Improvement of Cottonseed Oil and Fatty Acids Through Introgression Breeding in Upland Cotton
Abstract
1. Introduction
2. Results
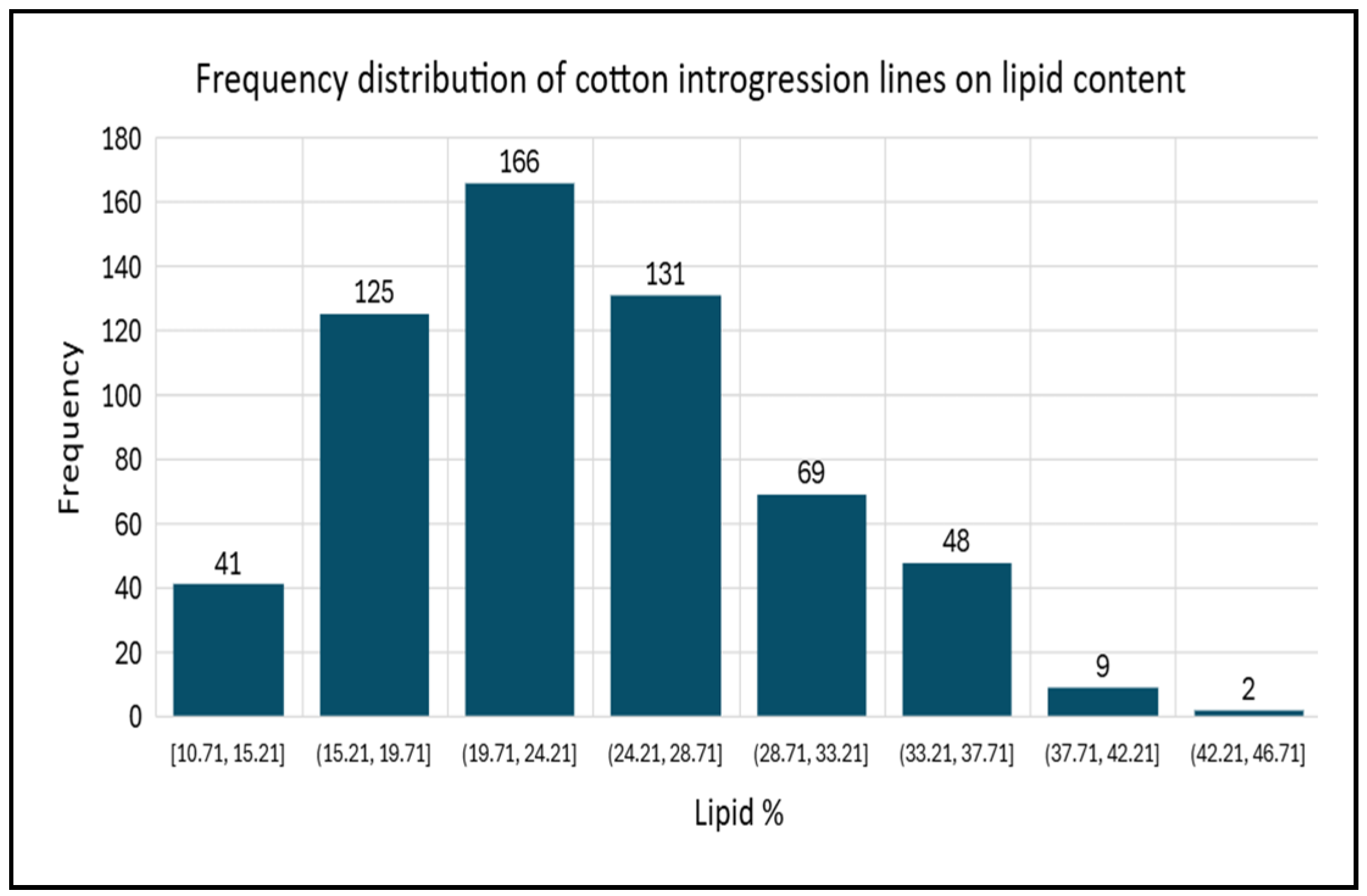
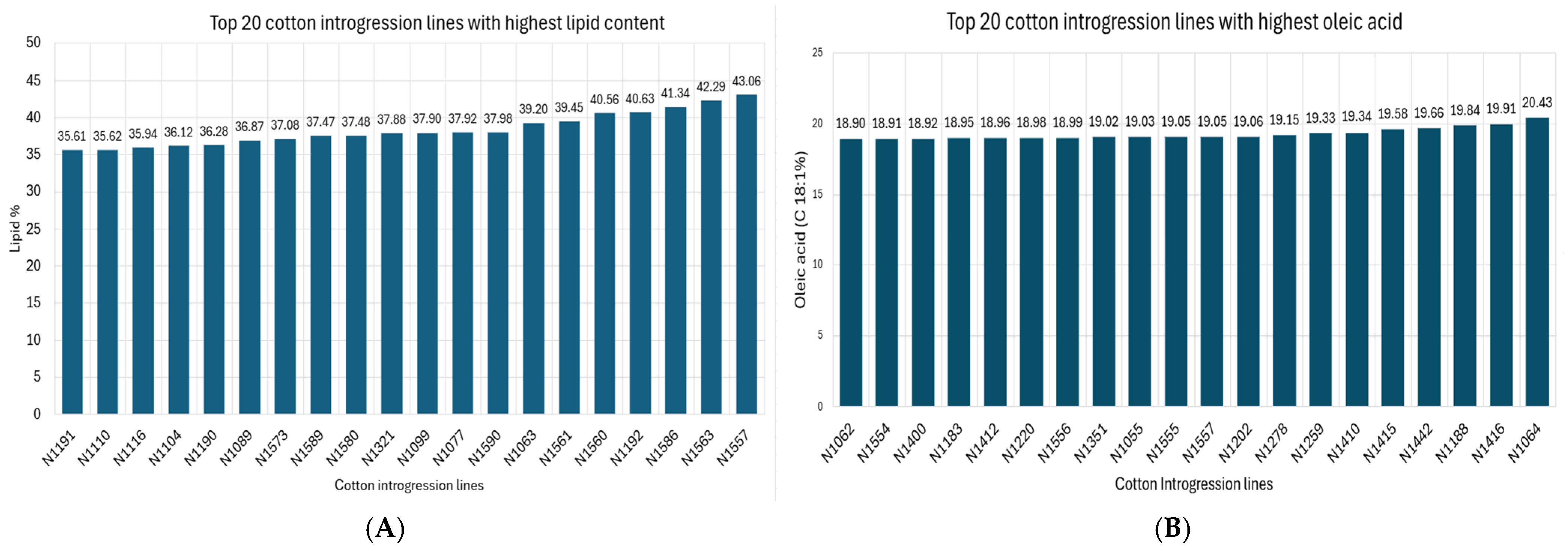
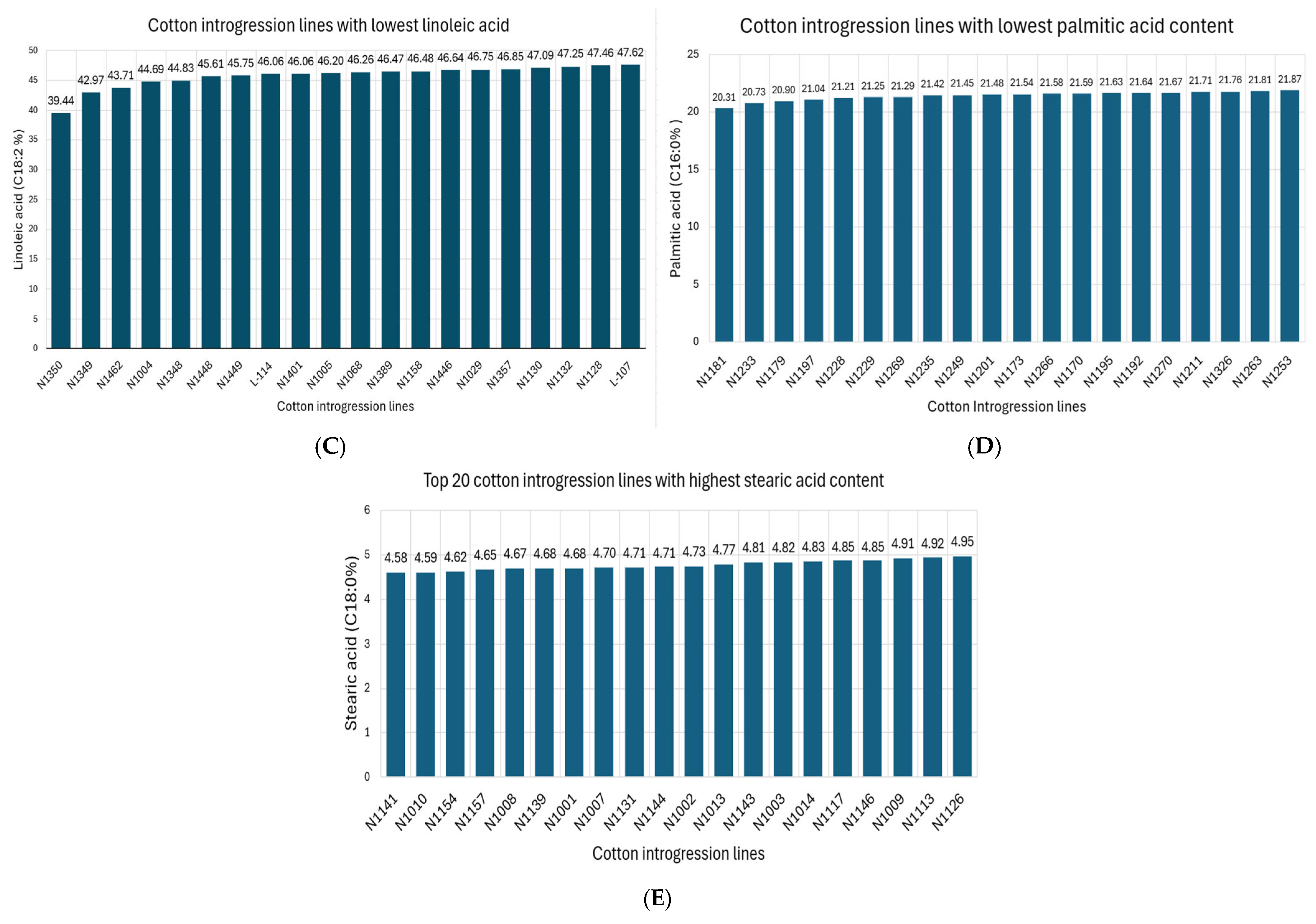
2.1. Cottonseed Oil and Fatty Acids
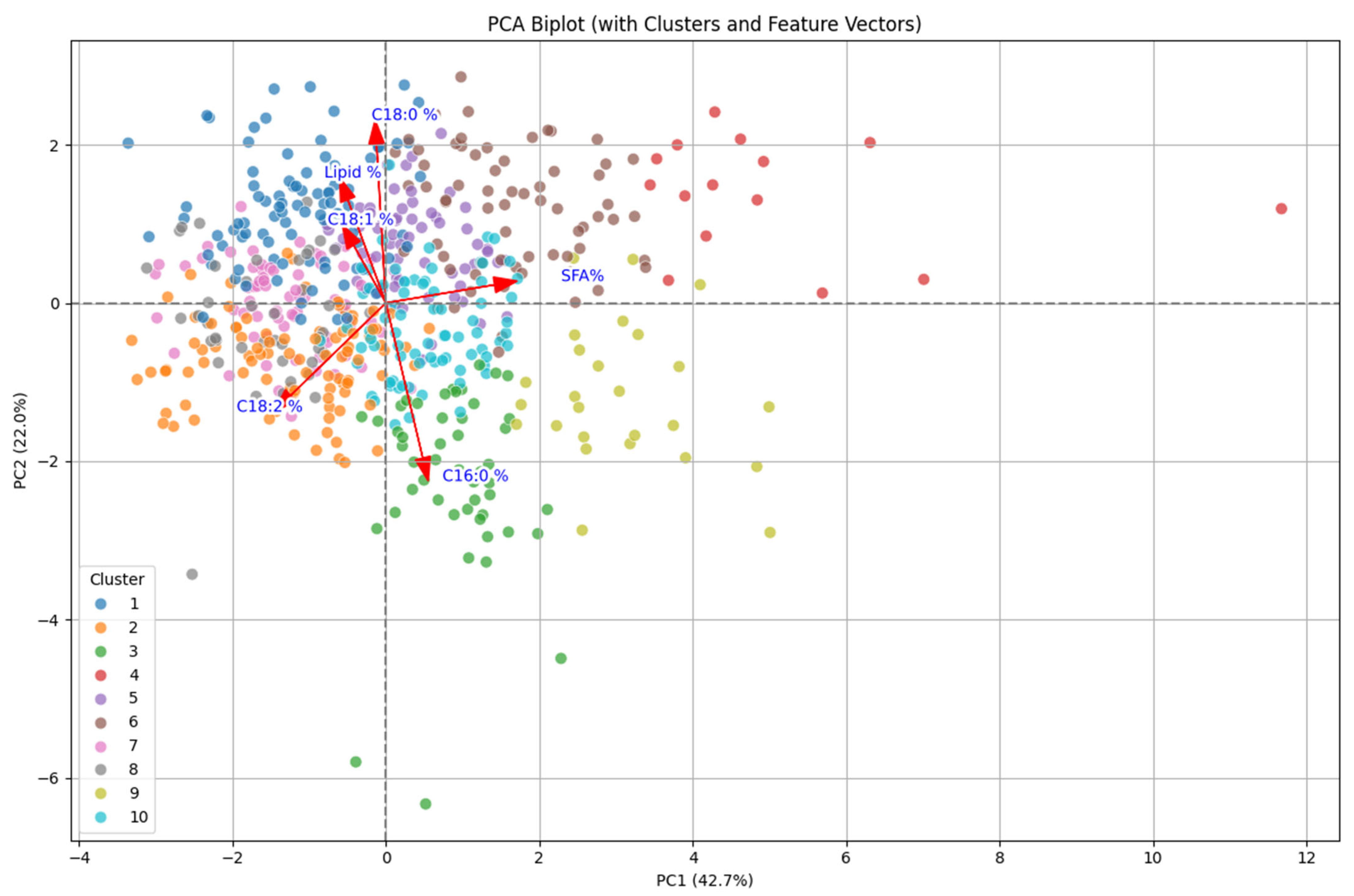
2.2. Principal Component Analysis
2.3. Biplot Analysis
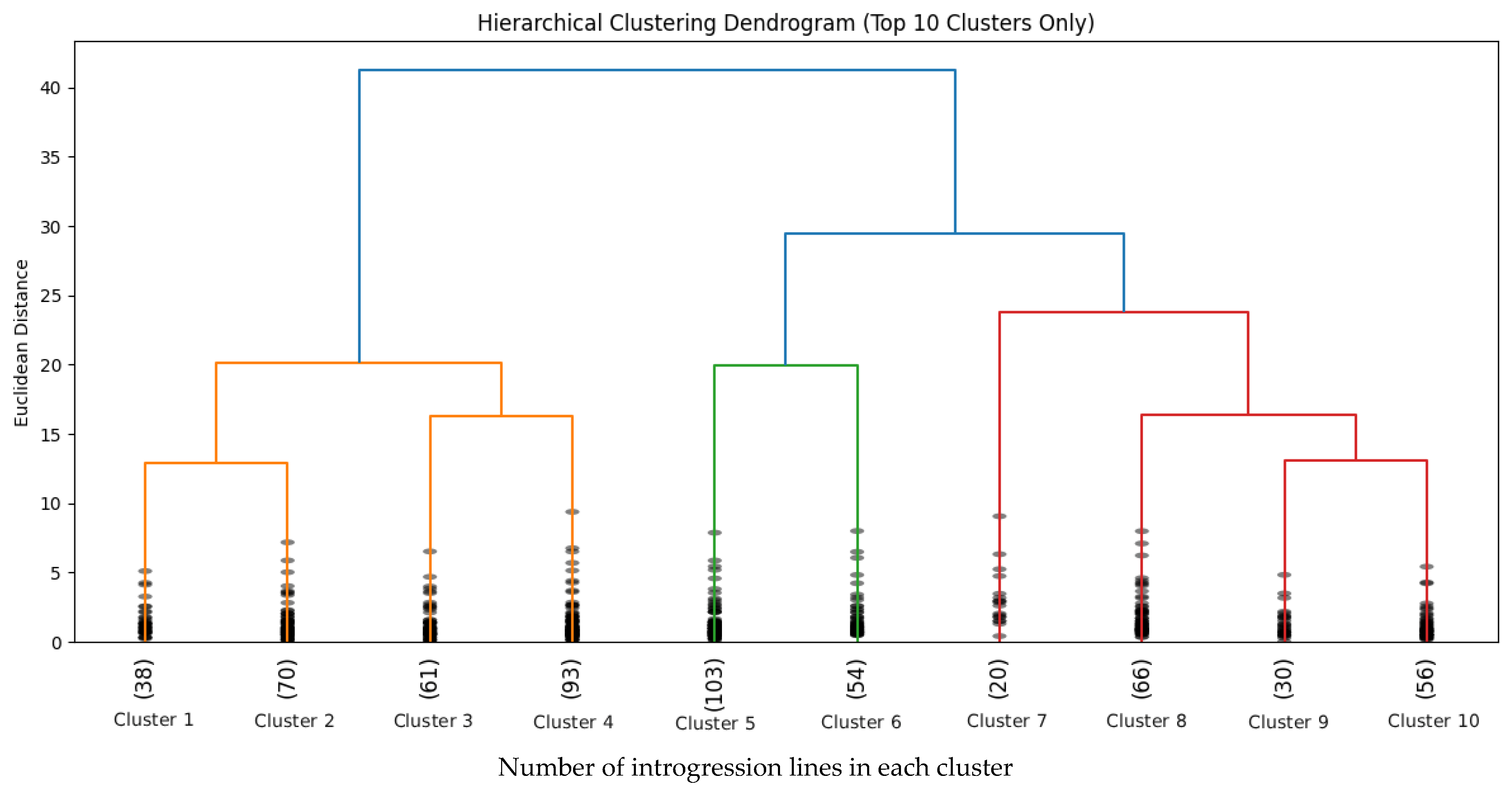
2.4. Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Field Trials and Seed Samples
4.2. Fatty Acid Methyl Ester Sample Preparation
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| USD | United States Dollar |
| MUFAs | Mono-unsaturated fatty acids |
| PUFAs | Poly-unsaturated fatty acids |
| SFAs | Saturated fatty acids |
| USFAs | Unsaturated fatty acids |
| ILs | Introgression Lines |
| FAME | Fatty Acid Methyl Ester |
| GC-FID | Gas Chromatography–Flame Ionization Detector |
| LDL | Low Density Lipoprotein |
| SOC | Seed Oil Content |
| PCA | Principal Component Analysis |
References
- Malik, T.H.; Ahsan, M.Z. Review of the cotton market in Pakistan and its future prospects. Oilseed Fat Crops Lipids 2016, 23, D606. [Google Scholar] [CrossRef]
- Agarwal, D.; Singh, P.; Chakrabarty, M.; Shaikh, A.; Gayal, S. Cottonseed oil quality, utilization and processing. Cicr. Tech. Bull. 2003, 25, 5. [Google Scholar]
- Zion Market Research. Available online: https://www.zionmarketresearch.com/report/cottonseed-oil-market (accessed on 1 June 2025).
- Lindsey, S.; Benattar, J.; Pronczuk, A.; Hayes, K. Dietary palmitic acid (16:0) enhances high density lipoprotein cholesterol and low-density lipoprotein receptor mRNA abundance in hamsters. Proc. Soc. Exp. Biol. Med. 1990, 195, 261–269. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.P.; Zhu, H.G.; Zhu, Q.H.; Sun, J. Simultaneous silencing of GhFAD2-1 and GhFATB enhances the quality of cottonseed oil with high oleic acid. J. Plant Physiol. 2017, 215, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Singh, S.P.; Green, A.G. High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing. Plant Physiol. 2002, 129, 1732–1743. [Google Scholar] [CrossRef]
- Dani, R.G.; Kohel, R.J. Maternal effects and generation mean analysis of seed-oil content in cotton (Gossypium hirsutum L.). Theor. Appl. Genet. 1989, 77, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H.; Lu, Z.Z.; Zhu, J. Genetic analysis for developmental behavior of some seed quality traits in upland cotton (Gossypium hirsutum L.). Euphytica 2003, 129, 183–191. [Google Scholar] [CrossRef]
- Hom, N.H.; Schierholt, A.; Mollers, C.; Becker, H.C. Pollen genotype effects on seed quality traits in winter oil seed rape. Crop Sci. 2015, 55, 493–500. [Google Scholar] [CrossRef]
- Ecker, R.; Yaniv, Z. Genetic control of fatty acid composition in seed oil of Sinapis alba L. Euphytica 1993, 69, 45–49. [Google Scholar] [CrossRef]
- Velasco, L.; Vich, B.P.; Fernandez, M.J.M. Novel variation for tocopherol profile in sunflower created by mutagenesis and recombination. Plant Breed. 2004, 123, 490–492. [Google Scholar] [CrossRef]
- Gopalakrishnan, D. Seed Oil Improvement in Cotton; Central Institute for Cotton Research Regional Station C: Coimbatore, India, 2007; pp. 61–70. [Google Scholar]
- Pahlavani, M.; Miri, A.; Kazemi, G. Response of oil and protein content to seed size in cotton (Gossypium hirsutum L. cv. Sahel). Plant Breed Seed Sci. 2009, 59, 53–64. [Google Scholar] [CrossRef]
- Horn, P.J.; Neogi, P.; Tombokan, X.; Ghosh, S.; Campbell, B.T.; Chapman, K.D. Simultaneous quantification of oil and protein in cottonseed by low-field time-domain nuclear magnetic resonance. J. Am. Oil Chem. Soc. 2011, 88, 1521–1529. [Google Scholar] [CrossRef]
- Kaur, B.; Tyagi, P.; Kuraparthy, V. Genetic diversity and population structure in the landrace accessions of Gossypium hirsutum. Crop Sci. 2017, 57, 2457. [Google Scholar] [CrossRef]
- Li, B.; Shi, Y.; Gong, J.; Li, J.; Liu, A.; Shang, H.; Gong, W.; Yuan, Y. Genetic effects and heterosis of yield and yield component traits based on Gossypium barbadense chromosome segment substitution lines in two Gossypium hirsutum backgrounds. PLoS ONE 2016, 11, e0157978. [Google Scholar] [CrossRef]
- Jenkins, J.N.; McCarty, J.C.; Deng, D.; Geng, L.; Hayes, R.W.; Jones, D.C.; Mammadova, R. Introgression of Gossypium barbadense L. into upland cotton germplasm RMBUP-C4S1. Euphytica 2018, 214, 118. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Huang, Y.; Wang, Y.M.; Cui, Y.P.; Liu, Z.J.; Hua, J.P. RNA interference of GhPEPC2 enhanced seed oil accumulation and salt tolerance in Upland cotton. Plant Sci. 2018, 271, 52–61. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.M.; Wang, Z.W.; You, C.Y.; Nie, X.; Sun, J.; Lin, Z. Genetic dissection of an allotetraploid interspecific CSSLs guides interspecific genetics and breeding in cotton. BMC Genom. 2020, 21, 431. [Google Scholar] [CrossRef]
- Zhang, J.; Percy, R.G.; McCarty, J.C. Introgression genetics and breeding between upland and Pima cotton: A review. Euphytica 2014, 198, 1–12. [Google Scholar] [CrossRef]
- Wu, J.; Jenkins, J.N.; Mccarty, J.C.; Thaxton, P. Seed trait evaluation of Gossypium barbadense L. chromosomes/arms in a G. hirsutum L. background. Euphytica 2009, 167, 371–380. [Google Scholar] [CrossRef]
- Yu, J.; Yu, S.; Fan, S.; Song, M.; Zhai, H.; Li, X.; Zhang, J. Mapping quantitative trait loci for cottonseed oil, protein and gossypol content in a Gossypium hirsutum × Gossypium barbadense backcross inbred line population. Euphytica 2012, 187, 191–201. [Google Scholar] [CrossRef]
- Shockey, J.; Dowd, M.; Mack, B.; Gilbert, M.; Scheffler, B.; Ballard, L.; Frelichowski, J.; Mason, C. Naturally occurring high oleic acid cottonseed oil: Identification and functional analysis of a mutant allele of Gossypium barbadense fatty acid desaturase-2. Planta 2017, 245, 611–622. [Google Scholar] [CrossRef]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil content and fatty acids composition in Brassica species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Azhar, F.M.; Naeem, M. Assessment of cotton (Gossypium hirsutum) germplasm for combining abilities in fiber traits. J. Agric. Soc. Sci. 2008, 4, 129–131. [Google Scholar]
- Lee, D.; Hwang, W.; Artan, M.; Jeong, D.E.; Lee, S.J. Effects of nutritional components on aging. Aging Cell 2015, 14, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.A.; Abdel-Aal, M.H.; Khalil, M.M. Studies on Egyptian cottonseeds: Fatty acid composition and protein patterns. Nahrung 1988, 32, 255–259. [Google Scholar] [CrossRef]
- O’Brien, R.D.; Wakelyn, P.J.; Wan, P.J. Cottonseed oil: Alternative to trans fatty acid containing oils. Oil Mill Gazet. 2005, 111, 2–4. [Google Scholar]
- Dowd, M.K.; Boykin, D.L.; Meredith, W.R.; Campbell, B.T.; Bourland, F.M.; Gannaway, J.R.; Zhang, J.F. Fatty acid profiles of cottonseed genotypes from the national cotton variety trials. J. Cotton Sci. 2010, 14, 64–73. [Google Scholar]
- Hall, A.J. Evaluation of Fatty Acid Composition of Cotton Germplasm and Association with Cold Tolerance. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 2003. [Google Scholar]
- Wan, P.J.; Hron, R.J.; Dowd, M.K.; Kuk, M.S.; Conkerton, E.J. Alternative hydrocarbon solvents for cottonseed extraction: Plant trials. J. Am. Oil Chem. Soc. 1995, 72, 661–664. [Google Scholar] [CrossRef]
- Sekhar, S.; Rao, B.C. Cottonseed oil as health oil. Pertanika J. Trop. Agric. Sci. 2011, 34, 17–24. [Google Scholar]
- Sharma, D.; Pathak, D.; Atwal, A.K.; Sangha, M.K. Genetic variation for some chemical and biochemical characteristics in cotton seed oil. J. Cotton Res. Dev. 2009, 23, 1–7. [Google Scholar]
- Lukonge, E.; Labuschagne, M.T.; Hugo, A. The evaluation of oil and fatty acid composition in seed of cotton accessions from various countries. J. Sci. Food Agric. 2007, 87, 340–347. [Google Scholar] [CrossRef]
- Lawhon, J.T.; Cater, C.M.; Mattil, K.F. Evaluation of the food potential of sixteen varieties of cottonseed. J. Am. Oil Chem. Soc. 1977, 54, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Matthaus, B.; Ozcan, M.M. Oil content, fatty acid composition and distribution of vitamin-E-active compounds of some fruit seed oils. Antioxidants 2015, 4, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Shakeel, A.; Malik, T.; Saleem, M. Assessment of drought tolerance in some cotton genotypes based on drought tolerance indices. J. Animal Plant Sci. 2019, 29, 998–1009. [Google Scholar]

| Variable (%) | Mean | Minimum | Maximum |
|---|---|---|---|
| Lipid | 24.01 | 10.71 | 43.10 |
| Unsaturated fatty acids (USFA) | 68.92 | 57.24 | 73.20 |
| Saturated fatty acids (SFA) | 30.92 | 27.20 | 42.76 |
| Palmitic acids (C16:0) | 24.36 | 20.30 | 30.00 |
| Stearic acid (C18:0) | 3.38 | 0.8 | 5.80 |
| Oleic acid (C18:1) | 17.12 | 13.39 | 20.40 |
| Linoleic acid (C18:2) | 51.04 | 39.44 | 56.10 |
| Ratio of SFA to USFA | 0.45 | 0.37 | 0.75 |
| Variables | PC I | PC II | PC III | PC IV | PC V | PC VI | PC VII |
|---|---|---|---|---|---|---|---|
| Eigenvalue | 1.728 | 1.241 | 1.008 | 0.927 | 0.740 | 0.224 | 0.048 |
| Variability % | 0.427 | 0.220 | 0.145 | 0.123 | 0.078 | 0.006 | 0.001 |
| Cumulative % | 0.427 | 0.647 | 0.792 | 0.915 | 0.993 | 0.999 | 1.000 |
| Variables | PC I | PC II | PC III | PC IV | PC V | PC VI | PC VII |
|---|---|---|---|---|---|---|---|
| Lipid % | 0.200 | 0.397 | 0.012 | 0.798 | 0.406 | 0.011 | 0.002 |
| C16:0 % | −0.184 | −0.563 | 0.370 | −0.034 | 0.701 | −0.136 | −0.016 |
| C18:0 % | 0.045 | 0.578 | −0.170 | −0.576 | 0.548 | 0.050 | 0.002 |
| C18:1 % | 0.189 | 0.248 | 0.871 | −0.110 | −0.153 | 0.330 | −0.007 |
| C18:2 % | 0.487 | −0.342 | −0.270 | −0.028 | 0.138 | 0.744 | −0.024 |
| SFA % | −0.571 | 0.067 | −0.031 | 0.092 | 0.022 | 0.415 | 0.698 |
| SFA/USFA | 0.570 | 0.090 | −0.039 | 0.093 | 0.006 | 0.380 | −0.716 |
| Clusters | Lipid % | C16:0 % | C18:0 % | C18:1 % | C18:2 % | SFA % | SFA/USFA |
|---|---|---|---|---|---|---|---|
| 1 | 32.51 | 23.44 | 3.73 | 17.53 | 51.33 | 29.97 | 0.43 |
| 2 | 24.31 | 23.84 | 2.98 | 16.55 | 53.06 | 29.59 | 0.42 |
| 3 | 19.58 | 26.51 | 2.49 | 16.03 | 51.87 | 31.66 | 0.46 |
| 4 | 20.60 | 24.12 | 3.63 | 16.41 | 45.29 | 36.38 | 0.57 |
| 5 | 21.79 | 23.87 | 4.18 | 16.67 | 50.51 | 31.22 | 0.46 |
| 6 | 25.68 | 23.20 | 3.59 | 16.72 | 48.81 | 32.90 | 0.49 |
| 7 | 20.91 | 24.56 | 4.04 | 17.51 | 52.67 | 29.45 | 0.42 |
| 8 | 23.34 | 23.92 | 2.95 | 18.42 | 51.94 | 29.11 | 0.41 |
| 9 | 16.92 | 26.86 | 2.96 | 16.72 | 48.73 | 33.97 | 0.51 |
| 10 | 23.28 | 25.30 | 2.98 | 17.94 | 49.97 | 31.41 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandel, S.; Holguin, F.O.; Galvan, C.; Zhu, Y.; Dever, J.; Kelly, C.; Whitelock, D.; Zhang, J. Improvement of Cottonseed Oil and Fatty Acids Through Introgression Breeding in Upland Cotton. Plants 2025, 14, 3078. https://doi.org/10.3390/plants14193078
Kandel S, Holguin FO, Galvan C, Zhu Y, Dever J, Kelly C, Whitelock D, Zhang J. Improvement of Cottonseed Oil and Fatty Acids Through Introgression Breeding in Upland Cotton. Plants. 2025; 14(19):3078. https://doi.org/10.3390/plants14193078
Chicago/Turabian StyleKandel, Savyata, Francisco Omar Holguin, Claudia Galvan, Yi Zhu, Jane Dever, Carol Kelly, Derek Whitelock, and Jinfa Zhang. 2025. "Improvement of Cottonseed Oil and Fatty Acids Through Introgression Breeding in Upland Cotton" Plants 14, no. 19: 3078. https://doi.org/10.3390/plants14193078
APA StyleKandel, S., Holguin, F. O., Galvan, C., Zhu, Y., Dever, J., Kelly, C., Whitelock, D., & Zhang, J. (2025). Improvement of Cottonseed Oil and Fatty Acids Through Introgression Breeding in Upland Cotton. Plants, 14(19), 3078. https://doi.org/10.3390/plants14193078







