Tree Endotherapy: A Comprehensive Review of the Benefits and Drawbacks of Trunk Injection Treatments in Tree Care and Protection
Abstract
1. Introduction
2. Advantages of Endotherapy Treatments
3. Eco-Friendly and Safety Features
4. Endotherapy Techniques
4.1. Empirical Period
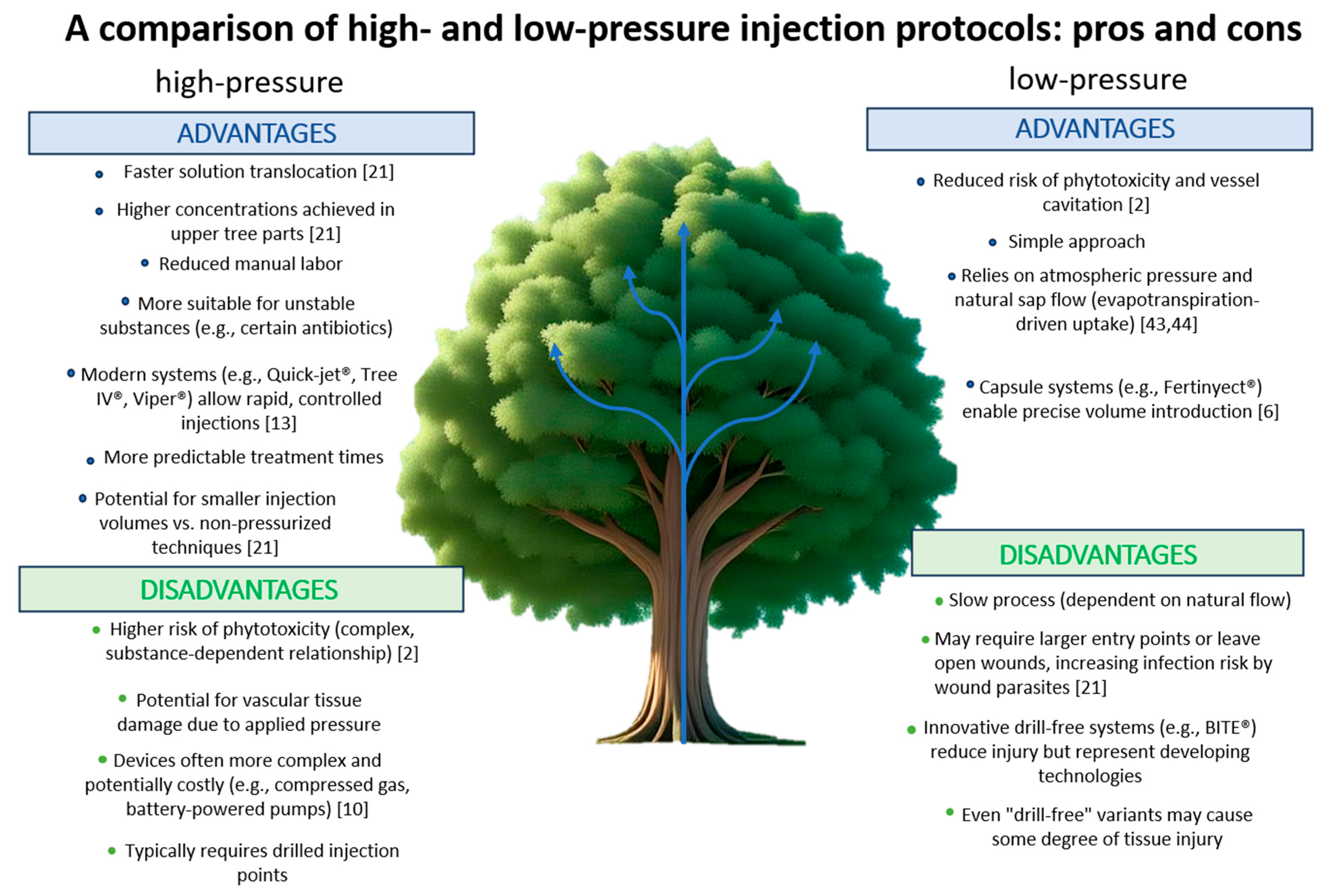
4.2. Low-Pressure Injection Phase
4.3. High-Pressure Phase
4.4. Passive Infusion and Non-Pressurized Capsule Systems
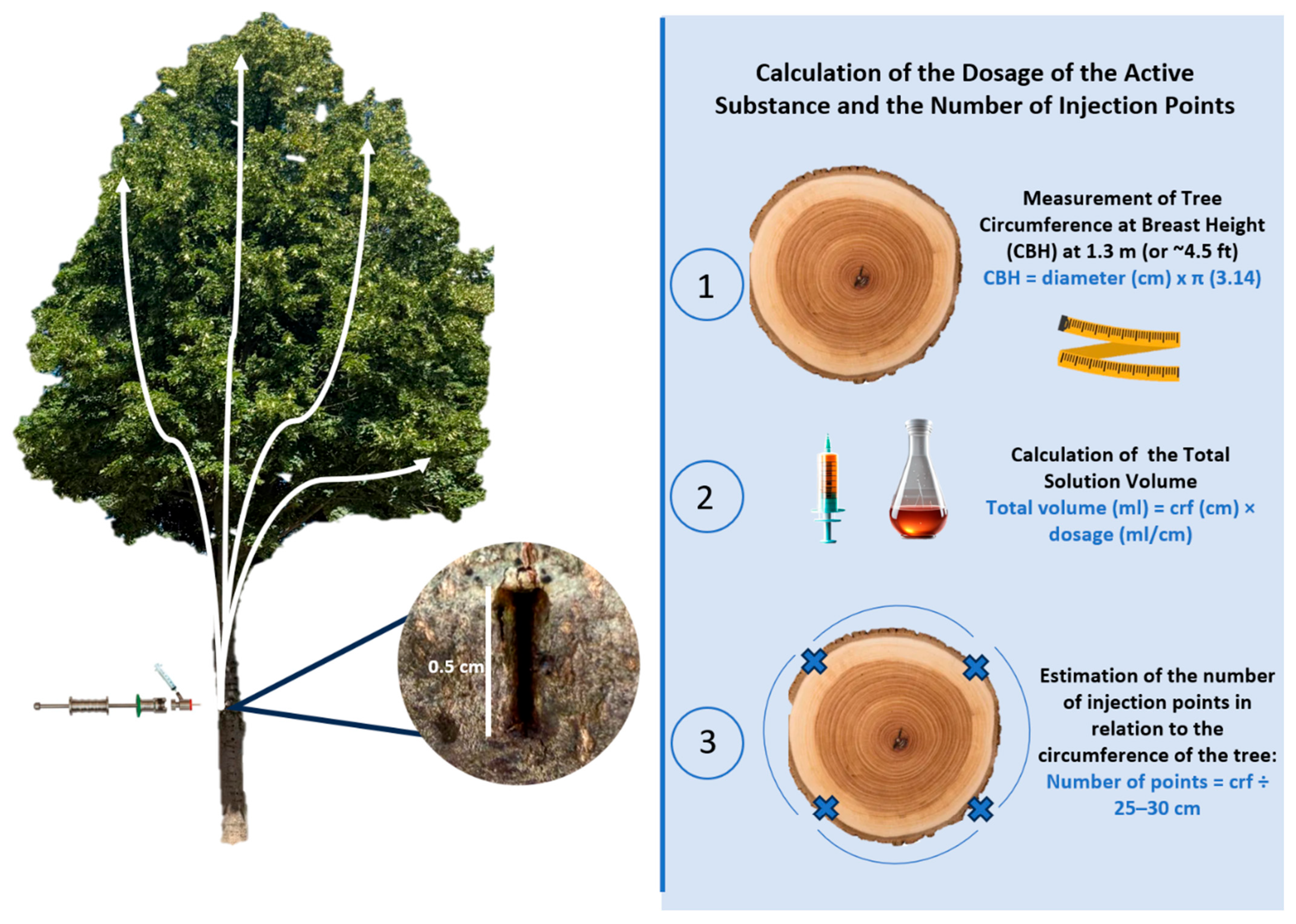
5. Endotherapy Application
5.1. Products Formulation
5.2. Digital Technologies for Endotherapy
5.3. Efficacy of Endotherapy
6. Drawbacks of Endotherapy
7. Future Perspectives
- Ameliorating the distribution uniformity;
- Applying less invasive, low-injury delivery methods;
- Refining injection technologies through more efficient operational throughput/automated dosing;
- Expanding the range and type of active ingredients used, moving towards more environmentally friendly options.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L. Novel endotherapy-based applications of ozonated water to Bobal grapevines: Effect on grape quality. Agronomy 2020, 10, 1218. [Google Scholar] [CrossRef]
- Grandi, L.; Oehl, M.; Lombardi, T.; De Michele, V.R.; Schmitt, N.; Verweire, D.; Balmer, D. Innovations towards sustainable olive crop management: A new dawn by precision agriculture including endo-therapy. Front. Plant Sci. 2023, 14, 1180632. [Google Scholar] [CrossRef]
- Moura, J.I.L.; da Costa Pereira, R.R.; Pereira, C.E. Trunk Injection as an Alternative Approach to Insecticide Spraying: An Experience with Cashew Trees. Sci. Plena 2022, 18. [Google Scholar] [CrossRef]
- Del Frari, G.; Costa, J.; Oliveira, H.; Ferreira, R.B. Endotherapy of infected grapevine cuttings for the control of Phaeomoniella chlamydospora and Phaeoacremonium minimum. Phytopathol. Mediterr. 2018, 57, 439–448. [Google Scholar] [CrossRef]
- Archer, L.; Kunwar, S.; Alferez, F.; Batuman, O.; Albrecht, U. Trunk injection of oxytetracycline for huanglongbing management in mature grapefruit and sweet orange trees. Phytopathology 2023, 113, 1010–1021. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Esparraguera, L.B.; Queiroz, S.C.N.; Bottoli, C.B.G. Vegetative endotherapy—Advances, perspectives, and challenges. Agriculture 2023, 13, 1465. [Google Scholar] [CrossRef]
- Di Ilio, V.; Metwaly, N.; Saccardo, F.; Caprio, E. Adult and egg mortality of Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae) induced by thiamethoxam and clothianidin. IOSR J. Agric. Vet. Sci. 2018, 11, 59–67. [Google Scholar]
- Panconesi, A. Canker stain of plane trees: A serious danger to urban plantings in Europe. J. Plant Pathol. 1999, 81, 3–15. [Google Scholar]
- Da Vinci, L. Codex Atlanticus; Veneranda Biblioteca Ambrosiana: Milan, Italy, 1490; pp. 12–76. [Google Scholar]
- Ojo, I.; Ampatzidis, Y.; Neto, A.D.O.C.; Bayabil, H.K.; Schueller, J.K.; Batuman, O. The development and evolution of trunk injection mechanisms-a review. Biosyst. Eng. 2024, 240, 123–141. [Google Scholar] [CrossRef]
- Pal, M.; Balint, J.; Balog, A. Using the technique of vegetal endoterapy against the horse chestnut’s leaf miner (Lepidoptera: Cameraria ohridella Deschka & Dimie). Sci. Pap. Ser. B Hortic. 2014, LVIII, 353–358. [Google Scholar]
- Di Sora, N.; Rossini, L.; Contarini, M.; Chiarot, E.; Speranza, S. Endotherapic treatment to control Toumeyella parvicornis Cockerell infestations on Pinus pinea L. Pest Manag. Sci. 2022, 78, 2443–2448. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Skourti, A.; Nika, E.P.; Papadoulis, G.T. Trunk injection with insecticides manages Xylotrechus chinensis (Chevrolat) (Coleoptera: Cerambycidae). Insects 2022, 13, 1106. [Google Scholar] [CrossRef]
- Chihaoui-Meridja, S.; Harbi, A.; Abbes, K.; Chaabane, H.; La Pergola, A.; Chermiti, B.; Suma, P. Systematicity, persistence and efficacy of selected insecticides used in endotherapy to control the red palm weevil Rhynchophorus ferrugineus (Olivier, 1790) on Phoenix canariensis. Phytoparasitica 2020, 48, 75–85. [Google Scholar] [CrossRef]
- Moll, L.; Baró, A.; Montesinos, L.; Badosa, E.; Bonaterra, A.; Montesinos, E. Induction of defense responses and protection of almond plants against Xylella fastidiosa by endotherapy with a bifunctional peptide. Phytopathology 2022, 112, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, A.; Aghamohammadi, N.; Aalipour, H.; Akhbarfar, G.; Fernández-Escobar, R. An Integrated Approach Employing Endotherapy Accompanied with Fertilization and Soil Mulching Recovered Plane Trees from Early Leaf Chlorosis in the Urban Landscape. J. Soil Sci. Plant Nutr. 2025, 25, 2156–2172. [Google Scholar] [CrossRef]
- Doccola, J.J.; Wild, P.M. Tree injection as an alternative method of insecticide application. In Insecticides—Basic and Other Applications; InTech: Rijeka, Croatia, 2012; pp. 61–78. [Google Scholar]
- Benigno, A.; Aglietti, C.; Cacciola, S.O.; Moricca, S. Trunk Injection Delivery of Biocontrol Strains of Trichoderma spp. Effectively Suppresses Nut Rot by Gnomoniopsis castaneae in Chestnut (Castanea sativa Mill.). Biology 2024, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, C.; Alma, A. How to preserve horse chestnut trees from Cameraria ohridella in the urban environment. Crop Prot. 2008, 27, 1251–1255. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Fassoni, A.C.; Ferreira, J.M.S.; Lins, P.M.P.; Bottoli, C.B.G. Cyproconazole translocation in coconut palm tree using vegetative endotherapy: Evaluation by LC-MS/MS and mathematical modeling. Horticulturae 2022, 8, 1099. [Google Scholar] [CrossRef]
- Vizzarri, V.; Ienco, A.; Benincasa, C.; Perri, E.; Pucci, N.; Cesari, E.; Novellis, C.; Rizzo, P.; Pellegrino, M.; Zaffina, F.; et al. Phenolic extract from olive leaves as a promising endotherapeutic treatment against Xylella fastidiosa in naturally infected Olea europaea (var. europaea) Trees. Biology 2023, 12, 1141. [Google Scholar] [CrossRef]
- VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Mota-Sanchez, D.; Vandervoort, C.; Wise, J.C. Trunk injection: An alternative technique for pesticide delivery in apples. Crop Prot. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Gyuris, R.; Szabó, Á.; László, A.M.; Gutermuth, Á.; Sörös, C. An Evaluation of Insecticidal Trunk Injections for the Control of the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae). Horticulturae 2024, 10, 278. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.K. Pesticide residues and risk assessment of trunk-injected pesticides in pine nut (Pinus koraiensis) seeds. Chemosphere 2024, 365, 143313. [Google Scholar] [CrossRef]
- May, C. Methods of tree injection. Trees Mag. 1941, 4, 7–16, 10–12, 14. [Google Scholar]
- Roach, W.A. Plant injection as a physiological method. Ann. Bot. 1939, 3, 155–226. [Google Scholar] [CrossRef]
- Wilson, J.M. The Rural Cyclopedia; Fullarton and Co.: Edinburgh, Scotland, 1847; p. 423. [Google Scholar]
- Himelick, E.B. High pressure injection of chemicals into trees. Arborists News 1972, 37, 97–103. [Google Scholar]
- Shevyrev, I. Extraradicate Nutrition of Diseased Trees; Records of the Botanical Division, St. Petersburg Imperial Society of Naturalists; Supplement to Extraradicate nutrition of diseased tree//I; Reprint from the Agricultural Gazette; St. Petersburg Imperial Society of Naturalists: St. Petersburg, Russia, 1894; pp. 3–6. [Google Scholar]
- Schreiber, L.R. A method for the injection of chemicals into trees. Plant Dis. Report. 1969, 53, 764–765. [Google Scholar]
- Schwarz, R.E.; Vuuren, S.V. Decrease in fruit greening of sweet orange by trunk injection of tetracyclines. Plant Dis. Report. 1971, 55, 747–750. [Google Scholar]
- Jones, T.W.; Gregory, G.F. An Apparatus for Pressure Injection of Solutions into Trees; Northeastern Forest Experiment Station, Forest Service, US Department of Agriculture: Washington, DC, USA, 1971; Volume 233.
- Pinkas, Y.; Shabi, E.; Solel, Z.; Cohen, A. Infiltration and translocation of thiabendazole in apple trees by means of a pressure injection technique. Phytopathology 1973, 63, 1166–1168. [Google Scholar] [CrossRef]
- Filer, T.H.J. Pressure apparatus for injecting chemicals into trees. Plant Dis. Report. 1973, 57, 338–341. [Google Scholar]
- Brown, G.K.; Bachelor, J.B. Engineering development of equipment for injecting chemicals into trees. Am. Soc. Agric. Eng. 1974, 74, 1520. [Google Scholar]
- Reil, W.O.; Beutel, J.A.A. pressure machine for injecting trees. Calif. Agric. 1976, 30, 4–5. Available online: https://californiaagriculture.org/article/111753-a-pressure-machine-for-injecting-trees (accessed on 23 April 2025).
- Navarro, C.; Fern’andez-Escobar, R.; Benlloch, M.A. Low-pressure, trunk injection method for introducing chemical formulations into olive trees. J. Am. Soc. Hortic. Sci. 1992, 117, 357–360. [Google Scholar] [CrossRef]
- Brown, G.K. Prototype equipment for commercial pressure-injection of aqueous growth regulators into trees. J. Arboric. 1978, 4, 7–13. [Google Scholar] [CrossRef]
- Sterrett, J.P.; Creager, R.A. A miniature pressure injector for deciduous woody seedlings and branches. HortScience 1977, 12, 156–158. [Google Scholar] [CrossRef]
- Wilson, C.L.; Spotts, R.A.; Semer, C.R.I. New injection equipment and a new fungicide for Dutch elm disease control. Plant Dis. Report. 1977, 61, 694–698. [Google Scholar]
- Sachs, R.M.; Nyland, G.; Hackett, W.P.; Coffelt, J.; Debie, J.; Giannini, G. Pressurised injection of aqueous solutions into tree trunks. Sci. Hortic. 1977, 6, 297–310. [Google Scholar] [CrossRef]
- Sano, Y.; Okamura, Y.; Utsumi, Y. Visualising water-conduction pathways of living trees: Selection of dyes and tissue preparation methods. Tree Physiol. 2005, 25, 269–275. [Google Scholar] [CrossRef]
- Montecchio, L.A. venturi effect can help cure our trees. J. Vis. Exp. JoVE 2013, 80, 1–8. [Google Scholar] [CrossRef]
- Wang, J.H.; Che, S.C.; Qiu, L.F.; Li, G.; Shao, J.L.; Zhong, L.; Zhang, G.F.; Xu, H. Efficacy of Emamectin Benzoate Trunk Injection Against the Asian Long-Horned Beetle [Anoplophora glabripennis (Coleoptera: Cerambycidae)]. J. Econ. Entomol. 2020, 113, 340–347. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Vanwoerkom, A.H.; Garavaglia, T.; Vandervoort, C.; Sundin, G.W.; Wise, J.C. Seasonal and Cross-Seasonal Timing of Fungicide Trunk Injections in Apple Trees to Optimize Management of Apple Scab. Plant Dis. 2016, 100, 1606–1616. [Google Scholar] [CrossRef]
- Gomez, S.; Ferry, M. A simple and low cost injection technique to protect efficiently ornamental Phoenix against the red palm weevil during one year. Arab. J. Plant Prot. 2019, 37, 124–129. [Google Scholar] [CrossRef]
- Mokhtaryan, A.; Sheikhigarjan, A.; Arbab, A.; Mohammadipour, A.; Ardestanirostami, H. The efficiency of systemic insecticides and complete fertilizer by trunk injection method against leopard moth in infested walnut trees. J. Basic Appl. Zool. 2021, 82, 55. [Google Scholar] [CrossRef]
- Gentile, S.; Valentino, D.; Tamietti, G. Control of ink disease by trunk injection of potassium phosphite. J. Plant Pathol. 2009, 91, 565–571. Available online: http://www.jstor.org/stable/41998673 (accessed on 11 April 2024).
- Akinsanmi, O.A.; Drenth, A. Phosphite and Metalaxyl Rejuvenate Macadamia Trees in Decline Caused by Phytophthora cinnamomi. J. Crop Prot. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- Scortichini, M.; Chen, J.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; L’Aurora, A.; Petriccione, M.; Zampella, L.; Mastrobuoni, F.; et al. A zinc, copper and citric acid biocomplex shows promise for control of Xylella fastidiosa subsp. pauca in olive trees in Apulia region (southern Italy). Phytopathol. Mediterr. 2018, 57, 48–72. [Google Scholar] [CrossRef]
- Fettig, C.J.; Burnside, R.E.; Schultz, M.E. Injection of emamectin benzoate protects paper birch from birch leafminer (Hymenoptera: Tenthredinidae) for two field seasons. J. Entomol. Sci. 2013, 48, 166–168. [Google Scholar]
- Dembilio, Ó.; Riba, J.M.; Gamón, M.; Jacas, J.A. Mobility and efficacy of abamectin and imidacloprid against Rhynchophorus ferrugineus in Phoenix canariensis by different application methods. Pest. Manag. Sci. 2015, 71, 1091–1098. [Google Scholar] [CrossRef]
- Shin, K.; Ascunce, M.S.; Narouei-Khandan, H.A.; Sun, X.; Jones, D.; Kolawole, O.O.; Goss, E.M.; van Bruggen, A.H. Effects and side effects of penicillin injection in huanglongbing affected grapefruit trees. Crop Prot. 2016, 90, 106–116. [Google Scholar] [CrossRef]
- Badosa, E.; Moiset, G.; Montesinos, L.; Talleda, M.; Bardají, E.; Feliu, L.; Planas, M.; Montesinos, E. Derivatives of the antimicrobial peptide BP100 for expression in plant systems. PLoS ONE 2013, 8, e85515. [Google Scholar] [CrossRef]
- Baró, A.; Mora, I.; Montesinos, L.; Montesinos, E. Differential susceptibility of Xylella fastidiosa strains to synthetic bactericidal peptides. Phytopathology 2020, 110, 1018–1026. [Google Scholar] [CrossRef]
- Baró, A.; Saldarelli, P.; Saponari, M.; Montesinos, E.; Montesinos, L. Nicotiana benthamiana as a model plant host for Xylella fastidiosa: Control of infections by transient expression and endotherapy with a bifunctional peptide. Front. Plant Sci. 2022, 13, 1061463. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, L. Rice Seeds as Biofactories of the Production of Antimicrobial Peptides. Ph.D. Thesis, University of Girona, Girona, Spain, 16 May 2014. [Google Scholar]
- Montesinos, L.; Bundo, M.; Badosa, E.; San Segundo, B.; Coca, M.; Montesinos, E. Production of BP178, a derivative of the synthetic antibacterial peptide BP100, in the rice seed endosperm. BMC Plant Biol. 2017, 17, 63. [Google Scholar] [CrossRef]
- Montesinos, L.; Gascon, B.; Badosa, E.; Planas, M.; Feliu, L.; Montesinos, E.A. bifunctional synthetic peptide with antimicrobial and plant elicitation properties that protects tomato plants from bacterial and fungal infections. Front. Plant Sci. 2021, 12, 756357. [Google Scholar] [CrossRef]
- Moll, L.; Badosa, E.; De La Fuente, L.; Montesinos, E.; Planas, M.; Bonaterra, A.; Feliu, L. Mitigation of almond leaf scorch by a peptide that inhibits the motility of Xylella fastidiosa. Plant Dis. 2025, 109, 327–340. [Google Scholar] [CrossRef]
- Montesinos, L.; Baró, A.; Gascón, B.; Montesinos, E. Bactericidal and plant defense elicitation activities of Eucalyptus oil decrease the severity of infections by Xylella fastidiosa on almond plants. Front. Plant Sci. 2023, 14, 1122218. [Google Scholar] [CrossRef] [PubMed]
- Bahadou, S.A.; Ouijja, A.; Boukhari, M.A.; Tahiri, A. Development of field strategies for fire blight control integrating biocontrol agents and plant defense activators in Marocco. J. Plant Pathol. 2017, 99, 51–58. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Zeng, Q.; McGhee, G.C.; Sundin, G.W.; Wise, J.C. Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Front. Plant Sci. 2015, 6, 16. [Google Scholar] [CrossRef]
- Dal Maso, E.; Linaldeddu, B.T.; Fanchin, G.; Faccoli, M.; Montecchio, L. The potential for pesticide trunk injections for control of thousand cankers disease of walnut. Phytopathol. Mediterr. 2019, 58, 73–80. [Google Scholar]
- Ojo, I.; Ampatzidis, Y.; Neto, A.D.O.C.; Batuman, O. Development of an automated needle-based trunk injection system for HLB-affected citrus trees. Biosyst. Eng. 2024, 240, 90–99. [Google Scholar] [CrossRef]
- Ojo, I.; Vijayakumar, V.; Ampatzidis, Y.; Batuman, O.; Kunwar, S.; Albrecht, U.; Archer, L.; Alferez, F.; Bayabil, H.; Schueller, J.K. Prototype development of an automated needle-based injection system to treat HLB affected citrus trees. In Proceedings of the 2022 ASABE Annual International Meeting, Houston, TX, USA, 17–20 July 2022; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2022. [Google Scholar] [CrossRef]
- da Silva, D.Q.; dos Santos, F.N.; Filipe, V.; Sousa, A.J.; Oliveira, P.M. Edge AI-based tree trunk detection for forestry monitoring robotics. Robotics 2022, 11, 136. [Google Scholar] [CrossRef]
- Ampatzidis, Y.; Partel, V.; Costa, L. Agroview: Cloud-based application to process, analyze and visualize UAV-collected data for precision agriculture applications utilizing artificial intelligence. Comput. Electron. Agric. 2020, 174, 105157. [Google Scholar] [CrossRef]
- Partel, V.; Costa, L.; Ampatzidis, Y. Smart tree crop sprayer utilizing sensor fusion and artificial intelligence. Comput. Electron. Agric. 2021, 191, 106556. [Google Scholar] [CrossRef]
- Hu, W.; Kuang, F.; Chun, J.; Lu, Z.; Li, X.; Zhao, Q.; Zhong, B.; Su, H.; Zhang, Z.; Zhang, N. Uptake of soil-applied thiamethoxam in orange and its effect against Asian citrus psyllid in different seasons. Pest Manag. Sci. 2018, 75, 1339–1345. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Santiago-Aliste, A.; Torres-Sánchez, S.; Martín-Ramos, P. Lignin–Chitosan Nanocarriers for the Delivery of Bioactive Natural Products against Wood-Decay Phytopathogens. Agronomy 2022, 12, 461. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Talamine, V.; Facco, J.F.; Rizzetti, T.M.; Ferreira, J.M.S.; Oliveira, F.A.; Prestes, O.D.; Zanella, R.; Martins, M.L.; Adaime, M.B.; et al. Determination of Pesticide Residues in Coconut Tree Trunks by Modified QuEChERS Method and Ultra-High-Performance Liquid Chromatography Coupled to Triple Quadrupole Tandem Mass Spectrometry. Anal. Methods 2015, 7, 4237–4245. [Google Scholar] [CrossRef]
- Berger, G.; Czarnocka, K.; Cochard, B.; Oszako, T.; Lefort, F. Biocontrol endotherapy with Trichoderma spp. and Bacillus amyloliquefaciens against Phytophthora spp.: A comparative study with phosphite treatment on Quercus robur and Fagus sylvatica. J. Agric. Sci. Technol. 2015, 5, 409–420. [Google Scholar] [CrossRef]
- Darvas, J.M.; Toerien, J.C.; Milne, D.L. Control of avocado root rot by trunk injection with phosethyl-A1. Plant Dis. 1984, 68, 691–693. [Google Scholar] [CrossRef]
- Amiri, A.; Bussey, K.E.; Riley, M.B.; Schnabel, G. Propiconazole inhibits Armillaria tabescens in vitro and translocates into peach roots following trunk infusion. Plant Dis. 2008, 92, 1293–1298. [Google Scholar] [CrossRef]
- McCoy, R.E. Uptake, translocation, and persistence of oxytetracycline in coconut palm. Phytopathology 1976, 66, 1038–1042. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Cregg, B.M.; McCullough, D.G.; Poland, T.M.; Hollingworth, R.M. Distribution of trunk-injected 14C-imidacloprid in ash trees and effects on emerald ash borer (Coleoptera: Buprestidae) adults. Crop Prot. 2009, 28, 655–661. [Google Scholar] [CrossRef]
- Hu, J.; Wang, N. Evaluation of the spatiotemporal dynamics of oxytetracycline and its control effect against Citrus Huanglongbing via trunk injection. Phytopathology 2016, 106, 1495–1503. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Wang, N. Control of citrus huanglongbing via trunk injection of plant defense activators and antibiotics. Phytopathology 2017, 108, 186–195. [Google Scholar] [CrossRef]
- Dal Maso, E.; Cocking, J.; Montecchio, L. Efficacy tests on commercial fungicides against ash dieback in vitro and by trunk injection. Urban For. Urban Green. 2014, 13, 697–703. [Google Scholar] [CrossRef]
- Pierron, R.J.G.; Pages, M.; Couderc, C.; Compant, S.; Jacques, A.; Violleau, F. In vitro and in planta fungicide properties of ozonated water against the esca-associated fungus Phaeoacremonium aleophilum. Sci. Hortic. 2015, 189, 184–191. [Google Scholar] [CrossRef]
- Rumbold, C. Effect on chestnuts of substances injected into their trunks. Am. J. Bot. 1920, 7, 45–56. [Google Scholar] [CrossRef]
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.; Barrett, G.A.; Jackson, R.W. Endophytes vs tree pathogens and pests: Can they be used as biological control agents to improve tree health? Eur. J. Plant Pathol. 2019, 155, 711–729. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; Lins, P.M.P.; Farias, S.C.C.; Bottoli, C.B.G. Translocation of pesticides in coconut palm by endotherapy with the addition of different adjuvants. Ciência E Nat. 2020, 42, e56. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk Injection: A Discriminating Delivering System for Horticulture Crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 2161-0983. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of Hairpin Rnas and Small Rnas into Woody and Herbaceous Plants by Trunk Injection and Petiole Absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef]



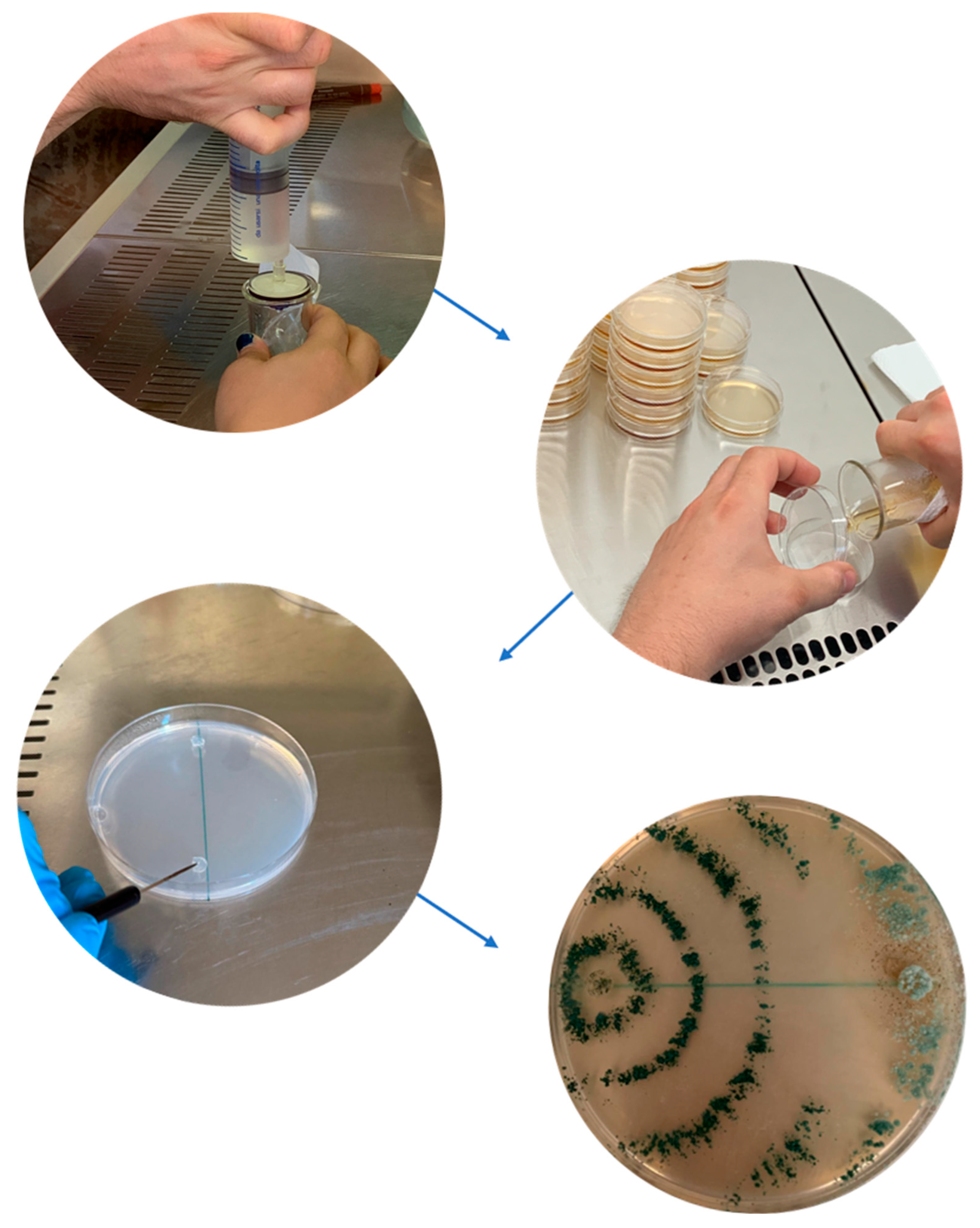
| Agent Name | Product Formulation | Host | Endotherapic Treatment Efficacy | Reference |
|---|---|---|---|---|
| Fungi and oomycetes | ||||
| Armillaria tabescens | Propiconazole | Prunus persica | Evaluated only in vitro | Amiri et al. [77] |
| Geosmithia morbida | Tiabendazole, Procloraz, Allicin, Tetraconazole | Juglans nigra | 28–31% | Dal Maso et al. [65] |
| Gnomoniopsis castaneae | Trichoderma spp. | Castanea sativa | 26–40% | Benigno et al. [19] |
| Hymenoscyphus fraxineus | Allicin, Thiabendazole | Fraxinus spp. | 56–67% | Dal Maso et al. [81] |
| Ophiostoma ulmi | 2-(3′,4′-dichlorophenol)-1,3-dioxolan-2-ylmethyl)imidazole | Ulmus spp. | 44% | Wilson et al. [41] |
| Phaeoacremonium aleophilum | Ozonated water | Vitis vinifera | 50% | Pierron et al. [82] |
| Phaeoacremonium minimum | Blad-containing oligomer (BCO), Elemental silver, Fosetyl-Al, Glutaraldehyde, Hydrogen peroxide | Vitis vinifera | 0–90% | Del Frari et al. [4] |
| Phaeomoniella chlamydospora | Blad-containing oligomer (BCO), Elemental silver, Fosetyl-Al, Glutaraldehyde, Hydrogen peroxide | Vitis vinifera | 0–90% | Del Frari et al. [4] |
| Phytophthora spp. | Phosphite, Trichoderma spp.; Bacillus amyloliquefaciens | Quercus robur; Fagus sylvatica | 31–86% | Berger et al. [74] |
| Phytophthora cambivora | Potassium phosphite | Castanea sativa | Up to 90% | Gentile et al. [49] |
| Phytophthora cinnamomi | Potassium phosphite, metalaxyl | Castanea sativa, Macadamia spp. | Up to 90% | Gentile et al. [49]; Akinsanmi and Drenth [50] |
| Bacteria | ||||
| Candidatus Liberibacter asiaticus | Oxytetracycline | Citrus spp. | c.a 15% reduction in fruits drop | Archer et al. [5] |
| Erwinia amylovora | Eucalyptus essential oil | Pyrus spp. | 30–39% | Montesinos et al. [62] |
| Xylella fastidiosa | Synthetic peptides | Nicotiana benthamiana, Prunus dulcis | 70–74% | Barò et al. [57]; Moll et al. [16]; Moll et al. [61] |
| Zinc and copper complexed with citric-acid hydracids (Dentamet®) | Olea europaea | Decrease in pathogen DNA concentration (from 27 ng in untreated samples to 3 ng in treated samples) | Scortichini et al. [51] | |
| Eucalyptus essential oil | Prunus dulcis | 52–68% | Montesinos et al. [62] | |
| Phenolic extract from olive leaves | Olea europaea | Increase in leaf area index (2–10%) and leaf area density (6–9%) | Vizzarri et al. [22] | |
| Insects | ||||
| Cameraria ohridella | Imidacloprid, Abamectin, Avermectin | Aesculus hippocastanum | Up to 82% | Ferracini and Alma [20]; Pal et al. 2018 [11] |
| Rhagoletis cerasi | Acetamiprid | Prunus spp. | 95% | Gyuris et al. [24] |
| Rhynchophorus ferrugineus | Emamectin benzoate, Thiamethoxam, Imidacloprid, Clothianidin, Fipronil | Phoenix canariensis | 20–80% | Chihaoui-Meridja et al. [15]; Di Ilio et al. [7]; Gomez and Ferry [47] |
| Toumeyella parvicornis | Abamectin | Pinus spp. | From 67 (untreated) to 20 (treated) individuals recorded at the peak of population | Di Sora et al. [12] |
| Xylotrechus chinensis | Spirotetramat, Fipronil, Imidacloprid, Abamectin | Morus spp. | 8–85% | Kavallieratos et al. [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benigno, A.; Aglietti, C.; Papini, V.; Riolo, M.; Cacciola, S.O.; Moricca, S. Tree Endotherapy: A Comprehensive Review of the Benefits and Drawbacks of Trunk Injection Treatments in Tree Care and Protection. Plants 2025, 14, 3108. https://doi.org/10.3390/plants14193108
Benigno A, Aglietti C, Papini V, Riolo M, Cacciola SO, Moricca S. Tree Endotherapy: A Comprehensive Review of the Benefits and Drawbacks of Trunk Injection Treatments in Tree Care and Protection. Plants. 2025; 14(19):3108. https://doi.org/10.3390/plants14193108
Chicago/Turabian StyleBenigno, Alessandra, Chiara Aglietti, Viola Papini, Mario Riolo, Santa Olga Cacciola, and Salvatore Moricca. 2025. "Tree Endotherapy: A Comprehensive Review of the Benefits and Drawbacks of Trunk Injection Treatments in Tree Care and Protection" Plants 14, no. 19: 3108. https://doi.org/10.3390/plants14193108
APA StyleBenigno, A., Aglietti, C., Papini, V., Riolo, M., Cacciola, S. O., & Moricca, S. (2025). Tree Endotherapy: A Comprehensive Review of the Benefits and Drawbacks of Trunk Injection Treatments in Tree Care and Protection. Plants, 14(19), 3108. https://doi.org/10.3390/plants14193108








