-nor-Sesquiterpenes, an Intriguing Class of Bioactive Metabolites. Plants 2022, 11, 769. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a Key Floral and Foliar Volatile Involved in Multiple Interactions between Plants and Other Organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef]
- Tran, L.T.T.; Nguyen, T.K.; Pham, T.V.; Ha, T.P.; Tran, P.T.D.; Tam, V.T.T.; Dat, T.T.H.; Thai, P.H.; Cuong, L.C.V. Chemical Composition, Anti-α-Glucosidase Activity, and Molecular Modelling Studies of Cleistocalyx operculatus Essential Oil. Appl. Sci. 2023, 13, 11224. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral. Scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Calva, J.; Celi, J.; Benítez, Á. Analysis of the Volatile and Enantiomeric Compounds Emitted by Plumeria rubra L. Flowers Using HS-SPME–GC. Plants 2024, 13, 2367. [Google Scholar] [CrossRef]
- Calva, J.; Cuenca, M.B.; León, A.; Benítez, Á. Chemical Composition, Acetylcholinesterase-Inhibitory Potential and Antioxidant Activity of Essential Oils from Three Populations of Parthenium hysterophorus L. in Ecuador. Molecules 2025, 30, 2712. [Google Scholar] [CrossRef]
- NIST. NIST Chemistry WebBook: Nist Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 8 August 2025).
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T. Morphometrics. In Paleontological Data Analysis; Wiley: Hoboken, NJ, USA, 2008; pp. 78–156. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).