Seasonal, Organ-, and Location-Dependent Variations in the Alkaloid Content of Pachysandra terminalis Investigated by Multivariate Data Analysis of LC-MS Profiles
Abstract
1. Introduction
2. Results
2.1. Sampling, LC/MS-Analysis, and Pretreatment for Data Analysis and Statistical Methods Used
2.2. Optimum Harvesting Periods for Previously Isolated Pachysandra Alkaloids
2.3. Mass Spectrometric Characterization of Pachysandra Aminosteroids: Characteristic Fragments
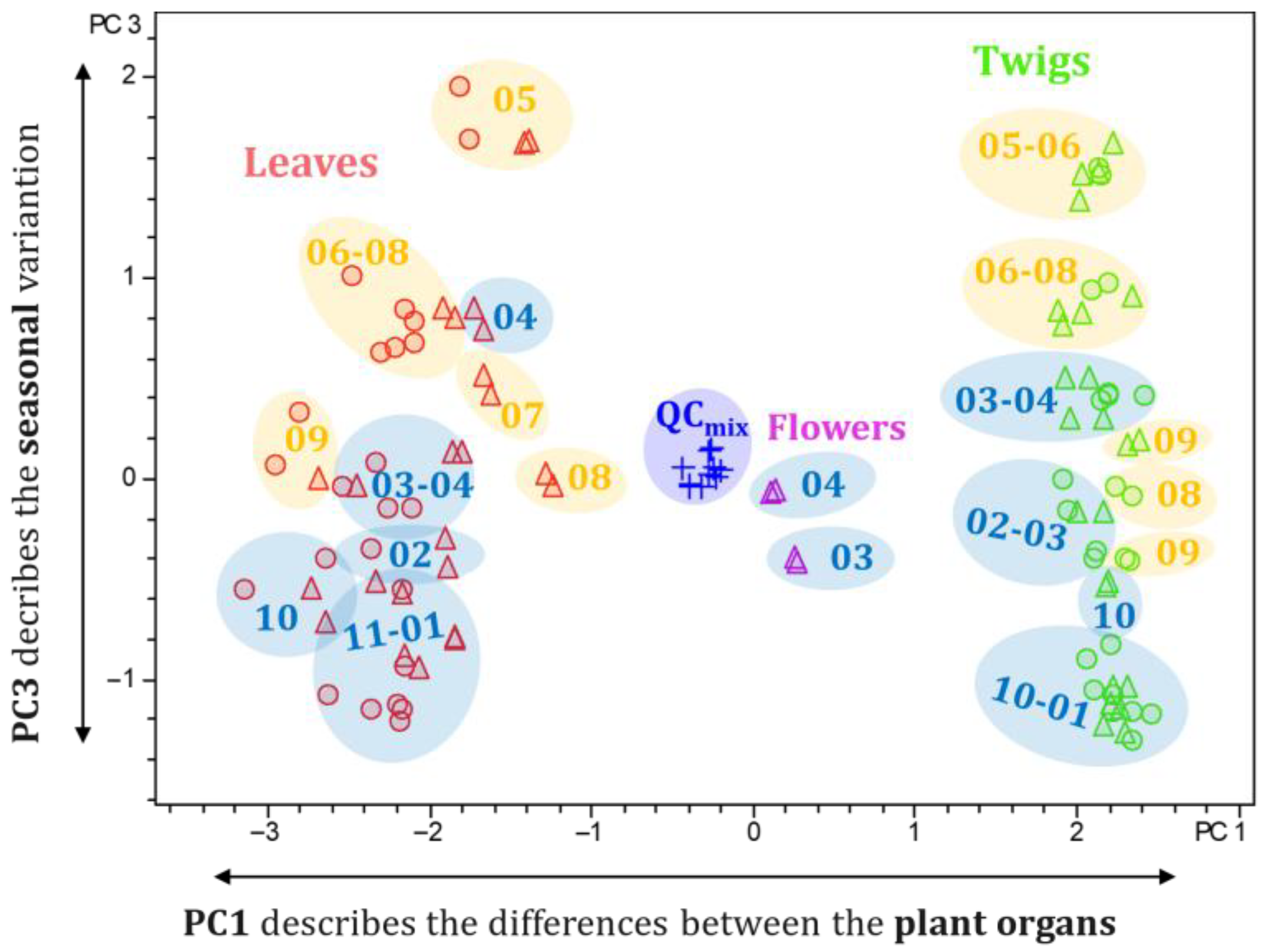
2.4. Principal Component Analysis (PCA)
2.4.1. Principal Components 1 and 2: Differences in the Alkaloid Profile of the Plant Organs and Populations from Distinct Locations
2.4.2. Principal Component 3: Seasonal Differences in the Alkaloid Profile of Pachysandra terminalis
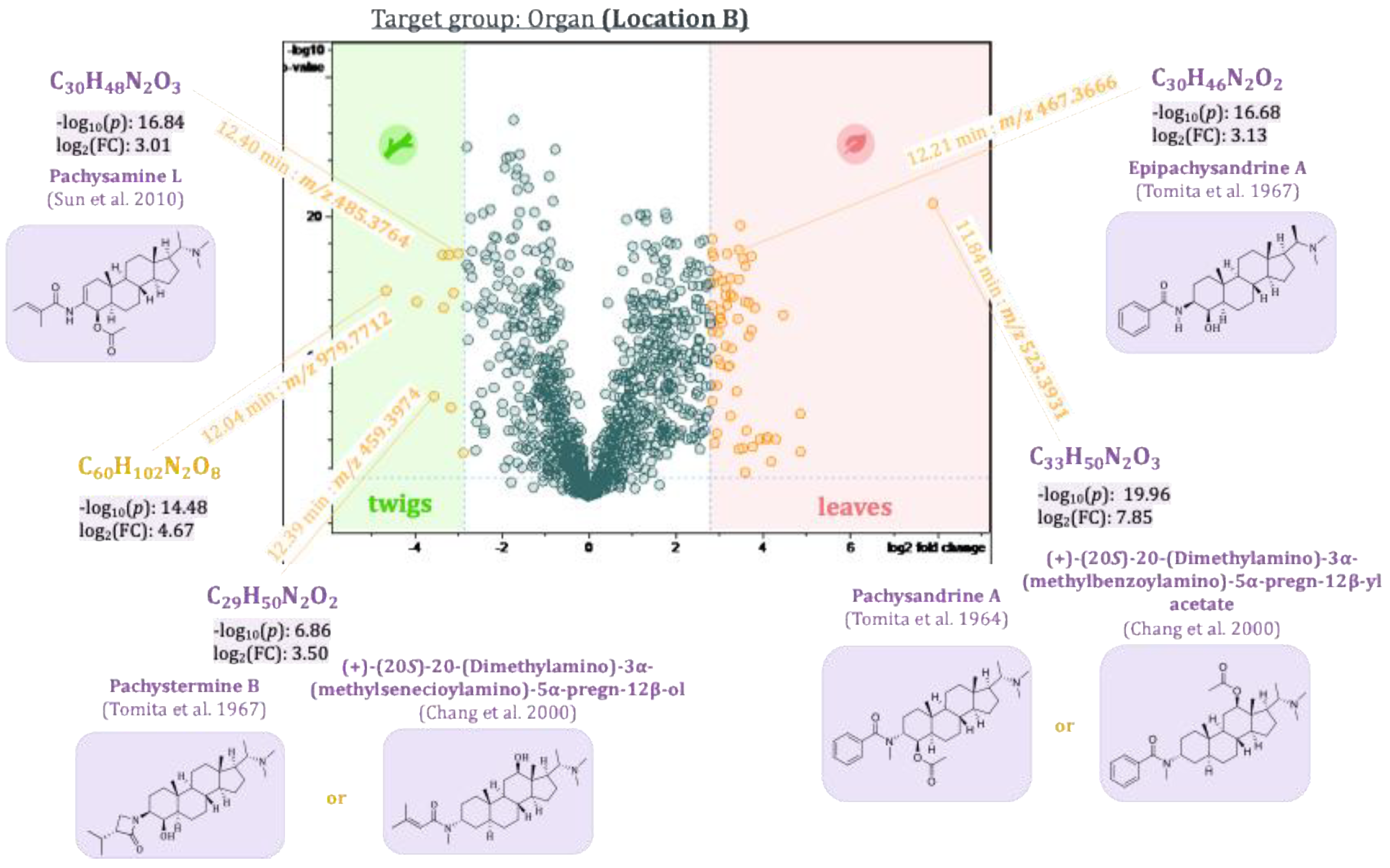
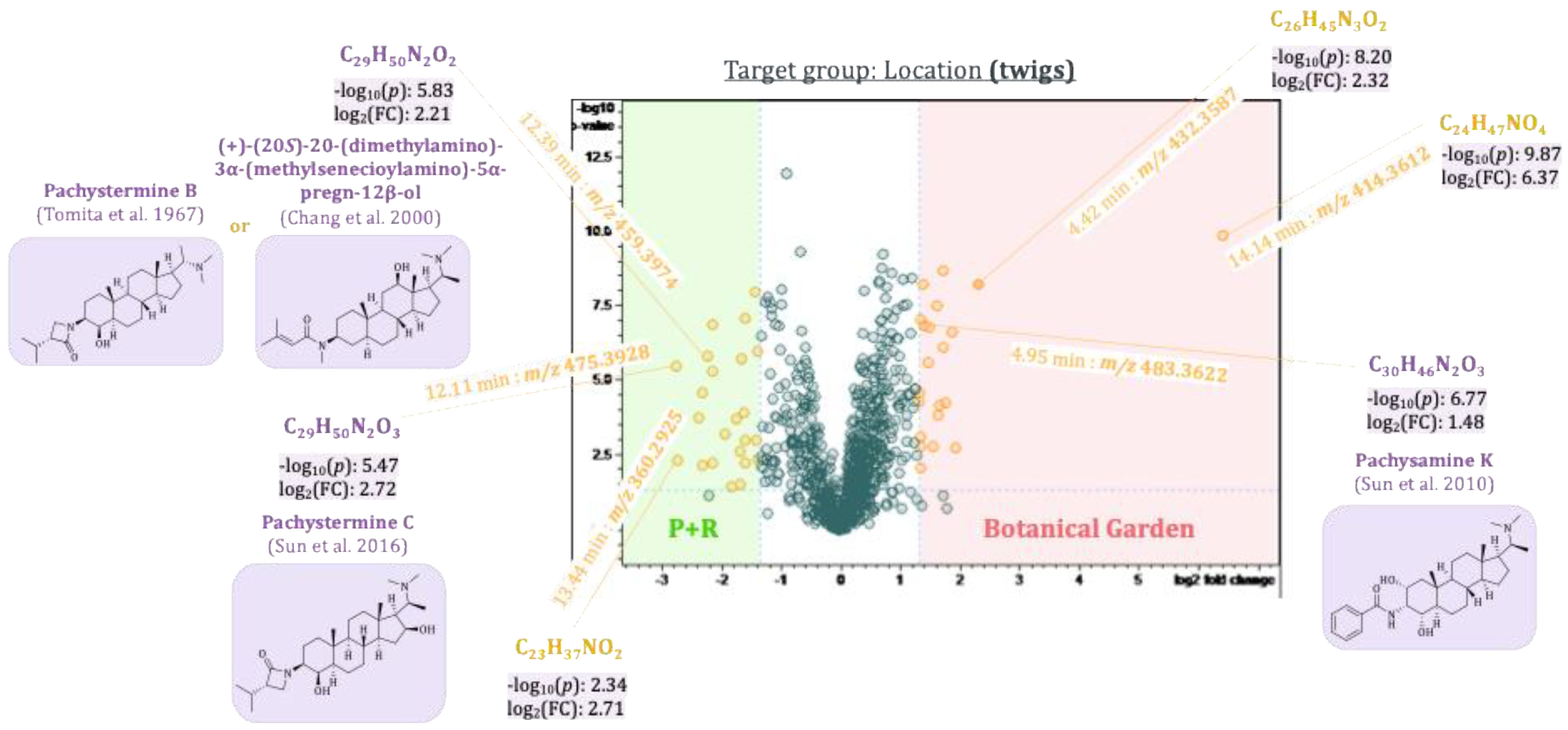
2.5. Pairwise Comparison of Sample Groups Using Volcano Plots
2.5.1. Differences Between the Leaves and Twigs of Pachysandra terminalis
2.5.2. Differences Between Plants of Two Pachysandra terminalis Populations
3. Materials and Methods
3.1. Preparation of the Plant Material
3.2. UHPLC/+ESI-QqTOF-MS/MS-Analysis
3.3. Multivariate Data Analysis
3.4. Principal Component Analysis (PCA)
3.5. Volcano Plot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Author Statement
Abbreviations
| P. terminalis | Pachysandra terminalis |
| MVDA | Multivariate data analysis |
| UHPLC/+ESI-QqTOF-MS/MS | Ultra-high-performance liquid chromatography electrospray ionization quadrupole time of flight massspektrometer |
| LC/MS | short for UHPLC/+ESI-QqTOF-MS/MS |
| PCA | Principal component analysis |
| tR | Retention time |
| PC | Principal component |
| QC | Quality control |
References
- Knaack, W.; Geissman, T. Steroidal alkaloids of Pachysandra terminalis (Buxaceae). Tetrahedron Lett. 1964, 5, 1381–1385. [Google Scholar] [CrossRef]
- Tomita, M.; Uyeo, S.; Kikuchi, T. Studies on the alkaloids of Pachysandra terminalis Sieb. et Zucc.: Structure of Pachysandrine A and B. Tetrahedron Lett. 1964, 5, 1053–1061. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uyeo, S.; Ando, M.; Yamamoto, A. Studies on the alkaloids of Pachysandra terminalis sieb. et zucc. (3): Systematic separation and characterization of alkaloids. Tetrahedron Lett. 1964, 5, 1817–1823. [Google Scholar] [CrossRef]
- Xiang, M.; Hu, B.; Qi, Z.; Wang, X.; Xie, T.; Wang, Z.; Ma, D.; Zeng, Q.; Luo, X. Chemistry and bioactivities of natural steroidal alkaloids. Nat. Prod. Bioprospecting 2022, 12, 23. [Google Scholar] [CrossRef]
- Zhai, H.Y.; Zhao, C.; Zhang, N.; Jin, M.N.; Tang, S.A.; Qin, N.; Kong, D.X.; Duan, H.Q. Alkaloids from Pachysandra terminalis Inhibit Breast Cancer Invasion and Have Potential for Development As Antimetastasis Therapeutic Agents. J. Nat. Prod. 2012, 75, 1305–1311. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, C.; Wang, F.; Zhang, Y.; Huang, W.; Zhang, H.; Li, Y.; Zhang, D.; Song, X. Pregnane alkaloids with BRD4 inhibitory and cytotoxic activities from Pachysandra terminalis. Phytochem. Lett. 2021, 45, 63–67. [Google Scholar] [CrossRef]
- Li, X.-Y.; Yu, Y.; Jia, M.; Jin, M.-N.; Qin, N.; Zhao, C.; Duan, H.-Q.; Terminamines, K.-S. Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis. Molecules 2016, 21, 1283. [Google Scholar] [CrossRef] [PubMed]
- Flittner, D.; Kaiser, M.; Mäser, P.; Lopes, N.P.; Schmidt, T.J. The Alkaloid-Enriched Fraction of Pachysandra terminalis (Buxaceae) Shows Prominent Activity against Trypanosoma brucei rhodesiense. Molecules 2021, 26, 591. [Google Scholar] [CrossRef]
- Schäfer, L.; Cal, M.; Kaiser, M.; Mäser, P.; Schmidt, T.J. Antiprotozoal Aminosteroids from Pachysandra terminalis. Molecules 2025, 30, 1093. [Google Scholar] [CrossRef] [PubMed]
- Sengnon, N.; Vonghirundecha, P.; Chaichan, W.; Juengwatanatrakul, T.; Onthong, J.; Kitprasong, P.; Sriwiriyajan, S.; Chittrakarn, S.; Limsuwanchote, S.; Wungsintaweekul, J. Seasonal and Geographic Variation in Alkaloid Content of Kratom (Mitragyna speciosa (Korth.) Havil.) from Thailand. Plants 2023, 12, 949. [Google Scholar] [CrossRef]
- Demir, S.C.; Yildirim, A.B.; Turker, A.U.; Eker, I. Seasonal variation in alkaloid content, phenolic constituent and biological activities of some Leucojum aestivum L. populations in Turkey. S. Afr. J. Bot. 2022, 147, 713–723. [Google Scholar] [CrossRef]
- Mahar, R.; Manivel, N.; Kanojiya, S.; Mishra, D.K.; Shukla, S.K. Assessment of Tissue Specific Distribution and Seasonal Variation of Alkaloids in Alstonia scholaris. Metabolites 2022, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.U.; Schmidt, T.J. Investigation of the Variability of Alkaloids in Buxus sempervirens L. Using Multivariate Data Analysis of LC/MS Profiles. Molecules 2022, 27, 82. [Google Scholar] [CrossRef] [PubMed]
- Deutscher Wetterdienst, Stationsmessungen Münster/Osnabrück (ID1766), Climate Data Center. 2024. Available online: www.dwd.de (accessed on 3 September 2024).
- Musharraf, S.G.; Goher, M.; Ali, A.; Adhikari, A.; Choudhary, M.I.; Atta-ur-Rahman. Rapid characterization and identification of steroidal alkaloids in Sarcococca coriacea using liquid chromatography coupled with electrospray ionization quadropole time-of-flight mass spectrometry. Steroids 2012, 77, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Reh, E. Chemometrie: Grundlagen der Statistik, Numerischen Mathematik und Software Anwendungen in der Chemie; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Reaxys Database; Elsevier: Amsterdam, The Netherlands. 2025. Available online: https://www.reaxys.com/ (accessed on 29 September 2025).
- Tomita, M.; Kikuchi, T.; Uyeo, S.; Nishinaga, T.; Yasunishi, M.; Yamamoto, A. Pachysandra Alkaloids I. Systematic Isolation and Characterization of Alkaloids. Yakugaku Zasshi 1967, 87, 215–227. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Chen, J.X.; Chen, J.C.; Li, Z.R.; Zhou, L.; Li, Y.; Qiu, M.H. Three New Pregnane Alkaloids from Pachysandra terminalis. Helv. Chim. Acta 2016, 99, 513–517. [Google Scholar] [CrossRef]
- Qiu, M.; Nie, R.; Li, Z.; Zhou, J. Three New Steroidal Alkaloids from Pachysandra axillaris. J. Integr. Plant Biol. 1990, 32, 626–630. [Google Scholar]
- Kikuchi, T.; Uyeo, S. Pachysandra Alkaloids. IV. Structure of Pachysamine-A and -B. Chem. Pharm. Bull. 1967, 15, 302–306. [Google Scholar] [CrossRef][Green Version]
- Li, W. Volcano plots in analyzing differential expressions with nRNA microarrays. J. Bioinform. Comput. Biol. 2012, 10, 1231003. [Google Scholar] [CrossRef]
- Chang, L.C.; Bhat, K.P.L.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Novel bioactive steroidal alkaloids from Pachysandra procumbens. Tetrahedron 2000, 56, 3133–3138. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, Y.X.; Chen, J.C.; Lu, L.; Zhang, X.M.; Li, Y.; Qiu, M.H. Pregnane alkaloids from Pachysandra axillaris. Steroids 2010, 75, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, L.; Schmidt, T.J. Investigating seasonal changes in the alkaloid profile of Pachysandra terminalis using multivariate data analysis. In Conference Book—Pharmaceutical Sciences: Emerging Technologies for Healthy Individuals, Proceedings of the Annual Meeting of the German Pharmaceutical Society, Münster, Germany, 23–26 September 2024; Deutsche Pharmazeutische Gesellschaft (DPhG): Frankfurt am Main, Germany, 2024. [Google Scholar] [CrossRef]
- Schäfer, L. Isolierung, Strukturaufklärung und Struktur-Wirkungs-Beziehungen antiprotozoaler Alkaloide aus Pachysandra terminalis sowie deren saisonale, organ- und standortbedingte Variabilität. Doctoral Thesis, University of Münster, Münster, Germany, 2025. Available online: https://miami.uni-muenster.de/Record/c3c334c4-4a90-4d96-963e-d1c895882ca1 (accessed on 29 September 2025). [CrossRef]

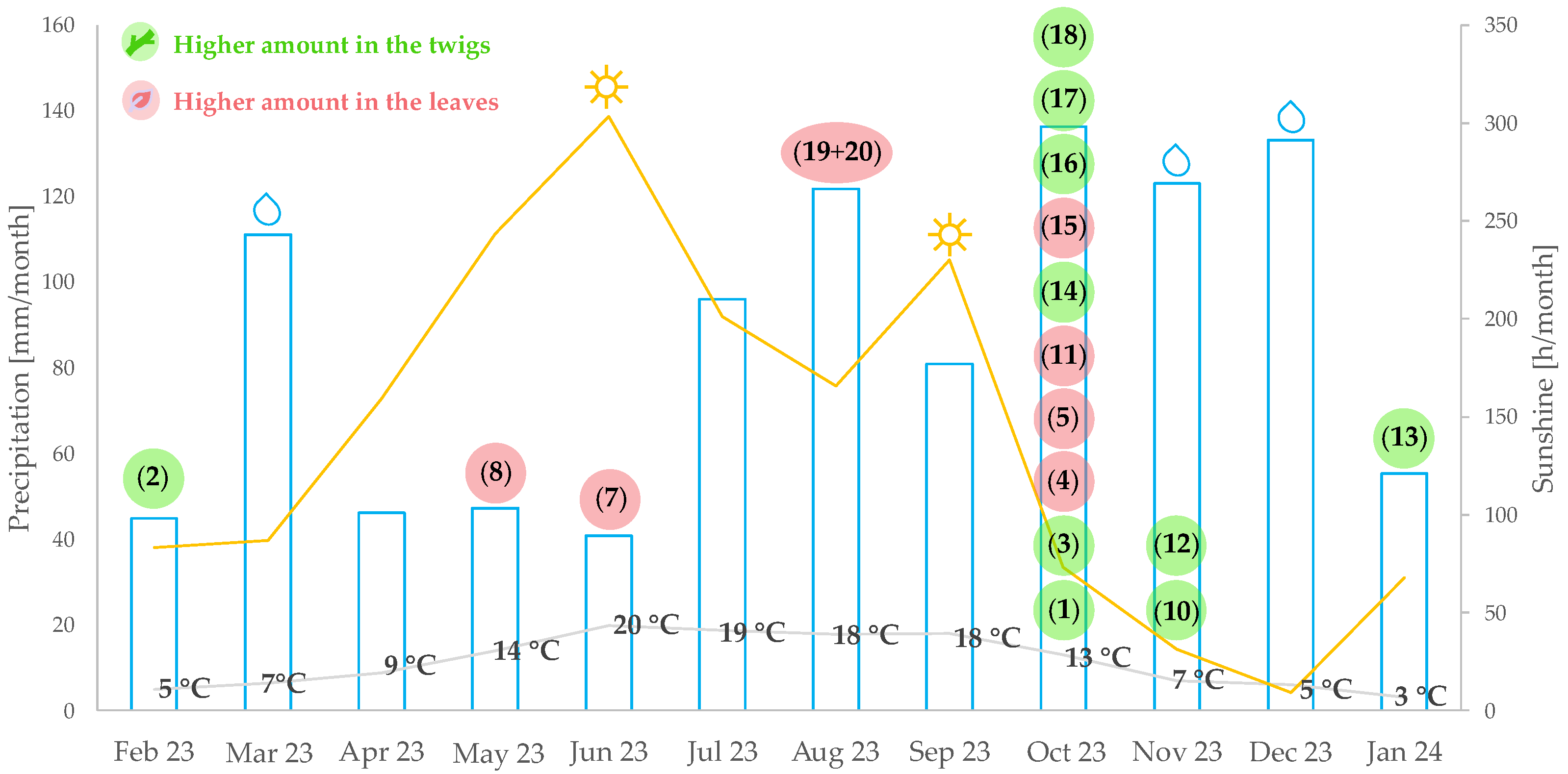
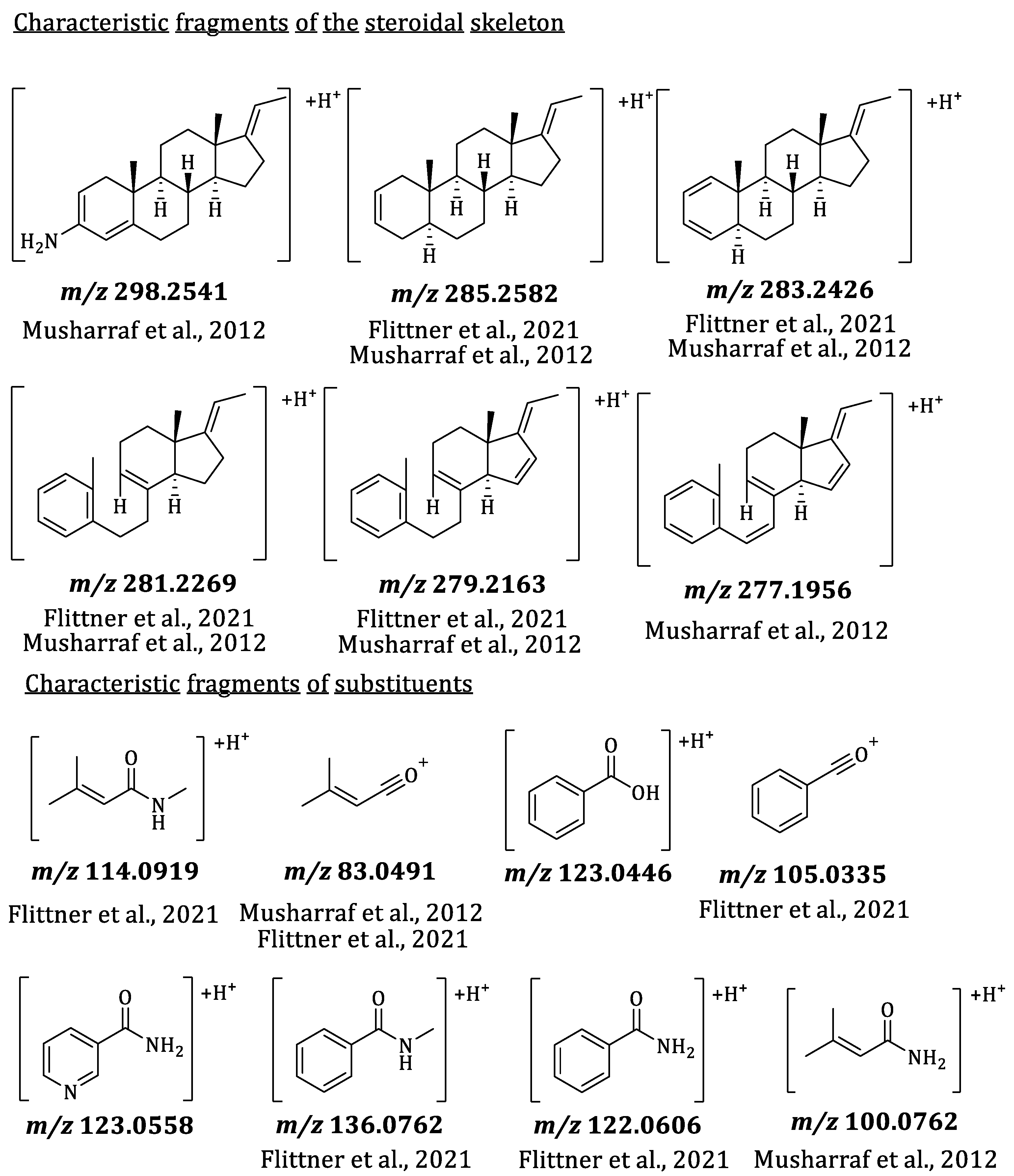

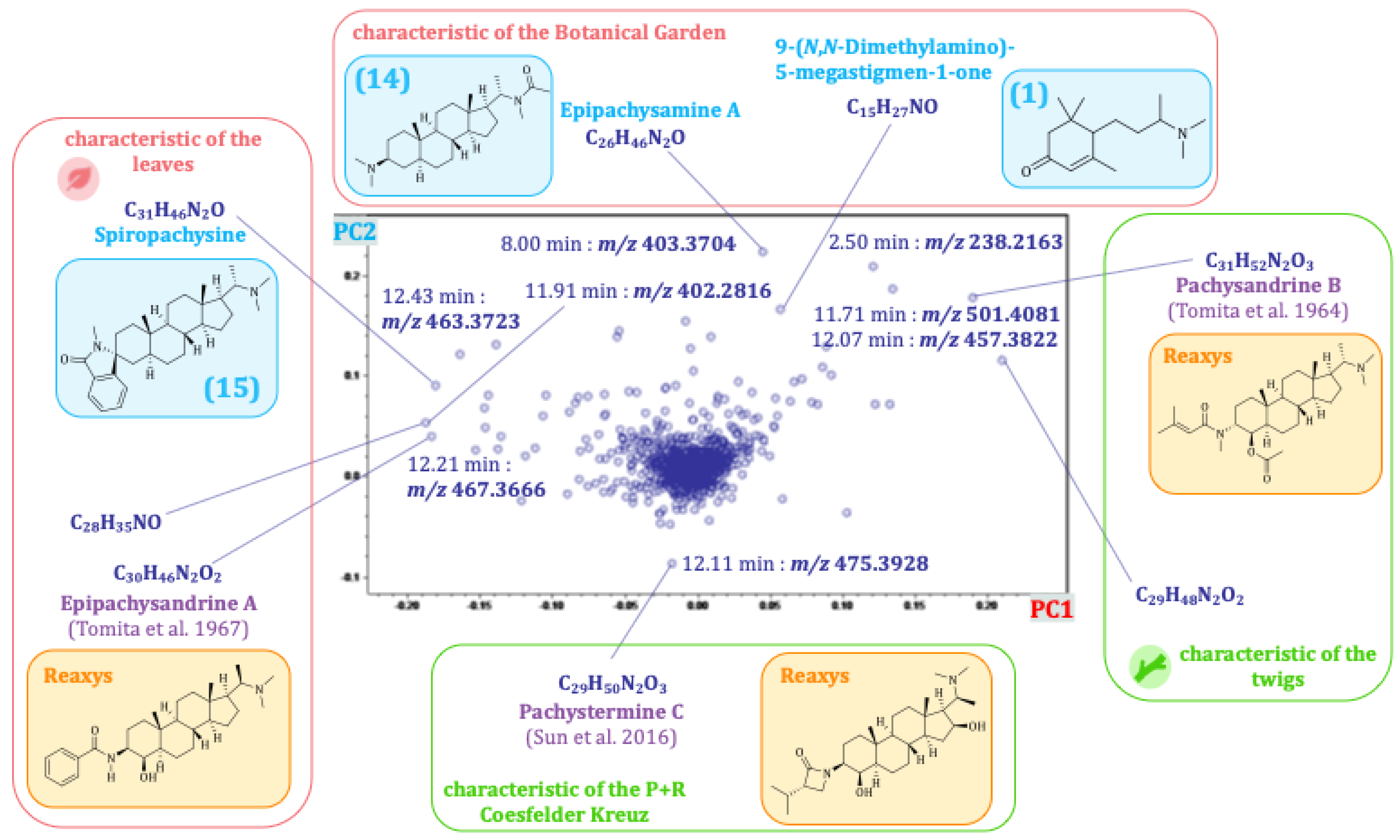
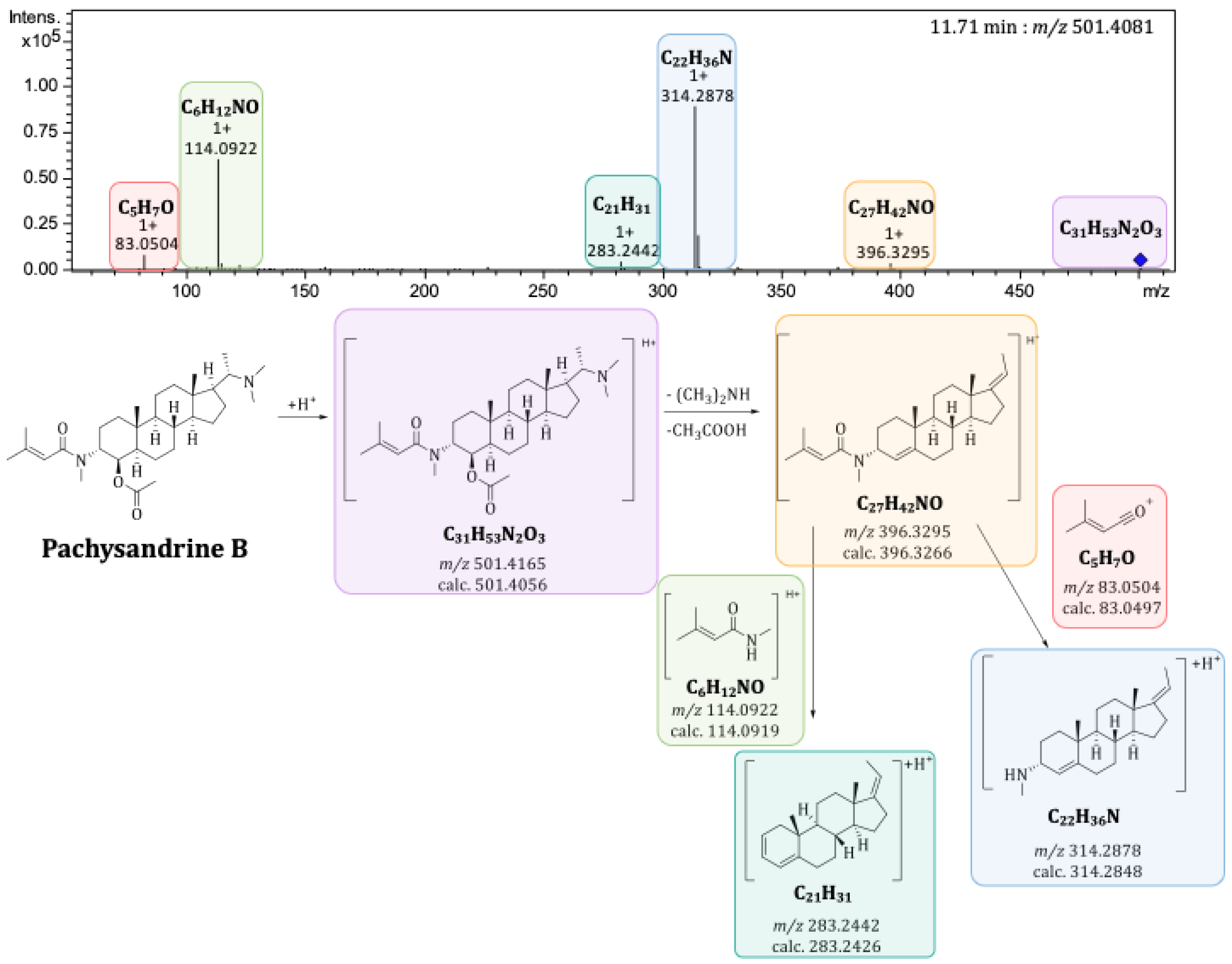



| Alkaloid | Bucket <tR:m/z> | Type of Ion | Optimum Harvesting Period | Location | Organ |
|---|---|---|---|---|---|
| 9-(N,N-Dimethylamino)-5-megastigmen-1-one (1) | 2.50 min:m/z 238.2163 | [M + H]+ | October | A > B | twigs > leaves |
| 5,6-Dehydro-desacyl-epipachysamine A (2) | 2.78 min:m/z 180.1789 | [M + 2H]2+ | January, February, May | A > B | twigs ≈ leaves |
| Desacyl-epipachysamine A (3) | 3.28 min:m/z 181.1848 | [M + 2H]2+ | October, November | A > B | twigs > leaves |
| Epipachysamine B (4) | 6.64 min:m/z 226.6883 | [M + 2H]2+ | October | A > B | A: leaves > twigs B: twigs > leaves |
| Pactermine A (5) | 6.12 min:m/z 225.6803 | [M + 2H]2+ | October | A > B | A: leaves >> twigs B: twigs ≈ leaves |
| 3α,4α-Diapachsanaximine A (7) | 5.24 min:m/z 481.3444 | [M + H]+ | June | A > B | leaves > twigs |
| Pachysamine A (8) | 4.08 min:m/z 181.1847 | [M + 2H]2+ | April, May | A > B | twigs > leaves |
| Pachysandrine D (10) | 5.74 min:m/z 459.3918 | [M + H]+ | October, November | A > B | twigs > leaves |
| Terminaline (11) | 4.32 min:m/z 364.3219 | [M + H]+ | October | A > B | twigs ≈ leaves |
| N-methyl-desacyl-epipachysamine A (12) | 3.21 min:m/z 188.1925 | [M + 2H]2+ | October–December | A > B | twigs >> leaves |
| Sarcodinine (13) | 2.69 min:m/z 187.1832 | [M + 2H]2+ | January–March | A ≈ B | twigs > leaves |
| Epipachysamine A (14) | 8.00 min:m/z 403.3704 | [M + H]+ | October | A > B | twigs > leaves |
| Spiropachysine (15) | 12.43 min:m/z 463.3723 | [M + H]+ | October | A ≈ B | twigs < leaves |
| 4β-Hydroxy-hookerianamide N (16) | 5.67 min:m/z 461.3757 | [M + H]+ | October | A > B | twigs > leaves |
| 5α-Hydroxy-3α,4α-diapachysanaximine A (17) | 5.49 min:m/z 249.1928 | [M + 2H]2+ | October | A > B | twigs > leaves |
| 2β,3β,4β-Diapachysamine K (18) | 6.54 min:m/z 483.3607 | [M + H]+ | October | A > B | twigs > leaves |
| 3β-Dimethylamino-pregnane-20-oxime (19) | 8.98 min:m/z 361.3224 | [M + H]+ | June–August | A > B | twigs ≈ leaves |
| 3β-Dimethylamino-pregn-5,6-ene-20-oxime (20) | 8.05 min:m/z 359.3070 | [M + H]+ | June–August | A > B | twigs ≈ leaves |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, L.; Sendker, J.; Schmidt, T.J. Seasonal, Organ-, and Location-Dependent Variations in the Alkaloid Content of Pachysandra terminalis Investigated by Multivariate Data Analysis of LC-MS Profiles. Plants 2025, 14, 3060. https://doi.org/10.3390/plants14193060
Schäfer L, Sendker J, Schmidt TJ. Seasonal, Organ-, and Location-Dependent Variations in the Alkaloid Content of Pachysandra terminalis Investigated by Multivariate Data Analysis of LC-MS Profiles. Plants. 2025; 14(19):3060. https://doi.org/10.3390/plants14193060
Chicago/Turabian StyleSchäfer, Lizanne, Jandirk Sendker, and Thomas J. Schmidt. 2025. "Seasonal, Organ-, and Location-Dependent Variations in the Alkaloid Content of Pachysandra terminalis Investigated by Multivariate Data Analysis of LC-MS Profiles" Plants 14, no. 19: 3060. https://doi.org/10.3390/plants14193060
APA StyleSchäfer, L., Sendker, J., & Schmidt, T. J. (2025). Seasonal, Organ-, and Location-Dependent Variations in the Alkaloid Content of Pachysandra terminalis Investigated by Multivariate Data Analysis of LC-MS Profiles. Plants, 14(19), 3060. https://doi.org/10.3390/plants14193060








