ZnONPs Alleviates Salt Stress in Maize Seedlings by Improving Antioxidant Defense and Photosynthesis Potential
Abstract
1. Introduction
2. Results
2.1. Combined Analysis of Different Treatments and Genotypes of Various Traits in Maize
2.2. Phenotypic Changes in Two Maize Genotypes Under Different Treatments
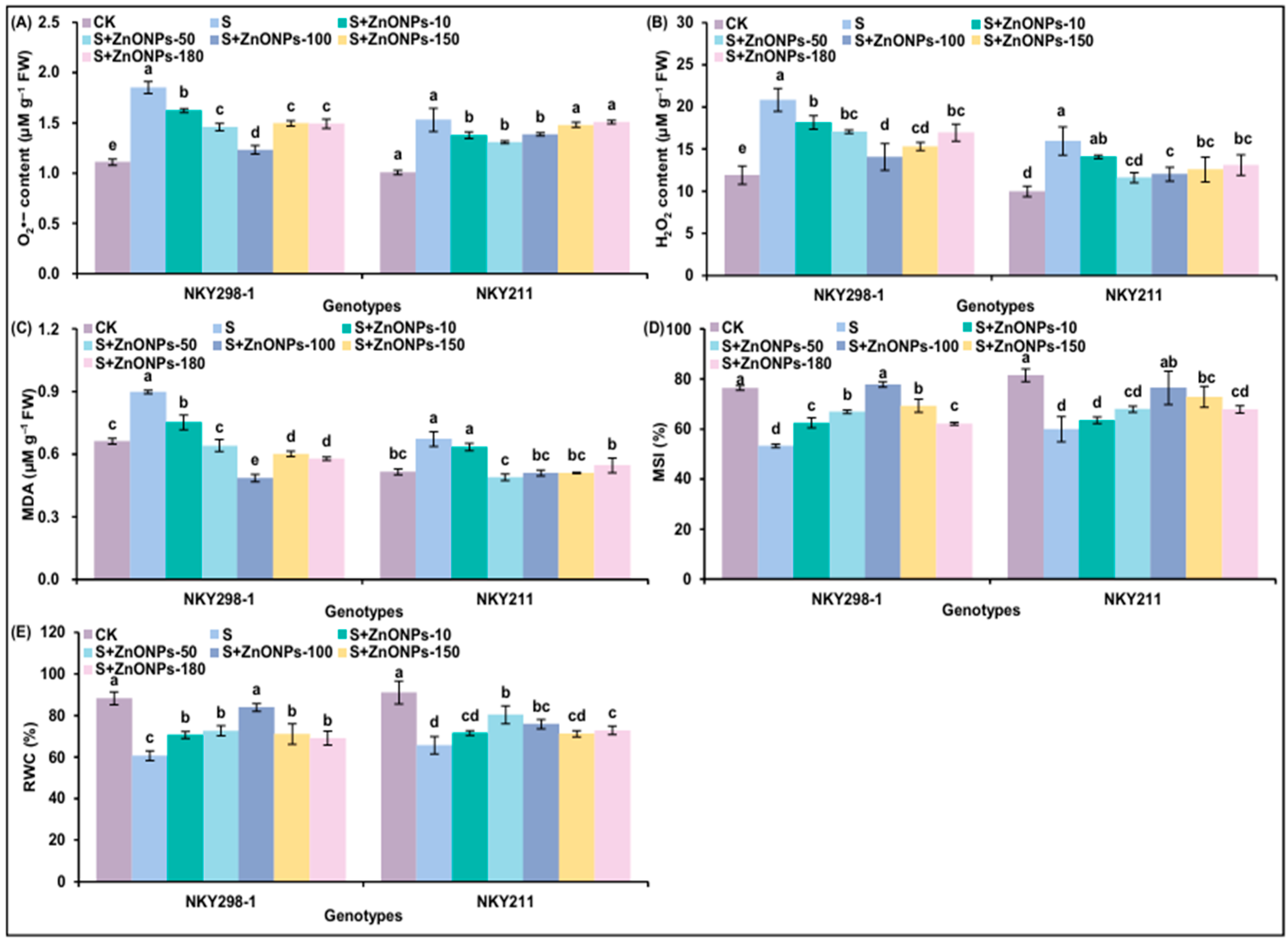
2.3. The Responses in Oxidative Stress and Membrane Stablity of Two Maize Genotypes Under Different Treatments
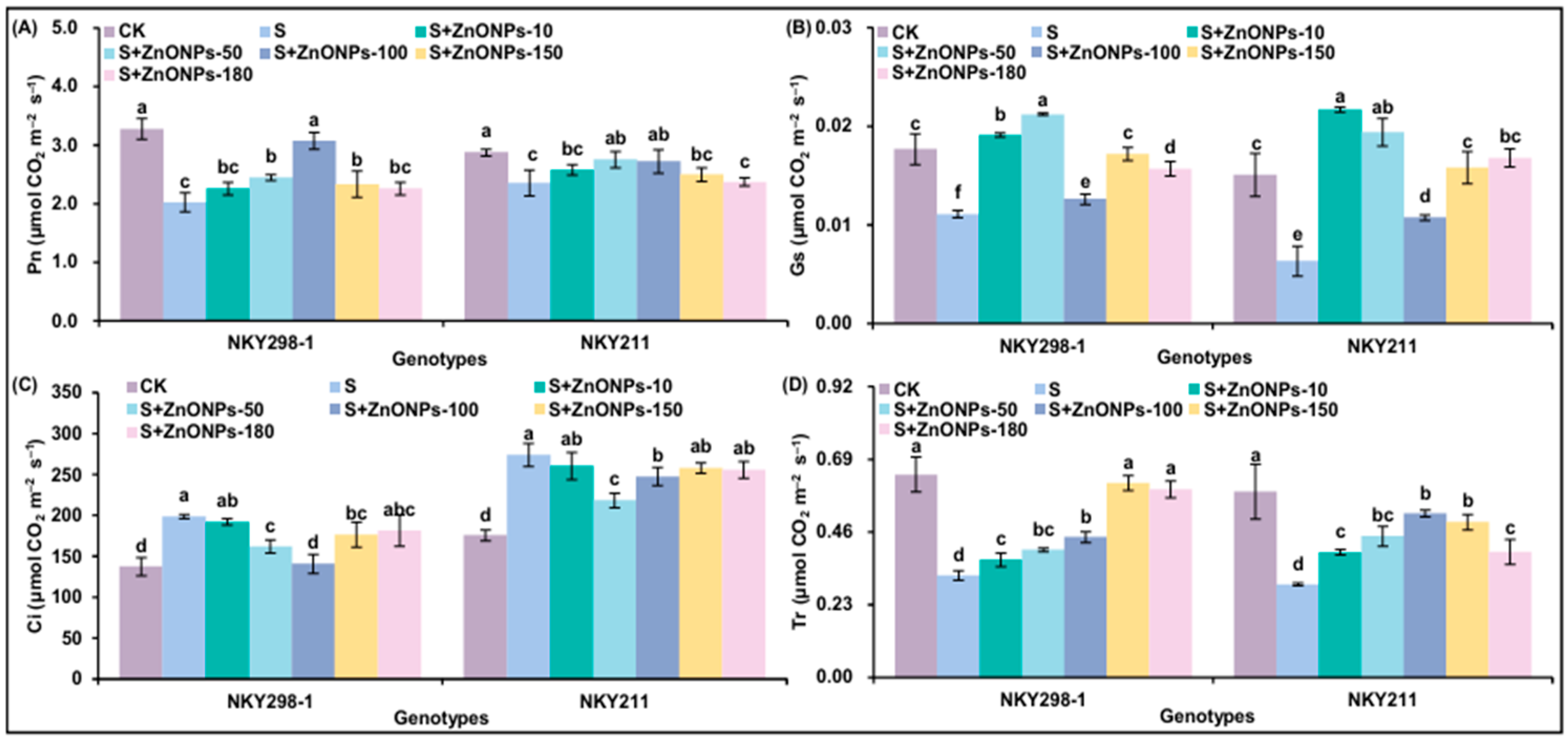
2.4. The Changes in Photosynthetically Related Parameters of Two Maize Genotypes Under Different Treatments
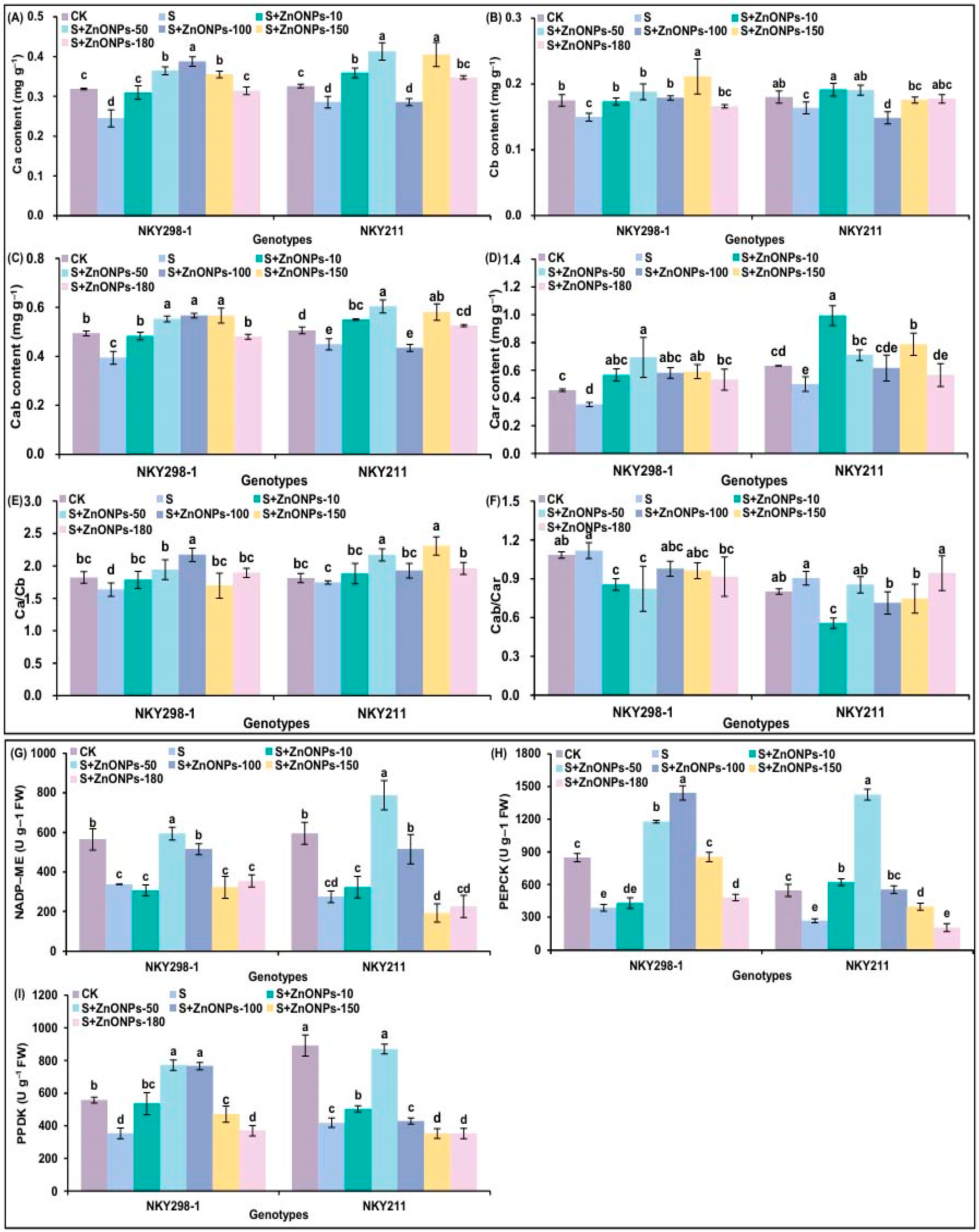
2.5. The Changes in Antioxidant Enzyme Activity of Two Maize Genotypes Under Different Treatments
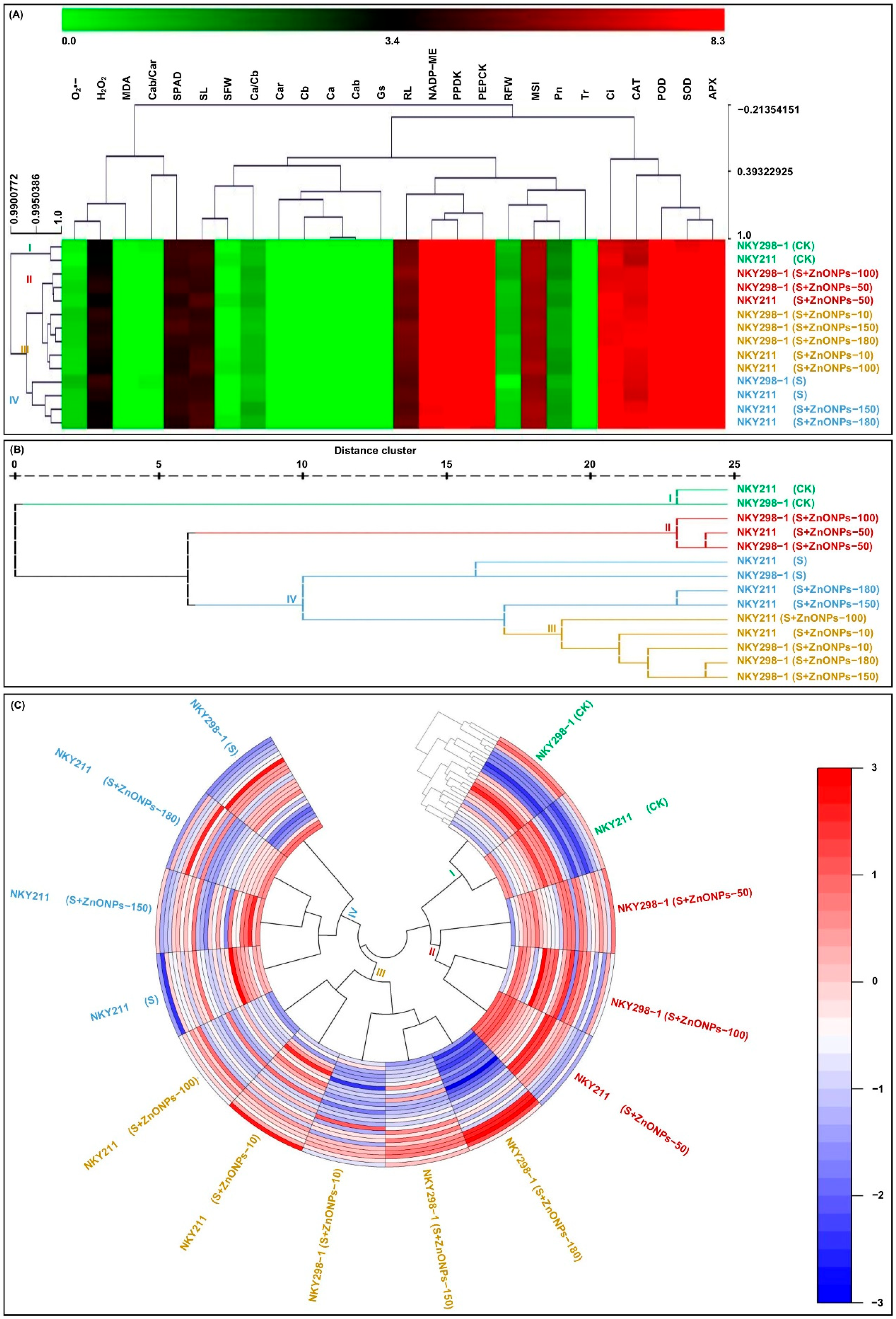
2.6. Cluster Analysis
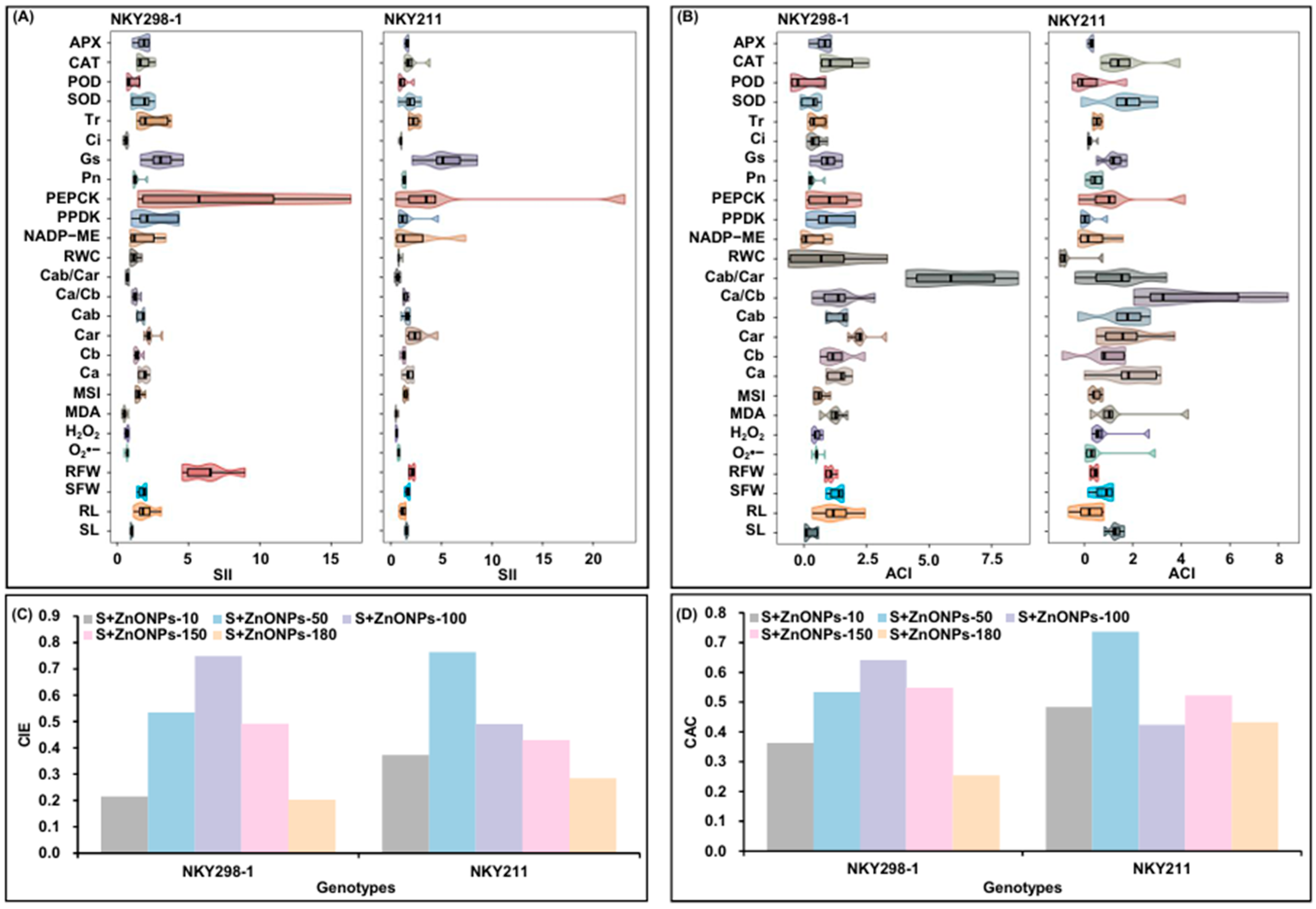
2.7. Comprehensive Evaluation
3. Discussion
4. Materials and Methods
4.1. Maize Materials and Experimental Design
4.2. Phenotypic and Physiological Assays
4.3. Comprehensive Evaluation Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.M.; Chen, Z.T.; Sui, N. Sensitivity and response of chloroplasts to salt stress in plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, B.S. Protection of halophytes and their uses for cultivation of saline-alkali soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar]
- Zhu, Z.Y.; Dai, Y.; Yu, G.R.; Zhang, X.; Chen, Q.; Kou, X.B.; Mehareb, E.M.; Raza, G.; Zhang, B.H.; Wang, B.H.; et al. Dynamic physiological and transcriptomic changes reveal memory effects of salt stress in maize. BMC Genom. 2023, 24, 726. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Yao, T.; Chai, J.L.; Han, J.R. Adsorption of sodium ions by exopolysaccharides from pseudomonas simiae MHR6 and its improving of Na+/K+ homeostasis in maize under salt stress. J. Agric. Food Chem. 2023, 71, 19949–19957. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, W.Z.; Lei, G.Q.; Hou, Y.L.; Ao, C.; Chen, H.R.; Gaiser, T.; Srivastava, A.K. The effects of multiwalled carbon nanotubes and Bacillus subtilis treatments on the salt tolerance of maize seedlings. Front. Plant Sci. 2022, 13, 1093529. [Google Scholar] [CrossRef]
- Araújo, G.D.S.; Lopes, L.D.S.; Paula-Marinho, S.D.O.; Mesquita, R.O.; Nagano, C.S.; Vasconcelos, F.R.; Carvalho, H.H.D.; Moura, A.D.A.A.N.; Marques, E.C.; Gomes-Filho, E. H2O2 priming induces proteomic responses to defense against salt stress in maize. Plant Mol. Biol. 2021, 106, 33–48. [Google Scholar] [CrossRef]
- Shelke, D.B.; Pandey, M.; Nikalje, G.C.; Zaware, B.N.; Suprasanna, P.; Nikam, T.D. Salt responsive physiological, photosynthetic and biochemical attributes at early seedling stage for screening soybean genotypes. Plant Physiol. Biochem. 2017, 118, 519–528. [Google Scholar] [CrossRef]
- Fang, H.; Fu, X.Y.; Ge, H.Q.; Jia, M.X.; Ji, J.; Zhao, Y.Z.; Qu, Z.J.; Cui, Z.Q.; Zhang, A.X.; Wang, Y.D.; et al. Genetic analysis and candidate gene identification of salt tolerance-related traits in maize. J. Integr. Agr. 2024, 23, 2196–2210. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhou, X.Y.; Song, H.F.; Zhang, M.; Jiang, C.F. Advances in deciphering salt tolerance mechanism in maize. Crop J. 2023, 11, 1001–1010. [Google Scholar] [CrossRef]
- Buriak, J.M.; Liz-Marzán, L.M.; Parak, W.J.; Chen, X.D. Nano and plants. ACS Nano 2022, 16, 1681–1684. [Google Scholar] [CrossRef]
- Alsaeedi, A.; EI-Ramady, H.; Alshaal, T.; EI-Garawany, M.; Elhawat, N.; AI-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Seleiman, F.M.; Ahmad, A.; Alhammad, A.B.; Tola, E. Exogenous application of zinc oxide nanoparticles improved antioxidants, photosynthetic, and yield Traits in salt-stressed maize. Agronomy 2023, 13, 2645. [Google Scholar] [CrossRef]
- Tessia, R.; Nzumbululo, N.; Takalani, M.; Emmanuel, I.; Noluthando, M.; Fanelwa, A.R. Green-synthesized zinc oxide nanoparticles mitigate salt stress in Sorghum bicolor. Agriculture 2022, 12, 597. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Alhmad, M.F.A.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Tiago, F.J.; Takayuki, T.; Regina, W.; José, C.R.; Fernando, C.L.; Ana, I.R.; Alisdair, R.F.; Carla, A. Salt-stress secondary metabolite signatures involved in the ability of Casuarina glauca to mitigate oxidative stress. Environ. Exp. Bot. 2019, 166, 103808. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhao, C.; Niu, Y.N.; Chao, W.; He, W.; Wang, Y.F.; Mao, T.T.; Bai, X.D. Understanding and comprehensive evaluation of cold resistance in the seedlings of multiple maize genotypes. Plants 2022, 11, 1881. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Z.B.; Yuan, L.Y.; Zhou, H.F.; Hou, X.L.; Liu, T.K. Cold acclimation can specifically inhibit chlorophyll biosynthesis in young leaves of Pakchoi. BMC Plant Biol. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Bajguz, A.; Lobato, A.K.D.S. 24-Epibrassinolide simultaneously stimulates photosynthetic machinery and biomass accumulation in tomato plants under lead stress: Essential contributions connected to the antioxidant system and anatomical structures. Agronomy 2022, 12, 1985. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Ebadi, A.; Habibi, H.; Nabizadeh, E.; Jahanbakhsh, S. Enhancing enzymatic and nonenzymatic response of Echinacea purpurea by exogenous 24-epibrassinolide under drought stress. Ind. Crops Prod. 2020, 146, 112045. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles and 24-epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Merwad, A.-R.M.; Abo El-Maati, M.F.; Mansour, E.; Arnaout, S.M.; Awad, M.F.; Ramadan, M.F.; Ibrahim, S.A. Physiological and biochemical mechanisms of exogenously applied selenium for alleviating destructive impacts induced by salinity stress in bread wheat. Agronomy 2021, 11, 926. [Google Scholar] [CrossRef]
- Medhat, R.; Mohamed, M.K.; Hassan, B. Comparative analysis of physiological parameters, antioxidant defense, ion regulation, and gene expression in two distinct maize hybrids under salt stress at seedling Stage. Life 2025, 15, 591. [Google Scholar] [CrossRef]
- Wang, M.; Guo, W.; Li, J.; Pan, X.; Pan, L.; Zhao, J.; Zhang, Y.; Cai, S.; Huang, X.; Wang, A.; et al. The miR528-AO module confers enhanced salt tolerance in rice by modulating the ascorbic acid and abscisic acid metabolism and ROS scavenging. J. Agric. Food Chem. 2021, 69, 8634–8648. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Ai, P.; Cui, X.; Zhang, Z. ALM1, encoding a Fe-superoxide dismutase, is critical for rice chloroplast biogenesis and drought stress response. Crop J. 2021, 9, 1018–1029. [Google Scholar] [CrossRef]
- Singh, R.B.; Rao, V.P.; Sengar, R.S. Study of salinity induced oxidative stress and antioxidant responses in callus cultures of sugarcane. Ecol. Genet. Genom. 2023, 26, 100164. [Google Scholar] [CrossRef]
- Qi, G.X.; Zhao, X.Q.; He, F.Q.; Sun, S.Q.; Shi, Z.Z.; Niu, Y.N. Exogenous melatonin reinforces photosynthesis, antioxidant defense and gene expression to ameliorate Na2CO3 stress in maize. Plants 2024, 13, 2844. [Google Scholar] [CrossRef]
- Bichara, L.; Slimani, A.; Raklami, A.; Sahlaoui, T.; Tahiri, A.I.; Taoufiq, K.; Biari, K.E.; Faghire, M.; Oufdou, K. Inoculation with rizobacterial consortia from argan tree rhizosphere mitigates salt stress and enhances growth in alfalfa (Medicago sativa L.). J. Crop Health 2025, 77, 135. [Google Scholar] [CrossRef]
- Kumari, R.; Rakhra, G.; Alsahli, A.A.; Bhat, J.A.; Ahmad, P. Exploring the potential of signalling molecules hydrogen sulfide and nitric oxide in augmenting salt stress resilience in bitter gourd. BMC Plant Biol. 2025, 25, 1008. [Google Scholar] [CrossRef]
- Mishra, M.; Afzal, S.; Yadav, R.; Singh, N.K.; Zarbakhsh, S. Salinity stress amelioration through selenium and zinc oxide nanoparticles in rice. Sci. Rep. 2025, 15, 27554. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.Z.; Shahzad, M.; Nauman, M.; Abbasi, A.M.; Reichelt, M.; Mithöfer, A. Silicon-mediated mitigation of salt stress in maize plants. Plant-Environ. Interact. 2025, 6, e70073. [Google Scholar] [CrossRef] [PubMed]
- Rozita, A.K.; Sahebali, B.; Fariborzm, N.Z. Exogenous nitric oxide protect garlic plants against oxidative stress induced by salt stress. Plant Stress. 2022, 5, 100101. [Google Scholar]
- Lin, X.H.; Zhou, M.; Yao, J.; Li, Q.S.; Zhang, Y.Y. Phenotypic and methylome responses to salt stress in arabidopsis thaliana natural accessions. Front. Plant Sci. 2022, 13, 841154. [Google Scholar] [CrossRef]
- Qian, J.J.; Shan, R.; Shi, Y.Q.; Li, H.Z.; Xue, L.S.; Song, Y.; Zhao, T.L.; Zhu, S.J.; Chen, J.H.; Jiang, M. Zinc oxide nanoparticles alleviate salt stress in cotton (Gossypium hirsutum L.) by adjusting Na+/K+ ratio and antioxidative ability. Life 2024, 14, 595. [Google Scholar] [CrossRef]
- Muhammad, A.; Safdar, B.; Saqib, B.; Zubair, A.; Niaz, A.; Tasaddaq, Y.; Ammar, A.R.M.; Jawaher, A.; Yheni, D.; Mohamed, E.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Ahmed, M.; Marrez, A.D.; Rizk, R.; Zedan, M.; Hamid, A.D.; Decsi, K.; Kovács, P.G.; Tóth, Z. The influence of zinc oxide nanoparticles and salt stress on the morphological and some biochemical characteristics of Solanum lycopersicum L. plants. Plants 2024, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Lovely, M.; Marek, Ž.; Maria, B.; Marek, K.; Andrej, F.; Jana, F.; Mlynáriková, D.V.; Marián, B. Effect of copper oxide and zinc oxide nanoparticles on photosynthesis and physiology of Raphanus sativus L. under salinity stress. Plant Physiol. Biochem. 2023, 206, 108281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Li, H.; Li, X.N.; Xin, C.Y.; Si, J.S.; Li, S.D.; Li, Y.J.; Zheng, X.X.; Li, H.W.; Wei, X.H.; et al. Nano-ZnO priming induces salt tolerance by promoting photosynthetic carbon assimilation in wheat. Arch. Agron. Soil Sci. 2020, 66, 1259–1273. [Google Scholar] [CrossRef]


| Variation Source | Genotype (G) | NaCl Treatments (Na) | ZnONPs Treatments (Zn) | G*Na Interaction | G*Zn Interaction |
|---|---|---|---|---|---|
| SL | F = 238.924 *** | F = 43.881 *** | F = 13.281 *** | F = 0.475 | F = 7.928 *** |
| RL | F = 30.596 *** | F = 39.334 *** | F = 15.619 *** | F = 1.028 | F = 35.111 *** |
| SFW | F = 120.192 *** | F = 29.595 *** | F = 14.817 *** | F = 0.838 | F = 5.075 *** |
| RFW | F = 2.973 | F = 184.176 *** | F = 46.861 *** | F = 13.256 ** | F = 21.488 *** |
| O2− | F = 13.553 ** | F = 619.889 *** | F = 51.973 *** | F = 18.392 *** | F = 24.471 *** |
| H2O2 | F = 61.992 *** | F = 151.111 *** | F = 19.576 *** | F = 5.916 * | F = 2.244 |
| MDA | F = 144.284 *** | F = 256.253 *** | F = 152.402 *** | F = 10.394 ** | F = 25.714 *** |
| MSI | F = 8.361 ** | F = 184.790 *** | F = 37.383 *** | F = 0.228 | F = 1.839 |
| Chl a | F = 5.365 * | F = 45.360 *** | F = 54.054 *** | F = 3.877 | F = 25.267 *** |
| Chl b | F = 0.836 | F = 12.326 ** | F = 12.089 *** | F = 0.554 | F = 7.824 *** |
| Car | F = 37.387 *** | F = 9.077 ** | F = 20.926 *** | F = 0.144 | F = 8.105 *** |
| Chl a + b | F = 1.657 | F = 50.035 *** | F = 54.181 *** | F = 3.670 | F = 23.215 *** |
| Chl a/b | F = 5.289 * | F = 3.560 | F = 8.887 *** | F = 0.809 | F = 8.380 *** |
| Cab/Car | F = 31.818 *** | F = 1.722 | F = 7.644 *** | F = 0.426 | F = 3.975 ** |
| RWC | F = 1.470 | F = 208.415 *** | F = 19.345 *** | F = 0.380 | F = 4.451 ** |
| NADP-ME | F = 0.075 | F = 96.247 *** | F = 75.305 *** | F = 2.802 | F = 9.410 *** |
| PPDK | F = 13.716 ** | F = 249.301 *** | F = 131.605 *** | F = 39.676 *** | F = 26.825 *** |
| PEPCK | F = 309.647 *** | F = 254.963 *** | F = 563.513 *** | F = 15.325 ** | F = 168.660 *** |
| Pn | F = 1.342 | F = 112.291 *** | F = 17.573 *** | F = 19.124 *** | F = 4.838 ** |
| Gs | F = 4.235 * | F = 117.556 *** | F = 87.524 *** | F = 2.339 | F = 6.670 *** |
| Ci | F = 239.532 *** | F = 148.079 *** | F = 15.226 *** | F = 8.028 ** | F = 3.293 * |
| Tr | F = 11.146 ** | F = 242.406 *** | F = 39.524 *** | F = 0.422 | F = 14.484 *** |
| SOD | F = 21.755 *** | F = 162.378 *** | F = 97.001 *** | F = 47.710 *** | F = 5.457 ** |
| POD | F = 12.210 ** | F = 126.359 *** | F = 92.638 *** | F = 0.111 | F = 40.249 *** |
| CAT | F = 6.578 * | F = 40.942 *** | F = 106.514 *** | F = 0.001 | F = 5.732 ** |
| APX | F = 34.835 *** | F = 1111.396 *** | F = 148.302 *** | F = 36.445 *** | F = 38.032 *** |
| Treatments | NaCl Concentrations (mmol/L) | ZnONPs Concentrations (μmol/L) |
|---|---|---|
| CK | 0 | 0 |
| S | 120 | 0 |
| S + ZnONPs-10 | 120 | 10 |
| S + ZnONPs-50 | 120 | 50 |
| S + ZnONPs-100 | 120 | 100 |
| S + ZnONPs-150 | 120 | 150 |
| S + ZnONPs-180 | 120 | 180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Zhao, X.; Li, X.; He, M.; Wang, J.; Xiang, X.; Niu, Y. ZnONPs Alleviates Salt Stress in Maize Seedlings by Improving Antioxidant Defense and Photosynthesis Potential. Plants 2025, 14, 3104. https://doi.org/10.3390/plants14193104
Sun S, Zhao X, Li X, He M, Wang J, Xiang X, Niu Y. ZnONPs Alleviates Salt Stress in Maize Seedlings by Improving Antioxidant Defense and Photosynthesis Potential. Plants. 2025; 14(19):3104. https://doi.org/10.3390/plants14193104
Chicago/Turabian StyleSun, Siqi, Xiaoqiang Zhao, Xin Li, Meiyue He, Jing Wang, Xinxin Xiang, and Yining Niu. 2025. "ZnONPs Alleviates Salt Stress in Maize Seedlings by Improving Antioxidant Defense and Photosynthesis Potential" Plants 14, no. 19: 3104. https://doi.org/10.3390/plants14193104
APA StyleSun, S., Zhao, X., Li, X., He, M., Wang, J., Xiang, X., & Niu, Y. (2025). ZnONPs Alleviates Salt Stress in Maize Seedlings by Improving Antioxidant Defense and Photosynthesis Potential. Plants, 14(19), 3104. https://doi.org/10.3390/plants14193104








