Ethnoveterinary Potential of Acacia (Vachellia and Senegalia) Species for Managing Livestock Health in Africa: From Traditional Uses to Therapeutic Applications
Abstract
1. Introduction
2. Results
2.1. Geographical Occurrrence and Ethnoveterinary Practices
2.2. Biological Activity and Safety
2.3. Phytochemical Profiles
3. Discussion
3.1. Ethnoveterinary Practices Associated with Acacia/Vachellia/Senegalia Species in Africa
3.2. Antimicrobial Activity of Acacia/Vachellia/Senegalia Species
3.3. Antiparasitic Activity
3.4. Antioxidant Activity
3.5. Anti-Inflammatory Activity
3.6. Toxicity Evaluation of Acacia/Vachellia/Senegalia Species
3.7. Phytochemicals in Acacia/Vachellia/Senegalia Species
3.8. Limitations
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion/Exclusion Criteria and Data Extraction
4.3. Quality Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 15-LOX | 15-Lipoxygenases |
| ABTS | 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| COX | Cyclooxygenase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| HPLC | High-performance liquid chromatography |
| HepG2 | Hepatocellular carcinoma |
| IC50 | Inhibitory concentration 50% |
| LD50 | Lethal dosage 50% |
| LDH | Lactate dehydrogenase |
| MBc | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MS | Mass spectrometry |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| TAC | Total antioxidant capacity |
| TLC | Thin layer chromatography |
| UV | Ultraviolet spectroscopy |
References
- Ibeagha-Awemu, E.M.; Peters, S.O.; Bemji, M.N.; Adeleke, M.A.; Do, D.N. leveraging available resources and stakeholder involvement for improved productivity of African livestock in the era of genomic breeding. Front. Genet. 2019, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Dassou, G.H.; Ouachinou, J.M.S.; Adomou, A.C.; Yedomonhan, H.; Tossou, M.; Favi, A.; Djidohokpin, D.; Gbedolo, E.; Akoegninou, A. Plant and natural product based homemade remedies for veterinary uses by the Peul community in Benin. J. Ethnopharmacol. 2020, 261, 113107. [Google Scholar] [CrossRef]
- Mapiye, O.; Chikwanha, O.C.; Makombe, G.; Dzama, K.; Mapiye, C. Livelihood, food and nutrition security in Southern Africa: What role do indigenous cattle genetic resources play? Diversity 2020, 12, 74. [Google Scholar] [CrossRef]
- Molina-Flores, B.; Manzano-Baena, P.; Coulibaly, M.D. The Role of Livestock in Food Security, Poverty Reduction and Wealth Creation in West Africa; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Ekwem, D.; Morrison, T.A.; Reeve, R.; Enright, J.; Buza, J.; Shirima, G.; Mwajombe, J.K.; Lembo, T.; Hopcraft, J.G.C. Livestock movement informs the risk of disease spread in traditional production systems in East Africa. Sci. Rep. 2021, 11, 16375. [Google Scholar] [CrossRef]
- Birhanu, T.; Abera, D. Survey of ethno-veterinary medicinal plants at selected Horro Gudurru Districts, Western Ethiopia. Afr. J. Plant Sci. 2015, 9, 185–192. [Google Scholar] [CrossRef]
- Yirga, G.; Teferi, M.; Brhane, G.; Amare, S. Plants used in ethnoveterinary practices in Medebay-Zana district, northern Ethiopia. J. Med. Plants Res. 2012, 6, 433–438. [Google Scholar] [CrossRef]
- Tolossa, K.; Debela, E.; Athanasiadou, S.; Tolera, A.; Ganga, G.; Houdijk, J.G. Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in South Omo, Southern Ethiopia. J. Ethnobiol. Ethnomed. 2013, 9, 32. [Google Scholar] [CrossRef]
- van Wyk, A.S.; Prinsloo, G. Health, safety and quality concerns of plant-based traditional medicines and herbal remedies. S. Afr. J. Bot. 2020, 133, 54–62. [Google Scholar] [CrossRef]
- Chaachouay, N.; Azeroual, A.; Bencharki, B.; Douira, A.; Zidane, L. Ethnoveterinary medicinal plants for animal therapy in the Rif, North of Morocco. S. Afr. J. Bot. 2022, 147, 176–191. [Google Scholar] [CrossRef]
- Uprety, Y.; Karki, S.; Poudel, R.C.; Kunwar, R.M. Ethnoveterinary use of plants and its implication for sustainable livestock management in Nepal. Front. Vet. Sci. 2022, 9, 930533. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Rangra, N.K. Antimicrobial activities of Acacia genus: A review. Asian Pac. J. Trop. Biomed. 2023, 13, 45–59. [Google Scholar] [CrossRef]
- Dyer, C. New names for the African Acacia species in Vachellia and Senegalia. South. For. 2014, 76, iii. [Google Scholar] [CrossRef]
- Shinwari, Z.K.; Jamil, K.; Zahra, N.B. Molecular systematics of selected genera of subfamily Mimosoideae-Fabaceae. Pak. J. Bot. 2014, 46, 591–598. [Google Scholar]
- Assefa, A.; Bahiru, A. Ethnoveterinary botanical survey of medicinal plants in Abergelle, Sekota and Lalibela districts of Amhara region, Northern Ethiopia. J. Ethnopharmacol. 2018, 213, 340–349. [Google Scholar] [CrossRef]
- Barbosa, F.M.A.; Cala, A.C.; Sevastyanov, V.; Boane, E.; Hlashwayo, D.F. Ethnoveterinary study of plant-based remedies for treating diseases in small ruminants in Maputo Province, Mozambique. Evid. Based Complement. Altern. Med. 2023, 2023, 1842870. [Google Scholar] [CrossRef] [PubMed]
- Amoussa, A.M.; Lagnika, L.; Bourjot, M.; Vonthron-Senecheau, C.; Sanni, A. Triterpenoids from Acacia ataxacantha DC: Antimicrobial and antioxidant activities. BMC Complement. Altern. Med. 2016, 16, 284. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Kumar, S.; Arora, S. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food Chem. Toxicol. 2007, 45, 1216–1223. [Google Scholar] [CrossRef]
- Badar, N.; Iqbal, Z.; Khan, M.N.; Akhtar, M.S. In vitro and in vivo anthelmintic activity of Acacia nilotica (L.) Willd. Ex Delile bark and leaves. Pak. Vet. J. 2011, 31, 185–191. [Google Scholar]
- Obid Allah, N.; Elsayed, M.; Ahmed, M.; Elgohary, F. Evaluation of antimicrobial effect of Acacia nilotica plant extract and selected commercial disinfectants against some pathogens causing mastitis. Mansoura Vet. Med. J. 2020, 21, 193–200. [Google Scholar] [CrossRef]
- Paswan, J.K.; Kumar, K.; Kumar, S.; Chandramoni; Kumar, A.; Kumar, D.; Kumar, A. Effect of feeding Acacia nilotica pod meal on hematobiochemical profile and fecal egg count in goats. Vet. World 2016, 9, 1400–1406. [Google Scholar] [CrossRef]
- Zabre, G.; Tindano, B.; Dicko, A.; Bayala, B.; Kabore, A.; Belem, A.; Tamboura, H. In vitro anthelmintic efficacy of Acacia nilotica pods on eggs and adult worms of Haemonchus contortus. Indian J. Vet. Sci. Biotechnol. 2023, 19, 90–94. [Google Scholar] [CrossRef]
- Zahid, M.U.; Khalique, A.; Qaisrani, S.N.; Ashraf, M.; Sheikh, A.A.; Yaqoob, M.U. Efficacy of Acacia nilotica bark extract as an alternative to antibiotics in broilers challenged with Escherishia coli from 15 to 28 days. Turk. J. Vet. Anim. Sci. 2023, 47, 100–107. [Google Scholar] [CrossRef]
- Elshamy, S.; Kuhnert, N.; El-Shazly, M.; Ziemah, J.; Handoussa, H. Comparative metabolomic study of twelve Acacia species by UHPLC-q-tof-ESI-MS coupled with chemometrics in correlation with antibacterial activity. Fitoterapia 2025, 181, 106378. [Google Scholar] [CrossRef]
- Alajmi, M.F.; Alam, P.; Alqasoumi, S.I.; Siddiqui, N.A.; Basudan, O.A.; Hussain, A.; Husain, F.M.; Khan, A.A. Comparative anticancer and antimicrobial activity of aerial parts of Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis grown in Saudi Arabia. Saudi Pharm. J. 2017, 25, 1248–1252. [Google Scholar] [CrossRef]
- Singh, B.; Prashad, J.; Sharma, R.A. A systematic review on Indian Acacia species. Curr. Res. Biotechnol. 2025, 9, 100274. [Google Scholar] [CrossRef]
- Suriyamoorthy, S.; Subramaniam, K.; Durai, S.J.R.; Wahaab, F.; Chitraselvi, R.P.E. Evaluation of wound healing activity of Acacia caesia in rats. Wound Med. 2014, 7, 1–7. [Google Scholar] [CrossRef]
- Nakhate, V.P.; Akojwar, N.S.; Sinha, S.K.; Lomte, A.D.; Dhobi, M.; Itankar, P.R.; Prasad, S.K. Wound healing potential of Acacia catechu in streptozotocin-induced diabetic mice using in vivo and in silico approach. J. Tradit. Complement. Med. 2023, 13, 489–499. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Wanzala, W. A survey of plants and plant products traditionally used in livestock health management in Buuri district, Meru County, Kenya. J. Ethnobiol. Ethnomed. 2012, 8, 39. [Google Scholar] [CrossRef]
- Hilou, A.; Florence, R.; Pierre, D. Identification and quantification of phenolic compounds and flavonoids in anthelmintic ethnoveterinary plants used among Fulani and Mosse, central Burkina Faso. Int. J. Phytomed. 2014, 6, 87. [Google Scholar]
- Berhanu, M.; Tintagu, T.; Fentahun, S.; Giday, M. Ethnoveterinary survey of medicinal plants used for treatment of animal diseases in Ambo District of Oromia Regional State of Ethiopia. Evid. Based Complement. Altern. Med. 2020, 2020, 8816227. [Google Scholar] [CrossRef] [PubMed]
- Eiki, N.; Maake, M.; Lebelo, S.; Sakong, B.; Sebola, N.; Mabelebele, M. Survey of ethnoveterinary medicines used to treat livestock diseases in Omusati and Kunene Regions of Namibia. Front. Vet. Sci. 2022, 9, 762771. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, D.; Swan, G.E.; Botha, C.J. Use of ethnoveterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province of South Africa. J. S. Afr. Vet. Assoc. 2001, 72, 189–196. [Google Scholar] [CrossRef]
- Moichwanetse, B.I.; Ndhlovu, P.T.; Sedupane, G.; Aremu, A.O. Ethno-veterinary plants used for the treatment of retained placenta and associated diseases in cattle among Dinokana communities, North West Province, South Africa. S. Afr. J. Bot. 2020, 132, 108–116. [Google Scholar] [CrossRef]
- Usman, I. Ethno-veterinary care amongst the nomadic Fulani herdsmen in southern zone of Adamawa State, Nigeria. J. Anim. Sci. Vet. Med. 2016, 1, 108–117. [Google Scholar] [CrossRef]
- Hassan, M.; Yuguda, U.A. Study of ethnoveterinary medicinal plants used by pastoralists in Northern Gombe State, Nigeria. Asian J. Plant Sci. 2021, 3, 1–5. [Google Scholar] [CrossRef]
- Mengistu, M.; Kebede, E.; Serda, B. Ethnobotanical knowledge of pastoral community for treating livestock diseases in Shinle Zone, Somali Regional State, Eastern Ethiopia. J. Vet. Sci. Technol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Feyera, T.; Mekonnen, E.; Wakayo, B.U.; Assefa, S. Botanical ethnoveterinary therapies used by agro-pastoralists of Fafan zone, Eastern Ethiopia. BMC Vet. Res. 2017, 13, 232. [Google Scholar] [CrossRef]
- Eloff, J. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Bamidele, O.; Nnana, M.; Imelda, L.; Sekomeng, M. Acacia decurrens (Wild) an invasive South Africa tree: Chemical profile, antibacterial and antioxidant activities. Org. Med. Chem. Int. J. 2017, 3, 555611. [Google Scholar] [CrossRef]
- Mbolekwa, B.; Kambizi, L.; Songca, S.; Oluwafemi, O. Antibacterial activity of the stem bark extracts of Acacia mearnsii De Wild. Afr. J. Microbiol. Res. 2014, 8, 211–216. [Google Scholar] [CrossRef]
- van Vuuren, S.F.; Nkwanyana, M.N.; de Wet, H. Antimicrobial evaluation of plants used for the treatment of diarrhoea in a rural community in northern Maputaland, KwaZulu-Natal, South Africa. BMC Complement. Altern. Med. 2015, 15, 53. [Google Scholar] [CrossRef]
- Negi, B.S.; Dave, B.P. In vitro antimicrobial activity of Acacia catechu and its phytochemical analysis. Indian J. Microbiol. 2010, 50, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Alain, K.Y.; Pascal, A.D.C.; Boniface, Y.; Paul, T.F.; Alain, A.G.; Felicien, A.; Akpovi, A.; Dominique, S.K.C. Chemical characterization and biological activities of extracts from two plants (Cissus quadrangularis and Acacia polyacantha) used in veterinary medicine in Benin. J. Pharmacogn. Phytochem. 2015, 3, 91–96. [Google Scholar]
- Mollel, E.; Runyoro, D.K.B.; Ngassapa, O.D. Antimicrobial activity and phytochemical profile of Acacia drepanolobium Harms Ex Sjostedt (Fabaceae) and Solanum arundo Mattei (Solanaceae). J. Pharm. Sci. Res. 2020, 12, 734–739. [Google Scholar]
- Jodi, S.; Umar, N.; Ungo-kore, H.; Ngwai, Y. Antimicrobial activity of Acacia nilotica against some bacterial isolates associated with wound infections. FUDMA J. Sci. 2020, 4, 807–811. [Google Scholar] [CrossRef]
- Solomon-Wisdom, G.; Shittu, G. In vitro antimicrobial and phytochemical activities of Acacia nilotica leaf extract. J. Med. Plants Res. 2010, 4, 1232–1234. [Google Scholar] [CrossRef]
- Sserunkuma, P.; McGaw, L.; Nsahlai, I.; Van Staden, J. Selected southern African medicinal plants with low cytotoxicity and good activity against bovine mastitis pathogens. S. Afr. J. Bot. 2017, 111, 242–247. [Google Scholar] [CrossRef]
- Gmaraldeen, S.M.; Magzoub, A.A.; Badri, A.M.; Garbi, M.I.; Saleh, M.S. Antibacterial activity of Acacia nilotica fruits extract against pathogenic bacteria. Int. J. Appl. Res. 2016, 2, 103–106. [Google Scholar]
- Aliero, A.A.; Mang, S.; Musa, I. Antimicrobial activities of bioactive compounds isolated from Acacia nilotica against multi-drug resistant bacteria. Microbes Infect. Dis. 2023, 5, 1217–1227. [Google Scholar] [CrossRef]
- Mwakalesi, A.J. Green synthesis of silver nanoparticles using aqueous extract of Vachellia xanthophloea and their potential use for antibacterial and sensing of mercury ions. Plasmonics 2023, 18, 2077–2090. [Google Scholar] [CrossRef]
- Alsadeg, A.M.; Koko, W.S.; Osman, E.; Kabbashi, A.; Dahab, M.; Garbi, M. In vitroanthelminthic activity of the methanol stem bark extract of Acacia senegal against Fasciola gigantica. Inter. Inven. J. Biochem. Bioinform. 2015, 3, 18–22. [Google Scholar]
- Ndlela, S.Z.; Mkwanazi, M.V.; Chimonyo, M. In vitro efficacy of plant extracts against gastrointestinal nematodes in goats. Trop. Anim. Health Prod. 2021, 53, 295. [Google Scholar] [CrossRef]
- Lebeloane, M.; Famuyide, I.; Kgosana, K.; Elgorashi, E.; Ndivhuwo, K.; Maharaj, V.; McGaw, L. Anti-inflammatory activity of seven plant species with potential use as livestock feed additives. S. Afr. J. Bot. 2024, 167, 322–332. [Google Scholar] [CrossRef]
- Gabr, S.; Nikles, S.; Wenzig, E.M.P.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Amoussa, A.M.O.; Sanni, A.; Lagnika, L. Antioxidant activity and total phenolic, flavonoid and flavonol contents of the bark extracts of Acacia ataxacantha. J. Pharmacogn. Phytochem. 2015, 4, 172–178. [Google Scholar]
- Idamokoro, E.M.; Masika, P.J.; Muchenje, V. A report on the in vitro antioxidant properties of Vachellia karroo leaf extract: A plant widely grazed by goats in the Central Eastern Cape of South Africa. Sustainability 2017, 9, 164. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. Pharmacological assessment of the medicinal potential of Acacia mearnsii De Wild.: Antimicrobial and toxicity activities. Int. J. Mol. Sci. 2012, 13, 4255–4267. [Google Scholar] [CrossRef]
- Abd’quadri-Abojukoro, A.N.; Nkadimeng, S.M.; McGaw, L.J.; Nsahlai, I.V. Phytochemical composition and cytotoxicity of ethanolic extracts of some selected plants. J. Appl. Anim. Res. 2022, 50, 656–665. [Google Scholar] [CrossRef]
- Ahmed, E.G.M.; Basher, A.W.; Ahmed, M.; Seddek, A.-L.; Morad, S.A.; Abdelmageed, N. The evaluation of the safety and toxicological characteristics of Acacia nilotica in broiler. SVU Int. J. Vet. Sci. 2023, 6, 124–135. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Sofidiya, M.O.; Masika, P.J.; Afolayan, A.J. Anti-inflammatory and analgesic activities of the aqueous extract of Acacia karroo stem bark in experimental animals. Basic Clin. Pharmacol. Toxicol. 2008, 103, 397–400. [Google Scholar] [CrossRef]
- Alli, L.A.; Adesokan, A.A.; Salawu, O.A.; Akanji, M.A. Toxicological studies of aqueous extract of Acacia nilotica root. Interdiscip. Toxicol. 2015, 8, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gabi, B.; Sharif, H.B.; Umar, H.; Umar, I. Toxicity study and effect of the leaf extract of Acacia nilotica on some biochemical parameters of Wistar albino rats. Sci. World J. 2022, 17, 390–397. [Google Scholar]
- Isah, J.; Ibrahim, Y.; Tytler, B.; Mujahid, H. Phytochemical screening and acute toxicity studies on Acacia nilotica stem bark methanol extract. J. Sci. Agric. 2021, 4, 1–7. [Google Scholar]
- Jurbe, G.; Ntim, P.; Ajayi, T.; Chuwkuka, J.; Dawurung, C.; Makoshi, M.; Elisha, I.; Oladipo, O.; Lohlum, A. Phytochemical screening and antidiarrheal evaluation of acetone extract of Acacia sieberiana var woodii (Fabaceae) stem bark in Wistar rats. Australas. J. Psychoanal. Psychother. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Mambe, F.T.; Tchinda, C.F.; Wamba, B.E.; Nayim, P.; Ashu, F.; Manekeng, H.T.; Veronique, P.; Kuete, V. Modes of action of the methanol extract and 3-O-[β-galactopyranosyl-(1 → 4)-β-D-galactopyranosyl]-oleanolic acid from Acacia polyacantha against multidrug-resistant Gram-negative bacteria. Investig. Med. Chem. Pharmacol. 2022, 5, 60. [Google Scholar] [CrossRef]
- Abbas, M.Y.; Ejiofor, J.I.; Yakubu, M.I. Acute and chronic toxicity profiles of the methanol leaf extract of Acacia ataxacantha DC (Leguminosae) in Wistar rats. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 185–189. [Google Scholar] [CrossRef]
- Gedara, S.R.; Galala, A.A. New cytotoxic spirostane saponin and biflavonoid glycoside from the leaves of Acacia saligna (Labill.) HL Wendl. Nat. Prod. Res. 2014, 28, 324–329. [Google Scholar] [CrossRef]
- Ashu, F.A.; Na-Iya, J.; Wamba, B.E.N.; Kamga, J.; Nayim, P.; Ngameni, B.; Beng, V.P.; Ngadjui, B.T.; Kuete, V. Antistaphylococcal activity of extracts, fractions, and compounds of Acacia polyacantha Wild (Fabaceae). Evid. Based Complement. Altern. Med. 2020, 2020, 2654247. [Google Scholar] [CrossRef]
- Mambe, F.T.; Na-Iya, J.; Fotso, G.W.; Ashu, F.; Ngameni, B.; Ngadjui, B.T.; Beng, V.P.; Kuete, V. Antibacterial and antibiotic modifying potential of crude extracts, fractions, and compounds from Acacia polyacantha Willd. against MDR Gram-negative bacteria. Evid. Based Complement. Altern. Med. 2019, 2019, 7507549. [Google Scholar] [CrossRef]
- Tajuddeen, N.; Swart, T.; Hoppe, H.C.; van Heerden, F.R. Antiplasmodial activity of Vachellia xanthophloea (Benth.) PJH Hurter (African fever tree) and its constituents. Pharmaceuticals 2022, 15, 470. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, J.; He, Q.; Wang, F. Characterization and potential antidiabetic activity of proanthocyanidins from the barks of Acacia mangium and Larix gmelinii. J. Chem. 2019, 2019, 4793047. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; Al-Yafrsi, M.A. Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants 2020, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Roy, U.S. Studies on phytochemical analysis, antioxidant, antibacterial and larvicidal properties of the Acacia nilotica fruit extracts. Mapana J. Sci. 2022, 21, 47–72. [Google Scholar] [CrossRef]
- Diab, K.A.; Fahmy, M.A.; Hassan, E.M.; El-Toumy, S.A. Evaluation of the cytotoxic, anticancer, and genotoxic activities of Acacia nilotica flowers and their effects on N-methyl-N-nitrosourea-induced genotoxicity in mice. Mol. Biol. Rep. 2022, 49, 8439–8448. [Google Scholar] [CrossRef] [PubMed]
- Oda, B.K.; Lulekal, E.; Warkineh, B.; Asfaw, Z.; Debella, A. Ethnoveterinary medicinal plants and their utilization by indigenous and local communities of Dugda District, Central Rift Valley, Ethiopia. J. Ethnobiol. Ethnomed. 2024, 20, 32. [Google Scholar] [CrossRef]
- Rao, P.K.; Hasan, S.S.; Bhellum, B.L.; Manhas, R.K. Ethnomedicinal plants of Kathua district, J&K, India. J. Ethnopharmacol. 2015, 171, 12–27. [Google Scholar] [CrossRef]
- Chahad, A.M.; Michalet, S.; Bechir, A.B.; Tidjani, A.; Nkongmeneck, B.A.; Dijoux-Franca, M.G. Medicinal plants from the Ouaddai Province (Chad): An ethnobotanical survey of plants used in traditional medicine. J. Altern. Complement. Med. 2015, 21, 569–577. [Google Scholar] [CrossRef]
- Koubé, J.; Dongmo, S.S.; Guiama, V.D.; Bum, E.N. Ethnomedicinal survey of Gavdé (Acacia nilotica): A medicinal plant used in sahelian zone of Cameroon, Central Africa. Int. J. Innov. Appl. Stud. 2016, 16, 820. [Google Scholar]
- Subhan, N. Phytochemical and Pharmacological Investigations of Australian Acacia: An Ethnomedicine-Guided Bioprospective Approach. Ph.D. Thesis, Charles Sturt University, Bathurst, Australia, 22 March 2016. [Google Scholar]
- Dogara, M.; Umar, U.; Usman, M.; Sunusi, N.; Ladan, S.; Lema, A. Survey of medicinal plants used for the treatment of malaria in Kaduna State, Nigeria. Katsina J. Nat. Appl. Sci. 2021, 10, 14–24. [Google Scholar]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef]
- Mpude, L.; Yendze, A.W.; Assonfack, D.J.; Cadet, E.; Matieta, V.Y.; Megaptche, J.F.; Mbaveng, A.T.; Kuete, V. Antibacterial activity of Sarcocephalus latifolius and Acacia sieberiana and the effect of their association with antibiotics against multidrug-resistant Staphylococcus aureus. Investig. Med. Chem. Pharmacol. 2024, 7, 96. [Google Scholar] [CrossRef]
- Kirabo, I.; Mabiki, F.P.; Mdegela, R.H.; Obbo, C.J. In vitro antibacterial potential of extracts of Sterculia africana, Acacia sieberiana, and Cassia abbreviata ssp. abbreviata used by yellow baboons (Papio cynocephalus) for possible self-medication in Mikumi National Park, Tanzania. Int. J. Zool. 2018, 2018, 9407962. [Google Scholar] [CrossRef]
- Abdalla, A.A.; Mustafa, M.I.; Makhawi, A.M. Phytochemical screening and antimicrobial activities studies of Acacia nilotica fruit cover. bioRxiv 2020. [Google Scholar] [CrossRef]
- Fatimah, A.-O. Antifungal activity and fourier transform infrared spectrometric characterization of aqueous extracts of Acacia senegal and Acacia tortilis on phytopathogenic fungi. J. Pharm. Res. Int. 2019, 31, 1–11. [Google Scholar] [CrossRef]
- Mutai, C.; Bii, C.; Rukunga, G.; Ondicho, J.; Mwitari, P.; Abatis, D.; Vagias, C.; Roussis, V.; Kirui, J. Antimicrobial activity of pentacyclic triterpenes isolated from Acacia mellifera. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 42–48. [Google Scholar] [CrossRef]
- Thanish Ahamed, S.; Lakshmi, T. Antibacterial activity of taxifolin isolated from Acacia catechu leaf extract–An in vitro study. Int. J. Pharm. Res. Allied Sci. 2018, 7, 133–137. [Google Scholar]
- Sanchez, E.; Heredia, N.; Camacho-Corona Mdel, R.; Garcia, S. Isolation, characterization and mode of antimicrobial action against Vibrio cholerae of methyl gallate isolated from Acacia farnesiana. J. Appl. Microbiol. 2013, 115, 1307–1316. [Google Scholar] [CrossRef]
- Attah, F. Antimicrobial effects of honey and its specific actions on cell walls, membranes and enzymes of some microbial pathogens. Master’s Thesis, Federal University of Technology, Minna, Nigeria, 2021. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Zabre, G.; Kabore, A.; Bayala, B.; Katiki, L.M.; Costa-Junior, L.M.; Tamboura, H.H.; Belem, A.M.G.; Abdalla, A.L.; Niderkorn, V.; Hoste, H.; et al. Comparison of the in vitro anthelmintic effects of Acacia nilotica and Acacia raddiana. Parasite 2017, 24, 44. [Google Scholar] [CrossRef]
- Zarza-Albarran, M.A.; Olmedo-Juarez, A.; Rojo-Rubio, R.; Mendoza-de Gives, P.; Gonzalez-Cortazar, M.; Tapia-Maruri, D.; Mondragon-Ancelmo, J.; Garcia-Hernandez, C.; Ble-Gonzalez, E.A.; Zamilpa, A. Galloyl flavonoids from Acacia farnesiana pods possess potent anthelmintic activity against Haemonchus contortus eggs and infective larvae. J. Ethnopharmacol. 2020, 249, 112402. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalo, J.; Manolaraki, F.; Frutos, P.; Hervas, G.; Celaya, R.; Osoro, K.; Ortega-Mora, L.M.; Hoste, H.; Ferre, I. In vitro effect of heather (Ericaceae) extracts on different development stages of Teladorsagia circumcincta and Haemonchus contortus. Vet. Parasitol. 2013, 197, 235–243. [Google Scholar] [CrossRef]
- Botura, M.B.; dos Santos, J.D.; da Silva, G.D.; de Lima, H.G.; de Oliveira, J.V.; de Almeida, M.A.; Batatinha, M.J.; Branco, A. In vitro ovicidal and larvicidal activity of Agave sisalana Perr. (sisal) on gastrointestinal nematodes of goats. Vet. Parasitol. 2013, 192, 211–217. [Google Scholar] [CrossRef]
- de Medeiros, M.L.S.; de Moura, M.C.; Napoleao, T.H.; Paiva, P.M.G.; Coelho, L.; Bezerra, A.; da Silva, M.D.C. Nematicidal activity of a water soluble lectin from seeds of Moringa oleifera. Int. J. Biol. Macromol. 2018, 108, 782–789. [Google Scholar] [CrossRef]
- Castillo-Mitre, G.F.; Rojo-Rubio, R.; Olmedo-Juárez, A.; Mendoza de Gives, P.; Vázquez-Armijo, J.F.; Zamilpa, A.; Lee-Rangel, H.A.; Avendano-Reyes, L.; Macias-Cruz, U. In vivo anthelmintic activity of Acacia cochliacantha leaves against Haemonchus contortus in Boer goat kids. Rev. Mex. Cienc. Pecu. 2021, 12, 138–150. [Google Scholar] [CrossRef]
- Minho, A.P.; Bueno, I.C.d.S.; Louvandini, H.; Jackson, F.; Gennari, S.M.; Abdalla, A.L. Effect of Acacia molissima tannin extract on the control of gastrointestinal parasites in sheep. Anim. Feed. Sci. Technol. 2008, 147, 172–181. [Google Scholar] [CrossRef]
- Borges, D.; Borges, F.d.A. Plants and their medicinal potential for controlling gastrointestinal nematodes in ruminants. Nematoda 2016, 3, e92106. [Google Scholar] [CrossRef]
- Martin, R.J. Modes of action of anthelmintic drugs. Vet. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar]
- El-Aal, H.A.H.M.A. Lipid Peroxidation End-Products as a Key of Oxidative Stress: Effect of Antioxidant on Their Production and Transfer of Free Radicals. Lipid Peroxidation; InTech: Buenos Aires, Argentina, 2012. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Konvičná, J.; Vargová, M.; Paulíková, I.; Kováč, G.; Kostecká, Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno 2015, 84, 133–140. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Abdoul, G.L.B.; Traore, A.; Ouedraogo, M.; Belemlilga, M.; Traore, T.K.; Belemnaba, L.; Ouedraogo, N.; Lupu, A.; Ouedraogo, S.; Guissou, I.P. Pharmacological study of trunk bark of Acacia nilotica var adansonii (Guill et Perr). o Ktze (Mimosaceae): Assays, antioxidant and antispasmodic activities. J. Drug Deliv. Ther. 2019, 9, 524–530. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kang, S.C. Protective effect of ethyl acetate fraction of Acacia ferruginea DC. against ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2013, 148, 175–181. [Google Scholar] [CrossRef]
- Delgadillo Puga, C.; Cuchillo Hilario, M.; Espinosa Mendoza, J.G.; Medina Campos, O.; Molina Jijon, E.; Diaz Martinez, M.; Alvarez Izazaga, M.A.; Ledesma Solano, J.A.; Pedraza Chaverri, J. Antioxidant activity and protection against oxidative-induced damage of Acacia shaffneri and Acacia farnesiana pods extracts: In vitro and in vivo assays. BMC Complement. Altern. Med. 2015, 15, 435. [Google Scholar] [CrossRef]
- Hasib, M.S.; Bari, M.S.; Chowdhury, A.; Hossain, M.A.; Rashid, M.A. Vachellia farnesiana (L.) Wight & Arn. Growing in Bangladesh exerts in-vitro antioxidant and in-vivo analgesic and anti-diarrheal activities. Bangladesh Pharm. J. 2020, 23, 181–186. [Google Scholar] [CrossRef]
- Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Vargas-Hernández, M.; Munguía-Fragozo, P.V.; Loarca-Piña, G.F.; Mendoza-Díaz, S.O.; Ocampo-Velázquez, R.V.; Rico-García, E.; Guevara-Gónzalez, R.G. Antioxidant and antimutagenic activities of Acacia pennatula pods. J. Sci. Ind. Res. 2011, 70, 859–864. [Google Scholar]
- Gowri, S.S.; Pavitha, S.; Vasantha, K. Free radical scavenging capacity and antioxidant activity of young leaves and barks of Acacia nilotica (L.) Del. Int. J. Pharm. Pharm. Sci. 2011, 3, 160–164. [Google Scholar]
- Sulaiman, C.; Gopalakrishnan, V.; Balachandran, I. Phenolic compounds and antioxidant properties of selected Acacia species. J. Biol. Act. Prod. Nat. 2014, 4, 316–324. [Google Scholar]
- Yadav, A.; Yadav, M.; Kumar, S.; Sharma, D.; Yadav, J. In vitro antioxidant activities and GC-MS analysis of different solvent extracts of Acacia nilotica leaves. Indian J. Pharm. Sci. 2018, 80, 892–902. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Hanpithakpong, W.; Tarning, J.; Anal, A.K. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind. Crops Prod. 2015, 77, 873–882. [Google Scholar] [CrossRef]
- Kwon, Y.-R.; Youn, K.-S. Antioxidant activity and physiological properties of moringa (Moringa oleifera Lam.) leaves extracts with different solvents. Korean J. Food Preserv. 2014, 21, 831–837. [Google Scholar] [CrossRef]
- Fitri, A.; Toharmat, T.; Astuti, D.; Tamura, H. The potential use of secondary metabolites in Moringa oleifera as an antioxidant source. Media Peternak. 2015, 38, 169–175. [Google Scholar] [CrossRef]
- Hossain, M.D.; Sarwar, M.S.; Dewan, S.M.; Hossain, M.S.; Shahid-Ud-Daula, A.; Islam, M.S. Investigation of total phenolic content and antioxidant activities of Azadirachta indica roots. Avicenna J. Phytomed. 2014, 4, 97–102. [Google Scholar]
- Kiranmai, M.; Kumar, M.; Ibrahim, M. Free radical scavenging activity of neem tree (Azadirachta indica a. Juss var., Meliaceae) root bark extract. Asian J. Pharm. Clin. Res. 2011, 4, 134–136. [Google Scholar]
- Ahmed, M.; Ahmad, S.; Aati, H.Y.; Sherif, A.E.; Ashkan, M.F.; Alrahimi, J.; Motwali, E.A.; Tousif, M.I.; Khan, M.A.; Hussain, M. Phytochemical, antioxidant, enzyme inhibitory, thrombolytic, antibacterial, antiviral and in silico studies of Acacia jacquemontii leaves. Arab. J. Chem. 2022, 15, 104345. [Google Scholar] [CrossRef]
- Arjabi, A.; Anarjan, N.; Jafarizadeh-Malmiri, H. Effects of extracting solvent composition on antioxidant and antibacterial activities of Alhagi maurorum extracts. J. Food Process Preserv. 2021, 45, e15300. [Google Scholar] [CrossRef]
- Koochak, H.; Seyyednejad, S.M.; Motamedi, H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran). Asian Pac. J. Trop. Med. 2010, 3, 180–184. [Google Scholar] [CrossRef]
- Benmoussa, H.; Elfalleh, W.; Farhat, A.; Bachoual, R.; Nasfi, Z.; Romdhane, M. Effect of extraction methods on kinetic, chemical composition and antibacterial activities of Tunisian Thymus vulgaris. L. essential oil. Sep. Sci. Technol. 2016, 51, 2145–2152. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011, 5, 120. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef]
- Xiong, J.; Graceb, M.H.; Esposito, D.; Wang, F.; Lila, M.A. Phytochemical characterization and anti-inflammatory properties of Acacia mearnsii leaves. Nat. Prod. Commun. 2016, 11, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, S.; Mownika, S.; Dhivya, S. Anti-inflammatory activity of the leaves of Acacia caesia (L.) Willd. against carrageenan induced paw edema in Wistar rats. J. Adv. Sci. Res. 2020, 11 (Suppl. S5), 154–160. [Google Scholar]
- de Oliveira Silva, D.; Conceição Santos, M.F.; de Jesus Nicácio, K.; Neto, A.K.; Ghilardi Lago, J.H.; Chagas-Paula, D.A.; Dias, D.F.; Soares, M.G. Evaluation of the anti-inflammatory activity of Acacia polyphylla and identification of a new apigenin-3-C-glycosylated type flavonoid. Nat. Prod. Res. 2024, 38, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Veronica, S.A.; Cheruiyot, K.S.; Bosibori, M.J.; Munene, I.M.; Murugi, J.; Piero, N.M. Antiinflammatory, analgesic and antipyretic effects of dichloromethane stem bark extract of Acacia mellifera. J. Phytopharmacol. 2017, 6, 239–246. [Google Scholar] [CrossRef]
- Safari, V.; Kamau, J.; Nthiga, P.; Ngugi, M.; Orinda, G.; Njagi, E. Antipyretic, antiinflammatory and antinociceptive activities of aqueous bark extract of Acacia nilotica (L.) Delile in albino mice. Pain Manag. Med. 2016, 2, 113. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Mishra, A. Anti-inflammatory activity of methanolic (seed) extract of Acacia nilotica L. Del mediated through cyclooxygenase (cox) inhibition. Plant Arch. 2020, 20, 776–780. [Google Scholar]
- Devaraj, E.; Lakshmi, T. Cytotoxic and apoptotic effects of acacia catechu in hepatocellular CARCINOMA HepG2 cells. J. Clin. Exp. Hepatol. 2017, 7, S76. [Google Scholar] [CrossRef]
- Mattana, C.M.; Cangiano, M.A.; Alcaraz, L.E.; Sosa, A.; Escobar, F.; Sabini, C.; Sabini, L.; Laciar, A.L. Evaluation of cytotoxicity and genotoxicity of Acacia aroma leaf extracts. Sci. World J. 2014, 2014, 380850. [Google Scholar] [CrossRef]
- Manzo, L.M.; Moussa, I.; Ikhiri, K. Cytotoxic effect of various solvent extracts of Acacia nilotica pods on human erythrocyte cells. Int. J. Biol. Chem. Sci. 2022, 16, 2911–2915. [Google Scholar] [CrossRef]
- Buttner, D.H.; Reddy, S.; Koekemoer, T.; van de Venter, M. An in vitro assessment of the potential antidiabetic activity and cytotoxic effects of ethanolic and aqueous extracts from three invasive Australian acacias. S. Afr. J. Bot. 2021, 141, 1–11. [Google Scholar] [CrossRef]
- Mohan, S.; Thiagarajan, K.; Chandrasekaran, R.; Arul, J. In vitro protection of biological macromolecules against oxidative stress and in vivo toxicity evaluation of Acacia nilotica (L.) and ethyl gallate in rats. BMC Complement. Altern. Med. 2014, 14, 257. [Google Scholar] [CrossRef]
- Adewale, A.L. Evaluation of root extract of acacia nilotica on haematological and lipid profile in rats. Eur. J. Med. Plants. 2016, 17, 1–7. [Google Scholar] [CrossRef]
- Umaru, B.; Saka, S.; Mahre, M.; Ojo, N.; Dogo, H.; Onyeyili, P. Acute toxicity and effects of aqueous pod extract of Acacia nilotica on some haematological parameters and body weight in rats. Int. J. Health Med. Inf. 2015, 4, 37–42. [Google Scholar]
- Sarkiyayi, S.; Abubakar, M. Effect of ethanol leaf extract of Acacia nilotica on phenyl hydrazine induced anemia in rats. IOSR J. Pharm. Biol. Sci. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Thangavelu, L.; Balusamy, S.R.; Shanmugam, R.; Sivanesan, S.; Devaraj, E.; Rajagopalan, V.; Veeraiyan, D.N.; Chellappan, D.K.; Dua, K.; Kim, Y.J.; et al. Evaluation of the sub-acute toxicity of Acacia catechu Willd seed extract in a Wistar albino rat model. Regul. Toxicol. Pharmacol. 2020, 113, 104640. [Google Scholar] [CrossRef] [PubMed]
- Magnini, R.D.; Nitiema, M.; Ouedraogo, G.G.; Ilboudo, S.; Bance, A.; Millogo-Kone, H.; Giorgio, C.D.; Pages, J.M.; Hilou, A.; Davin-Regli, A. Toxicity and bacterial anti-motility activities of the hydroethanolic extract of Acacia senegal (L.) Willd (Fabaceae) leaves. BMC Complement. Med. Ther. 2021, 21, 178. [Google Scholar] [CrossRef]
- Selogatwe, K.M.; Asong, J.A.; Struwig, M.; Ndou, R.V.; Aremu, A.O. A review of ethnoveterinary knowledge, biological activities and secondary metabolites of medicinal woody plants used for managing animal health in South Africa. Vet. Sci. 2021, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K.; Wilson, I.D. Directly coupled HPLC–NMR and HPLC–NMR–MS in pharmaceutical research and development. J. Chromatogr. B Biomed. Sci. Appl. 2000, 748, 233–258. [Google Scholar] [CrossRef]
- Parys, W.; Dołowy, M.; Pyka-Pająk, A. Significance of chromatographic techniques in pharmaceutical analysis. Processes 2022, 10, 172. [Google Scholar] [CrossRef]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial activity of quercetin, naringenin and catechin: Flavonoids inhibit Staphylococcus aureus-induced hemolysis and modify membranes of bacteria and erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef]
- Wang, S.; Chang, Y.; Xu, J.; Zhao, C.; Song, Y.; Liu, M.; Tian, C. Comparative study of anti-inflammatory, antioxidant and antibacterial activities of epigallocatechin gallate, epigallocatechin, epicatechin gallate and epicatechin in vitro. Pharmacol. Res. Mod. Chin. Med. 2025, 15, 100599. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Akhtar, N.; Alsayegh, A.A.; Abusudah, W.F.; Almohmadi, N.H.; Shaheen, H.M.; Singh, T.G.; De Waard, M. Bioactive compounds, pharmacological actions, and pharmacokinetics of genus Acacia. Molecules 2022, 27, 7340. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; Li, J.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary β-sitosterol regulates serum lipid level and improves immune function, antioxidant status, and intestinal morphology in broilers. Poult. Sci. 2020, 99, 1400–1408. [Google Scholar] [CrossRef]
- Rodríguez, B.; Pacheco, L.; Bernal, I.; Piña, M. Mechanisms of action of flavonoids: Antioxidant, antibacterial and antifungal properties. Cienc. Ambiente Clima 2023, 6, 33–66. [Google Scholar] [CrossRef]
- Pikhtirova, A.; Pecka-Kiełb, E.; Zigo, F. Antimicrobial activity of saponin-containing plants: Review. J. Dairy Vet. Anim. Res. 2023, 2, 121–127. [Google Scholar] [CrossRef]
- Hossain, M.T.; Furhatun-Noor, A.; Matin, A.; Tabassum, F.; Ar Rashid, H. A review study on the pharmacological effects and mechanism of action of tannins. Eur. J. Pharm. Med. Res. 2021, 8, 5–10. [Google Scholar]
- Shiu, W.K.; Malkinson, J.P.; Rahman, M.M.; Curry, J.; Stapleton, P.; Gunaratnam, M.; Neidle, S.; Mushtaq, S.; Warner, M.; Livermore, D.M. A new plant-derived antibacterial is an inhibitor of efflux pumps in Staphylococcus aureus. Int. J. Antimicrob. Agents. 2013, 42, 513–518. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, D.; Saini, M.; Jain, A.; Chaudhary, J.; Kaur, N.; Sahoo, S.; Singh, S.K.; Goyal, K.; Rekha, A. Flavonoids as antimicrobial agents: A comprehensive review of mechanisms and therapeutic potential. Curr. Pharm. Biotechnol. 2025, 26, 1–19. [Google Scholar] [CrossRef] [PubMed]

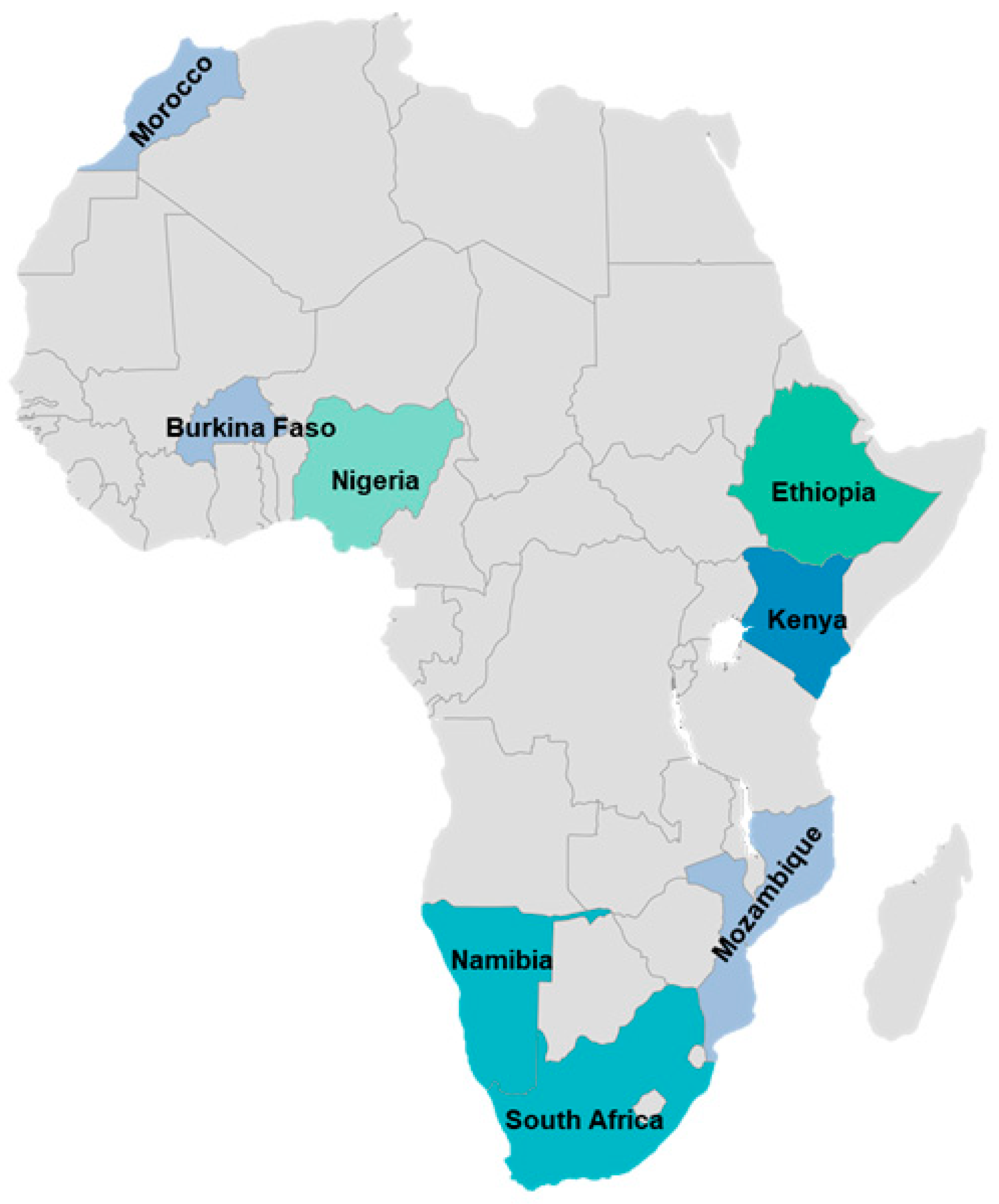
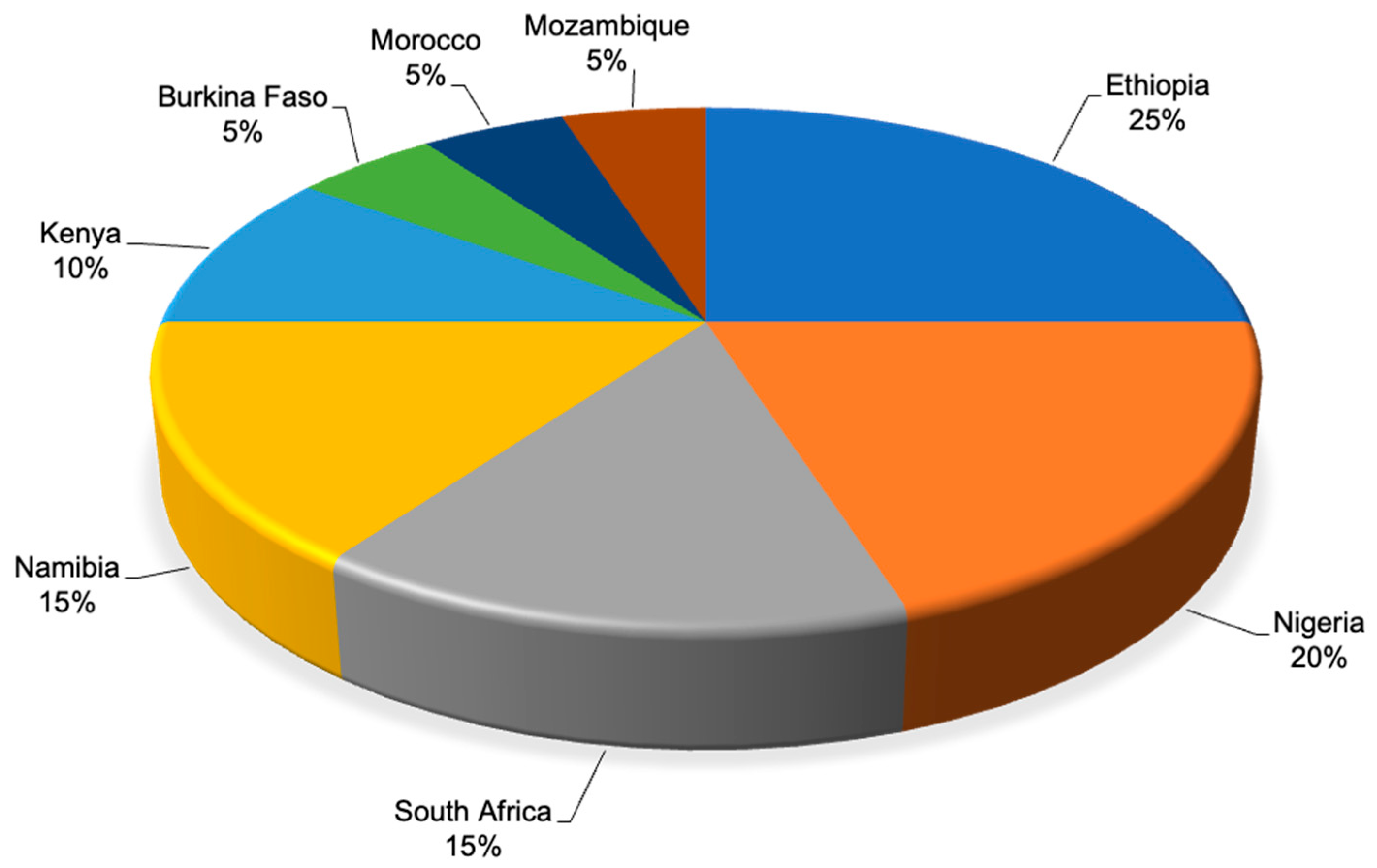
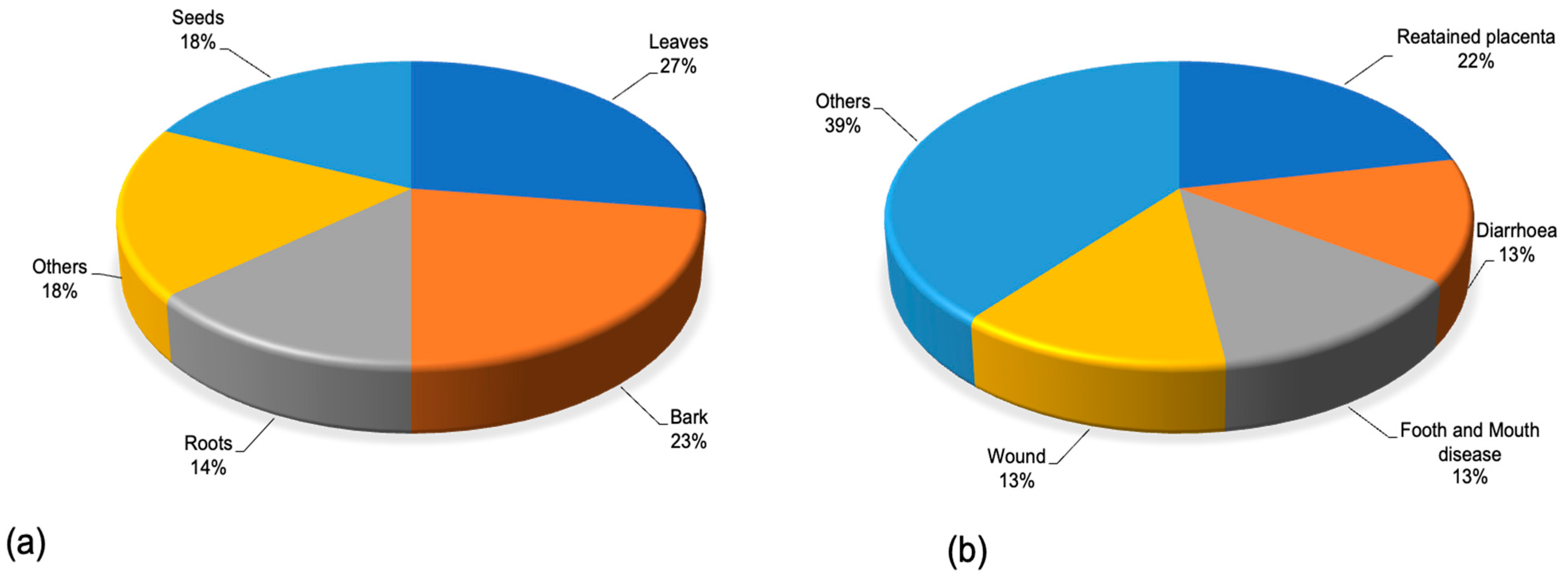
| Plant Species (Accepted Name) | Plant Species (Synonym) | Plant Part Used | Disease/Condition | Livestock | Country | Preparation Method | Dosages and Mode of Application | Reference |
|---|---|---|---|---|---|---|---|---|
| Acacia mearnsii De Wild | - | Leaves | Coughing | Ruminants, pigs, poultry | Kenya | Infusion | Not specified. | [29] |
| Acacia sp. | - | Leaves | Wound | Small ruminants | Mozambique | Leaves are ground along with salt | Ground material is topically applied. | [16] |
| Faidherbia albida (Delile) A. Chev. | Roots | Urinary problems | Sheep | Morocco | Decoction | Root decoction is administered orally to the diseased animal. | [10] | |
| Senegalia asak (Forssk.) Kyal. & Boatwr. | Acacia asak (Forssk.) Willd. | Bark | Dystocia and retained placenta | Large and small ruminants | Ethiopia | Bark is used as fresh material | Bark is tied to the neck of the diseased animal. | [15] |
| Senegalia macrostachya Rchb. ex DC. | Acacia macrostachya Rchb. ex DC. | Leaves | Anthelmintic | Cattle | Burkina Faso | Maceration | Leaves are given to the cattle orally (two doses) on two consecutive days. | [30] |
| Vachellia abyssinica Benth. | Vachellia abyssinica Benth. | Leaves | Snakebite | Bovine | Ethiopia | Not specified | Freshly ground leaf juice is applied topically (daily) until the wound heals. | [31] |
| Vachellia drepanolobium (Harms ex siostedt) | Acacia drepanolobium Harms ex siostedt | Bark | Retained placenta | Cattle | Kenya | Decoction | Not specified. | [29] |
| Vachellia erioloba E. Mey | Acacia erioloba E. Mey | Bark | Retained placenta | Not specified | Namibia | Infusion | Infusion is given orally to the diseased animal. | [32] |
| Vachellia karoo Hayne | Acacia karoo Hayne | Bark | Fractures and Diarrhea | Cattle | South Africa | Not specified | Not specified. | [33] |
| Vachellia karoo Hayne | Acacia karoo Hayne | Roots | Eye problems | All livestock | Namibia | Infusion | Infusion is applied topically to treat eye inflammation. | [32] |
| Vachellia karroo Hayne | Acacia karroo Hayne | Bulb | Retained placenta and bacterial infection | Cattle | South Africa | Maceration | Bulbs are given orally to the diseased animal. | [34] |
| Vachellia nilotica (L.) Wild | Acacia nilotica (L.) Wild | Seed | Diarrhea | Cattle | Nigeria | Seeds are soaked in water for 24 h | The mixture is administered orally to the diseased animals. | [35] |
| Vachellia nilotica (L.) Wild | Acacia nilotica (L.) Wild | Bark | Retained placenta | Cattle | Namibia | Infusion | Infusion is administered orally to the diseased animal. | [32] |
| Vachellia nilotica (L.) Wild | Acacia nilotica (L.) Wild | Seeds | Foot and mouth disease | Cattle and sheep | Nigeria | Infusion | Not specified. | [36] |
| Vachellia nilotica (L.) | Acacia nilotica (L.) | Seeds | Foot and mouth disease | Cattle | Nigeria | Seeds are boiled with salt | Mixture is used to wash the affected areas. | [35] |
| Vachellia nilotica (L.) | Acacia nilotica (L.) | Seeds | Foot and mouth disease | Cattle | Nigeria | The seeds are soaked in cow urine for 24 h | Infusion is used to wash the affected areas. | [35] |
| Vachellia oerfota Forssk. | Acacia oerfota (Forssk.) | Leaves, stem, roots | Kidney problem | Cattle | Ethiopia | Decoction | Decoction is administered orally (1–2 cups). | [37] |
| Vachellia sieberiana DC. | Acacia sieberiana DC. | Leaves | Wound | Large ruminants | Ethiopia | Leaves are crushed and mixed with water | Mixture is applied as a topical ointment to the wounded area. | [15] |
| Vachellia tortilis (Forssk.) Galasso & Banfi | Acacia tortilis (Forssk.) Hayne | Branch tips | Diarrhea | Cattle | South Africa | Not specified | Not specified. | [33] |
| Vachellia tortilis (Forssk.) Hayne. | Acacia tortilis (Forssk.) Galasso & Banfi | Gum | Wound | All livestock | Ethiopia | Not specified | Gum is applied topically to the wounded area. | [38] |
| Plant Species (Accepted Name) | Plant Species (Synonym) | Plant Part | Extraction Solvent | Strain(s) Tested | Extract(s) Concentration | Summary of the Tested Extracts | Reference |
|---|---|---|---|---|---|---|---|
| Acacia decurrens Willd. | Mimosa decurrens J.C. Wendl. | Stem bark | Ethyl acetate, methanol | Staphylococcus aureus, Escherichia coli, Salmonella typhi, Klebsiella pneumoniae, Micrococcus luteus, Shigella sonnei, Staphylococcus epidermis, listeria monocytogenes, Enterococcus faecalis | 0.0125–0.05 mg/mL | Ethyl acetate and methanol fractions showed excellent activity against the tested microorganisms with MIC value of 0.0125 mg/mL, comparable to the standard antibiotic ampicillin. | [40] |
| Acacia mearnsii De Wild. | Racosperma mearnsii (De wild.) Pedley | Stem bark | Hexane, methanol, ethyl acetate, dichloromethane | S. aureus, S. epidermidis, B. cereus, Micrococcus kristinae, S. faecalis, E. coli, Pseudomonas aeruginosa, Shigella flexneri, Klebsiella pneumonia, Serratia marcescens | 1–10 mg/mL | Hexane extract showed relatively weak activity against S. aureus and E. coli (MIC = 10 mg/mL), with no activity observed against Shigella Flexneri and Klebsiella pneumonia. Methanol extracts had moderate activity when tested against Gram-positive strains and E. coli (MIC = 5 mg/mL). The MIC values of ethyl acetate extracts ranged from 1 to 10 mg/mL against all tested bacterial strains. | [41] |
| Senegalia burkei Benth. | Acacia burkei (Benth.) | Bark | Dichloromethane: methanol, water | Bacillus cereus, Enterococcus faecalis, Escherichia coli, Salmonella typhimurium, Shigella flexneri and Staphylococcus aureus | 0.25–3 mg/mL | Organic extract displayed noteworthy activity against P. vulgarius and S. aureus, 0.50 and 0.25 mg/mL, respectively. Water extracts showed mainly moderate activity (1–3 mg/mL) against tested strains with exception observed for B. cereus (0.75 mg/mL). | [42] |
| Senegalia catechu (L.f.) | Acacia catechu (L.f.) | Leaves | Methanol | B. subtilis, S. aureus, S. typhi, E. coli, P. aeruginosa and Candida. albicans | 1–2 mg/mL | Leaf extract exhibited good activity against S. typhi (0.7 mg/mL), with moderate (1–2 mg/mL) microbial activity against the other tested microorganisms. | [43] |
| Senegalia polyacantha Willd. | Acacia polyacantha (Willd.) | Trunk bark | Ethanol, hydroethanol, aqueous | S. aureus, E. coli, S. typhi, K. pneumoniae | 1.56–>100 mg/mL | Ethanolic stem bark extract displayed moderate activity against S. aureus (MIC = 1.56 mg/mL, MBC = 6.25 mg/mL) and E. coli (MIC = 3.13 mg/mL, MBC = 25 mg/mL), while the aqueous extract demonstrated weak activity against S. typhi with MIC and MBC values of 12.50 mg/mL and 100 mg/mL, respectively. | [44] |
| Vachellia drepanolobium Harms ex Y.sjostedt | Acacia drepanolobium Harms ex Y.sjostedt | Stem bark | Methanol | E. coli, S. aureus, K. pneumoniae, P. aeruginosa, S. typhi, P. vulgaris, Candida albicans | 0.31–5 mg/mL | Stem bark extract exhibited good to moderate antimicrobial activity against all tested microorganisms with MIC ranging from 0.3125 to 5 mg/mL. | [45] |
| Vachellia nilotica (L.) | Acacia nilotica (L.) | Leaves Stem bark | Ethanol | S. aureus, B. subtilis, P. aeruginosa, E. coli | 15.6–125 mg/mL | Ethanolic leaf extracts showed weak activity against the test pathogens, with MIC values ranging from 15.6 to 31.3 mg/mL and values at 31.3 mg/mL. For stem bark extracts, MIC was 125 mg/mL and MBC was 250 mg/mL for all test pathogens. | [46] |
| Vachellia nilotica (L.) | Acacia nilotica (L.) | Leaves | Ethanol | Campylobacter coli | 3 mg/mL, 30 mg/mL and 70 mg/mL | Ethanolic leaf extract exhibited weak activity against C. coli with MIC value of 70 mg/mL. | [47] |
| Vachellia nilotica (L.) | Acacia nilotica (L.) | Bark, leaves | Acetone, water | S. aureus, S. uberis, S. agalactiae, S. chromogenes, S. epidermidis, Klebsiella pnuemoniae, E. coli, Pseudomonas aeruginosa, P. vulgaris, E. aerogenes and Proteus mirabilis | 0.039 to 0.625 mg/mL | Acetone bark extract demonstrated noteworthy activity against all the tested bacterial strains (clinical isolates, ATCC and field strains) with an MIC ranging between 0.039 and 0.3130 mg/mL. Bark water extracts also showed strong activity amongst majority of the tested strains. Leaf extracts (acetone and water) showed good to moderate activity. | [48] |
| Vachellia nilotica (L.) | Acacia nilotica (L.) | Fruits | Methanol | Salmonella typhi, P. aeruginosa, B. cereus, E. coli, K. pneumonia and Shigella flexneri | 100 mg/mL | Fruit extracts showed the largest inhibition zone against S. typhi (39 mm) and B. cereus (30 mm) at 100 mg/mL concentration relative to gentamicin, zone of inhibition (10 μg/disc). | [49] |
| Vachellia nilotica (L.) | Acacia nilotica (L.) | Leaves, pods, bark | Ethanol | S. typhimurium, S. paratyphi, Salmonella sp., S. dysenteriae, Shigella sp., and S. flexneri | 50–200 mg/mL | Leaves, pods, bark displayed a synergistic effect when tested against multi-drug-resistant bacteria, with an inhibition zone ranging from 15 to 22.7 mm and MIC values between 100 and 200 mg/mL. | [50] |
| Vachellia xanthophloea Benth. | Acacia xanthophloea (Benth.) Banfi & Galasso | Leaf (Ag-NPs) | Aqueous | Staphylococcus aureus, E. coli | Silver nanoparticles showed excellent activity against S. aereus (MIC = 0.04 mg/mL, MBC = 0.04 mg/mL) and weak activity for E. coli (MIC = 0.33 mg/mL, MBC = 0.66 mg/mL). | [51] |
| Bioassay | Plant Species (Accepted Name) | Plant Species (Synonym) | Plant Part | Extraction Solvent | Concentration Tested | Summary of the Tested Extracts | Reference |
|---|---|---|---|---|---|---|---|
| In vitro—Anthelmintic | Senegalia senegal (L.) | Acacia senegal (L.) | Stem bark | Methanol | 0.25–1 mg/mL | Methanol stem bark extract demonstrated significant anthelmintic activity against Fasciola gigantica. The extract achieved 100% mortality at concentrations of 1 mg/mL and 0.5 mg/mL within 6 to 12 h, while 0.25 mg/mL resulted in 100% mortality within 24 h. | [52] |
| In vitro—Anthelmintic | Vachellia xanthophloea Benth. | Acacia xanthophloea (Benth.) | Bark | Methanol, cold water, boiled water | 8, 16, 24, 32, 40% v/v | Methanolic extract demonstrated significant anthelmintic activity against L3 larvae of gastrointestinal nematodes in goats. | [53] |
| In vitro—Anti-inflammatory | Vachellia Sieberiana DC. | Acacia Sieberiana (DC.) | Leaves | Methanol, acetone | 0.39–2 mg/mL | Methanol extracts inhibited NO (0.094 mg/mL) with the acetone extract exhibiting moderate inhibition of 15-LOX (IC50—2 mg/mL). | [54] |
| In vitro—Anti-inflammatory | Vachellia farnesiana (L.) | Acacia farnesiana (L.) | Leaves, bark | Ethanol | 0.05 mg/mL | In most cases, bark extract inhibited COX-1 more than COX-2 enzyme, whereas leaf extract demonstrated higher anti-inflammatory activity against COX-2 relative to COX-1 enzyme. | [55] |
| In vitro—Antioxidant | Senegalia ataxacantha DC. | Acacia ataxacantha (DC.) | Bark | Hexane, methanol, dichloromethane, ethyl acetate, 70% ethanol | 74.18 mg, GAE/100 mg, 23.14–26.65 mg QE/100 mg, 0.1 mg/mL | Ethyl acetate extract demonstrated antioxidant activity with DPPH radical scavenging inhibition of 92.62% and a FRAP value of 1273.63 µmol AAE/g. | [56] |
| In vitro—Antioxidant | Vachellia karroo Hayne. | Acacia karroo (Hayne.) | Leaves | Aqueous, acetone | 0.2–1 mg/mL | Acetone and aqueous leaf extracts showed good antioxidant activity with observed IC50 values of 0.62 and 0.67 mg/mL (DPPH), 0.56 and 0.59 mg/mL (FRAP), as well as 0.43 and 0.60 mg/mL (NO), respectively. | [57] |
| In vitro—Antioxidant | Vachellia eriolaba | Acacia eriolaba | Leaves | Methanol, acetone | 0.001–1 mg/mL | Acetone extract demonstrated strong antioxidant activity using the ABTS (0.001–0.14 mg/mL) and DPPH (0.003–1 mg/mL) assays. | [54] |
| In vitro—Antioxidant | Acacia decurrens Willd. | Mimosa decurrens J.C. Wendl. | Stem bark | Ethyl acetate, methanol | 0.0125–0.05 mg/mL | The ethyl acetate and methanol fractions showed good antioxidant activity with IC50 values of 42.2–49.6 mg/mL for ABTS and 0.0378–0.075 mg/mL for DPPH compared to the standard ascorbic acid. | [40] |
| In vitro—Cytotoxicity | Vachellia gerrardii Benth. | Acacia gerrardii (Benth.) | Leaves | Methanol, acetone | 0.039 mg/mL to 2.5 mg/mL | Methanol extract showed moderate cytotoxicity against RAW 264.7 macrophages with an LC50 value of 78.22 mg/mL. | [54] |
| In vitro—Cytotoxicity | Vachellia nilotica (L.) | Acacia nilotica (L.) | Bark, leaves | Acetone, water | 0.0097 to >2.5 mg/mL | The cytotoxicity assays revealed that both bark and leaf extracts showed toxicity to mammalian cells, with bark extract showing a high level of toxicity (LC50 value of 0.029 mg/mL) and leaf extract displaying moderate toxicity with an LC50 value of 0.069 mg/mL. | [48] |
| In vitro—Cytotoxicity | Vachellia eriolaba E. Mey. | Acacia eriolaba E. Mey. | Leaves | Methanol, acetone | 0.039 mg/mL to 2.5 mg/mL | Methanol extract demonstrated low cytotoxicity, >1000 µg/mL. | [54] |
| In vitro—Cytotoxicity | Acacia mearnsii De Wild. | Racosperma mearnsii (De Wild.) Pedley | Stem bark | Acetone | 0.031–0.50 ug/mL | Extract showed non-toxic effects with LC50 > 0.1 mg/mL in the brine shrimp lethality assay. | [58] |
| In vitro—Cytotoxicity | Vachellia nilotica (L.) | Acacia nilotica (L.) | Pods with seeds | Ethanol | 0.0101–0.016 mg/mL | Pod extract exhibited significant cytotoxicity with an LC50 value of 0.0101 mg/mL, indicating potential toxicity. | [59] |
| In vivo—Antioxidant | Vachellia nilotica (L.) | Acacia nilotica (L.) | Pods | Aqueous | 1, 3, 5, 7.5, 10, 15 g/kg body weight | Aqueous extracts were reported to be toxic to broilers at the highest tested dose, 15 g/kg, causing liver damage. | [60] |
| In vivo—Anti-inflammatory | Vachellia karroo Hayne. | Acacia karroo (Hayne.) | Stem bark | Aqueous | 100–200 mg/kg | Aqueous extract exhibited good anti-inflammatory and analgesic activities at doses of 100 and 200 mg/kg in the animal model. | [61] |
| In vivo—Toxicity | Vachellia nilotica (L.) | Acacia nilotica (L.) | Roots | Aqueous | 50, 300, 500, 2000 mg/kg (acute), 125, 250, 500 mg/kg (sub-acute) | Aqueous extract was safe in single-dose administration but repeated doses above 250 mg/kg led to hepatotoxicity. | [62] |
| In vivo—Toxicity | Vachellia nilotica (L.) | Acacia nilotica (L.) | Leaves | Aqueous | 250, 500, 1000 mg/kg | LD50 (3808 mg/kg) showed safety in acute exposure. The sub-acute exposure (28 days) at 500 and 1000 mg/kg caused mild hepatic and nephron toxicity. | [63] |
| In vivo—Toxicity | Vachellia nilotica (L.) | Acacia nilotica (L.) | Stem bark | Methanol | 600, 800, 1000, 1200 mg/kg | Extract was safe at LD50 (1200 mg/kg) as no mortality was observed, and the animals did not exhibit signs of toxicity. | [64] |
| In vivo—Toxicity | Vachellia sieberiana DC. | Acacia Sieberiana (DC.) | Stem bark | Acetone | 300, 600, 1200 mg/kg | No mortality or abnormal behaviour observed in rats at LD50 > 2000 mg/kg. | [65] |
| In vivo—Toxicity | Senegallia polyacantha (Willd.) | Acacia polyacantha Willd. | Leaves | Methanol | 5000 mg/kg | LD50 > 5000 mg/kg showed no extract toxicity. | [66] |
| In vivo—Toxicity | Senegalia ataxacantha (DC.) Kyal. & Boatwr. | Senegalia ataxacantha (DC.) Kyal. & Boatwr. | Leaves | Methanol | 50, 200, 400 mg/kg | Methanol extract was safe on acute exposure. However, prolonged use may produce harmful effects on the liver, kidney and stomach. | [67] |
| Plant Species | Synonym | Plant Part | Extraction Solvent | Isolated Compound | Method | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| Acacia saligna (Labill.) H.L Wendl | Mimosa Saligna Labill. | Leaves | Methanol | (25S)-5b-spirostan-3β-yl-3-O-b-Dxylopyranosyl(1–3)-O-β-D-xylopyranosyl(1–4)-β-D-galactopyranoside, myricetin-3-O-rhamnoside (C7-O-C7) myricetin-3-O-rhamnoside, 3β-O-trans-pcoumaroyl-erythrodiol, quercetin-3-O-α-L-rhamnoside and myricetin-3-O-β-Lrhamnoside | TLC, UV, NMR | Antioxidant, Cytotoxicity | [68] |
| Senegalia ataxacantha (DC.) Kyal. & Boatwr. | Acacia ataxacantha DC. | Bark | Hexane, Dichloromethane, ethyl acetate, methanol | Lupeol, betulinic acid, betulinic acid-3-trans-caffeate | Column Chromatography (silica gel), TLC, HPLC, UV, NMR, Mass Spectrometry | Antimicrobial, Antioxidant | [17] |
| Senegalia polyacantha (Willd.) Seigler & Ebinger | Acacia polyacantha Willd. | Leaves | Methanol | Stigmasterol, β-Amyrin, 3-O-Methyl-D-chiro-inositol, Epicatechin, Quercetin-3-O-galactoside, 3-O-[β-D-xylopyranosyl-(1⟶4)-β-D-galactopyranosyl]-oleanolic acid, 3-O-[β-galactopyranosyl-(1⟶ 4)-β-D-galactopyranosyl]-oleanolic acid | Column chromatography (silica gel), TLC, Sephadex LH-20 chromatography, UV, NMR, Mass Spectrometry | Antibacterial | [69] |
| Senegalia polyacantha (Willd.) Seigler & Ebinger | Acacia polyacantha Willd. | Stem Bark | Methanol | Lupeol, 2,3-Dihydroxypropyltetracosanoate, Methyl Gallate | Column chromatography (silica gel), TLC, Sephadex LH-20 chromatography, UV, NMR, Mass Spectrometry | Antibacterial | [69] |
| Senegalia polyacantha (Willd.) Seigler & Ebinger | Acacia polyacantha Willd. | Leaves | Methanol | Stigmasterol, β-Amyrin, 3-O-β-D-glucopyranosylstigmasterol, 3-O-methyl-D-chiro-inositol, Epicatechin, Quercetin-3-O-glucoside, 3-O-[β-D-xylopyranosyl-(1⟶4)-β-D-galactopyranosyl]-oleanolic acid, 3-O-[β-galactopyranosyl-(1⟶4)-β-D-galactopyranosyl]-oleanolic acid | Column chromatography (silica gel), TLC, Sephadex LH-20 chromatography, UV, NMR, Mass Spectrometry | Antibacterial | [70] |
| Vachellia xanthophloea (Benth.) Banfi & Galasso | Acacia xanthophloea Benth. | Leaves | Dichloromethane/methanol (1:1) | Methyl Gallate, 3-O-Methylquercetin, Kaempferol, Apigenin, Pinoresinol, Lupeol, Phytol | Column chromatography (silica gel), TLC, Sephadex LH-20 chromatography, HPLC, UV, NMR | Antiplasmodial | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msimango, N.N.P.; Aremu, A.O.; Amoo, S.O.; Masondo, N.A. Ethnoveterinary Potential of Acacia (Vachellia and Senegalia) Species for Managing Livestock Health in Africa: From Traditional Uses to Therapeutic Applications. Plants 2025, 14, 3107. https://doi.org/10.3390/plants14193107
Msimango NNP, Aremu AO, Amoo SO, Masondo NA. Ethnoveterinary Potential of Acacia (Vachellia and Senegalia) Species for Managing Livestock Health in Africa: From Traditional Uses to Therapeutic Applications. Plants. 2025; 14(19):3107. https://doi.org/10.3390/plants14193107
Chicago/Turabian StyleMsimango, Nokwethemba N. P., Adeyemi O. Aremu, Stephen O. Amoo, and Nqobile A. Masondo. 2025. "Ethnoveterinary Potential of Acacia (Vachellia and Senegalia) Species for Managing Livestock Health in Africa: From Traditional Uses to Therapeutic Applications" Plants 14, no. 19: 3107. https://doi.org/10.3390/plants14193107
APA StyleMsimango, N. N. P., Aremu, A. O., Amoo, S. O., & Masondo, N. A. (2025). Ethnoveterinary Potential of Acacia (Vachellia and Senegalia) Species for Managing Livestock Health in Africa: From Traditional Uses to Therapeutic Applications. Plants, 14(19), 3107. https://doi.org/10.3390/plants14193107








